Current myeloablative conditioning regimens for hematopoietic stem cell (HSC) transplantation are associated with significant morbidity and mortality. Thus, alternative strategies to promote engraftment of infused HSCs with increased safety warrant investigation. Using parabiotic mice, we determined that, after mobilization with AMD3100 (a CXCR4 antagonist), HSCs exited from marrow, transited blood, and engrafted in open niches in partner marrow. We then hypothesized that mobilization before transplantation might vacate niches and improve HSC engraftment. When PeP3b mice were treated with AMD3100 at 2 hours before the transplantation of 4 × 107 marrow cells, donor cell engraftment was higher (4.6% ± 1.1%) than in control animals (no AMD3100; 1.0% ± 0.24%, P < .001). When mice received weekly injections of AMD3100 on 3 consecutive weeks and marrow cells were transplanted 2 hours after each mobilization, donor cell engraftment further increased (9.1% ± 1.7%, P = .001). In contrast, in similar experiments with Balb/cByJ mice that mobilize poorly, there was no difference between the donor cell engraftment of AMD3100-treated and control recipients. These results indicate that the number of available niches regulates the number of HSCs. In addition, mobilization with AMD3100 may provide a safer preparative approach for HSC transplantation in genetic and other nonmalignant disorders.
Introduction
The preparative or conditioning regimen is a critical element in the hematopoietic stem cell (HSC) transplantation procedure. The purpose of the preparative regimen in allogeneic transplantation is both to provide adequate immunosuppression to prevent rejection of the allogeneic graft and to eradicate the disease for which the transplantation is being performed. Conditioning regimens also damage (or destroy) endogenous stem cells and provide a competitive advantage to the infused HSCs.1,2 Several decades ago, Micklem et al3 observed a very small level of engraftment of chromosomally tracked hematopoietic cells in unconditioned mouse recipients. Better engraftment was achieved in the absence of myeloablative conditioning by infusing large numbers of stem cells, proving that engraftment is a competitive process between endogenous and infused stem cells.4-6
Conditioning has traditionally been achieved by delivering maximally tolerated doses of chemotherapeutic agents with nonoverlapping toxicities, with or without radiation. These regimens result in the impairment of host immune function and are associated with significant morbidity and mortality.7 Although myeloablative regimens are justified in stem cell transplantation for malignant disorders, many of the diseases that could be targeted in allogeneic transplantation applications are chronic, indolent disorders in which the risks of myeloablative regimens outweigh the potential benefits. Also, clinically relevant engraftment of genetically modified HSCs in the absence of myeloablation remains a highly desirable goal for the therapy of genetic disorders in which the corrected cells lack a growth advantage.8-13
In this study, we investigated whether HSC mobilization can function as a preparative regimen for HSC transplantation. Mobilizing agents release HSCs from marrow to blood and thus facilitate their collection from blood. Cytokines, such as G-CSF, are the most widely used mobilizing agents. Recently, AMD3100, a specific CXCR4 antagonist, has been shown to be a powerful mobilizer of HSCs in mice,14 dogs (personal oral communication, Rainer Storb, Fred Hutchinson Cancer Research Center, Seattle, WA, January 2005), rhesus macaques (see the accompanying paper by Larochelle et al,33 beginning on page 3772), and humans.14-18
Using a model of hematopoiesis in parabiotic mice,19 we first demonstrated that exposure of each parabiont to one or multiple cycles of AMD3100 or cytokines (G-CSF + stem cell factor [SCF]) results in increased percentages of partner HSCs, CFU-GMs, and granulocytes within the marrow of each parabiont compared with baseline. Our data, therefore, implied that HSCs exited marrow, transited blood, engrafted in open niches in partner marrow, and contributed normally to partner hematopoiesis. We therefore hypothesized that mobilization before HSC transplantation might vacate niches and lead to the improved engraftment of donor HSCs. We tested this hypothesis by using AMD3100 as the preparative regimen before marrow cell transplantation in mice.
Materials and methods
Mice
Heterozygous ROSA26 (C57BL/6J-Gtrosa26.Ly5.2), PeP3b (B6 SJL/.Ly5.1), and C57BL/6J (B6.Ly5.2) mice were purchased from Jackson Laboratories (Bar Harbor, ME) and were bred at the University of Washington under specific pathogen-free conditions. ROSA26 homozygous offspring of heterozygous matings were identified by polymerase chain reaction (PCR) typing of tail-tip gDNA.20 PeP3b mice express the CD45.1 antigen on their hematopoietic cells, whereas hematopoietic cells in ROSA26 mice express CD45.2, phenotypes readily distinguishable by flow cytometry. In addition, all cells in ROSA26 mice express a transgene encoding β-galactosidase that allows ROSA26 CFU-GMs to be distinguished from PeP3b CFU-GMs using β-galactosidase staining of colonies in agar cultures.
Balb/cByJ mice were also purchased from Jackson Laboratories and maintained under specific pathogen-free conditions at the National Institutes of Health.
Parabiosis
Pairs of 6- to 10-week-old, sex- and weight-matched ROSA26 and PeP3b mice were housed together in a single cage for 1 to 2 weeks and then subjected to parabiotic surgery using methods adapted from Bunster and Meyer.19 Mice were anesthetized with 0.020 to 0.026 mL 2.5% tribromoethanol (Avertin; Aldrich, Milwaukee, WI) per gram body weight. Operative sides were shaved and sterilized. Lateral skin was opened from hip to shoulder and freed of attached tissue. Opposing muscle and perineum were sutured with 4.0 chromic gut (Roboz, Rockville, MD), and corresponding skin was joined with 9-mm wound clips (Fisher Scientific, Houston, TX). Parabiotic mice were provided with water bottles with extra-long necks for easy access. Half the wound clips (alternating) were removed after 1 week of parabiosis, and the remaining clips were removed after 2 weeks. Blood (100 μL) was collected from the orbital sinus (after sedation with a 2.5%-4.0% isoflurane inhalant anesthetic; Summit Medical, Portland, OR) to document joint circulation by flow cytometry of granulocytes and lymphocytes. At the conclusion of experiments, parabiotic mice were anesthetized with tribromoethanol and separated through transection at the anastomosis site. Before separation, 0.6 to 1.0 mL blood was obtained by eye puncture. After separation, 2 to 4 × 107 marrow cells were obtained by dissection of femurs, and 3 to 6 × 107 spleen cells were obtained by the passage of minced spleen through nylon mesh.
Cytokine and AMD3100 mobilization
Recombinant human G-CSF and SCF were provided by Amgen (Thousand Oaks, CA). Each day from days 17 to 20, days 24 to 27, and days 31 to 34 of parabiosis, after light sedation with isoflurane inhalant, each parabiont (average weight, 25 g) was give subcutaneous injections with 200 μg/kg recombinant human G-CSF and 25 μg/kg recombinant human SCF. Mice were separated and blood, marrow, and spleen granulocytes, progenitors (CFU-GMs), and HSCs from 3 pairs were analyzed on day 35 (ie, 1 day after last cytokine cycle and 5 weeks after joining) and 3 additional pairs were analyzed on day 42 (ie, 1 week after last cytokine cycle and 6 weeks after joining).
AMD3100 was provided by AnorMED (Langley, BC, Canada). Each mouse received AMD3100 (5 mg/kg) subcutaneously on day 21 of parabiosis. Three pairs of ROSA26/PeP3b mice were studied. Mice were separated and blood, marrow, and spleen granulocytes, progenitors (CFU-GMs), and HSCs were analyzed on day 42 (ie, 3 weeks after AMD3100 and 6 weeks after joining). For analysis of kinetics of cell mixing in blood and marrow, additional pairs were separated at 2 or 4 hours after AMD3100 administration.
Transplantation for determination of the percentage of HSCs of partner phenotype
Before transplantation of 5 to 10 × 106 marrow or blood mononuclear cells or 3 × 107 spleen mononuclear cells, recipient mice received 1100 cGy radiation (from a dual cesium source). Cells from the ROSA26 and PeP3b parabionts were infused into 2 to 3 C57BL/6J and 2 to 3 PeP3b recipients, respectively. For some experiments, blood cells from 2 parabionts were pooled to allow sufficient numbers of HSCs for transplantation into one to 2 irradiated recipients. Three months after transplantation, the percentages of blood and marrow granulocytes of ROSA26 versus PeP3b phenotype were determined by flow cytometry, and the percentage of marrow CFU-GMs of ROSA26 versus PeP3b phenotype was determined by analysis of agar cultures.21 The average of these 3 values was considered the percentage of HSCs of partner phenotype in each secondary recipient. For each parabiont, the percentage of partner HSCs was set equal to the average (± SD) of values from all secondary recipients. Transplanting cells from the ROSA26 parabiont into C57BL/6J recipients allowed us to quantify any recovery of endogenous hematopoiesis in the secondary host and thus served as an internal methodologic control. If inadequate numbers of HSCs were transferred and recipient hematopoiesis recovered, the percentage of PeP3b partner cells, as estimated by the GM colony assay, would have been greater than the value derived by measuring the percentage of granulocytes or marrow cells with a CD45.1 phenotype. The percentages of blood and marrow lymphocytes of ROSA26 versus PeP3b phenotype were also determined by flow cytometry in secondary recipients; and in all studies, HSC engraftment as defined by lymphoid chimerism was equivalent to HSC engraftment as defined by granulocytic chimerism.
Progenitor cell culture
Methods for CFU assays and for quantitating the number of colonies containing β-galactosidase have been described.21
Transplantation studies after mobilization with AMD3100
PeP3b (n = 3 or 6 mice/group) and female Balb/cByJ mice (n = 6 or 7/group) were treated with AMD3100 and 4 × 107 donor marrow cells (from ROSA26 and male Balb/cByJ mice, respectively) were transplanted 2 hours, 6 hours, or both 2 and 6 hours later without prior irradiation. Control animals received donor cells but no AMD3100. Donor cell engraftment was assayed at 3 months using flow cytometry (PeP3b mice) or quantitative real-time PCR using the male-specific sex-determining region Y chromosome (Sry) gene (Balb/cByJ mice).
Flow cytometry
In parabiotic mouse studies and transplantation studies, percentages of partner and transplanted cells in PeP3b and ROSA26 mice were determined by staining with partner or graft-specific antibody (FITC anti-mouse CD45.1 or CD45.2) coupled with antibodies to Gr-1 (granulocytes, phycoerythrin [PE] labeled) and CD3 (T lymphocytes, Cy-chrome labeled; PharMingen, San Diego, CA). Gates were set so that less than 0.5% of cells from control mice were considered positive.
Real-time PCR for SRY gene
To determine the percentages of cells in female Balb/cByJ mice given transplants with marrow from male Balb/cByJ mice, 300 to 500 μL peripheral blood was collected from each mouse by retro-orbital bleeding, suspended in ACK lysing buffer (BioWhittaker, Walkersville, MD) to lyse red blood cells and washed with PBS. Male-specific SRY gene real-time PCR was performed on DNA extracted from these cells using an ABI Prism 7700 Sequence Detector (Applied Biosystems, Foster City, CA). The primers used were specific for the mouse SRY gene: SRY forward primer: 5′-GTACAACCTTCTGCAGTGGGACAGG-3′; SRY reverse primer: 5′-GCTGGTTTTTGGAGTACAGGTGTGC-3′; SRY probe: 5′-/FAM/CCATCACATACAGGCAAGACTGGAGTAGAGC/TAMRA/-3′. Real-time PCR results were normalized to the amounts of mouse β2-microglobulin DNA: β2-microglobulin forward primer: 5′-CTTCAGCAAGGACTGGTCTTTC-3′; β2-microglobulin reverse primer: 5′-CGGCCATACTGTCATGCTTAAC-3′; β2-microglobulin probe: 5′-/FAM/TGAATTCACCCCCACTGAGACTG/TAMRA/-3′.
Statistical analysis
Results are presented as mean plus or minus standard deviation (SD). Statistical analysis was performed using the Student t test.
Results
Cytokine and AMD3100 mobilization induces exit of HSCs from marrow microenvironmental niches, their transit through blood, and their re-engraftment in open sites
Experiments were performed to study the effect of cytokine mobilization on HSC trafficking. For these experiments, PeP3b and ROSA26 mice were joined in parabiosis. These mice are congenic on a C57BL/6J background but express distinct hematopoietic cell antigens (CD45.1 and CD45.2, respectively) readily distinguishable by flow cytometry. Blood was obtained at day 14 and Gr-1+ granulocytes were phenotyped to ensure that the circulations were shared. The mean percentage of partner granulocytes was 46.2% ± 8.0% (n = 24), demonstrating full potency of this model for cell trafficking. In a previous study, each parabiont was treated with one cycle of cytokine combination G-CSF (200 μg/kg subcutaneously daily) and SCF (25 μg/kg subcutaneously daily) on day 17 to 20 of parabiosis.22 In the current set of experiments, each mouse received 3 cycles of G-CSF and SCF subcutaneously on days 17 to 20, days 24 to 27, and days 31 to 34 of parabiosis. Six pairs of ROSA26/PeP3b mice were studied. Blood, marrow, and spleen granulocytes, progenitors (CFU-GMs), and HSCs from 3 pairs were analyzed at 5 weeks of parabiosis (ie, 1 day after last cytokine cycle). Three additional pairs were analyzed at 6 weeks of parabiosis (ie, 1 week after last cytokine cycle) to allow migrating HSCs to re-engraft and contribute to hematopoiesis at their adopted site.
As shown in Table 1, the percentage of partner HSCs in marrow, as determined by transplantation of marrow cells into irradiated secondary recipients, increased significantly from baseline values of 1.0% ± 0.8% to 1.4% ± 0.4% (without mobilization22 ) to 18.1% ± 10.6% (P = .003) at 5 weeks of parabiosis. The percentage of partner marrow HSCs remained significantly increased at 13.4% ± 9.9% (P = .03) at 6 weeks of parabiosis. In comparison, 10.1% ± 6.2% marrow HSCs were of partner origin after one cycle of mobilization at 6 weeks of parabiosis.22 For this significant increase in partner HSCs to occur after one or 3 cycles of mobilization, there must be empty niches in the marrow, that is, HSCs must vacate microenvironmental niches in the marrow, transit through blood, and engraft in open niches in partner marrow. Also, because the percentage of granulocytes (16.4% ± 4.1% and 18.7% ± 9.1%) and CFU-GMs (17.6% ± 4.7% and 20.7% ± 9.7%) of partner origin in each parabiont marrow was similar to the percentage of engrafted HSCs (18.1% ± 10.6% and 13.4% ± 9.9%) at 5 and 6 weeks of parabiosis, respectively (Table 1), HSCs differentiated and contributed normally to hematopoiesis at their adopted site.
Comparative studies were performed with AMD3100. For these experiments, each mouse received AMD3100 (5 mg/kg) subcutaneously on day 21 of parabiosis. Three pairs of ROSA26/PeP3b mice were studied. Blood, marrow, and spleen granulocytes, progenitors (CFU-GMs), and HSCs were analyzed at 6 weeks of parabiosis (ie, 3 weeks after AMD3100). As shown in Table 1, the percentage of partner HSCs in marrow increased significantly from the baseline value of 1.4% ± 0.4% to 5.5% ± 2.3% (P = .05). Similar percentages of marrow CFU-GMs (5.7% ± 2.3%) and marrow granulocytes (5.6% ± 2.8%) were also of partner origin (Table 1), implying mobilized HSCs had returned to marrow and a new steady state had been achieved by 3 weeks after AMD3100 exposure.
To demonstrate that HSC mobilization, and not proliferation of HSCs, was responsible for the results obtained after mobilization with AMD3100, 5 additional pairs of ROSA26/PeP3b mice were joined in parabiosis. At 3 weeks of parabiosis, each parabiont was treated with one cycle of ADM3100 and killed 2 hours (3 pairs) or 4 hours (2 pairs) after AMD3100 administration. Mixing of HSCs in the blood of each parabiont was not complete at 2 hours (only 7.5% ± 1.9% HSCs were of partner origin), but complete mixing of HSCs was seen in blood as early as 4 hours after administration of ADM3100 (52.8% ± 29.7% HSCs were of partner origin). No significant changes in partner marrow HSCs were detected at these early time points after mobilization (1.9% ± 1.0% and 1.6% ± 1.0% at 2 and 4 hours, respectively). These data clearly demonstrate mobilization of HSCs to blood in a time frame (4 hours) where stem cell proliferation is unlikely.
To calculate the percentages of HSCs that were exchanged, as presented in Table 1, data from ROSA26 and PeP3b parabiont were averaged. Theoretically, mobilized marrow HSCs should re-engraft equivalently in partner marrow as self marrow. This was observed in our parabiotic mouse studies22 and after exposure to AMD3100 (Table 2). However, trafficking of HSCs was not symmetric after G-CSF and SCF exposure, and especially after repeated cytokine cycles, because significantly more HSCs exited from ROSA26 marrow and engrafted in the PeP3b parabiont than migrated in the opposite direction (Table 2). As an example, 1 week after the third cycle of cytokine mobilization (week 6 of parabiosis), only 5.0% ± 0.9% of blood HSCs were of partner phenotype in the ROSA26 parabiont, but 37.8% ± 1.7% blood HSCs were of partner phenotype in the PeP3b parabiont (P = .002).
HSC trafficking in spleen after mobilization with cytokines and AMD3100
Studies of HSC trafficking to the spleen after AMD3100 mobilization showed that 6.8% ± 2.3% of splenic HSCs had a partner phenotype 3 weeks after AMD3100 (Table 3). In contrast, 40.0% ± 6.1% of splenic granulocytes, and 44.0% ± 3.2% of splenic CFU-GMs were of partner origin, values comparable to those of granulocytes (46.9% ± 9.3%) and CFU-GMs (46.0% ± 5.1%) in blood, but dissimilar to those of granulocytes (5.6% ± 2.8%) and CFU-GMs (5.7% ± 2.3%) in marrow. Similar results were obtained after one or 3 cycles of mobilization with G-CSF plus SCF. These data indicate that splenic granulocytes do not derive exclusively from HSCs resident in spleen but derive primarily from progenitors or directly traffic from blood. Therefore, the spleen appears conceptually as a sieve in continual equilibrium with blood, not an intact compartment where progenitors and granulocytes differentiate from HSCs resident in the spleen.
Donor cell engraftment is higher in PeP3b mice conditioned with AMD3100 compared with control (no AMD3100) mice
The data in parabiotic mice suggest that because microenvironmental niches are vacated after cytokines or AMD3100 exposure, donor HSCs might engraft better in hosts treated with a mobilizing agent compared with nonmobilized controls. Although our studies in parabiotic mice indicate that cytokines (G-CSF and SCF) induced superior trafficking of HSCs compared with AMD3100, we chose AMD3100, not cytokines, for these studies because mobilization with cytokines may stimulate proliferation of HSCs and their progeny,23 impede the engraftment of transplanted cells, and complicate our analysis. The studies in parabiotic mice indicated complete mixing of HSCs in blood as early as 4 hours after administration of ADM3100. These data, combined with the known rapid mobilization kinetics (1-2 hours)14 and short half-life (0.9 hours)24 of this agent, demonstrate that AMD3100 is unlikely to result in HSC proliferation.
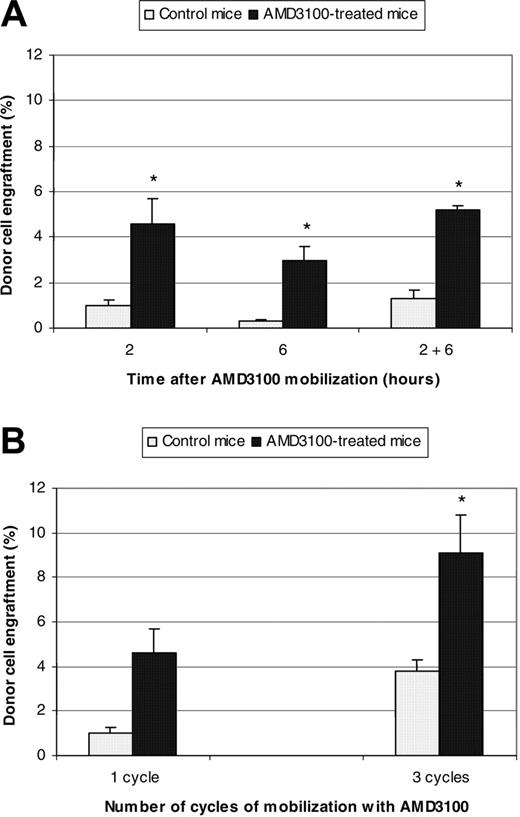
Therefore, PeP3b mice were treated with AMD3100, and 4 × 107 donor marrow cells (from ROSA26 mice) were transplanted 2 hours (n = 6 mice), 6 hours (n = 3 mice), or both 2 and 6 hours later (n = 3 mice). Control animals (n = 3 mice/condition) received donor cells but no AMD3100. Donor cell engraftment was assayed at 3 months and was significantly higher in the experimental animals than controls (Figure 1A). Engraftment in AMD3100-treated mice receiving transplants 2 hours, 6 hours, or both 2 and 6 hours later was 4.6% ± 1.1% (P < .001), 3.0% ± 0.6% (P = .02), and 5.2% ± 0.2% (P = .02), respectively; engraftment was 1.0% ± 0.2%, 0.3% ± 0.1%, and 1.3% ± 0.4% in the concurrent control studies.
The increased percentage of partner marrow HSCs observed in parabiotic experiments after 3 cycles of mobilization (13.4% ± 9.9%) compared with one cycle (10.1% ± 6.2%) indicated that additional microenvironmental niches become available with repeated mobilization cycles. Therefore, we investigated whether repeated cycles of AMD3100 mobilization, each followed by donor cell transplantation, could result in increased engraftment. PeP3b mice (n = 3 mice) received one weekly injection of AMD3100 (5 mg/kg) on 3 consecutive weeks and 4 × 107 donor marrow cells (from ROSA26 mice) were transplanted 2 hours after each mobilization. Control animals (n = 3 mice) also received 3 weekly infusions of 4 × 107 donor marrow cells but no AMD3100. Donor cell engraftment was assayed at 3 months after transplantation and was significantly higher in the experimental animals than controls (Figure 1B). Engraftment in AMD3100-treated mice was 9.1% ± 1.7% compared with 3.8% ± 0.5% in controls (P = .006). Multiple cycles of AMD3100 mobilization also resulted in a statistically significant increase in engraftment (9.1% ± 1.7%) compared with one cycle (4.6% ± 1.1%; P = .001).
Donor cell engraftment in AMD3100-conditioned Balb/cByJ mice, where mobilization is poor compared with PeP3b mice, is not statistically different compared with control, no AMD3100, mice
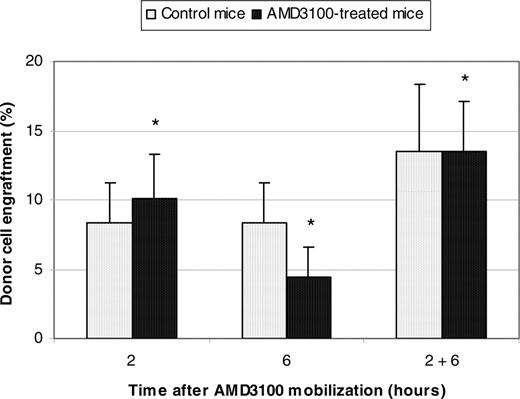
To exclude the possibility of strain-specific results, experiments were repeated with Balb/cByJ mice where higher engraftment levels were anticipated in control animals.25 Female Balb/cByJ mice were treated with AMD3100 and donor marrow cells from male Balb/cByJ mice were transplanted 2 hours, 6 hours, or both 2 and 6 hours later. Control animals received donor cells but no AMD3100. Donor cell engraftment was assayed 3 months after transplantation by quantitative real-time PCR using the male-specific SRY gene and was not statistically different compared with control animals. Engraftment in AMD3100-treated mice given transplants 2 hours, 6 hours, or 2 and 6 hours later was 10.1% ± 3.2%, 4.4% ± 2.2%, and 13.5% ± 3.6%, respectively; engraftment was 8.3% ± 2.9% (P = .42), 8.3% ± 2.9% (P = .11), and 13.5% ± 4.9% (P = .34) in the concurrent control studies (Figure 2).
Summary donor cell engraftment in PeP3b mice conditioned with AMD3100. (A) PeP3b mice were treated with one cycle of AMD3100 (5 mg/kg) and 4 × 107 donor marrow cells (from ROSA26 mice) were transplanted 2 hours (n = 6 mice), 6 hours (n = 3 mice), or both 2 and 6 hours later (n = 3 mice). Control animals (n = 3 mice/condition) also received 4 × 107 donor marrow cells but no AMD3100. Donor cell engraftment was assayed at 3 months. *P values for donor cell engraftment of AMD3100-treated mice compared with control mice at 2 hours, 6 hours, and 2 and 6 hours were < .001, .02, and .02, respectively. (B) PeP3b mice (n = 3 mice) received one weekly injection of AMD3100 (5 mg/kg) on 3 consecutive weeks and 4 × 107 donor marrow cells (from ROSA26 mice) were transplanted 2 hours after each mobilization. Control animals (n = 3 mice) also received 3 weekly infusions of 4 × 107 donor marrow cells but no AMD3100. Donor cell engraftment was assayed at 3 months. *P values for donor cell engraftment of mice treated with 3 cycles of AMD3100 compared with control mice and mice treated with only one cycle of AMD3100 were .006 and .001, respectively.
Summary donor cell engraftment in PeP3b mice conditioned with AMD3100. (A) PeP3b mice were treated with one cycle of AMD3100 (5 mg/kg) and 4 × 107 donor marrow cells (from ROSA26 mice) were transplanted 2 hours (n = 6 mice), 6 hours (n = 3 mice), or both 2 and 6 hours later (n = 3 mice). Control animals (n = 3 mice/condition) also received 4 × 107 donor marrow cells but no AMD3100. Donor cell engraftment was assayed at 3 months. *P values for donor cell engraftment of AMD3100-treated mice compared with control mice at 2 hours, 6 hours, and 2 and 6 hours were < .001, .02, and .02, respectively. (B) PeP3b mice (n = 3 mice) received one weekly injection of AMD3100 (5 mg/kg) on 3 consecutive weeks and 4 × 107 donor marrow cells (from ROSA26 mice) were transplanted 2 hours after each mobilization. Control animals (n = 3 mice) also received 3 weekly infusions of 4 × 107 donor marrow cells but no AMD3100. Donor cell engraftment was assayed at 3 months. *P values for donor cell engraftment of mice treated with 3 cycles of AMD3100 compared with control mice and mice treated with only one cycle of AMD3100 were .006 and .001, respectively.
We then hypothesized that the difference in donor cell engraftment between AMD3100-treated PeP3b and Balb/cByJ mice might relate to a difference in their mobilization potential with AMD3100. To address this question, peripheral blood from 4 mice of each strain was collected at baseline (time = 0 hour) and 2 hours after mobilization with a single dose of AMD3100, and cells were plated in progenitor (CFU) assays. Overall, the increase in circulating levels of myeloid and erythroid progenitor cells compared with baseline (time = 0 hour) was significantly higher in PeP3b (28.2 ± 5.3) compared with Balb/cByJ (9.9 ± 1.8) mice (P = .03; Table 4). These results imply that the inefficient mobilization of Balb/cByJ compared with PeP3b mice resulted in a lower increment in available niches after mobilization and, consequently, lower HSC engraftment compared with PeP3b mice.
Summary donor cell engraftment in female Balb/cByJ mice conditioned with AMD3100. Female Balb/cByJ mice were treated with AMD3100 and 4 × 107 donor marrow cells from male Balb/cByJ mice were transplanted 2 hours, 6 hours, or both 2 and 6 hours later. Control animals also received 4 × 107 donor marrow cells but no AMD3100. Donor cell engraftment was assayed 3 months after transplantation by quantitative real-time PCR using the male-specific SRY gene. *P values for donor cell engraftment of AMD3100-treated mice compared with control mice at 2 hours, 6 hours, and 2 and 6 hours were .42, .11, and .34, respectively.
Summary donor cell engraftment in female Balb/cByJ mice conditioned with AMD3100. Female Balb/cByJ mice were treated with AMD3100 and 4 × 107 donor marrow cells from male Balb/cByJ mice were transplanted 2 hours, 6 hours, or both 2 and 6 hours later. Control animals also received 4 × 107 donor marrow cells but no AMD3100. Donor cell engraftment was assayed 3 months after transplantation by quantitative real-time PCR using the male-specific SRY gene. *P values for donor cell engraftment of AMD3100-treated mice compared with control mice at 2 hours, 6 hours, and 2 and 6 hours were .42, .11, and .34, respectively.
Discussion
Cytokines (eg, G-CSF) and AMD3100, a CXCR4 antagonist, are used for the recruitment of HSCs from bone marrow to peripheral blood. Cytokine-mobilized peripheral blood stem cells have replaced bone marrow as the preferred source of HSCs for transplantation in patients receiving high-dose chemotherapy because of their ease of collection and their rapid engraftment following transplantation, resulting in less morbidity and mortality.26 Our studies using the parabiotic mouse model provide new insights into the physiologic fate of mobilized HSCs.
One day and one week after mobilization with 3 cycles of G-CSF and SCF, 18.1% ± 10.6% and 13.4% ± 9.9% of marrow HSCs were of partner origin, respectively (Table 1). These results imply that HSCs exited the marrow, transited the blood, and entered the marrow of the opposite parabiont. Because similar percentages of marrow CFU-GMs and granulocytes were also of partner origin, the HSCs that circulated and re-engrafted contributed to hematopoiesis equivalently to endogenous (nontrafficking) HSCs. The percentage of partner marrow HSCs after 3 cycles of cytokines was higher than the percentage after only one cycle,22 indicating that the exit and return of marrow HSCs can be increased with additional cycles of mobilization.
A statistically significant increase in partner marrow HSCs was also obtained after administration of AMD3100 (Table 1). A stable reconstitution of marrow with 5.5% ± 2.3% partner cells 3 weeks after AMD3100 administration was noted, which implies that 11% of marrow HSCs mobilized after a single dose of AMD3100, that is, on average 5.5% HSCs returned to host and 5.5% HSCs returned to partner marrow. The apparent superiority of the mobilizing potential of G-CSF plus SCF (10.1% ± 6.2% of HSCs were exchanged after one cycle [4 days] of G-SCF plus SCF22 compared with 5.5% ± 2.3% after AMD3100 administration) could be due to cytokine-induced proliferation23 of an equivalent number of HSCs mobilized with G-CSF plus SCF and AMD3100. In contrast, proliferation of HSCs with AMD3100 is unlikely; our studies in parabiotic mice demonstrated AMD3100 mobilization of HSCs to blood in a time frame (4 hours) where stem cell proliferation is unlikely, given the known short half-life of this agent (0.9 hours).24 Alternatively, the cytokine combination G-CSF plus SCF may open more niches in this mouse model and be a better mobilizer than AMD3100 at the doses used in this study.
Trafficking of HSCs was not symmetric after cytokine exposure; the percentage of ROSA26-derived HSCs in Pep3b parabiont was significantly higher than the percentage of HSCs that migrated in the opposite direction (Table 2). These data suggest that ROSA26-origin HSCs may preferentially survive in the circulation after cytokine mobilization, and thus out-compete PeP3b-origin cells for re-engraftment. This could reflect functional contributions of the CD45 antigen27 or minor histocompatibility differences between PeP3b and ROSA26 mice leading to immunologic rejection of PeP3b-origin HSCs.28 This difference in engraftment suggests that PeP3b and ROSA26 mice are not functionally congenic despite the extensive backcrossing onto a C57BL/6J background and argues that careful controls are required before attributing a mobilization/homing result to a specific intervention.
Our previous studies of HSC trafficking in nonmobilized parabiotic mice indicated that, although half the granulocytes and CFU-GMs in peripheral blood were from each partner, the percentages of marrow granulocytes and CFU-GMs of partner origin at the time of separation of the parabiotic pairs were low and similar to those of marrow HSCs.22 These results demonstrated that marrow, despite its geographic disparity, functioned as an intact compartment in which granulocytes and progenitors directly derive from marrow HSCs, and not from the engraftment of progenitors and granulocytes circulating in blood.22 In this study, mobilization experiments with 3 cycles of G-CSF plus SCF or one cycle of AMD3100 confirm these marrow compartment dynamics (Table 3). The mobilization experiments in this study also confirm that the spleen, in contrast to marrow, is not an intact compartment (Table 3). Instead, because the percentages of partner progenitors and granulocytes in spleen are equivalent to those in the blood with and without mobilization, the spleen functions as a sieve in constant equilibrium with the blood.22
Extrapolation from murine studies3 suggests that, in the absence of conditioning, the number of cells required to achieve significant engraftment levels in humans is prohibitively large. Thus, safe strategies that promote the engraftment of infused HSCs warrant investigation.
The results of our studies in parabiotic mice argued that mobilization might facilitate engraftment. We chose AMD3100, not cytokines, as the preparative agent because AMD3100-induced HSC replication is less likely, thus preserving the competitive dynamic of engraftment. When PeP3b mice were treated with AMD3100 and donor marrow cells from ROSA26 mice were transplanted 2 hours later, donor cell engraftment was significantly higher in the experimental animals (4.6% ± 1.1%) than controls (1.0% ± 0.24%) (Figure 1A). We mathematically confirmed that this observed level of donor cell engraftment is what was expected after AMD3100 mobilization. From our parabiotic mouse studies, we estimate that 11% of HSCs mobilized after a single dose of AMD3100, consequently opening 11% of microenvironmental niches for engraftment. We estimate there are 11 000 to 22 000 HSCs in mice.29 Hence, after a single dose of AMD3100, PeP3b mice mobilized approximately 1200 to 2400 HSCs. Because PeP3b mice were infused with 4 × 107 donor marrow cells (with 4-8 HSCs/105 marrow cells30,31 ), 1600 to 3200 infused donor HSCs competed with an equivalent dose (1200-2400) of endogenous mobilized HSCs for the 11% of marrow niches open for engraftment. Therefore, approximately half of the available niches would be expected to be occupied by each population of HSCs (ie, 5.5%), correlating well with the observed donor cell engraftment of 4.6% ± 1.1%. Similar experiments in Balb/cByJ mice (Figure 2), where mobilization with AMD3100 is poor compared with PeP3b mice (Table 4), did not show a statistically significant difference compared with the control groups, as anticipated.
When Pep3b mice were treated with 3 weekly injections of AMD3100 each followed by donor cell transplantation, increased engraftment was observed (9.1% ± 1.7%) compared with mice subjected to only one cycle of mobilization and transplantation (4.6% ± 1.1%) (Figure 1B). These results indicate that additional endogenous HSCs mobilize with repeated administrations of AMD3100 and donor cell infusions, opening new niches and resulting in increased engraftment. The minimal toxicity following one or multiple administrations of AMD3100, including mild gastrointestinal side effects and erythema at the injection site, observed in over 100 patients with multiple myeloma or non-Hodgkin lymphoma,15,16 40 patients with HIV,32 and 60 healthy volunteers,18 indicates that repeated cycles of mobilization and cell infusion are clinically feasible.
Overall, our data argue that the number of available niches determines the number of HSCs that engraft. Equally important, mobilization with AMD3100 could provide a nontoxic preparative approach, alone or in combination with reduced-intensity conditioning, to improve HSC engraftment in transplantation for genetic and other nonmalignant disorders.
Prepublished online as Blood First Edition Paper, January 26, 2006; DOI 10.1182/blood-2005-09-3593.
Supported by grant RO1 HL46598 from the National Institutes of Health (J.L.A.).
S.F. and G.B. are employed by a company, AnorMED Inc, whose product, AMD3100, was studied in the present work.
J.C. designed and performed experiments related to Figure 1 and Tables 1, 2, 3 and analyzed data; A.L. designed and performed experiments related to Figure 2 and Table 4, analyzed data, and wrote the manuscript; S.F. supplied AMD3100 and edited the manuscript; G.B. supplied AMD3100 and edited the manuscript; C.E.D. designed and supported research, provided oversight, and edited the manuscript; and J.L.A. designed and supported research, provided oversight, and edited the manuscript.
J.C. and A.L. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to the veterinary support staff at the University of Washington and the National Heart, Lung, and Blood Institute animal facilities for excellent mouse care.