Abstract
Fanconi anemia (FA) is a heterogeneous genetic disorder characterized by bone marrow (BM) failure and cancer susceptibility. Identification of the cDNAs of FA complementation types allows the potential of using gene transfer technology to introduce functional cDNAs as transgenes into autologous stem cells and provide a cure for the BM failure in FA patients. However, strategies to enhance the mobilization, transduction, and engraftment of exogenous stem cells are required to optimize efficacy prior to widespread clinical use. Hypersensitivity of Fancc–/– cells to interferon-gamma (IFN-γ), a nongenotoxic immune-regulatory cytokine, enhances engraftment of syngeneic wild-type (WT) cells in Fancc–/– mice. However, whether this phenotype is of broad relevance in other FA complementation groups is unresolved. Here we show that primitive and mature myeloid progenitors in Fanca–/– and Fancg–/– mice are hypersensitive to IFN-γ and that in vivo infusion of IFN-γ at clinically relevant concentrations was sufficient to allow consistent long-term engraftment of isogenic WT repopulating stem cells. Given that FANCA, FANCC, and FANCG complementation groups account for more than 90% of all FA patients, these data provide evidence that IFN-γ conditioning may be a useful nongenotoxic strategy for myelopreparation in FA patients.
Introduction
Fanconi anemia (FA) is a heterogeneous genetic disorder characterized by a progressive bone marrow aplasia, chromosomal instability, and the acquisition of malignancies. The progressive bone marrow failure in FA and the late developing myeloid malignancies account for 90% of the mortality in FA.1 Currently, the only cure for the hematopoietic manifestations of FA is HLA-identical allogeneic bone marrow transplantation, a therapy available to only about 30% of patients.2 Twelve FA complementation types (FANCA, FANCB, FANCC, FANCD1, FANCD2, FANCE, FANCF, FANCG, FANCI, FANCJ, FANCL, and FANCM) have been identified and the cDNAs of 11 FA genes have been sequenced.3-10 The identification of these genes raises the potential of using gene transfer technology to express the functional cDNA into autologous stem cells. The bone marrow hypoplasia commonly observed in FA patients1 and the reduced repopulating ability of stem cells in mice containing a disruption of the murine homologue of an FA gene11,12 led to the hypothesis that autologous, genetically-corrected stem cells would have an engraftment and proliferation advantage. However, ex vivo culture is known to reduce stem cell engraftment in both normal murine and human cells,13-15 and in an initial phase 1 clinical trial in FA patients where myelopreparation was not routinely performed16 the detection of transduced cells was low in all patients. Furthermore, the most efficient engraftment of transduced cells in the clinical FA trial was observed in a single patient who received nonlethal ionizing radiation. Collectively, these data provide rationale for considering nonmyeloablative therapy prior to transplantation of autologous genetically-corrected stem cells.
FA cells exhibit increased chromosomal breaks in response to DNA-damaging agents in vitro.3,17 Thus there is a theoretical potential of FA patients having an increased incidence for secondary malignancies following myelopreparation with genotoxic agents. Due to the low frequency of FA in the general population18 and the high incidence of malignant predisposition of FA patients, it is difficult to fully ascertain the risk associated with the genotoxic myelopreparation regimen in patients. However, several studies suggest an increased risk for hematopoietic and nonhematopoietic secondary malignancies19-22 after hematopoietic stem cell transplantation in FA patients. For these reasons, a nongenotoxic myelopreparative regimen would be ideal in selecting genetically-corrected stem cells in vivo.
Interferon-gamma (IFN-γ) is a nongenotoxic immunoregulatory lymphokine with a high therapeutic index23 that has been used to treat a number of malignancies.24-26 Rathbun et al provided the first evidence that murine (Fancc–/–) and human (FANCC) hematopoietic cells were hypersensitive to IFN-γ via an increase in apoptosis.27 These results were subsequently confirmed in an independent Fancc–/– murine model in vitro and in vivo.28,29 Previous work indicates that a mechanism for the hypersensitivity of FANCC-deficient cells to IFN-γ is mediated by FANCC physically interacting with heat shock protein 70 to inhibit PKR activation and apoptosis.30 Subsequent studies showed that FANCA and FANCG also participate in that complex.31 FANCA progenitors were found to be hypersensitive to IFN-γ via an increase in apoptosis in that same study.31 However, one in vitro study in a murine model of FANCA (Fanca–/–) was unable to detect an increase in the sensitivity of Fanca–/– myeloid progenitors to IFN-γ compared with wild-type (WT) controls.32 Whether the differing results of the human FANCA and murine (Fanca–/–) studies reflect differences in in vitro protocols or, alternatively, species-specific differences remains unclear. Studies to determine whether Fanca–/– or Fancg–/– progenitors are hypersensitive to IFN-γ in vivo have not been attempted. Since FANCA, FANCG, and FANCC patients collectively account for 90% of all FA patients,1 determination of the hypersensitivity of hematopoietic progenitors from these complementation groups to IFN-γ could have broad relevance to the potential of IFN-γ in nongenotoxic myelopreparation. Here we tested the hypothesis that IFN-γ can be used as a single myelopreparative agent in Fanca–/– and Fancg–/– mice prior to transplantation of isogenic repopulating stem cells. Our results demonstrate that both primitive and mature Fanca–/– and Fancg–/– myeloid progenitors are hypersensitive to IFN-γ treatment in vitro and in vivo compared with syngeneic WT progenitors, similar to Fancc–/– mice. In addition, continuous subcutaneous infusion of IFN-γ facilitated the engraftment of consistently high levels of exogenous, isogeneic WT hematopoietic stem/progenitor cells. These data support the hypothesis that IFN-γ may be useful as a nongenotoxic, myelopreparative regimen in multiple FA complementation groups.
Materials and methods
Mice
Fanca+/– mice (C57Bl/6 × SV129), Fancg+/– mice, and Fancc+/– mice were backcrossed into a C57Bl/6J strain (CD45.2+) and then bred to generate Fanca–/– or Fancg–/– and WT mice. Congenic C57Bl/6J (CD45.2+) and B6.SJL-PtrcaPep3b/BoyJ (B6.BoyJ) mice (CD45.1+) were purchased from Jackson Laboratories (Bar Harbor, ME) and maintained in our animal facility.12
Treatment and monitoring
All experimental mice were housed in the Indiana University School of Medicine Laboratory Animal Resource Center and examined regularly by one of the investigators. Murine IFN-γ was purchased from Peprotech (Rocky Hill, NJ), reconstituted as recommended by the manufacturer, and placed within micro-osmotic pumps to administer a defined volume (0.5 μL/h for 7 days; DURECT, Cupertino, CA). Micropumps containing either IFN-γ or phosphate-buffered saline (PBS) were implanted subcutaneously into the backs of WT, Fancc–/–, Fanca–/–, and Fancg–/– mice and removed following a 7-day infusion. IFN-γ was administered at a dosage of 0 or 400 μg/kg per day. These studies were reviewed and approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee.
Complete peripheral blood counts were obtained using an automated cell counter (Sysmex, Kobe, Japan) 7 days after treatment. The accuracy of abnormal blood counts was verified by direct examination of blood smears. Renal and hepatic functions of the mice were also examined. Spleen weight and bone marrow (BM) cellularity were determined. The hematopoietic tissues were collected for clonogenic assays and histologic analysis.
Isolation of bone marrow
Bone marrow cells were flushed from the tibiae and femurs using Iscoves modified Dulbecco media (IMDM; Gibco-BRL, Gaithersburg, MD) containing 5% fetal calf serum (FCS; Hyclone Laboratories, Logan, UT). Low-density mononuclear cells (LDMNCs) were prepared by centrifugation on ficoll-hypaque (density, 1.119; Sigma, St Louis, MO).28
Hematopoietic progenitor growth
Recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin 3 (IL-3), and stem cell factor (SCF) were obtained from Peprotech; recombinant murine IL-1 (mIL-1) and macrophage CSF (M-CSF) were purchased from R&D Research Laboratories (Minneapolis, MN). LDMNCs were placed into culture in triplicate 35-mm plates (Becton Dickinson, Franklin Lakes, NJ) at a final concentration of 2 × 104 LDMNCs or 5 × 105 spleen LDMNCs per plate. To culture low proliferating potential colony-forming cells (LPP-CFCs) and high proliferating potential colony-forming cells (HPP-CFCs), LDMNCs and recombinant growth factors were added to (0.66%-1.0%) agar, and the solution was thoroughly mixed before plating as previously described.35 Growth factors used for culture of HPP-CFCs and LPP-CFCs included SCF, IL-1, M-CSF, GM-CSF, and IL-3.35 To evaluate growth-inhibitory effects of IFN-γ on progenitor cells, LDMNCs were plated in the absence or presence of IFN-γ (0.3-1 ng/mL) in clonogenic assays as described elsewhere.28
Evaluation of IFN-γ as a myelopreparative conditioning agent
Fancc–/–, Fanca–/–, Fancg–/–, and WT mice (CD45.2+) were treated with 400 μg/kg per day IFN-γ or PBS for 7 days as described in “Treatment and monitoring” and then received transplants of 1 × 107 BM nucleated B6.BoyJ cells (CD45.1+). The percentage of CD45.1+ cells in the peripheral blood was analyzed at 1, 4, and 6 months after transplantation as described previously.11,36 To evaluate multilineage reconstitution of donor CD45.1+ cells, peripheral blood of IFN-γ–treated or untreated mice was costained with phycoerythrin (PE)–conjugated lineage marker antibodies (Gr1, Mac1, B220, and CD3; BD PharMingen, San Diego, CA) and fluorescein isothiocyanate (FITC)–conjugated CD45.1 antibody (BD PharMingen) and analyzed by fluorescence cytometry 6 months following transplantation. Six months following transplantation, secondary transplantation studies were conducted using BM cells from IFN-γ–treated or untreated recipients as donor cells.
Statistical analyses
Computations were carried out using the Graph Pad Prism software package (San Diego, CA). Statistical comparisons among all groups were compared using analysis of variance and Student t test. A P value less than .05 was considered statistically significant.
Results
Fanca–/– and Fancg–/– progenitors are hypersensitive to IFN-γ treatment in vitro
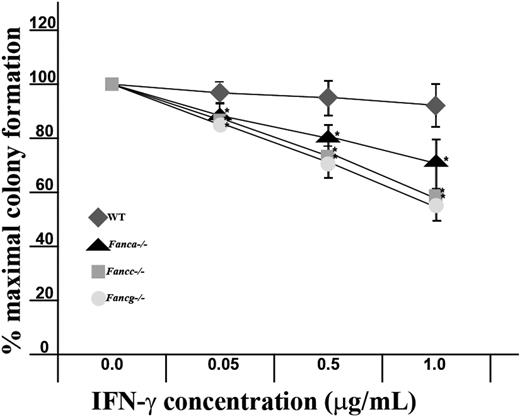
To compare the sensitivity of Fanca–/– and Fancg–/– myeloid progenitors to Fancc–/– and WT myeloid progenitors, clonogenic assays for the growth of CFU-GMs were established in a range of IFN-γ concentrations. The mean ± the standard error of the mean of 4 independent experiments is shown in Figure 1. Consistent with previous studies,27,28 Fancc–/– myeloid progenitors were hypersensitive to IFN-γ compared with WT control cells. Fanca–/– and Fancg–/– progenitors exhibited a sensitivity to IFN-γ that was comparable with Fancc–/– myeloid progenitors.
In vivo administration of IFN-γ results in a reduction of primitive and mature hematopoietic progenitor cell populations in Fanca–/– and Fancg–/– mice
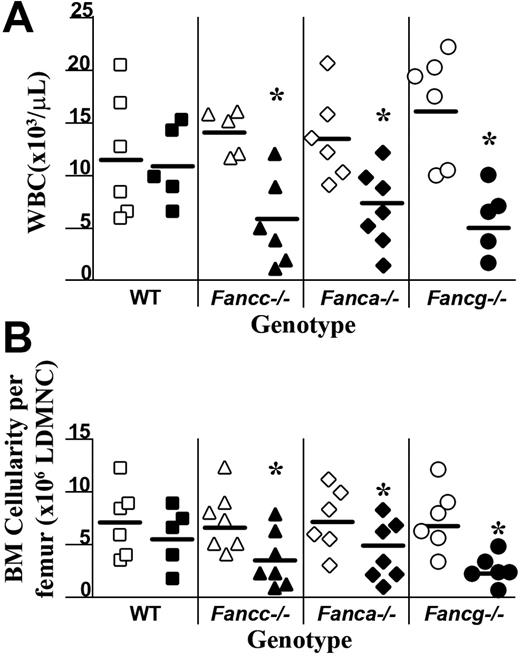
To determine whether Fanca–/– and Fancg–/– myeloid progenitors are hypersensitive to IFN-γ in vivo comparable with concentrations used clinically,23,24 osmotic micropumps containing IFN-γ were implanted subcutaneously into the backs of age-matched Fanca–/–, Fancg–/–, Fancc–/–, and WT mice to deliver 400 μg/kg per day IFN-γ or PBS alone for 7 days. The peripheral white blood cell (WBC) counts of all genotypes were similar following the administration of PBS (Figure 2A). Administration of IFN-γ had no significant reduction in the peripheral WBC count of WT mice following a 7-day infusion of 400 μg/kg per day IFN-γ. Similarly, IFN-γ treatment had no effect on the hematocrit or platelet counts in any genotype compared with mice treated with vehicle control (data not shown). However, the peripheral WBC counts of Fanca–/– and Fancg–/– mice had a significant reduction in peripheral WBC counts, similar to that observed in Fancc–/– mice. A similar decrease in bone marrow cellularity was observed in all complementation groups of FA-deficient mice (Figure 2B).
Evaluation of the sensitivity of Fanca–/– and Fancg–/– myeloid progenitors to IFN-γ. Methylcellulose cultures that promote the growth of myeloid progenitors from FA-deficient bone marrow were established containing a range of concentrations of IFN-γ in triplicate wells. The respective genotypes are indicated. Data represent the mean ± standard error of the mean (SEM) of 4 independent experiments. *P < .01 comparing the IFN-γ–dependent reduction in myeloid progenitors of Fancc–/–, Fanca–/–, and Fancg–/– myeloid progenitors to wild-type controls using analysis of variance.
Evaluation of the sensitivity of Fanca–/– and Fancg–/– myeloid progenitors to IFN-γ. Methylcellulose cultures that promote the growth of myeloid progenitors from FA-deficient bone marrow were established containing a range of concentrations of IFN-γ in triplicate wells. The respective genotypes are indicated. Data represent the mean ± standard error of the mean (SEM) of 4 independent experiments. *P < .01 comparing the IFN-γ–dependent reduction in myeloid progenitors of Fancc–/–, Fanca–/–, and Fancg–/– myeloid progenitors to wild-type controls using analysis of variance.
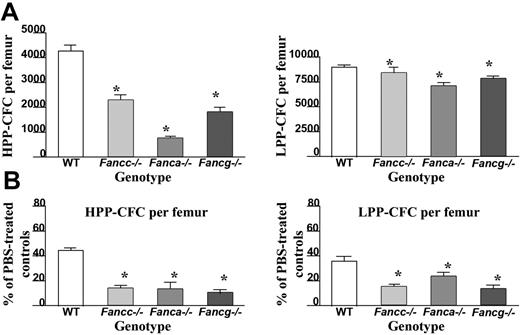
In order to determine the effect of IFN-γ treatment on primitive and mature hematopoietic progenitors in vivo, high proliferative potential colony-forming cells (HPP-CFC) and low proliferative potential colony-forming cells (LPP-CFC) were cultured from bone marrow of the respective genotypes. Even in the absence of IFN-γ treatment, Fanca–/– and Fancg–/– mice, similar to Fancc–/– mice, had a reduction in the number of HPP-CFCs per femur compared with WT mice (Figure 3A). Of importance, following administration of IFN-γ, a dramatic reduction in the number of HPP-CFCs and LPP-CFCs was observed in all 3 FA complementation groups compared with equivalently-treated WT mice (Figure 3B). Similar reductions were observed in burst-forming unit–erythroid (BFU-E) and multipotent myeloid progenitors (CFU-GEMM) (data not shown). Collectively, these data indicate that in vivo IFN-γ treatment preferentially reduces the number of primitive and mature myeloid progenitors in all 3 genotypes of FA-deficient mice.
Effects of in vivo IFN-γ treatment on peripheral white blood cell (WBC) counts and bone marrow cellularity. (A) Peripheral nucleated cell counts were obtained from experimental mice following completion of IFN-γ treatment (closed symbols) or the phosphate-buffered saline vehicle, PBS (open symbols). Each symbol represents total nucleated white blood cells from individual mice. Bars represent the mean WBC count of all experimental mice in the respective treatment group. *P < .01 comparing white blood cell counts of IFN-γ–treated mice to vehicle-treated control mice with the same genotype. (B) BM cellularity following IFN-γ treatment. Symbols represent bone marrow cellularity of individual mice. Bars represent the mean BM cellularity of all mice in the experimental group. *P < .01 comparing bone marrow cellularity of IFN-γ–treated mice versus vehicle-treated controls of the same genotype.
Effects of in vivo IFN-γ treatment on peripheral white blood cell (WBC) counts and bone marrow cellularity. (A) Peripheral nucleated cell counts were obtained from experimental mice following completion of IFN-γ treatment (closed symbols) or the phosphate-buffered saline vehicle, PBS (open symbols). Each symbol represents total nucleated white blood cells from individual mice. Bars represent the mean WBC count of all experimental mice in the respective treatment group. *P < .01 comparing white blood cell counts of IFN-γ–treated mice to vehicle-treated control mice with the same genotype. (B) BM cellularity following IFN-γ treatment. Symbols represent bone marrow cellularity of individual mice. Bars represent the mean BM cellularity of all mice in the experimental group. *P < .01 comparing bone marrow cellularity of IFN-γ–treated mice versus vehicle-treated controls of the same genotype.
Enhanced engraftment of WT hematopoietic cells in Fanca–/– and Fancg–/– mice pretreated with IFN-γ
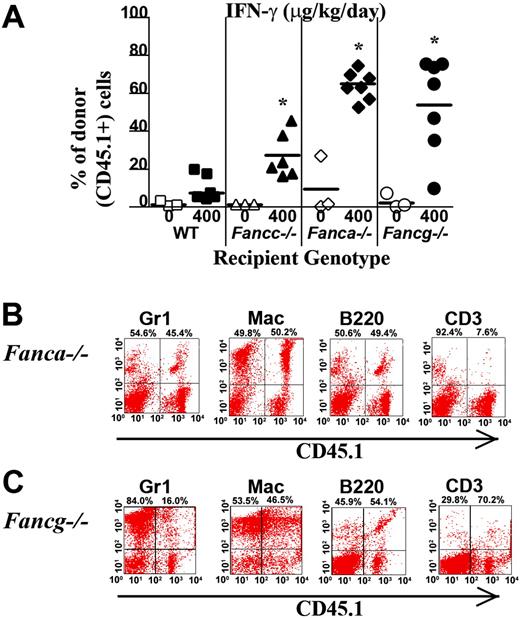
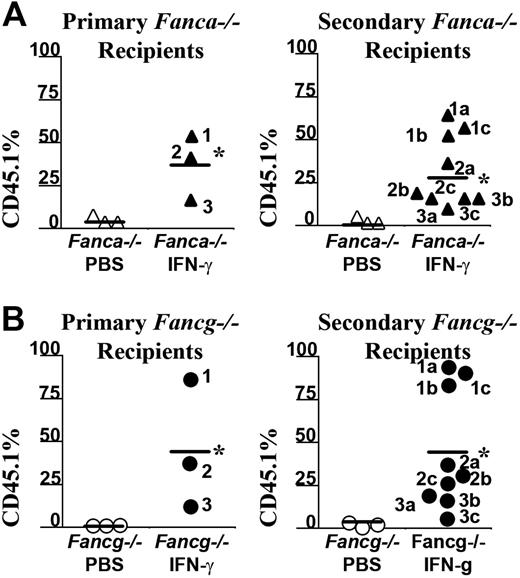
To determine whether the myelopreparative protocol with IFN-γ is sufficient to allow engraftment and proliferation of isogenic WT repopulating cells, 107 WT CD45.1+ BM nucleated cells were transplanted into Fanca–/–, Fancg–/–, Fancc–/–, and WT animals pretreated with IFN-γ or PBS. Figure 4A shows the chimerism of individual recipients in 1 of 2 independent experiments with similar results. The percentage of CD45.1+ donor cells in peripheral blood was examined using fluorescence cytometry 4 months following transplantation. Isogenic cells failed to engraft and contribute to hematopoiesis in any of the 3 respective FA or WT experimental groups following treatment with vehicle only (Figure 4A). IFN-γ was not sufficient to allow engraftment of exogenous, isogenic BM cells into WT recipients (Figure 4A). However, both Fanca–/– and Fancg–/– recipients treated with IFN-γ prior to transplantation had a significant donor chimerism in peripheral blood 4 months following transplantation comparable with the donor chimerism of Fancc–/– mice. Representative FACS analyses showing multilineage reconstitution in Fanca–/– and Fancg–/– mice (Figure 4B-C) are shown.
Evaluation of IFN-γ on primitive and mature myeloid progenitor numbers isolated from the BM of Fanca–/–, Fancg–/–, and Fancc–/– mice. (A) Evaluation of myeloid progenitors from WT, Fancc–/–, Fanca–/–, and Fancg–/– bone marrow. Hematopoietic cells derived from the bone marrow LDMNCs from mice of each respective genotype were cultured in triplicate to evaluate growth of high proliferating potential colony-forming cells (HPP-CFCs) and low proliferating potential colony-forming cells (LPP-CFCs). Data represent the mean ± standard error of the mean (SEM) of 3 independent experiments. (B) Bone marrow cells from WT, Fancc–/–, Fanca–/–, and Fancg–/– mice previously treated with a 7-day course of 400 μg/kg per day IFN-γ or vehicle control were cultured at 2 × 104 BM LDMNCs/mL to determine the reduction in the respective populations of progenitors. Data represent the mean ± standard error of the mean (SEM) of 3 independent experiments.
Evaluation of IFN-γ on primitive and mature myeloid progenitor numbers isolated from the BM of Fanca–/–, Fancg–/–, and Fancc–/– mice. (A) Evaluation of myeloid progenitors from WT, Fancc–/–, Fanca–/–, and Fancg–/– bone marrow. Hematopoietic cells derived from the bone marrow LDMNCs from mice of each respective genotype were cultured in triplicate to evaluate growth of high proliferating potential colony-forming cells (HPP-CFCs) and low proliferating potential colony-forming cells (LPP-CFCs). Data represent the mean ± standard error of the mean (SEM) of 3 independent experiments. (B) Bone marrow cells from WT, Fancc–/–, Fanca–/–, and Fancg–/– mice previously treated with a 7-day course of 400 μg/kg per day IFN-γ or vehicle control were cultured at 2 × 104 BM LDMNCs/mL to determine the reduction in the respective populations of progenitors. Data represent the mean ± standard error of the mean (SEM) of 3 independent experiments.
IFN-γ treatment of Fanca–/– and Fancg–/– recipients is sufficient to allow engraftment of syngeneic WT bone marrow cells. (A) CD45.1+ WT BM nucleated cells (107) were injected into the tail vein of Fanca–/– and Fancg–/– C57Bl/6 recipients that express the CD45.2 antigen. Recipients were pretreated with IFN-γ or vehicle control. The percentage of CD45.1+ cells in the peripheral blood was determined by fluorescence cytometry 6 months following transplantation. WT and Fancc–/– mice treated with PBS or IFN-γ were used as controls. Data points represent CD45.1+ chimerism of individual mice. Bars represent the mean CD45.1+ chimerism. *P < .001 comparing chimerism of Fanca–/– or Fancg–/– recipients treated with IFN-γ versus vehicle-treated Fanca–/– recipients and Fancg–/– recipients. Multilineage analysis of donor CD45.1 cells in representative (B) Fanca–/– and (C) Fancg–/– mice. The percentage of WT CD45.1+ lymphoid (CD3 and B220) and myeloid (Gr1 and Mac1) cells is shown in the top right corner of each fluorescence-activated cell sorter (FACS) profile.
IFN-γ treatment of Fanca–/– and Fancg–/– recipients is sufficient to allow engraftment of syngeneic WT bone marrow cells. (A) CD45.1+ WT BM nucleated cells (107) were injected into the tail vein of Fanca–/– and Fancg–/– C57Bl/6 recipients that express the CD45.2 antigen. Recipients were pretreated with IFN-γ or vehicle control. The percentage of CD45.1+ cells in the peripheral blood was determined by fluorescence cytometry 6 months following transplantation. WT and Fancc–/– mice treated with PBS or IFN-γ were used as controls. Data points represent CD45.1+ chimerism of individual mice. Bars represent the mean CD45.1+ chimerism. *P < .001 comparing chimerism of Fanca–/– or Fancg–/– recipients treated with IFN-γ versus vehicle-treated Fanca–/– recipients and Fancg–/– recipients. Multilineage analysis of donor CD45.1 cells in representative (B) Fanca–/– and (C) Fancg–/– mice. The percentage of WT CD45.1+ lymphoid (CD3 and B220) and myeloid (Gr1 and Mac1) cells is shown in the top right corner of each fluorescence-activated cell sorter (FACS) profile.
To confirm that IFN-γ myelopreparation was sufficient to allow engraftment of donor long-term repopulating stem cells, 2 million BM LDMNCs from selected primary recipients of a second cohort were transplanted into irradiated, secondary recipients and their chimerism was evaluated 6 months following transplantation (Figure 5A-B). The chimerism of recipients that received BM cells from Fanca–/– or Fancg–/– mice previously given the vehicle control only as a myelopreparative agent was low as expected. However, the chimerism of secondary recipients who received bone marrow cells from IFN-γ–treated Fanca–/– (Figure 5A) and Fancg–/– (Figure 5B) recipients consistently retained high chimerism. The bone marrow and splenic cellularity of these recipients at postmortem exam was normal comparable with previous studies in Fancc–/– mice (data not shown). Collectively, these data indicate that in vivo administration of IFN-γ in Fanca–/– and Fancg–/– mice is sufficient as a myelopreparative agent to enhance the engraftment of repopulating syngeneic WT cells in Fanca–/– and Fancg–/– mice.
Discussion
Although palliative therapy has improved the survival of patients with Fanconi anemia over the past 10 years,1 bone marrow failure remains the leading cause of death.37 Allogenic bone marrow transplantation or cord blood transplantation is available to a subset of these patients.2 However, for individuals who lack an HLA-compatible donor, non–HLA-matched transplantation is rarely used because of the particularly high morbidity of this therapy in FA patients.38 Thus transplantation of genetically-corrected autologous stem cells remains an important potential therapy for this uniformly fatal disease.
In addition to the usual challenges in gene transfer protocols, FA disease itself creates an additional set of potential obstacles. Patients that are considered for gene transfer often have bone marrow hypoplasia.37 In addition, emerging data in FA patients39 and in Fancc–/– mice40 suggests that granulocyte colony-stimulating factor, the current cornerstone of stem cell mobilization, inefficiently mobilizes FA-deficient stem/progenitor cells. Further, the propensity of Fancc–/– cells to apoptosis ex vivo12 and the known challenges in homing of ex vivo–cultured cells41 are additional challenges in having an adequate graft of genetically-transduced cells. These observations, together with previous clinical data showing that patients with FA have an increased risk for secondary malignancies following genotoxins,19,20,42-44 have provided the impetus for considering IFN-γ as a myelopreparative conditioning agent in FA preclinical models as a proof of concept.
Given previous in vitro and in vivo studies demonstrating that Fancc–/– cells are hypersensitive to IFN-γ, we evaluated whether a similar phenotype is present in other FA complementation groups. In 2 independent studies (6-7 mice per study group), we found that continuous subcutaneous infusion of IFN-γ enhances the engraftment of WT cells in Fanca–/– and Fancg–/– mice. The high level of engraftment was maintained in secondary recipients, indicating the engraftment of long-term repopulating stem cells. The treatment of IFN-γ was well tolerated in all the genotyped mice tested in the study. No severe hematologic sequelae were observed in the IFN-γ–treated mice in either primary or secondary recipients. The establishment of the IFN-γ BM conditioning in Fanca–/–, Fancg–/–, and Fancc–/– models could have implications in using this approved drug in the clinic as a means to enhance the engraftment of exogenous genetically-corrected stem/progenitor cells.
IFN-γ treatment of Fanca–/– recipients is sufficient to allow long-term engraftment of syngeneic WT bone marrow cells. Six months following transplantation with CD45.1+ WT donor BM nucleated cells, selected recipients from a second cohort of (A) Fanca–/– or (B) Fancg–/– mice received transplants from secondary recipients to verify that the engrafting CD45.1 donor cells were long-term repopulating stem cells. The CD45.1+ chimerism of each primary recipient (left panels) and the chimerism of 3 secondary recipients (right panels) from each respective primary recipient are indicated. Bars represent the mean CD45.1+ chimerism. *P < .001 comparing chimerism of Fanca–/– and Fancg–/– recipients treated with IFN-γ versus vehicle-treated Fanca–/– and Fancg–/– recipients.
IFN-γ treatment of Fanca–/– recipients is sufficient to allow long-term engraftment of syngeneic WT bone marrow cells. Six months following transplantation with CD45.1+ WT donor BM nucleated cells, selected recipients from a second cohort of (A) Fanca–/– or (B) Fancg–/– mice received transplants from secondary recipients to verify that the engrafting CD45.1 donor cells were long-term repopulating stem cells. The CD45.1+ chimerism of each primary recipient (left panels) and the chimerism of 3 secondary recipients (right panels) from each respective primary recipient are indicated. Bars represent the mean CD45.1+ chimerism. *P < .001 comparing chimerism of Fanca–/– and Fancg–/– recipients treated with IFN-γ versus vehicle-treated Fanca–/– and Fancg–/– recipients.
Authorship
Conflict-of-interest disclosure: the authors declare no competing financial interests.
Prepublished online as Blood First Edition Paper, August 31, 2006; DOI 10.1182/blood-2006-03-007997.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
The authors would like to acknowledge Dr Alan D'Andrea at the Dana Farber Cancer Research Institute at Harvard University for providing Fancg–/– mice. We also thank Natascha Karlova for preparing the paper.
This work was supported by National Institutes of Health (NIH) grant P01HL053586.