Abstract
We evaluated the relative contribution of the humoral and cellular arms of the immune response to bone marrow cells transplanted into sensitized recipients. We report here for the first time that humoral immunity contributes predominantly to allosensitization. Although the major role for nonmyeloablative conditioning is to control alloreactive host T cells in nonsensitized recipients, strikingly, none of the strategies directed primarily at T-cell alloreactivity enhanced engraftment in sensitized mice. In evaluating the mechanism behind this barrier, we found that humoral immunity plays a critical role in the rejection of allogeneic marrow in sensitized recipients. Adoptive transfer of as little as 25 μL serum from sensitized mice abrogated engraftment in secondary naive recipients. With the use of μMT mice as recipients, we found that T-cell-mediated immunity plays a secondary but still significant role in allorejection. Targeting of T cells in sensitized B-cell-deficient μMT mice enhanced alloengraftment. Moreover, both T- and B-cell tolerance were achieved in sensitized recipients when allochimerism was established, as evidenced by the acceptance of second donor skin grafts and loss of circulating donor-specific Abs. These findings have important implications for the management of sensitized transplant recipients and for xenotransplantation in which B-cell reactivity is a predominant barrier.
Introduction
Sensitization to MHC antigens because of transfusion, pregnancy, previous failed grafts, and ventricular assist devices is among the most critical challenges to clinical transplantation.1 Sensitization increases the risk for bone marrow and solid organ graft rejection and sometimes causes patients to be excluded as candidates for transplantation. Mixed chimerism has been suggested as an approach to induce donor-specific tolerance in sensitized recipients2,3 and may even allow the prolonged acceptance of xenografts.4 A better understanding of the role that innate and adaptive immune responses play in allosensitization will allow a mechanistically driven approach to establish chimerism in sensitized recipients.
We previously demonstrated that 700 cGy total body irradiation (TBI) is sufficient to achieve mixed chimerism in 100% of nonsensitized, MHC-disparate allogeneic mouse recipients.5 The addition of cyclophosphamide (CyP) 2 days after bone marrow infusion reduces the TBI requirement to 500 cGy.6 Pretreatment with anti-CD8 and anti-αβ-T-cell receptor (TCR) mAbs decreased the TBI dose to as low as 300 cGy,7 and the addition of CyP to this mAb preconditioning allowed the TBI to be decreased to as low as 50 cGy TBI (H.X., unpublished data, August 2001). Taken together, these findings suggest that T-cell-mediated cellular immunity is the primary barrier for bone marrow allorejection in nonsensitized recipients.
Recently, mixed allogeneic chimerism was demonstrated to reverse sensitization in allosensitized recipients.8,9 Previously sensitized recipients rendered chimeric did not produce antidonor antibody and accepted donor-specific skin grafts, confirming reversal of the antigen-familiar state. However, ablative conditioning and significantly higher numbers of allogeneic cells were required to induce chimerism in sensitized mice compared with nonsensitized recipients.8 In the present studies, we have defined the hierarchical contribution of components of the innate and adaptive arms of the immune response to sensitization. We found that, in sensitized mice, it is almost impossible to achieve allogeneic chimerism with nonmyeloablative conditioning strategies targeting T cells and NK-cell activity. Our present findings demonstrate that the humoral arm of the immune response plays a previously unappreciated and dominant role in the rejection of allogeneic marrow in sensitized recipients. Passive transfer of as little as 25 μL sensitized serum to naive secondary recipients resulted in bone marrow graft failure. We found that, with B-cell-deficient μMT mice as recipients, T-cell-mediated cellular immunity also plays a significant but less formidable role in allorejection in sensitized recipients. Targeting T cells in sensitized μMT mice reduced the requirement for TBI and higher bone marrow cell (BMC) doses to achieve alloengraftment, but not to levels comparable to those of nonsensitized controls. Our findings characterize for the first time the critical effector cells that contribute to allosensitization. A better understanding of the immune mechanisms that contribute to allogeneic sensitization is important for the development of mechanistically based therapeutic approaches for the conditioning of sensitized patients for transplantation and reversing the sensitized state.
Materials and methods
Animals
Male C57BL/10SnJ (B10, H-2b), B10.BR/SgSnJ (B10.BR, H-2k), C57BL/6 (B6, H-2b), BALB/cJ (BALB/c, H-2d), and B-cell-deficient (C57BL/6-129S2-Igh-6tm1Cgn [μMT, H-2b]) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Animals were housed in the barrier facility at the Institute for Cellular Therapeutics and were cared for according to National Institutes of Health guidelines.
Skin grafting
Flow cross-match assay
Anti-donor Abs were measured by flow cross-match (FCXM) assay. Splenocytes (0.5 × 106) or BMCs from naive B10.BR or BALB/c mice were incubated with 5 μL sera for 30 minutes. Cells were washed and incubated with fluorescein isothiocyanate (FITC)-conjugated polyclonal goat antimouse immunoglobulin and the goat anti-mouse isotype Abs of IgM, IgG, IgG1, IgG2a, and IgG2b (Immunology Consultants Laboratory, Newberg, OR), followed by incubation with phycoerythrin (PE)-conjugated anti-mouse CD4 plus CD8. Levels of circulating alloantibodies were determined by FACSCalibur (BD Biosciences, Mountain View, CA) gating on the CD4+ and CD8+ T-cell fractions and were reported as mean fluorescence intensity (MFI). For hematopoietic stem-cell (HSC) staining, c-Kit PE Cy5 (Biosciences, San Diego, CA), Sca-1-PE, and allophycocyanin (APC)-labeled lineage markers (B220, αβ-TCR, γδ-TCR, Gr-1, Mac-1) were used. Unless indicated, all mAbs were purchased from PharMingen (San Diego, CA).
Chimera preparation
Recipient mice were preconditioned with mAbs of anti-αβ-TCR (H57-597), anti-γδ-TCR (UC7-13D5), anti-CD4 (GK1.5), anti-CD8 (53-6.7), anti-class I (28-8-6S), anti-class II (25-9-175II), anti-CD45RB (MB23G2), anti-NK1.1 (PK136), or anti-CD154 (MR1); cytotoxic or immunosuppressive drugs of CyP (Sigma, St Louis, MO), fludarabine (Fluda; Berlex, Seattle, WA), rapamycin (Rapa; Wyeth Laboratories, Philadelphia, PA), mycophenolate mofetil (MMF; Roche, Nutley, NJ), or cyclosporin A (CyA; Novartis, East Hanover, NJ); cobra venom factor (CVF; Quidel, San Diego, CA); or TBI (γ-cell 40; Nordion, Ontario, Canada) alone or in different combinations. All mAbs used in vivo, except anti-CD154 (Bioexpress, Lebanon, NH), were produced in our laboratory. Chimeras were prepared using 15 × 106 to 80 × 106 untreated donor BMCs injected by lateral tail vein injection 4 to 6 hours after irradiation, as previously described.5,7
One-way mixed lymphocyte reaction
Chimeras were evaluated for donor-specific tolerance in vitro by mixed lymphocyte reaction (MLR), as previously described.7 Responder splenocytes (2.5 × 105) were cultured 1:1 with irradiated host, donor, and third-party stimulator cells (2000 cGy) for 5 days at 37° in 5% CO2. Each well was pulsed with 1 μCi (0.037 MBq) of [3H] thymidine (DuPont-NEN, Boston, MA) for 16 hours before harvesting with an automated harvester (PHD Cell Harvester Technology, Cambridge, MA).
Serum transfer
Serum was collected from presensitized mice 4 to 6 weeks after skin grafting, pooled, heat inactivated (56°C for 30 minutes) and filtered through a 0.2-μm syringe filter. On the day before bone marrow transplantation (BMT), 10 to 500 μL serum was injected intravenously into naive mice.
Statistical analysis
Data are presented as the mean plus or minus SD. The 2-tailed t test (2-sample, assuming unequal variances) was used to evaluate statistical differences. The difference between groups was considered significant at P less than .05.
Results
Kinetics and isotype of antidonor antibody production
Sensitization was induced by skin grafting from B10.BR or BALB/c to B10 or B6 recipients. Donor skin grafts were rejected by naive recipients (n = 50) with a mean survival time (MST) of 13.0 ± 1.2 days. To document allosensitization, recipient serum was collected before and weekly up to 13 weeks after skin transplantation and tested for donor-specific Abs by FCXM assay, which is more sensitive than lymphocytotoxicity.11 Antidonor Abs were present in all B6 or B10 mice at 4 weeks after skin grafting, with titers of MFI 280.4 ± 99.7 and 229.3 ± 56.0, respectively (Figure 1A). Antidonor IgM appeared early and peaked at week 2, then decreased to almost undetectable levels by week 4 (Figure 1B). Donor-specific IgG was detected starting at week 2 and reached a peak at week 6 (MFI, 326.2 ± 63.8), then decreased with time, persisting at relatively high levels (MFI, 70.2 ± 28.7) even after week 11 (Figure 1B). All sensitized mice produced high levels of anti-donor total IgG, including IgG subclass IgG1, IgG2a, and IgG2b (Figure 1C). The IgG in sera from week 4 and up to week 12 directly bound donor c-Kit+/Sca-1+/Lin- (KSL) hematopoietic stem cells (Figure 1D). The IgM in sera from week 2 also bound to donor KSL cells (data not shown).
Memory T- and B-cell populations do not change after sensitization
To define the influence of sensitization on the relative proportion of cells from the adaptive arm of the immune response, splenocytes from sensitized B10 mice were compared with age-matched naive mice. There were no significant changes (P > .05) in the percentage of memory T cells (CD44+/CD8+, 30.3% ± 1.5% vs 35.6% ± 3.1%; CD44+/CD4+, 29.2% ± 2.67% vs 24.1% ± 1.9%) or memory B cells (IgD-/CD19+, 42.2% ± 3.9% vs 39.4% ± 1.5%) in allosensitized mice 4 weeks after skin grafting compared with naive mice. Moreover, there was no significant (P > .05) difference in the percentage of activated T cells (CD69+/CD8+, 0.67% ± 0.1% vs 0.71% ± 0.1%; CD69+/CD4+, 2.3% ± 0.2% vs 2.1% ± 0.1%) or activated B cells (CD86+/CD19+, 2.2 ± 0.3% vs 2.0 ± 0.2%) between the 2 groups.
Sensitized recipients resist engraftment of donor BMCs
Our recent findings in a nonsensitized mouse model demonstrated that the primary role for conditioning is to control host-reactive T cells.7 Therefore, we examined whether engraftment could be similarly enhanced in sensitized recipients by targeting these populations. Five to 7 weeks after sensitization, recipients were conditioned with 950 cGy TBI plus different conditioning strategies shown to enhance engraftment in nonsensitized recipients (Table 1) and were reconstituted with 80 × 106 allogeneic BMCs (B10.BR → B10 or BALB/c → B6). As expected, engraftment was not achieved when sensitized recipients were conditioned with 950 cGy TBI alone.8 Engraftment was not significantly enhanced in sensitized recipients with the following conditioning approaches, used alone or in combination: (1) T-cell-targeting mAbs: anti-CD8 and anti-αβ-TCR; (2) costimulatory blockade: anti-CD154; (3) anti-MHC host mAbs: anti-class I and anti-class II; (4) anti-NK-cell mAb: anti-NK1.1; (5) cytotoxic and immunosuppressive drugs: CyP, CyA, fludarabine, MMF, and rapamycin; (6) cardiovascular failure (CVF); (7) anti-B-cell mAb: anti-B220; (8) anti-CD45RB; (9) splenectomy; (10) subtractive immunization using donor splenocytes + CyP (effector cells reactivated by exposure to donor antigens should be killed by CyP); and (11) multiple large doses of BMC infusion: 3 × 80 × 106 BMCs (Table 1).
Flow cytometric analysis of the kinetics of immunoglobulin subclasses in presensitized mice. Donor splenocytes (0.5 × 106) from naive B10.BR or BALB/c mice were incubated with 5 μL sera from sensitized B10 or B6 recipients and age-matched naive controls for 30 minutes, then incubated with FITC-conjugated goat anti-mouse immunoglobulin and goat anti-mouse IgM, IgG, IgG1, IgG2a, or IgG2b, followed by incubation with PE-conjugated anti-mouse CD4 plus CD8. Levels of circulating alloantibodies were determined by FACSCalibur gating on the CD4+ and CD8+ T cells and were reported as MFI. (A) Antidonor Ab titers were detected with staining of goat anti-mouse immunoglobulin in B6 or B10 mice before placement of the donor skin graft (week 0) or at 4 weeks after skin grafting. (B) The kinetics of IgM and IgG were analyzed in presensitized mice up to 13 weeks. (C) Isotypes of IgG (IgG2a, IgG2b, IgG1) were tested with sera collected before (week 0 [w0]) and 4 to 6 weeks after skin grafting. Results from sera tested at week 5 are presented. IgM was tested with sera collected before and 2 weeks (w2) after skin grafting. Data are representative of 1 of 4 separate experiments. (D) Sera were incubated with donor BMCs, and the expression of IgG on KSL cells from sensitized mice at 4 to 12 weeks was compared with the control collected before graft placement. Data are representative of 1 of 3 separate experiments. Error bars indicate SD.
Flow cytometric analysis of the kinetics of immunoglobulin subclasses in presensitized mice. Donor splenocytes (0.5 × 106) from naive B10.BR or BALB/c mice were incubated with 5 μL sera from sensitized B10 or B6 recipients and age-matched naive controls for 30 minutes, then incubated with FITC-conjugated goat anti-mouse immunoglobulin and goat anti-mouse IgM, IgG, IgG1, IgG2a, or IgG2b, followed by incubation with PE-conjugated anti-mouse CD4 plus CD8. Levels of circulating alloantibodies were determined by FACSCalibur gating on the CD4+ and CD8+ T cells and were reported as MFI. (A) Antidonor Ab titers were detected with staining of goat anti-mouse immunoglobulin in B6 or B10 mice before placement of the donor skin graft (week 0) or at 4 weeks after skin grafting. (B) The kinetics of IgM and IgG were analyzed in presensitized mice up to 13 weeks. (C) Isotypes of IgG (IgG2a, IgG2b, IgG1) were tested with sera collected before (week 0 [w0]) and 4 to 6 weeks after skin grafting. Results from sera tested at week 5 are presented. IgM was tested with sera collected before and 2 weeks (w2) after skin grafting. Data are representative of 1 of 4 separate experiments. (D) Sera were incubated with donor BMCs, and the expression of IgG on KSL cells from sensitized mice at 4 to 12 weeks was compared with the control collected before graft placement. Data are representative of 1 of 3 separate experiments. Error bars indicate SD.
We previously reported that BMT performed 5 to 7 weeks after sensitization was associated with a higher rate of graft failure than when performed 12 weeks after sensitization.8 In the present studies, we found that when BMT was performed 12 weeks after sensitization, conditioning with anti-CD8 plus anti-CD154 significantly increased the rate of donor engraftment to 85.7% (n = 6 of 7) in sensitized recipients that underwent ablation with 950 cGy TBI and reconstitution with 80 × 106 allogeneic BM cells (Table 2). However, only 25% (n = 8) of the sensitized mice achieved engraftment when the TBI dose was decreased to 850 cGy, and none achieved engraftment when the BMC dose was decreased to 40 × 106. Forty-four percent (n = 9) of sensitized mice achieved engraftment with 3 doses of 40 × 106 BMCs infused at days 0, 2, and 4 after conditioning with 950 cGy TBI. The level of donor chimerism in animals after engraftment was relatively high, with 96.3% ± 4.4% donor chimerism 1 month after reconstitution. In all the mice tested, donor-derived lymphoid lineages (T and B cells), NK cells, dendritic cells, and myeloid lineages (macrophages and granulocytes) were detected (data not shown).
Sensitized chimeras are functionally tolerant
Reversal of sensitization was assessed in vitro and in vivo in chimeric, presensitized recipients to determine whether donor-specific tolerance had been achieved. Chimeras tested were those shown in Table 2. Lymphocytes from previously sensitized allogeneic chimeras (B10.BR → B10 or BALB/c → B6) were tolerant to both donor (B10.BR or BALB/c) and host-strain (B10 or B6) alloantigens, irrespective of conditioning approach, but were reactive to MHC-disparate, third-party (BALB/c or B10.BR) alloantigens in 1-way MLR assays (Figure 2A). In these MLR assays, 1 chimera had been conditioned with anti-CD8 plus 950 cGy TBI and the other 2 with anti-CD8, anti-CD154 plus 950 cGy TBI. Presensitized recipients exhibiting donor chimerism accepted donor-type skin grafts placed 2 to 4 months after BMT (MST, more than 200 days; Figure 2B). MHC-disparate third-party grafts (BALB/c), placed simultaneously, were rejected promptly (MST, 14.1 ± 1.3 days). In this group, 1 chimera was conditioned with anti-CD8 plus 950 cGy TBI, 2 with anti-CD8, anti-CD154 plus 950 cGy TBI, 2 with anti-CD8, anti-CD154 plus 850 cGy TBI, and 2 with 3 doses of 40 × 106 BMCs plus 950 cGy TBI. For all conditioning approaches, chimerism directly correlated with reversal of sensitization.
Sensitized chimeras exhibit donor-specific humoral tolerance
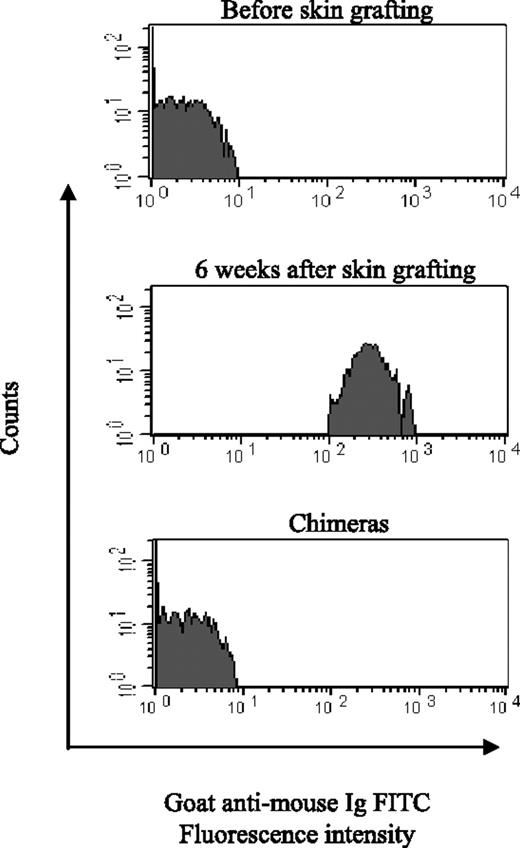
We previously reported that sera from sensitized mice in which chimerism was established lost anti-donor cytotoxic activity using the complement-mediated microcytotoxicity assay.8 To confirm the reversal of humoral immunity by chimerism, we used the FCXM assay (Figure 3) to test the sera from the chimeras generated (Table 2). The FCXM assay has two advantages: greater sensitivity and specificity and the ability to detect noncomplement-fixing antibody.11 Sera from naive mice (before skin grafting; n = 10) and sensitized mice (before BMT; n = 14) and chimeras made from sensitized mice (n = 14) were incubated with donor splenocytes. Antidonor antibody was detected in sensitized mice before BMT (MFI, 124.4 ± 84.8). In contrast, antidonor antibody was not detected in serum from chimeras; MFI (3.5 ± 1.3) was not significantly different from that before skin grafting (2.9 ± 0.5; P = .15). The absence of antidonor Abs persisted for up to 6 months after BMT (data not shown). These findings confirm that establishing chimerism in allosensitized recipients induces humoral tolerance.
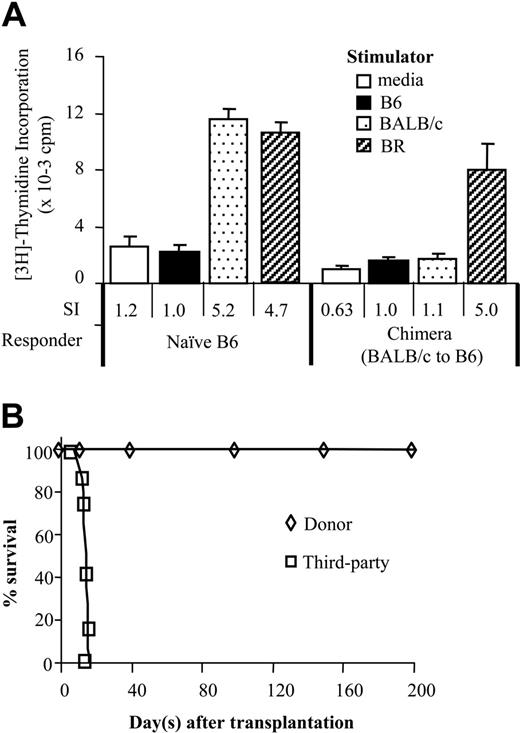
Donor-specific tolerance in vitro and in vivo in chimeric sensitized recipients. Presensitized chimeras (B10.BR → B10 and BALB/c → B6) were evaluated for donor-specific tolerance in vitro by MLR and in vivo by allogeneic skin grafts. (A) Splenocytes from chimeras (n = 3) were stimulated with irradiated host, donor, and third-party stimulator cells in MLR assay. Values are shown as the mean ± SD of triplicate cultures in a 1:1 responder/stimulator ratio from representative chimeras (BALB/c → B6). The stimulation index (SI) reflecting the ratio of the value to the autoresponse is shown at the bottom of the figure. (B) Life table survival of donor and third-party skin grafts in sensitized chimeras. B10.BR → B10 or BALB/c → B6 chimeras each received skin grafts from B10.BR and BALB/c donors as donor-specific (n = 7) or third-party (n = 7) grafts 2 to 4 months after BMT. Grafts were monitored for 200 days.
Donor-specific tolerance in vitro and in vivo in chimeric sensitized recipients. Presensitized chimeras (B10.BR → B10 and BALB/c → B6) were evaluated for donor-specific tolerance in vitro by MLR and in vivo by allogeneic skin grafts. (A) Splenocytes from chimeras (n = 3) were stimulated with irradiated host, donor, and third-party stimulator cells in MLR assay. Values are shown as the mean ± SD of triplicate cultures in a 1:1 responder/stimulator ratio from representative chimeras (BALB/c → B6). The stimulation index (SI) reflecting the ratio of the value to the autoresponse is shown at the bottom of the figure. (B) Life table survival of donor and third-party skin grafts in sensitized chimeras. B10.BR → B10 or BALB/c → B6 chimeras each received skin grafts from B10.BR and BALB/c donors as donor-specific (n = 7) or third-party (n = 7) grafts 2 to 4 months after BMT. Grafts were monitored for 200 days.
Passive transfer of serum from sensitized mice induces graft failure
To test the role of antibody in bone marrow graft rejection, we performed passive transfer experiments in which serum harvested from mice 4 to 6 weeks after skin graft rejection was injected intravenously into naive mice 1 day before BMT. We previously reported that 700 cGy was the lowest irradiation dose to achieve 100% allogeneic BM engraftment in nonsensitized mice.5 Therefore, this model was used to investigate the effect of circulating antidonor antibody from infused sensitized sera on alloengraftment. Naive recipients were conditioned with 700 cGy TBI and underwent transplantation with 15 × 106 or 40 × 106 BMCs 1 day after the infusion of sera from sensitized mice. Engraftment failure occurred in all treated animals that received 50 to 500 μL serum (Table 3). Only 1 of 5 secondary recipients received 25 μL serum infusion (n = 5; group G). Engraftment occurred in 5 of 6 mice when 10 μL sensitized serum was injected (n = 6; group H). In contrast, normal serum (500 μL) did not prevent alloengraftment (n = 8; group B) compared with untreated controls (n = 12; group A). These results indicate that humoral immunity plays a dominant and critical role in establishing chimerism in sensitized recipients.
Loss of donor-specific Abs in mixed sensitized chimeras. Donor splenocytes were incubated with sera from naive mice (n = 10), sensitized mice (before BMT, n = 14), and chimeras (n = 14) prepared from sensitized mice 6 weeks to 6 months after BMT, followed by an anti-mouse immunoglobulin FITC secondary antibody, and then anti-mouse CD4 and CD8 PE. Levels of circulating alloantibodies were determined by FACSCalibur and by gating on the CD4+ and CD8+ T-cell fraction and were reported as MFI ± SD. One representative sample from each group is presented, and 3 separate experiments were performed.
Loss of donor-specific Abs in mixed sensitized chimeras. Donor splenocytes were incubated with sera from naive mice (n = 10), sensitized mice (before BMT, n = 14), and chimeras (n = 14) prepared from sensitized mice 6 weeks to 6 months after BMT, followed by an anti-mouse immunoglobulin FITC secondary antibody, and then anti-mouse CD4 and CD8 PE. Levels of circulating alloantibodies were determined by FACSCalibur and by gating on the CD4+ and CD8+ T-cell fraction and were reported as MFI ± SD. One representative sample from each group is presented, and 3 separate experiments were performed.
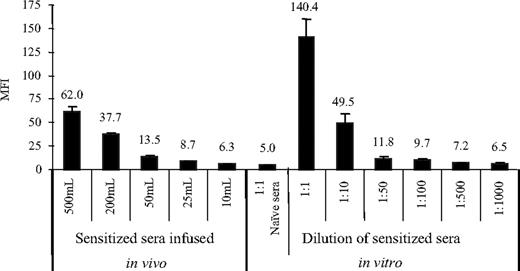
To confirm that antibody could be detected systemically in passive transfer recipients, sera pooled from sensitized recipients was injected intravenously into naive B10 recipients. Serum was collected from the peripheral blood on day 1 after passive serum infusion, analyzed by FCXM assay, and compared with levels detected after in vitro serial dilution of the sensitized serum (Figure 4). The antibody titer of pooled sensitized sera (without dilution) tested with goat anti-mouse immunoglobulin was 140.2 ± 19.2 MFI. Increased antidonor antibody was detected in the serum of naive recipients given between 500 and 25 μL of the sensitized serum (62.0 ± 4.4 and 8.7 ± 0.3 MFI, respectively). MFI decreased in a dose-dependent manner between these doses and correlated with the in vitro dilutions between 1:10 and 1:500, respectively (Figure 4). Antidonor MFI from mice infused with 10 μL sensitized sera was similar to the MFI value of 1:1000 dilution of the same sera in vitro. These values were not significantly different from each other (P = .8) or from the MFI value in naive sera (5.0 ± 0.6; P > .05). The serum was therefore diluted approximately 10-fold in vivo. Resistance to BMC engraftment from the infused antibody titrated out at 25 μL and correlated with a decrease in MFI in FCXM assay obtained from serum in vivo. The MFI level with transferring 25 μL sensitized sera was low but still detectable and was similar to the MFI value at a 1:500 dilution in vitro.
Serum antidonor Ab titers in passively transferred recipients. In vivo experiment: sera collected between 4 weeks to 6 weeks after skin grafting were pooled from B10 mice sensitized by B10.BR skin grafts, and 10 to 500 μL was injected intravenously into naive B10 mice (n = 4 mice/group). Sera were collected 1 day after serum infusion. In vitro experiment: pooled sensitized sera were serially diluted (1:1-1:1000). Each dilution had 3 samples. FCXM assays were performed for in vivo and in vitro experiments. Donor B10.BR splenocytes (0.5 × 106) were incubated with 5 μL serum and then incubated with FITC-conjugated goat anti-mouse immunoglobulin, followed by incubation with PE-conjugated anti-mouse CD4 plus CD8. Levels of antidonor Ab were determined by FACSCalibur and reported as MFI. Error bars indicate SD.
Serum antidonor Ab titers in passively transferred recipients. In vivo experiment: sera collected between 4 weeks to 6 weeks after skin grafting were pooled from B10 mice sensitized by B10.BR skin grafts, and 10 to 500 μL was injected intravenously into naive B10 mice (n = 4 mice/group). Sera were collected 1 day after serum infusion. In vitro experiment: pooled sensitized sera were serially diluted (1:1-1:1000). Each dilution had 3 samples. FCXM assays were performed for in vivo and in vitro experiments. Donor B10.BR splenocytes (0.5 × 106) were incubated with 5 μL serum and then incubated with FITC-conjugated goat anti-mouse immunoglobulin, followed by incubation with PE-conjugated anti-mouse CD4 plus CD8. Levels of antidonor Ab were determined by FACSCalibur and reported as MFI. Error bars indicate SD.
Dissection of the relative contribution of T- and B-cell responses in sensitization
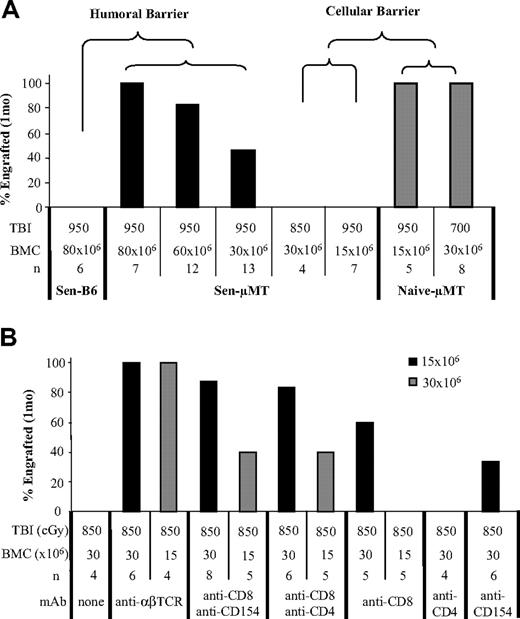
μMT mice were used as recipients to evaluate the contribution of cellular immunity in sensitization. μMT mice do not produce antibody, which allowed the role of T cells to be elucidated in sensitized recipients. μMT mice (n = 10) rejected allogeneic skin grafts with kinetics similar to those of wild-type (n = 16) controls (14.1 ± 2.2 days vs 13.5 ± 1.5 days; P = .52). As expected, no antidonor antibody was detected in μMT mice after skin graft rejection (data not shown). Sensitized μMT mice underwent BMT at 5 to 7 weeks; they received 950 to 850 cGy TBI and 80 to 15 × 106 BMCs. After 950 cGy TBI, all sensitized μMT mice (n = 7) achieved engraftment with 80 × 106, and 83.3% (n = 12) achieved engraftment with 60 × 106 donor BMCs (Figure 5A). Thirteen (46.2%) sensitized μMT mice achieved engraftment with 30 × 106 donor BMCs. In contrast, none of the sensitized control B6 mice achieved engraftment with 80 × 106 donor cells and 950 cGy TBI. The lower conditioning required in sensitized μMT mice compared with sensitized healthy mice further demonstrated that the role of the B-cell arm of adaptive immunity was the primary barrier in allosensitization, with T cells playing a less important role.
To further define the role of T cells in sensitized recipients, we compared alloengraftment between sensitized and naive μMT mice. All naive μMT mice achieved engraftment with 950 cGy TBI and 15 × 106 BMCs (n = 5), whereas none of the sensitized μMT mice achieved engraftment with 950 cGy TBI plus 15 × 106 BMCs or 850 cGy TBI plus 30 × 106 BMCs. All naive μMT mice (n = 6) achieved engraftment with lower TBI (700 cGy) and a lower BMC dose (30 × 106) than sensitized B6 controls (Figure 5A). However, more conditioning and higher cell doses were required in sensitized μMT recipients than in nonsensitized controls to establish engraftment. These data suggested that the increased barrier encountered must have been mediated by primed T cells.
Dissection of the relative contribution of T-cell responses using μMT mice. μMT mice (H-2b) underwent transplantation with BALB/c (H-2d) or B10.BR (H-2k) skin grafts. (A) BMT was performed in presensitized μMT mice 5 to 7 weeks after skin grafting. Recipients were conditioned with 950 to 850 cGy TBI, and 80 to 15 × 106 BMCs were transplanted between 4 and 6 hours after TBI. Sensitized normal B6 mice and naive μMT mice were used as controls. Engraftment was analyzed by flow cytometry 1 month after BMT. (B) Sensitized μMT mice were preconditioned with anti-CD8, anti-CD154, anti-αβ-TCR, or anti-CD4 mAb alone and in combination, followed by 850 cGy TBI, and then underwent transplantation with 30 × 106 or 15 × 106 BMCs matched to the skin graft donor. Chimerism was tested at 1 month and was observed monthly for at least 6 months.
Dissection of the relative contribution of T-cell responses using μMT mice. μMT mice (H-2b) underwent transplantation with BALB/c (H-2d) or B10.BR (H-2k) skin grafts. (A) BMT was performed in presensitized μMT mice 5 to 7 weeks after skin grafting. Recipients were conditioned with 950 to 850 cGy TBI, and 80 to 15 × 106 BMCs were transplanted between 4 and 6 hours after TBI. Sensitized normal B6 mice and naive μMT mice were used as controls. Engraftment was analyzed by flow cytometry 1 month after BMT. (B) Sensitized μMT mice were preconditioned with anti-CD8, anti-CD154, anti-αβ-TCR, or anti-CD4 mAb alone and in combination, followed by 850 cGy TBI, and then underwent transplantation with 30 × 106 or 15 × 106 BMCs matched to the skin graft donor. Chimerism was tested at 1 month and was observed monthly for at least 6 months.
We next examined whether targeting sensitized T-cell populations would enhance allogeneic engraftment. Given that all sensitized μMT mice failed to engraft after conditioning with 850 cGy TBI and transfusion with 30 × 106 donor BMCs, we used this as our starting dose. Sensitized recipient μMT mice were treated with anti-αβ-TCR, anti-CD8, anti-CD4, or anti-CD154 mAbs, alone or in combination, and were conditioned with 850 cGy TBI. Then they underwent transplantation with 30 to 15 × 106 B10.BR or BALB/c BMCs (Figure 5B). Without mAb pretreatment, engraftment did not occur in sensitized μMT mice. In striking contrast, almost all animals achieved engraftment after preconditioning with anti-αβ-TCR alone, anti-CD8 plus anti-CD154, or anti-CD8 plus anti-CD4 (100%, 87.5%, and 100%, respectively) mAbs after transplantation with 30 × 106 BMCs. Moreover, anti-αβ-TCR promoted engraftment in all sensitized μMT mice, even those with BMC levels as low as 15 × 106 (Figure 5B). These data showed that T-cell memory played an important but less dominant role in mediating bone marrow rejection in sensitized recipients.
Levels of circulating Abs cannot be significantly decreased to enhance engraftment in sensitized mice
Given that even low levels of circulating alloantibody pose a critical barrier to establishing chimerism, we explored approaches to neutralize or remove the preformed antibody. Apheresis and blood exchange techniques were not available in mice; therefore, we attempted to decrease the Ab titer with immunoadsorption agents or agents that target B-cell responses. First, immunoadsorption agents—including human intravenous immune globulin (IVIG, 2 g/kg intravenously [n = 4]; Baxter Healthcare, Westlake Village, CA), mouse gamma globulin (2 mg intravenously; Equitech-bio, Kerrville, TX), goat anti-mouse IgG (H+L) (0.2 mg intravenously; Chemicon, Temecula, CA), and naive mouse serum (0.5 mL; Harlan, Indianapolis, IN) were administered for 3 consecutive days. The IVIG preparation contained soluble HLA class I molecules that bound circulating anti-HLA antibodies. None of these agents had any significant effect on decreasing Ab levels or enhancing engraftment (data not shown). Multiple high-dose infusions of donor BMCs (3 doses of 100 × 106 BMCs injected every other day after TBI administration) were administered to consume the donor-specific Abs, but alloengraftment again failed in all animals (n = 5). We tried to decrease the level of circulating Abs by specifically targeting B cells. Unfortunately, no specific B-cell-depleting agents were available because anti-CD19 and anti-B220 are nondepleting12 (H.X., unpublished data, June 2003). Treatment with anti-B220 did not enhance engraftment with 950 cGy TBI and 80 × 106 donor BMCs. Finally, cyclophosphamide was tested based on a recent report indicating that it decreased circulating Ab levels.13 In our mouse model, CyP (200 mg/kg, intraperitoneally) depleted more than 99% of B cells within 4 days of the first dose of CyP treatment (Figure 6A). B-cell recovery began 15 days later, and B cells were almost fully recovered by 30 days (Figure 6A). Although B-cell populations were significantly reduced with CyP treatment, the antibody level in sensitized mice was not affected by CyP treatment compared with sensitized controls that did not receive CyP (Figure 6B). BMT was performed 35 days after treatment with 1 CyP dose on day 0 or with 2 doses on days 0 and 15. Recipients were conditioned with 950 cGy TBI and were administered 80 × 106 BMCs. None of the animals achieved engraftment (Figure 6C). These data suggest that at least 1 subset of the antibody-producing memory B cells were not affected by CyP treatment or that the graft failure resulted from the low levels of antidonor antibody that persisted.
Discussion
Until recently, most studies of the mechanism of allograft rejection have focused on T-cell-mediated responses. In the present report, we show for the first time that humoral adaptive immunity plays a dominant and critical role in sensitization. The role of B cells in sensitization is just now being characterized. Antibodies against MHC were recently shown to play a role in the rejection of solid organ transplants.14,15 Moreover, in xenotransplantation, anti-MHC antibody was recently shown to contribute in a dominant fashion to xenograft rejection.16,17 This antibody-mediated rejection is not readily controlled with conventional immunosuppressive drugs. Aggressive protocols to decrease the level of sensitization, including plasmapheresis, the administration of immunoglobulin, immunoadsorption, antilymphocyte Abs, and combination therapies using immunosuppression18,19 have provided limited, short-term success in improving outcomes of organ allografts in sensitized recipients. An understanding of the role of humoral adaptive immune responses could have a major impact on crossing the allosensitized barrier. For recipients of bone marrow and solid organ transplants, graft outcomes would be enhanced if sensitization could be reversed.
Effect of CyP treatment in sensitized recipients. Five to 7 weeks after sensitization, recipients were treated with CyP in a total dose of 200 mg/kg: 1 dose of 200 mg/kg and 2 doses of 100 mg/kg. Age-matched non-CyP treatment groups served as controls. Splenocytes were harvested and analyzed by flow cytometry on day 4. (A) Kinetics of B-cell populations (absolute number per microliter) were tested 4, 9, 15, and 30 days after CyP treatment. (B) Sera were collected from sensitized mice before treatment or 15 or 30 days after CyP treatment. FCXM assay was performed to test the change of donor-specific Abs in the sera. (C) Allogeneic BMT was performed 35 days after the first dose of CyP in 3 different groups: group 1, sensitized mice with 1 dose of CyP (200 mg/kg at day 0); group 2, sensitized mice with 2 doses of CyP (200 mg/kg at day 0 and 100 mg/kg at day 15); group 3, nonsensitized mice without CyP treatment. All recipients were conditioned with 950 cGy TBI and underwent transplantation with 80 × 106 BMCs for group 1 or 2 and 15 × 106 BMCs for group 3. BMCs were from the same donor of skin grafts. Engraftment was analyzed by flow cytometry 1 month later.
Effect of CyP treatment in sensitized recipients. Five to 7 weeks after sensitization, recipients were treated with CyP in a total dose of 200 mg/kg: 1 dose of 200 mg/kg and 2 doses of 100 mg/kg. Age-matched non-CyP treatment groups served as controls. Splenocytes were harvested and analyzed by flow cytometry on day 4. (A) Kinetics of B-cell populations (absolute number per microliter) were tested 4, 9, 15, and 30 days after CyP treatment. (B) Sera were collected from sensitized mice before treatment or 15 or 30 days after CyP treatment. FCXM assay was performed to test the change of donor-specific Abs in the sera. (C) Allogeneic BMT was performed 35 days after the first dose of CyP in 3 different groups: group 1, sensitized mice with 1 dose of CyP (200 mg/kg at day 0); group 2, sensitized mice with 2 doses of CyP (200 mg/kg at day 0 and 100 mg/kg at day 15); group 3, nonsensitized mice without CyP treatment. All recipients were conditioned with 950 cGy TBI and underwent transplantation with 80 × 106 BMCs for group 1 or 2 and 15 × 106 BMCs for group 3. BMCs were from the same donor of skin grafts. Engraftment was analyzed by flow cytometry 1 month later.
We previously demonstrated that the induction of mixed allogeneic chimerism in sensitized recipients induced tolerance to donor alloantigen and appeared to reverse immunologic memory.8 Our current study has confirmed these findings. After successful engraftment of BMCs from the donor, sensitized mice lost circulating antidonor Abs and accepted second donor-specific skin grafts. These findings provide evidence that immunologic memory can be altered after the induction of chimerism and that Ab responses are reeducated in sensitized recipients after alloengraftment. As a clinical correlate, alloantibodies were eliminated in sensitized patients when allogeneic chimerism was achieved after BMT.9 Thus, the induction of mixed hematopoietic chimerism may be a promising approach to control allosensitization.
A number of nonmyeloablative approaches developed to establish mixed chimerism in nonsensitized recipients have identified that host T cells play a critical role in HSC engraftment.5-7,20-22 Conditioning of recipients with TBI plus cyclophosphamide allowed mixed chimerism to be established with as little as 500 cGy TBI.5 The addition of antilymphocyte globulin to the regimen allowed the TBI dose to be reduced to as low as 300 cGy TBI.6 Pretreatment of the recipient with a combination of anti-CD8 and anti-αβ-TCR mAbs also achieved stable mixed chimerism with 300 cGy TBI.7 Blocking the CD154/CD40 costimulatory pathway with anti-CD154 decreased the TBI dose to 400 cGy,21 and adding cyclosporin A further decreased the TBI dose to 200 cGy.22 These data have led to the conclusion that the major mechanism for myelotoxic conditioning is to control host-versus-graft reactive cells rather than to prepare vacant niches.5-7,20
In mice sensitized by skin graft rejection, Colson et al8 achieved mixed chimerism with ablative doses of TBI (950 cGy) and 4-fold higher doses of allogeneic BMCs (80 × 106), but the engraftment was still poor. After fully allogeneic reconstitution, no animals achieved engraftment with a 5- to 7-week interval between sensitization and BMT, and only 37% of animals achieved engraftment with intervals exceeding 12 weeks. Interestingly, the coadministration of syngeneic and high-dose allogeneic marrow enhanced engraftment in sensitized recipients. However, for clinically applicability, a nonmyeloablative approach was required to establish chimerism. Because host T cells are the primary effector cells in allorejection and targeting host T cells can dramatically decrease the TBI requirement to achieve allogeneic BM engraftment in nonsensitized recipients,7 we hypothesized that more benign conditioning strategies could be developed in sensitized recipients with similar conditioning. Notably, the conditioning agents we tested, which primarily targeted T cells or their functional pathways in sensitized mice, had almost no effect on enhancing allogeneic BMC engraftment. These agents included mAbs against T cells (anti-CD8, anti-αβ-TCR), mAbs to block costimulatory pathways (anti-CD154), mAbs against MHC antigens (anti-mouse class I or II), cytotoxic and immunosuppressive drugs (CyP, rapamycin, fludarabine, CyA, MMF); complement-depleting agent (CVF, anti-CD45RB), and subtractive immunization using irradiated donor splenocytes plus CyP. That allogeneic engraftment is more difficult to achieve in sensitized mice suggests humoral immunity is more critical in sensitized recipients than was previously known. Secondary immune responses to immunologic memory are usually more rapid, vigorous, and qualitatively different from the primary immune responses to the same antigen.23
In the current study, we found that when BMT was performed 12 weeks after sensitization, engraftment was greater than when BMT was performed 5 to 7 weeks after sensitization. Antidonor Ab decreased with time after sensitization (Figure 1B), peaking at 6 weeks and declining to a persistently low level thereafter. At 12 weeks, preconditioning sensitized recipients by depleting CD8+ T cells plus blocking CD154/CD40 costimulatory molecule interactions significantly enhanced engraftment. It is likely that this outcome was caused by targeting of T- and B-cell function by anti-CD154.24 Adjunctive CD8 T-cell depletion in patients with CD154 blockade has been reported to prolong cardiac allograft survival because CD4+ cells are more susceptible to CD154 blockade than CD8+ T cells.25 Moreover, our recent finding that blocking CD154/CD40 interactions completely abrogates B-cell activation and germinal center formation in the response of naive mice to MHC alloantigens (H.X., J.Y., Y.H., Chuanlin Ding, C.L.S., Li Wang, and S.T.I.; “Co-stimulatory blockade of CD154: CD40 in combination with T-cell lymphodepletion results in prevention of allogeneic sensitization”; manuscript submitted, September 2006) further supports a role for B-cell activity in sensitized recipients. Our present findings demonstrate that CD8 T-cell depletion and CD154 blockade can also promote allogeneic BM engraftment in sensitized recipients.
The dominance of humoral immunity in sensitized recipients is reflected by the fact that allogeneic donor marrow engraftment was abrogated in naive mice with the passive transfer of as little as 25 μL pooled serum from sensitized donors. Antidonor Abs persisted in the circulation at a high level for a prolonged period after rejection of skin allografts. Efforts to decrease the level of circulating Ab in the blood with immunoadsorption using IVIG, mouse gamma globulin, goat anti-mouse IgG, and naive mouse serum did not have an effect. Subtractive immunization of memory T and B cells with infusions of multiple high doses of donor splenocytes or BMCs in combination with CyP to consume the donor-specific Abs and to eliminate activated memory cells also failed to enhance alloengraftment. Although we found that CyP eliminated more than 99% of B cells, it did not enhance engraftment. In the clinical setting, agents such as IVIG, immunosuppressive drugs, and Abs were used in combination with plasmapheresis.26,27 The technical impossibility of plasmapheresis in our mouse model almost certainly hampered the effects of these other treatments to decrease alloantibody and to enhance engraftment. Alternatively, it is possible that the antibody-producing memory B cells were resistant to elimination by these approaches.
Our present findings demonstrate that therapeutic interventions for sensitized transplant recipients must focus on targeting allospecific B cells and decreasing preformed alloantibodies. Although new alloimmune modulating protocols with IVIG plus immunosuppressive drugs combined with plasmapheresis have been successfully used for desensitization of patients with donor-specific anti-HLA antibody and improved transplantation outcomes have been observed,27,28 the effect is transient and B-cell tolerance does not occur. B-cell tolerance induced by chimerism is to date the only approach to reset immunologic memory and return the high circulating donor-specific Ab titer to levels similar to those before sensitization. Similarly, in xenotransplantation, mixed chimerism is associated with decreased natural anti-Gal antibody production29 and induction of B-cell tolerance to xenografts.30
An understanding of the mechanism of sensitization and control of humoral immunity will allow the development of approaches to induce immune deviation in sensitized recipients. Of note, we found a difference between the response of allosensitization to BMCs and the response to solid organ grafts. In sensitized rats, the administration of CTLA4 immunoglobulin markedly prolonged the survival of heart transplant recipients.31 Costimulatory blockade with anti-CD154 prolonged cardiac allograft survival in mice sensitized by skin grafting.32 Tacrolimus and MMF combined with plasmapheresis serves as rescue treatment for renal transplant recipients experiencing acute humoral rejection.33,34 In contrast to what occurs with solid organ grafts, we found that these agents did not significantly enhance BMC engraftment in sensitized murine recipients, suggesting that there is a difference between the sensitized host response to solid organ transplantation and to BMT. Because BMCs circulate in the blood and may be in more direct contact with recipient immune cells and preformed antidonor Abs, BMT may face stronger immune barriers than solid organ grafts. We previously reported that HSCs express a high density of MHC class I molecules on their cell surfaces.35 Our present data show that all donor BMCs, specifically HSCs, express antigens that are bound with high affinity by circulating donor-specific Abs from sensitized recipients.
To define the relative contribution of T cells to alloresistance to engraftment in sensitized recipients, we tested sensitized B-cell-deficient μMT mice incapable of producing antibody. Sensitized μMT mice achieved less engraftment than naive μMT mice, indicating that T cells also contribute to the sensitized barrier. This result is consistent with the observation made by Hancock et al,36 who showed that sensitized B-cell-deficient mice rejected hearts to an accelerated degree. An absolute requirement for the presence of functional T cells for rejection in sensitized mice was also shown in our study because the administration of T-cell-depleting Abs in sensitized μMT mice enhanced engraftment. Sensitized μMT mice achieved engraftment more readily than sensitized controls, further confirming that humoral immunity is the dominant arm of the immune response in antigen-familiar recipients. These results suggest that T-cell reactivity should also be targeted in developing nonmyeloablative conditioning approaches to establish engraftment in sensitized recipients.
Taken together, our data demonstrate that humoral immunity and, to a lesser extent, T-cell responses contribute to the sensitized state for BMT. Our data strongly indicate that to achieve mixed chimerism in sensitized recipients, nonmyeloablative conditioning must target T- and B-cell responses. If a mechanistically based, clinically acceptable strategy can be defined to establish chimerism in sensitized recipients, the sensitization barrier will be successfully crossed. Additionally, it would be of obvious clinical benefit if allosensitization could be prevented when recipients are exposed to donor alloantigens.
Prepublished online as Blood First Edition Paper, August 3, 2006; DOI 10.1182/blood-2006-04-017467.
Supported in part by National Institutes of Health grants R01 HL63442 and R01 DK069766, the Commonwealth of Kentucky Research Challenge Trust Fund, The Jewish Hospital Foundation, and the University of Louisville Hospital.
The authors declare no competing financial interests.
H.X. wrote the paper, helped design the experiments, performed the experiments, and analyzed the data. P.M.C. helped design the research and analyzed the data. M.K.T. performed and helped design the experiments. Y.H. performed and helped design the supporting experiments. C.L.S. performed the supporting experiments, edited, and gave feedback in the writing phase. M.D.-L. helped with design the experiments. J.Y. helped design the experiments. S.T.I. designed the experiments, helped interpret the data, and reviewed all the data.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We thank Haval Shirwan and Thomas C. Mitchell for review of the manuscript and helpful comments; Sharon S. Willer, Lala-Rukh Hussain, and Jennifer Tanner for technical assistance; Carolyn DeLautre and Kim Nichols for manuscript preparation; and the staff of the animal facility for outstanding animal care.

![Figure 1. Flow cytometric analysis of the kinetics of immunoglobulin subclasses in presensitized mice. Donor splenocytes (0.5 × 106) from naive B10.BR or BALB/c mice were incubated with 5 μL sera from sensitized B10 or B6 recipients and age-matched naive controls for 30 minutes, then incubated with FITC-conjugated goat anti-mouse immunoglobulin and goat anti-mouse IgM, IgG, IgG1, IgG2a, or IgG2b, followed by incubation with PE-conjugated anti-mouse CD4 plus CD8. Levels of circulating alloantibodies were determined by FACSCalibur gating on the CD4+ and CD8+ T cells and were reported as MFI. (A) Antidonor Ab titers were detected with staining of goat anti-mouse immunoglobulin in B6 or B10 mice before placement of the donor skin graft (week 0) or at 4 weeks after skin grafting. (B) The kinetics of IgM and IgG were analyzed in presensitized mice up to 13 weeks. (C) Isotypes of IgG (IgG2a, IgG2b, IgG1) were tested with sera collected before (week 0 [w0]) and 4 to 6 weeks after skin grafting. Results from sera tested at week 5 are presented. IgM was tested with sera collected before and 2 weeks (w2) after skin grafting. Data are representative of 1 of 4 separate experiments. (D) Sera were incubated with donor BMCs, and the expression of IgG on KSL cells from sensitized mice at 4 to 12 weeks was compared with the control collected before graft placement. Data are representative of 1 of 3 separate experiments. Error bars indicate SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/10/10.1182_blood-2006-04-017467/4/m_zh80220603890001.jpeg?Expires=1768044637&Signature=nqiISVu3u9zwruLeqsubcDTtKHfkMLmSLfAuZKLNZEdDHpsNtGdC37VVOwhV8gGgRdoAavZhlHqIRoBjCyK4VNuV~cOBuwY-J83vhvWJk1JBlRAwOcYW4VAHxXDJHDcCSJouS01SYo~ngybMNzNQ5LQG7nwqetOWmKDLl8VUa2qiRa0fDFjrxEtPBqG45BwtC5JeFioSsC9t4~AWApgq3XDH4TDlcPj7sFflG3Qkp4gN66D7Y7T3BVhRACDn8TfJbUCBQ1Sd8caBXS8YoK-4jL86hqFJMqts3C9YB3O0wyc7OVP47dUQFfvQc7srUu9HzyWv4E7yh2y~NVgZmJY1wg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)