Abstract
Standard first-line treatment for elderly multiple myeloma (MM) patients ineligible for stem cell transplantation is melphalan plus prednisone (MP). However, complete responses (CRs) are rare. Bortezomib is active in patients with relapsed MM, including elderly patients. This phase 1/2 trial in 60 untreated MM patients aged at least 65 years (half older than 75 years) was designed to determine dosing, safety, and efficacy of bortezomib plus MP (VMP). VMP response rate was 89%, including 32% immunofixation-negative CRs, of whom half of the IF– CR patients analyzed achieved immunophenotypic remission (no detectable plasma cells at 10–4 to 10–5 sensitivity). VMP appeared to overcome the poor prognosis conferred by retinoblastoma gene deletion and IgH translocations. Results compare favorably with our historical control data for MP—notably, response rate (89% versus 42%), event-free survival at 16 months (83% versus 51%), and survival at 16 months (90% versus 62%). Side effects were predictable and manageable; principal toxicities were hematologic, gastrointestinal, and peripheral neuropathy and were more evident during early cycles and in patients aged 75 years or more. In conclusion, in elderly patients ineligible for transplantation, the combination of bortezomib plus MP appears significantly superior to MP, producing very high CR rates, including immunophenotypic CRs, even in patients with poor prognostic features.
Introduction
For many years, the standard of care for patients with multiple myeloma (MM) has been melphalan plus prednisone (MP).1-5 Worldwide, MP is commonly used, with response rates of about 50%, although complete responses (CRs) are rare. Median duration of response is 1.5 years, median time to progression (TTP) is 18 months, and median overall survival (OS) is 2 to 3 years.4-6 The only treatment that has shown a significant survival advantage over conventional chemotherapy is high-dose therapy (HDT) supported by autologous stem cell transplantation (ASCT), which yields high CR or near CR (nCR) rates.7,8 In the Intergroupe Français du Myélome trial, 5-year survival rates were 52% with ASCT versus 12% with conventional chemotherapy.7 In the Medical Research Council Myeloma VII trial, median OS was 54 months versus 42 months, respectively.8 However, neither the Spanish (PETHEMA) myeloma trial nor the US Intergroup Trial 59321 demonstrated a significant difference in OS between ASCT and conventional chemotherapy after long-term follow-up.9,10
HDT with ASCT is now considered standard therapy for younger patients. However, the elderly, patients in poor physical condition, and those with comorbidities are often not candidates for ASCT because of increased toxicity and reduced yield of CD34+ cells.11-13 Because approximately half of MM patients are aged more than 70 years at diagnosis14 and therefore unlikely to be candidates for ASCT, there is an urgent need for more active and less toxic therapies for elderly patients. New treatments that produce high CR rates are needed, because CR is considered an important prognostic factor for survival.7,15-21 Recently, 2 randomized studies have shown significant benefits for the combination of MP plus thalidomide compared with MP, including higher CR rates and longer TTP and OS.22,23
Bortezomib (VELCADE; Millennium Pharmaceuticals, Cambridge, MA, and Johnson & Johnson Pharmaceutical Research and Development, Beerse, Belgium) is the first proteasome inhibitor to enter the clinic. It is a reversible inhibitor of the 26S proteasome, an enzyme involved in the catabolic pathway for numerous intracellular regulatory proteins.24-28 Based on the results of two phase 2 trials29,30 and a randomized phase 3 trial,31 bortezomib has become a standard of care in relapsed MM.
Preclinical trials have demonstrated in vitro synergy when bortezomib is administered in combination with a wide range of cytotoxic agents, including melphalan.32,33 The combination of bortezomib and melphalan, administered at doses lower than their single-agent doses, was active and well tolerated in a phase 1/2 trial in relapsed and/or refractory MM.34,35 In the first-line setting, bortezomib monotherapy and bortezomib-based combinations have yielded high CR/nCR rates, of up to 29%, when given prior to ASCT.36-39 Despite extensive clinical experience, there are no data from prospective trials evaluating bortezomib specifically in the elderly. A subgroup analysis of elderly patients (aged 65 years and older) in the phase 3 Assessment of Proteasome Inhibition for Extending Remissions (APEX) trial in relapsed/refractory MM following 1 to 3 prior therapies showed that bortezomib was significantly more active than dexamethasone and was as well tolerated as in younger patients.40
Because the efficacy of both bortezomib and MP is well established and both therapies are widely used in MM, it may be possible to improve the response rate, and ultimately survival, by combining these therapies. The differing safety profiles of bortezomib and melphalan, and the tolerability of bortezomib in elderly patients, lend further support to their investigation in combination.
The primary objectives of this phase 1/2 study were to identify the most appropriate dose of bortezomib in combination with a standard MP treatment regimen (phase 1) and to determine the efficacy of bortezomib plus MP (VMP) in terms of response rate (phase 2). Secondary objectives were to determine the safety and tolerability of VMP; assess efficacy in terms of OS, progression-free survival (PFS), and duration of response; and compare the efficacy of VMP with historical controls receiving MP alone.41 In addition, the study was designed to explore whether cytogenetic abnormalities, such as retinoblastoma (Rb) gene deletions and immunoglobulin heavy-chain (IgH) translocations, are predictive of response to VMP.
Patients, materials, and methods
Patient selection
Eligible patients were required to have the following: newly diagnosed symptomatic MM with measurable disease,42 age of at least 65 years, Karnofsky performance status (KPS) of at least 60%, and a life expectancy of more than 3 months. Patients were ineligible if they had previously received any anti-MM treatment including bortezomib, any investigational drugs within 14 days, or major surgery within 4 weeks of enrollment. Patients were also ineligible if they were HIV positive or hepatitis-B surface antigen positive or had active hepatitis C infection, myocardial infarction within 6 months of enrollment, or New York Heart Association class III or IV heart failure. Patients were excluded if, within 14 days prior to enrollment, they had a platelet count below 100 × 109/L, hemoglobin below 80 g/L (8 g/dL), absolute neutrophil count below 1.0 × 109/L, serum creatinine above 177 μM (2 mg/dL), corrected serum calcium above 3.5 mM (14 mg/dL), or at least grade 2 peripheral neuropathy.
Study design
This open-label, phase 1/2, dose-escalation study was carried out at 19 centers in Spain for the PETHEMA Foundation. The study was conducted in accordance with International Conference on Harmonisation Good Clinical Practice guidelines and the Declaration of Helsinki and was approved by the Institutional Review Board/Independent Ethics Committee at each participating center. All patients provided written informed consent before screening. Data were monitored by an independent/external contract research organization.
Treatment schedule
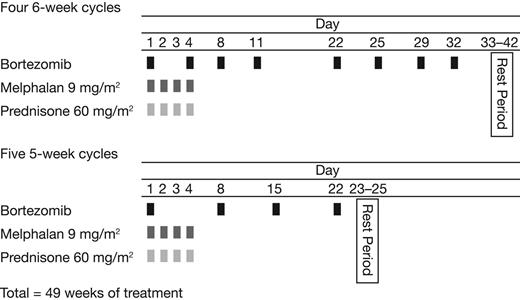
Treatment comprised an initial phase consisting of 4 6-week cycles of bortezomib 1.0 or 1.3 mg/m2 on days 1, 4, 8, 11, 22, 25, 29, and 32, followed by a 10-day rest period, in combination with oral melphalan 9 mg/m2 and oral prednisone 60 mg/m2, both on days 1 to 4 (Figure 1). Each cycle was equivalent to 2 standard bortezomib monotherapy cycles.31 This was followed by a maintenance phase comprising five 5-week cycles of bortezomib 1.0 or 1.3 mg/m2 on days 1, 8, 15, and 22, followed by a 13-day rest period, in combination with melphalan and prednisone as above (Figure 1).
Phase 1: identification of maximum tolerated dose (MTD)
Bortezomib was to be administered at a dose of 1.0 mg/m2 to the first cohort of 6 patients. If dose-limiting toxicities (DLTs) occurred in fewer than 2 of these patients, the next cohort of 6 patients was to receive a dose of 1.3 mg/m2. By contrast, if DLTs occurred in 2 or more of 6 patients or 4 or more of 12 patients, the previous dose level (or bortezomib 0.7 mg/m2 if DLTs occurred at the first dose level) would be identified as the MTD. Intrapatient dose escalation was not permitted. The starting dose of 1.0 mg/m2 bortezomib was selected on the basis of a phase 1 dose-escalation trial of bortezomib plus melphalan.34,35
The following were defined as DLTs: grade 3/4 peripheral neuropathy persisting for more than 3 weeks after discontinuation of bortezomib; any hematologic toxicity of grade 4 intensity or preventing administration of 3 or more of the 8 bortezomib doses of the first treatment cycle; grade 3/4 febrile neutropenia; grade 3/4 gastrointestinal toxicities (except for grade 3 nausea/vomiting if the patient had not received adequate antiemetic prophylaxis); and any other grade 3/4 nonhematologic toxicity considered related to VMP by the principal investigator. Adverse events (AEs) were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE, version 3.0). MTD determination was based on occurrence of DLTs during the first induction treatment cycle only.
Phase 2: expanded cohort
After identification of the MTD, it was planned for the dose level to be expanded to include up to a total of 60 patients at the MTD for the phase 2 part of the study. A full treatment course was the same as for phase 1 and is outlined in Figure 1. Treatment beyond 49 weeks was not permitted. Patients maintaining a confirmed CR for 2 treatment cycles beyond CR confirmation (minimum 10 weeks) were withdrawn from the study, as were patients who developed progressive disease (PD), experienced unacceptable toxicity, or withdrew consent.
The VMP schedule was based on the standard bortezomib monotherapy dosing schedule. The treatment consisted of 4 6-week cycles followed by the maintenance phase consisting of 5 5-week cycles, giving a total of 49 weeks of treatment.
The VMP schedule was based on the standard bortezomib monotherapy dosing schedule. The treatment consisted of 4 6-week cycles followed by the maintenance phase consisting of 5 5-week cycles, giving a total of 49 weeks of treatment.
Dose modifications
In patients with any grade 4 hematologic toxicity observed on day 43 of the 6-week cycle or day 36 of a 5-week cycle that was considered by the investigator to be study drug related, the cycle was delayed for up to 2 weeks until resolution to baseline or grade 1 or lower. In patients with at least grade 3 or nonhematologic toxicity that was considered by the investigator to be study drug related, chemotherapy was held until resolution to baseline or grade 1 or lower. Standard bortezomib dose reduction was applied in patients experiencing neuropathic pain and/or peripheral sensory neuropathy. During the phase 2 part of the study, the bortezomib dose was reduced (from 1.3 to 1.0 mg/m2 or from 1.0 to 0.7 mg/m2) if 3 or more of the 8 bortezomib doses in any of the 6-week cycles had to be missed. If patients developed any grade 4 hematologic toxicity during week 3 or 6 of the 6-week cycles, the melphalan dose was reduced by 25% for the subsequent cycle. If patients developed renal insufficiency (creatinine at least 177 μM [2 mg/dL]), the melphalan dose was reduced by 50%; patients were withdrawn if creatinine was above 354 μM [4 mg/dL]. Up to 2 dose reductions of melphalan or bortezomib were permitted for each drug. Prednisone dose reductions were permitted for grade 3 or 4 corticosteroid toxicities.
Concomitant medications
All patients received intravenous bisphosphonates every 4 weeks during the study. Supportive therapy for MM (eg, erythropoietin, G-CSF, platelet or red blood cell transfusions) was allowed.
Study assessments
Screening tests carried out 14 or fewer days before the study's start included medical history, physical examination, hematology, clinical chemistries, posteroanterior and lateral chest x-rays, electrocardiogram, bone scans, and bone marrow aspiration for morphology, flow cytometry (including S-phase analysis), and cytogenetic studies. Blood and 24-hour urine samples for quantitation of M protein and immunoglobulins (and assessment of M protein by immunofixation [IF] in serum) were collected from all patients.
Disease response was assessed according to the European Group for Blood and Marrow Transplantation criteria42 at the beginning of each treatment cycle (every 6 weeks during initial treatment and every 5 weeks during maintenance treatment), at the end-of-treatment visit, and at follow-up visits. In patients with secretory MM, blood and urine samples were collected at the start of each treatment cycle for quantitative M protein, immunoglobulin quantitation, and assessment of M protein by IF. KPS and clinical chemistry were assessed at the start of each treatment cycle. Hematologic parameters were assessed on bortezomib dosing days. All AEs (graded according to NCI CTCAE version 3.0) and use of concomitant medications and supportive therapies were recorded. All efficacy and safety assessments, including bone marrow analyses and skeletal surveys, were repeated at the end-of-treatment visit.
After completing treatment, all patients were monitored for response every 8 weeks for at least 6 months (follow-up period) and every 3 months thereafter for survival.
Minimal residual disease analysis by flow cytometry
As previously described, myelomatous plasma cells (PCs) can be unequivocally distinguished from normal PCs on the basis of aberrant expression of CD19, CD28, CD38, CD56, CD45, and CD117.43 This allows for the quantitation of residual myelomatous PCs following treatment. For this purpose we used a 2-step procedure in which up to 2 × 106 cells were acquired through a specific “live gate” drawn on a side scatter (SSC)/CD38strong+/CD138+ dot plot. A multiparametric analysis of antigenic expression was performed using Paint-A-Gate PRO software (Becton Dickinson, San Jose, CA). The sensitivity of this technique ranges between 10–4 and 10–5 (ie, identification of 1 residual PC among 10 000 to 100 000 normal cells). PC DNA ploidy status and cell cycle were analyzed as previously described using a double staining procedure for nuclear DNA (with propidium iodide) and surface PC antigen (CD38 and CD138).44
FISH analysis
Interphase fluorescence in situ hybridization (FISH) studies for the detection of IgH translocations were performed using LSI IGH Dual Color Break Apart Rearrangement Probe (Vysis, Downers Grove, IL). Patients with IgH translocations were analyzed for 11q13 partner (LSI IgH/CCND1 Dual Fusion Translocation Probe; Vysis), for 4p16 (BAC clones L75b9, L184d6, L190b4, L96a2; VH: cosmid; and IgH6-9/CH: B158 A2), and for 16q23 (BAC clones 356D21, 484H2, 10205 and 10206; kindly provided by R. Fonseca). The presence of 13q and 17p deletion was evaluated with a specific probe for RB LSI 13 (RB1) and P53-LSI P53 (17p13.1).45
Statistical analysis
A population of 60 patients provided 80% power to detect a 50% or higher response rate (CR + PR) with VMP versus 40% in historical controls receiving MP, tested at a 2-sided α level of 0.05.
The efficacy population (TTP, event-free survival [EFS], and OS) included all treated patients who received at least 1 dose of bortezomib per protocol. Patients who received at least 1 cycle of bortezomib were evaluable for response. Patients with a partial response (PR) were subdivided to show those with an nCR, defined as negative electrophoresis but IF positive. The secondary efficacy analysis was to determine whether VMP provided benefit over historical data for MP in a similar patient population in terms of response rate, PFS, and OS. Time-related end points were analyzed using the Kaplan-Meier method, adjusted for stratification factors (age, KPS, albumin, lactate dehydrogenase [LDH], C-reactive protein, β2-microglobulin, and bone marrow infiltration), and tested for a treatment difference versus the historical data using log-rank tests (2-sided, α= 0.05). Exploratory analyses were conducted to investigate whether potential prognostic variables, used as covariates in logistic regression (response rate), substantially affected the study conclusions.
TTP was calculated from the time of inclusion until the date of disease progression, with deaths due to causes other than progression not counted as an event but censored at that time point. EFS was calculated from the time of inclusion until the date of progression, relapse, death for any cause, or the date the patient was last known to be in remission. OS was calculated from the time of inclusion until the date of death for any cause or the date the patient was last known to be alive.
The historical control group consisted of 96 patients treated with MP. These correspond to patients in a randomized trial of MP versus melphalan plus dexamethasone (MD) recently reported by our group.41
The safety population included all patients who received at least 1 dose of bortezomib. Safety analyses were conducted based on incidence, intensity, and type of AE and on clinically significant changes in patients' physical examination findings, vital signs, and clinical laboratory results. All data were monitored by an independent/external contract research organization.
Results
Patient demographics and disposition
Between January 2004 and April 2005, 12 patients were enrolled in phase 1 (6 at bortezomib 1.0 mg/m2 and 6 at 1.3 mg/m2) and 48 patients in phase 2 (all at 1.3 mg/m2), giving a total of 60 patients for evaluation. Almost half of the patients were 75 years of age or older. Demographic and baseline characteristics, summarized in Table 1, were similar to the MP historical controls.41
All 60 patients received at least 1 dose of study drug; 7 failed to complete the first cycle of VMP (withdrawal of consent and early death in 3 patients each and diagnosis of lung cancer during the fourth week of the first cycle in 1 patient) and were therefore not evaluable for response. Nevertheless, all 60 patients received 1 dose and were evaluable for TTP, EFS, and OS as well as safety.
Identification of MTD
No DLTs occurred during phase 1. Two patients experienced grade 3 neutropenia, and 1 developed grade 3 thrombocytopenia, none of which were DLTs. Therefore, the 1.3 mg/m2 bortezomib dose level was expanded for phase 2.
Exposure to study drug
Delivery of planned doses was as follows: 37% of patients received all doses of bortezomib, 43% missed 2 to 4 doses, and 20% missed 5 or more doses. Mean duration of treatment was 7.5 months; at the data cutoff point for this report (April 2006), 79% of patients have completed at least the first 4 cycles of therapy, and 62% have received all planned cycles. The median number of cycles administered was 7 (range, 2-9 cycles), a duration of more than 9 months.
Efficacy
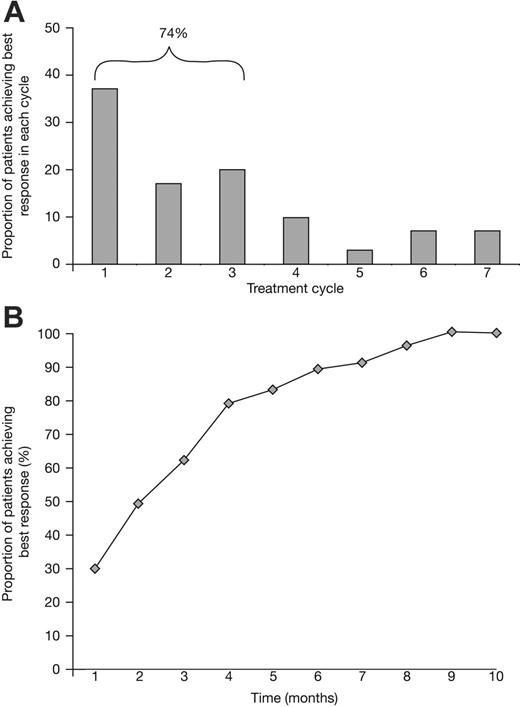
In the 53 patients who completed at least the first cycle, the response rate (CR + PR) was 89%, including 32% IF-negative CRs and 11% nCRs (Table 2). Responses were rapid: The response rate after the first cycle (6 weeks) was 70%, including 6% IF-negative CRs. Median time to response was 2.7 months (range, 1-10 months). The best response occurred within the first 3 cycles (18 weeks) in 74% of patients (Figure 2). Of 37 patients who completed scheduled treatment, 35% achieved CR, 11% nCR, and 46% PR for an overall 92% response rate. The patients who achieved CR underwent a median of 5 cycles of therapy (range, 3-8 cycles). Of 16 patients who discontinued treatment prematurely but were evaluable for response, 25% (n = 4) achieved CR, 12.5% nCR (n = 2), and 44% PR (n = 7) for an overall 81.5% response rate, similar to the total population. Notably, the response rate after just 1 cycle of VMP was higher than in the historical controls after 6 cycles of MP (42% response rate; 3% nCR, 39% PR) (Table 2).
Response to VMP was rapid. The percentage of responding patients achieving their best response to VMP is shown (A) by treatment cycle and (B) over time.
Response to VMP was rapid. The percentage of responding patients achieving their best response to VMP is shown (A) by treatment cycle and (B) over time.
After a median follow-up of 16 months (range, 11-24 months), median TTP was not reached. At 16 months, 91% of patients were free of disease progression, and the EFS rate was 83%. Six of the 11 events were disease progression, and 4 of these 6 patients were still alive. The projected overall 2-year survival rate is 86%. Median OS was not reached. As shown in Figure 3, these results compare favorably with PFS, EFS, and OS in our MP historical control (at 16 months, PFS was 91% versus 66% [P = .002]; EFS rate, 83% versus 51% [P < .001]; and OS rate, 90% versus 62% [P < .001]).
Time to events data in patients receiving VMP. (A) PFS, (B) EFS, and (C) OS of patients receiving VMP versus MP historical controls. The 16-month time point has been highlighted because it represents the median follow-up in patients treated with VMP.
Time to events data in patients receiving VMP. (A) PFS, (B) EFS, and (C) OS of patients receiving VMP versus MP historical controls. The 16-month time point has been highlighted because it represents the median follow-up in patients treated with VMP.
Among 17 patients achieving IF-negative CR, we assessed minimal residual disease in 12 using multiparametric flow cytometry. In 6 of these patients, no malignant PCs were detectable with a sensitivity level of 10–4 to 10–5, representing immunophenotypic remission. Exploratory logistic regression analyses demonstrated that none of the potential risk factors investigated (age older than 75 years, albumin below 35 g/L [3.5 g/dL], LDH above 460 U/L [460 mg/dL], C-reactive protein above 4 mg/L, β2-microglobulin 297 nM [3.5 mg/L] or more, and bone marrow infiltration 50% or more) was predictive of IF-negative CR. Further analyses using the same risk factors to compare patients with CR or PR (n = 47) versus those with stable disease (n = 6) demonstrated that only bone marrow infiltration of 50% or more was significantly associated with lack of response (P = .05).
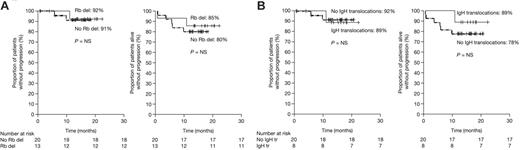
Cytogenetic information by FISH was available in 33 patients. To determine whether Rb gene deletion predicted response to VMP, we compared response rate among patients with (n = 13) and without (n = 20) Rb deletion. All patients with Rb deletion responded (Table 3). The response rate among patients with (n = 8) and without (n = 20) IgH translocations was almost identical (88% versus 82%, respectively; Table 3). Although the numbers are small, CRs/nCRs were observed not only in patients with t(11;14) (1 of 2) but also in patients with t(4;14) and t(14;16) (1 of 3, in each). PFS and EFS among patients with Rb deletion or IgH translocation were similar to those of patients without these cytogenetic alterations (Figure 4) and similar to those of the overall population. Finally, DNA ploidy status was analyzed in 35 patients; among 23 hyperdiploid and 12 diploid patients, the response rates were 87% (36% CR) and 100% (47% CR), respectively.
Safety
As mentioned in “Patient demographics and disposition,” of the 60 patients included in the study, 7 failed to complete the first cycle (early death in 3, withdrawal of informed consent in 3, and 1 diagnosis of lung cancer) and 37 patients (62%) completed treatment (24 received all planned cycles, and 13 achieved early CR and therefore stopped therapy after a median of 5 cycles; range, 3-8 cycles). The remaining 16 patients discontinued treatment due to the following reasons: withdrawal of consent in 3 patients (in cycles 2, 4, and 7) and AEs in 13 patients (peripheral neuropathy in 6 patients; severe infection, septic shock, grade 3 diarrhea, and sustained grade 4 thrombocytopenia that was possibly immune thrombocytopenic purpura in 1 patient each; and disease progression in 3 patients).
The most common AEs in the 60 safety-evaluable patients are shown in Table 4. The most common grade 3/4 AEs included hematologic toxicity (thrombocytopenia [51%] and neutropenia [43%]), peripheral neuropathy (17%), and diarrhea (16%). Despite the high rate of hematologic toxicity, the frequency of grade 3/4 infection was low (16%). Notably, the overall incidence of herpes zoster infection was 13% in the first 38 patients. Subsequently, the protocol was amended to recommend prophylactic acyclovir. After this, only 2 of 30 patients who followed protocol developed herpes zoster infection. Sixteen (27%) patients received G-CSF support. Most AEs occurred during the first 2 treatment cycles (Table 4).
The bortezomib dose was reduced in 14 (23%) patients and interrupted in 8 (13%). Among patients whose bortezomib dose was reduced, 10 required a single reduction to 1.0 mg/m2 (peripheral neuropathy in 5 patients, hematologic toxicity in 3 patients, nonhematologic toxicity in 2 patients) and 4 required a further reduction to 0.7 mg/m2, all for peripheral neuropathy. A further 2 patients required melphalan dose reductions to 6.75 mg/m2 for hematologic toxicity.
Seven (12%) patients died during study treatment. Of these, 4 were early deaths (during the first 6 weeks of treatment), in patients aged more than 75 years, due to pulmonary thromboembolism, septic shock with grade 3 neutropenia, pulmonary hypertension with right ventricular insufficiency, and lung cancer diagnosed during the fourth week of the first cycle. The remaining 3 deaths were from disease progression in 2 patients and septic shock in the third, who achieved CR and died after the third cycle of therapy. None of the deaths was considered related to any of the study drugs.
Influence of cytogenetic abnormalities on PFS and EFS. The graphs demonstrate the effect of (A) retinoblastoma gene deletion (Rb del) and (B) IgH translocations (IgH tr) on PFS and EFS. NS indicates not significant (P > .05).
Influence of cytogenetic abnormalities on PFS and EFS. The graphs demonstrate the effect of (A) retinoblastoma gene deletion (Rb del) and (B) IgH translocations (IgH tr) on PFS and EFS. NS indicates not significant (P > .05).
Discussion
Our study aimed to determine the recommended dose of bortezomib in combination with oral MP in elderly MM patients ineligible for ASCT and to investigate the efficacy and safety of VMP. The baseline characteristics of patients included in the present study appear to be broadly representative of this patient population. The inclusion of elderly patients is of particular interest: Almost half of the study population was aged more than 75 years, and 17% were older than 80. No patients in the dose-escalation phase of the study experienced DLTs, and therefore we used a bortezomib dose of 1.3 mg/m2 to further evaluate VMP. Our results demonstrate that in elderly untreated patients with MM who are not suitable for transplantation, VMP is highly active and well tolerated. The 32% IF-negative CR rate observed with VMP is noteworthy, because IF-negative CR is considered an important predictor for survival.7,43,46-51 Moreover, response was of high quality, as demonstrated by multiparametric flow cytometry, and responses were independent of cytogenetic abnormalities.
Consequently, VMP represents an attractive option for elderly patients, for whom new treatment strategies are clearly needed. In this setting, encouraging results have recently been reported for the combination of thalidomide plus MP (MPT) in newly diagnosed elderly MM patients.22,23,52 An Italian randomized study demonstrated significant benefit with MPT compared with MP, with a response rate of 76% (16% CR, 12% nCR) for the MPT arm versus 48% (2% CR, 5% nCR) for MP. This was associated with a higher EFS rate (54% versus 27% at 2 years) and OS rate (80% versus 64% at 3 years).22 Similarly, the Intergroupe Français du Myélome has reported benefits in terms of median PFS (28 versus 17 months) and median OS (not reached at 55 months versus 30 months) of MPT compared with MP in a randomized study.23
Results from the present study compare favorably with our MP historical control data. After only 1 cycle of VMP we obtained a higher response rate (70%, including 6% CR, 2% nCR, 62% PR) than with 6 cycles of MP in the historical controls (42%, including 3% nCR, 39% PR). Furthermore, the proportion of CRs increased with additional treatment cycles up to 32% CRs plus 11% nCRs in the current study. Moreover, VMP yielded a longer EFS (at 16 months, 83% versus 51%) and OS (at 16 months, 90% versus 62%) than our MP historical control data.
Another notable finding of the present study was the pattern of response to VMP. Although responses occurred rapidly, quality of responses (IF-negative CRs) improved with subsequent cycles. This observation is consistent with results from the recent update of the large international phase 3 study of bortezomib (APEX) in which it was seen that, despite rapid initial response, best response to single-agent bortezomib as measured by M-protein reduction continues to improve over the treatment course, with approximately 20% of patients achieving maximum M-protein reduction in 8 3-week cycles or later.53 The slow but continuous activity of melphalan could also play a role in the improved response over time. In the present study, patients maintaining a CR for 2 treatment cycles after confirmed CR discontinued treatment. Therefore, it is possible that in some responding patients a substantially shorter course of treatment may be possible when bortezomib is added to MP, although this has not been evaluated prospectively.
None of the potential factors evaluated in the present study, including cytogenetic abnormalities, was found to predict response to VMP therapy. A 100% response rate was seen in 13 patients with Rb deletion, which is an independent prognostic factor for shorter survival,54,55 and poor response to conventional chemotherapy and tandem transplantations.56 A similar pattern was seen with IgH translocations, including t(4;14) and t(14:16), which are also predictive of poor prognosis.57 It is possible that the unique mechanism of action of bortezomib overcomes the influence of these adverse prognostic factors; this is consistent with the results of a recent analysis of the APEX trial, in which bortezomib appeared to overcome the adverse impact of del(13) on response rate.58 In addition, our study shows no influence of cytogenetic abnormalities on the PFS and EFS of patients treated with VMP.
VMP was well tolerated, and toxicities were predictable and manageable. Thirteen (21%) patients discontinued from the study because of unacceptable toxicity; in most cases, AEs could be managed by dose modification. The frequency and severity of asthenia, rash, and gastrointestinal side effects were similar to those observed in the APEX,31 Study of Uncontrollable Multiple Myeloma managed with proteasome Inhibition Therapy (SUMMIT),29 and Clinical Response and Efficacy Study of bortezomib in the Treatment of relapsing multiple myeloma (CREST)30 studies. However, we observed higher incidences of hematologic toxicity and peripheral neuropathy. The former can be clearly related to the safety profile of melphalan, leading to a higher incidence of hematologic AEs than in other trials of bortezomib as first-line therapy.36,38 Melphalan is typically associated with a relatively high incidence of neutropenia and thrombocytopenia. Therefore, the incidence of thrombocytopenia in this trial was not unexpected and is consistent with the known side effects of melphalan.48 Nevertheless, it should be emphasized that in the present study patients were evaluated twice a week while, in studies of MP or MPT, patients were only assessed every 4 to 6 weeks; this less frequent reporting may underestimate the incidence of side effects, particularly hematologic toxicity, which is generally only detected through cell counts. Overall toxicity was higher in patients aged 75 years or more, particularly anemia, infection, neutropenia, asthenia, and peripheral neuropathy. This could be related to the physical condition of these elderly patients.
Peripheral neuropathy occurred in half of the patients in the present study and was of grade 3/4 intensity in 17%. This figure is higher than in the APEX trial31 and could be related to the advanced age of patients in the current study. Consistent with this hypothesis, the incidence of grade 3 peripheral neuropathy in patients aged 75 years or more was much higher than in those aged less than 75 years. The presence of peripheral neuropathy at diagnosis is often underestimated in MM patients59 ; better recognition of underlying peripheral neuropathy may facilitate prompt dose reductions when mild symptoms develop in this fragile population of patients.
Of concern was the potential for cumulative toxicity with additional cycles of VMP. Our results do not support such a hypothesis: Indeed, the frequency of side effects decreased after cycle 3. Again, this may be related to the physical condition of elderly patients, because it is well known that major toxicities, including deaths, are particularly common within the first 2 months with increased tumor burden. Additionally, the rapid responses obtained with VMP may have contributed to the subsequent decrease in side effects. Interestingly, only 4 (7%) early deaths occurred in this study, which is similar in frequency to that we previously observed with MP (8%) or MD (14%) in similar patient populations.41 Among 3107 MM patients treated in Medical Research Council trials between 1980 and 2002, 299 (10%) died within 60 days of trial entry,60 again illustrating that the early death rate in the present study is comparable to that seen in studies of conventional agents.
Our study demonstrates that VMP is a highly active regimen for newly diagnosed patients with MM aged at least 65 years who are not candidates for ASCT. The CR/nCR rate was 43%: Most of these were IF-negative CRs (32%), and half of the IF-negative CR patients who were analyzed achieved immunophenotypic remission. On the basis of these promising results, VMP is being compared with MP in an international, phase 3 randomized trial (Velcade as Initial Standard Therapy in multiple myeloma: Assessment with melphalan and prednisone [VISTA]) in patients at least 65 years old not suitable for transplantation. In conclusion, VMP is a more effective first-line regimen than MP in patients not eligible for transplantation, offering new hope to these difficult-to-treat patients.
Prepublished online as Blood First Edition Paper, June 13, 2006; DOI 10.1182/blood-2006-04-019778.
Supported by the PETHEMA (Programa para el Estudio de la Terapéutica en Hemopatía Maligna) Foundation, Spain, Millennium Pharmaceuticals, and Johnson & Johnson Pharmaceutical Research and Development.
Two of the authors (D.-L.E., and H.v.d.V.) are employed by companies (Millennium Pharmaceuticals, Inc, and Johnson & Johnson Pharmaceutical Research and Development, LLC, respectively) whose product (VELCADE [bortezomib]) was studied in the present work.
M.-V.M., R.G.-S., J.B., F.P., F.d.A., D.-L.E., and J.-F.S.M. were involved in conception and design of the study. M.-V.M., J.-M.H., M.-T.H., N.C.G., L.P., M.F., J.D.-M., J.-J.L., J.d.l.R., M.-J.T., A.S., J.B., P.R., F.d.A., A.A., A.O., D.C., J.G.-L., R.G.-S., and J.-F.S.M. performed the research. M.-V.M., A.S., J.M.H., and M.-J.T. collected data. M.-V.M., N.-C.G., R.G.-S., G.M., and J.-F.S.M. were involved in data analysis. M.-V.M., D.-L.E., and J.-F.S.M. were involved in writing the manuscript. All authors reviewed and gave final approval of the manuscript.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.