Abstract
Chemotactic responsiveness is crucial to neutrophil recruitment to sites of infection. During chemotaxis, highly divergent cytoskeletal programs are executed at the leading and trailing edge of motile neutrophils. The Rho family of small GTPases plays a critical role in cell migration, and recent work has focused on elucidating the specific roles played by Rac1, Rac2, Cdc42, and Rho during cellular chemotaxis. Rac GTPases regulate actin polymerization and extension of the leading edge, whereas Rho GTPases control myosin-based contraction of the trailing edge. Rac and Rho signaling are thought to crosstalk with one another, and previous research has focused on mutual inhibition of Rac and Rho signaling during chemotaxis. Indeed, polarization of neutrophils has been proposed to involve the activity of a negative feedback system where Rac activation at the front of the cell inhibits local Rho activation, and vice versa. Using primary human neutrophils and neutrophils derived from a Rac1/Rac2-null transgenic mouse model, we demonstrate here that Rac1 (and not Rac2) is essential for Rho and myosin activation at the trailing edge to regulate uropod function. We conclude that Rac plays both positive and negative roles in the organization of the Rhomyosin “backness” program, thereby promoting stable polarity in chemotaxing neutrophils.
Introduction
The Rho subfamily of ras-related small guanosine triphosphatases (GTPases) are important regulators of events downstream of membrane receptor activation, including the regulation of actin and myosin cytoskeletal dynamics and directed cell motility.1 Previous studies have confirmed that Cdc42 and Rac GTPases play crucial roles in neutrophil chemotaxis through the regulation of cell polarization and actin assembly, 2 critical events that occur at the leading edge during chemotaxis.1 Effective chemotaxis also requires the neutrophil to de-attach matrix contacts and retract the rear of the cell (the uropod).2 The GTPase Rho has been implicated in the regulation of uropod retraction through myosin-mediated contractility.3,4
The Rac1 and Rac2 isoforms have more than 90% homology at the amino acid level, and these GTPases are known to play multiple roles in hematopoietic cell function.5-9 Recent work has focused on the unique and separate roles of these closely related proteins in blood cell activity. Notably, neutrophils derived from Rac2-null mice exhibit marked decreases in their ability to chemotax in response to multiple chemoattractant stimuli.6-8 This defect is accompanied by a severe impairment in F-actin assembly at the leading edge. Rac1-deficient neutrophils, while able to undergo normal chemokinesis, exhibit a significant but more modest inability to migrate up chemoattractant gradients.9 These cells are characterized by the formation of multiple unstable pseudopods, as well as a substantial uropod retraction defect.
Neutrophils and related leukocytes show a strong inherent ability to polarize in the presence of chemoattractants10 and must maintain stable polarity for efficient directed migration. Xu et al11 have put forth the idea of locally incompatible Rac-organized leading edge “frontness” and rear Rho-orchestrated “backness” programs that generate cell polarization through mutual inhibition. These studies suggested that Rac-mediated frontness acts to locally suppress Rho activation and backness, leading to establishment of cell polarity. However, the mechanisms proposed to regulate frontness are inherently unstable, as formation of the pseudopod in an autocatalytic manner could potentially lead to the spread of frontness over the entire cell. Here we demonstrate that Rac not only locally inhibits the backness program at the leading edge, but is also globally required for generation of Rho-myosin mediated backness at the trailing edge of motile neutrophils. Further, this function appears to primarily be mediated by Rac1, but not Rac2, as determined in neutrophils genetically deficient in individual Rac isoforms. The regulation by Rac of uropod-associated Rho activity identifies a unique mechanism for the maintenance of stable cell polarity during chemotaxis.
Materials and methods
Mice
Previously generated and characterized Rac1, Rac2, and double Rac-null knock-out mice were bred as previously described at the University of Toronto Animal Care Facility.9 Briefly Rac1c/-LysMcre were bred with Rac2-/- mice and the resulting offspring were bred over at least 6 generations to generate optimal breeding pairs (Rac1c/-LysMcreRac2+/- × Rac1c/-LysM-Rac2+/-), which would enable the generation of Rac1-null (Rac1c/-LysMcreRac2+/+), Rac2-null (Rac1+/+LysMcreRac2-/-), Rac1/2-null (Rac1c/-LysMcreRac2-/-), and wild-type (Rac1+/+LysMcre Rac2+/+) mice from the same litters. Rac1 is disrupted in a granulocyte/neutrophil-specific fashion by breeding of mice carrying a loxP-flanked Rac1 allele with LysMcre mice in which the Cre-recombinase is expressed under control of the murine lysozyme M gene regulatory region, which is active during early embryogenesis.6 Using this approach, Rac1 is deleted in the neutrophils from birth. All experiments were performed with mice 6 to 13 weeks old. This breeding strategy allows for the controlling of background variations, and control experiments confirmed that LysMcre expression did not affect any neutrophil functions including bacterial killing when compared with controls. Genotyping for Rac1, Rac2, and LysM alleles was carried out as described previously.9 All procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals12 and were approved by the University of Toronto Animal Care Committee.
Mouse neutrophil preparations
Mice were killed by CO2 inhalation. Femurs and tibias were removed and bone marrow was isolated as described previously.9 Briefly, bone marrow cells were layered onto discontinuous Percoll (Sigma, Oakville, ON) gradients of 82%/65%/55%. Mature neutrophils were recovered at the 82%/65% interface and were positive for Gr-1 and Mac-1 as shown by flow cytometry. More than 85% of the isolated cells were neutrophils as assessed by Wright-Giemsa staining. Viability as determined by trypan blue exclusion was more than 90%.
Rhotekin assay for active Rho
The Rhotekin (Rho) binding domain (RBD) pulldown affinity assay was carried out as described previously.13 Briefly, isolated bone marrow (or human) neutrophils were exposed to fMLP at 37°C for the indicated times. They were immediately lysed and GST-RBD-glutathione beads were added to the lysates. The GST-RBD was recovered and immunoblotting was completed using RhoA (sc-418) antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Image J software version 1.31v (National Institutes of Health, Bethesda, MD) was used for blot densitometry. Data were normalized to resting wild-type neutrophils and are expressed as the mean from 3 separate experiments.
Human neutrophil chemotaxis and de-adhesion assays
Human neutrophils were isolated from blood collected from healthy human subjects as described earlier.14 The cells were suspended in chemotaxis buffer (140 mM KCl, 10 mM glucose, 10 mM HEPES, 1 mM EGTA, 1 mM MgCl2, and 189 μM CaCl2) and allowed to attach to coverslips coated with human serum albumin (0.2% in saline). Chemotaxis assays were carried out with live cells essentially as described.15 The percentage of cells able to migrate freely toward the point source over the 15-minute duration was used as a measure of chemotaxis. This ranged from 30% to 60% of the total cell population in various neutrophil preparations from distinct donors. Movements of 40 to 100 cells per field were tracked for each treatment. Values shown are an average from 4 to 8 experiments. Cells that were clearly able to sense the gradient but unable to move from the original spot due to their inability to retract their uropods were also counted. To study de-adhesion, neutrophils were allowed to attach to the bottom of plastic 96-well plates coated with human serum albumin and transduced with protein. After removing the buffer, the wells were gently washed once with chemotaxis buffer, and the adherent cells fixed with paraformaldehyde and then counted visually under the microscope.
Expression of proteins in human neutrophils
Rac1 and RhoA glutathione-S-transferase (GST)-fusion proteins were expressed in Escherichia coli and purified on glutathione beads, and the expressed protein was cleaved off using thrombin according to the manufacturer's instructions.15 The purified proteins were mixed with the appropriate concentration of Bioporter reagent and pipetted onto coverslips of spread neutrophils. Under these conditions, we determined that approximately 90% of the cells took up protein.15 Unless specified, a protein concentration of 9 μg/mL for the dominant-negative proteins, or 12 μg/mL for the constitutively active protein, was used in all the experiments described here. These concentrations gave cellular GTPase concentrations 1- to 2-fold the level of endogenous Rac2.15 The cells were incubated at room temperature with the mixture for 2 to 3 hours as per the manufacturer's instructions (Gene Therapy Systems, San Diego, CA). Neutrophils were either stimulated globally (10 μM fMLP, 5 minutes), then fixed with 4% paraformaldehyde and used for immunostaining, or mounted on Attofluor-live-cell chambers (Molecular Probes, Eugene, OR) and stimulated with an fMLP point source (supplied by micropipette) to measure chemotaxis, as described earlier.15
Staining with myosin antibodies and Rhotekin Rho-binding domain (RBD)
Immunostaining for myosin heavy chain (MHC) and phosphorylated myosin light chain (p-MLC) was carried out simultaneously with rabbit polyclonal antibodies to a synthetic peptide corresponding to amino acid residues 1950 to 1961 of the heavy chain of human myosin IIA (Sigma) and monoclonal phospho-myosin antibodies. The latter were a kind gift of Yasuharu Sasaki (Kitasato University, Japan).16 Biochemical analysis of MLC phosphorylation was as described in Chew et al.17
For Rhotekin RBD staining, the RBD domain of Rhotekin attached to agarose beads was labeled with Alexa 568 malemide according to the manufacturer's instructions (Molecular Probes). The beads were washed and suspended in thrombin cleavage buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 5 mM MgCl2, 1 mM DTT, and 2.5 mM CaCl2), and the RBD fragment was cleaved off the beads by incubation with thrombin. Thrombin was inactivated by adding PMSF and removed from solution using benzamidin-conjugated beads. Cells were fixed with cold 4% paraformaldehyde and incubated on ice for 60 minutes with labeled RBD and Alexa 488-conjugated phalloidin, washed briefly, then mounted on slides and observed under the microscope. Images were acquired on a Nikon TE300 microscope (Melville, NY) fitted with a Princeton MicroMax 5 MHz 12 bit cooled CCD camera using Metamorph imaging software. Staining intensities were compared by acquiring images for the same exposure times and by using the same scaling factors following background subtraction. Quantitation of fluorescence intensities in individual cells was carried out using region measurement and linescan tools in Metamorph (Photometrics, Tucson, AZ).
Results
Neutrophils deficient in Rac1 activity exhibit chemotaxis and tail retraction defects
We previously reported that in chemotactically responding primary human neutrophils, Rac activation occurs both at the leading edge and in the uropod of migrating cells.15 These results suggested the possibility that Rac could play a regulatory role in retraction of the uropod. Indeed, we showed that low-level expression of a dominant-negative Rac1T17N mutant (ie, at equal to or less than endogenous levels of Rac) decreased chemotactic motility (Figure 1A) in association with failure to efficiently retract the uropod (Figure 1B-C; Video S2) (Gardiner et al15 (Fig 3)). While the migration speed of these cells was decreased due to the observed defects in tail retraction, they still were apparently able to sense the gradient of chemoattractant, as evident by their orientation toward the point source of fMLP and their attempts to move toward this source (Video S2). The effects of Rac1T17N expression were concentration dependent, as expression at levels 2- to 3-fold greater than endogenous Rac induced a substantial loss of leading edge formation, associated with the redistribution of F-actin around the cell periphery.15 We cannot exclude that the effect of Rac1T17N is mediated via inhibition of Rac2, which is the predominant form of Rac in human neutrophils, representing 90% to 95% of total Rac.5
Rac1 and RhoA regulate neutrophil chemotaxis. (A-B) Chemotaxis in human neutrophils expressing dominant-negative Rac1. Human neutrophils were treated with GTPase mutants at 9 μg/mL (dominant negative) or 12 μg/mL (constitutively active) in the presence of Bioporter reagent as described in “Materials and methods.” The cells were then stimulated with a point source of chemoattractant, and the movement of the cells was recorded at 30-second intervals for 15 minutes. At these relatively low levels of expression of the dominant-negative mutants, inhibition of either Rac1 or RhoA activity results in marked reduction in the ability of human neutrophils to chemotax toward a point source of fMLP (A). Positive values represent the percentage of cells that moved freely toward the point source over a 15-minute interval. This was associated with a marked defect in tail retraction (quantified in B). Cells that sensed the gradient but exhibited elongated tails (inset in panel C; bar = 10 μM) and the inability to move toward the chemoattractant point source over the course of the 15-minute period were counted. Cells expressing Rac1T17N that were treated with RhoAG14V were now able to chemotax effectively (A), associated with a restoration of the cell's ability to retract the uropod, quantified in panel B. Data are derived from 4 to 8 experiments, and for each treatment in each experiment the movement of 40 to 100 cells was tracked. (C) Cell morphology during chemotaxis following Rac inhibition in human neutrophils. Cell length measurements were made on live human neutrophils undergoing chemotaxis as described in “Materials and methods.” The values are derived from 4 to 7 experiments and are an average ± standard error from 150 to 350 cells per condition. Measurements were made at random times during the 15-minute duration of the experiment, and thus represent an “average” over this time frame. The differences in cell length were highly significant (P < .001). Inset images were visualized using a 60×/1.45 NA objective lens. (D) Cell morphology during chemotaxis in Rac-deficient mouse neutrophils. Quantification of perturbed tail retraction in Rac1-null mouse neutrophils, as shown in the representative photomicrograph of neutrophils in an fMLP gradient (inset). Rac1-null neutrophils display poor tail retraction compared with wild-type and Rac2-null neutrophils. The average Rac1-null neutrophil length in fMLP-stimulated cells (head to tail) is more than twice as long as in stimulated wild-type cells (mean of 50 cells per genotype). Bar = 10 μm.
Rac1 and RhoA regulate neutrophil chemotaxis. (A-B) Chemotaxis in human neutrophils expressing dominant-negative Rac1. Human neutrophils were treated with GTPase mutants at 9 μg/mL (dominant negative) or 12 μg/mL (constitutively active) in the presence of Bioporter reagent as described in “Materials and methods.” The cells were then stimulated with a point source of chemoattractant, and the movement of the cells was recorded at 30-second intervals for 15 minutes. At these relatively low levels of expression of the dominant-negative mutants, inhibition of either Rac1 or RhoA activity results in marked reduction in the ability of human neutrophils to chemotax toward a point source of fMLP (A). Positive values represent the percentage of cells that moved freely toward the point source over a 15-minute interval. This was associated with a marked defect in tail retraction (quantified in B). Cells that sensed the gradient but exhibited elongated tails (inset in panel C; bar = 10 μM) and the inability to move toward the chemoattractant point source over the course of the 15-minute period were counted. Cells expressing Rac1T17N that were treated with RhoAG14V were now able to chemotax effectively (A), associated with a restoration of the cell's ability to retract the uropod, quantified in panel B. Data are derived from 4 to 8 experiments, and for each treatment in each experiment the movement of 40 to 100 cells was tracked. (C) Cell morphology during chemotaxis following Rac inhibition in human neutrophils. Cell length measurements were made on live human neutrophils undergoing chemotaxis as described in “Materials and methods.” The values are derived from 4 to 7 experiments and are an average ± standard error from 150 to 350 cells per condition. Measurements were made at random times during the 15-minute duration of the experiment, and thus represent an “average” over this time frame. The differences in cell length were highly significant (P < .001). Inset images were visualized using a 60×/1.45 NA objective lens. (D) Cell morphology during chemotaxis in Rac-deficient mouse neutrophils. Quantification of perturbed tail retraction in Rac1-null mouse neutrophils, as shown in the representative photomicrograph of neutrophils in an fMLP gradient (inset). Rac1-null neutrophils display poor tail retraction compared with wild-type and Rac2-null neutrophils. The average Rac1-null neutrophil length in fMLP-stimulated cells (head to tail) is more than twice as long as in stimulated wild-type cells (mean of 50 cells per genotype). Bar = 10 μm.
As noted, transduction of dominant-negative Rac1T17N versus Rac2T17N proteins into human neutrophils does not allow us to cleanly distinguish between possible differences in the contribution of Rac1 versus Rac2 to the uropod retraction defect (see “Discussion”). In order to assess whether Rac1 or Rac2 (or both) potentially regulates tail retraction, we took advantage of neutrophils derived from Rac1- and Rac2-null mice, genetically deficient in these individual GTPases. As noted previously,9 Rac1-null mouse neutrophils also exhibit an unusual elongated morphology during chemotaxis, remarkably similar to that of human neutrophils expressing dominant-negative Rac1 (Figure 1D). Also like human neutrophils expressing Rac1T17N, the Rac1-null leukocytes develop an oriented leading edge, but they exhibit an apparent defect in uropod retraction. This phenotype is not observed in the Rac2-null cells (Figure 1D).
Rho also regulates neutrophil tail retraction
Of interest, a similar effect on tail retraction was observed upon introduction of dominant-negative RhoT19N into human neutrophils: cells were no longer able to effectively chemotax in response to a point source of fMLP (Figure 1A). Like neutrophils expressing Rac1T17N, the cells appeared to respond to the chemotactic gradient (as evidenced by orientation toward the point source), but were unable to efficiently withdraw their tails, which became elongated in appearance (Figure 1B, C; Video S3). Cells expressing constitutively active RhoAG14V were also deficient in their ability to chemotax (Figure 1A). In this case, however, the cells appeared rounded and were characterized by formation of random, unstable pseudopods (Video S6). This inability to form stable pseudopods prevented the cells from maintaining a polarized morphology and inhibited effective directional migration. The latter phenotype is consistent with the proposed role of Rho in regulating backness, and Xu et al11 have reported a similar observation.
Rac1 regulates neutrophil Rho activity in the uropod
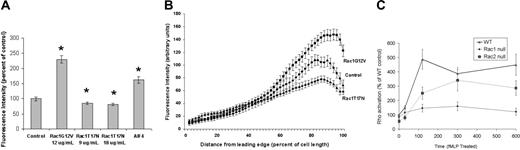
Since the small GTPase Rho has been implicated in uropod retraction during chemotaxis,2 we assessed Rho activation during fMLP stimulation in primary human neutrophils in which constitutively active or dominant-negative Rac1 mutants were expressed. A fluorescently tagged Rho GTP-binding domain (RBD) from Rhotekin was used to evaluate both Rho activation and the localization of active Rho. In control human neutrophils, we observed that approximately 50% of the fMLP-responsive cells showed readily detectable Rho activity, largely confined to the sides and uropod, following fMLP stimulation (Figure 2A-B). Xu et al11 and Li et al18 have both reported that total RhoA protein is deficient in the leading edge, and primarily localizes to the sides and tail of polarized HL-60 cells and mouse neutrophils, respectively. On expression of active Rac1G12V at levels similar to those of endogenous Rac,15 we observed a substantial increase in the intensity of active Rho staining (Figure 2A), as well as in the fraction of cells exhibiting detectable Rho activity (80%-90%), particularly within the uropod (Figure 2B). Higher amounts of Rac1G12V produced no further increase in the fraction of cells with active Rho or in the intensity of RBD staining. Rac1T17N caused no significant change in the proportion of cells showing some level of active Rho from the controls, although the amount of active Rho present within this population of cells was significantly decreased, particularly within the uropod (Figure 2A-B). This was determined more quantitatively using a pulldown assay based on the Rho binding domain of rhotekin fused to GST,13 which showed that RhoA activation induced by fMLP was inhibited by more than 50% to 70% by dominant-negative Rac1 mutants (data not shown), similar to results obtained in the Rac1-null neutrophils (see next paragraph). These results indicate that Rac activation in human neutrophils positively regulates uropod-localized Rho activity.
We then examined the Rac-deficient mouse neutrophil model to verify this observation and to determine whether there was any selectivity for Rac1 versus Rac2. Using the rhotekin pulldown assay, we observed that the level of activated Rho increased by more than 300% within 5 minutes of fMLP stimulation in Rac2-null and wild-type neutrophils (Figure 2C). In the Rac2-null cells, Rho activation was slightly delayed at short times (ie, 2 minutes) compared with the wild-type cells. However, in Rac1-null cells there was no detectable increase in fMLP-mediated Rho activation over a 10-minute time course (Figure 2C). This verifies the coupling of Rac activity to Rho activation observed in the human neutrophils and suggests that Rac1, but not Rac2, is essential for Rho activation, consistent with a role in stimulating uropod retraction during chemotaxis.
Rac regulates myosin II-mediated contractility through Rho in the uropod
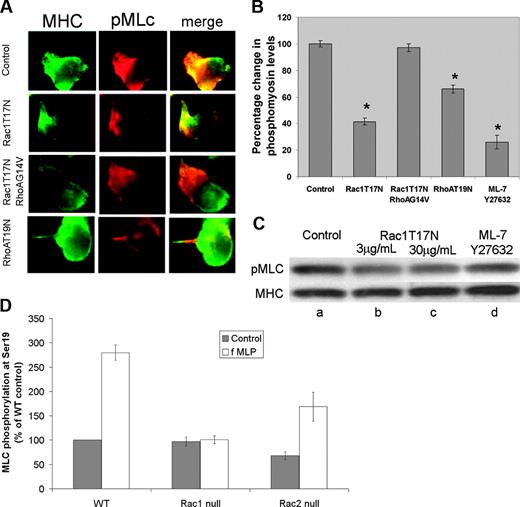
In order to determine whether the requirement for Rac1 signaling to RhoA could account for the observed tail retraction defect, we determined the localization and activation state of myosin using an antibody to phosphorylated (Ser19) myosin regulatory light chain (MLC). Myosin has been previously shown to function in uropod retraction through its role in regulating actin filament contraction. In human neutrophils, we observed that cells expressing Rac1T17N displayed significant inhibition of fMLP-stimulated MLC phosphorylation within the uropod (Figure 3A). This Rac1T17N-mediated suppression of MLC phosphorylation was verified at the biochemical level (Figure 3B-C). Overall levels of MLC phosphorylated at Ser19 were decreased by more than 30% at low levels of Rac1T17N (3-9 μg/mL), and by nearly 50% at 30 μg/mL Rac1T17N. Expression of active Rac1G12V did not significantly change the levels or distribution of neutrophil phospho-MLC relative to controls. This result was verified in the murine neutrophil model: the overall activation of myosin as measured by MLC phosphorylation at Ser19 was significantly increased in fMLP-stimulated wild-type and Rac2-null neutrophils, but was completely lacking in stimulated Rac1-null cells (Figure 3D). Immunolocalization of phospho-MLC in fMLP-activated mouse neutrophils demonstrated localization to the uropod in both wild-type and Rac2-null cells, but a more diffuse distribution of the remaining activity was observed in Rac1-null cells (not shown).
Rac1 regulates Rho activation. (A) Rac1 increases RhoA activity in human neutrophils. Constitutively active or dominant-negative Rac1 mutants were expressed in human neutrophils, stimulated with 1 μM fMLP, fixed, and stained with phalloidin (green) and the Rhotekin RBD (red), as described in “Materials and methods.” Rhotekin RBD mutated at R37,39A,D40A was used as an inactive control and showed only background staining (not shown). Fluorescence intensities of more than 300 cells from at least 3 samples were measured for each treatment. Error bars show standard error, and significant differences (at least P < .05) are indicated by asterisks. Cells were treated with 20μM aluminum fluoride (AIF4) for 15 minutes as a positive control for Rho activation. (B) Distribution of active Rho from front to back in human neutrophils. Rhotekin RBD staining was mostly confined to the uropod region in control cells following fMLP stimulation. The intensity of Rho activation was greater in the uropod of cells treated with Rac1G12V, and substantially less intense in Rac1T17N-treated cells. Linescans of control (vector treated), Rac1G12V-, and Rac1T17N-expressing cells reveal significant differences in levels of active Rho along the length of these cells, particularly at the rear, compared with controls. Fluorescence intensities were measured from the leading edge to the tail, with cell lengths normalized to 100% to allow comparison. Results shown are the mean ± standard error of at least 30 cells per condition. (C) Rac1-null mouse neutrophils fail to activate Rho GTPase following fMLP stimulation. FMLP-mediated Rho activation in murine neutrophils requires Rac1 but not Rac2. Using the Rhotekin assay (“Materials and methods”), we demonstrate that in cells stimulated globally with 1 μM fMLP, RhoA activation increases in a similar pattern in wild-type and Rac2-null neutrophils over 600 seconds, but is severely perturbed in Rac1-null neutrophils. Rac1 is significantly different from wild-type and Rac2 for all time points greater than 100 seconds (P < .01).
Rac1 regulates Rho activation. (A) Rac1 increases RhoA activity in human neutrophils. Constitutively active or dominant-negative Rac1 mutants were expressed in human neutrophils, stimulated with 1 μM fMLP, fixed, and stained with phalloidin (green) and the Rhotekin RBD (red), as described in “Materials and methods.” Rhotekin RBD mutated at R37,39A,D40A was used as an inactive control and showed only background staining (not shown). Fluorescence intensities of more than 300 cells from at least 3 samples were measured for each treatment. Error bars show standard error, and significant differences (at least P < .05) are indicated by asterisks. Cells were treated with 20μM aluminum fluoride (AIF4) for 15 minutes as a positive control for Rho activation. (B) Distribution of active Rho from front to back in human neutrophils. Rhotekin RBD staining was mostly confined to the uropod region in control cells following fMLP stimulation. The intensity of Rho activation was greater in the uropod of cells treated with Rac1G12V, and substantially less intense in Rac1T17N-treated cells. Linescans of control (vector treated), Rac1G12V-, and Rac1T17N-expressing cells reveal significant differences in levels of active Rho along the length of these cells, particularly at the rear, compared with controls. Fluorescence intensities were measured from the leading edge to the tail, with cell lengths normalized to 100% to allow comparison. Results shown are the mean ± standard error of at least 30 cells per condition. (C) Rac1-null mouse neutrophils fail to activate Rho GTPase following fMLP stimulation. FMLP-mediated Rho activation in murine neutrophils requires Rac1 but not Rac2. Using the Rhotekin assay (“Materials and methods”), we demonstrate that in cells stimulated globally with 1 μM fMLP, RhoA activation increases in a similar pattern in wild-type and Rac2-null neutrophils over 600 seconds, but is severely perturbed in Rac1-null neutrophils. Rac1 is significantly different from wild-type and Rac2 for all time points greater than 100 seconds (P < .01).
Chemoattractant-mediated phosphorylation of myosin light chain requires Rac1. (A) Effect of GTPase mutants on phosphorylation of myosin light chain in human neutrophils. Human neutrophils were transduced with the indicated GTPases using BioPorter reagent, globally stimulated with 1 μM fMLP, fixed, and simultaneously stained with antibodies to myosin heavy chain (green) and phosphorylated MLC (red). There is a significant reduction in myosin phosphorylation upon treatment with Rac1T17N compared with control. In contrast, phosphorylation is significantly restored by RhoAG14V. Inhibition of RhoA by RhoAT19N also suppresses MLC phosphorylation. Cells shown are representative of more than 20 cells screened per condition. (B) Suppression of myosin light chain phosphorylation by Rac1T17N. Human neutrophils transduced (using BioPorter reagent) with the dominant-negative Rac1T17N mutant (3 μg/mL), with dominant-negative RhoA (9 μg/mL), with Rac1T17N (3 μg/mL) plus constitutively active RhoAG14V (12 μg/mL), or with a combination of the drugs ML-7 and Y27632 (at 20 μM each) to simultaneously inactivate MLCK and Rho kinase, respectively, were stimulated with fMLP and stained with antibodies to MHC and pMLC. After normalization for total myosin, the relative intensity of pMLC, as determined from the immunofluorescent images, was compared with untreated control in the bar graph shown. Values represent an average of at least 100 cells. (C) Biochemical analysis of MLC Ser19 phosphorylation. In addition to single-cell measurements, overall changes in myosin phosphorylation were also measured by immunostaining of blots made from cell lysates. Lane a represents controls; b and c, cells treated with Rac1T17N at 3 and 30 μg/mL, respectively; and d, cells treated with a combination of ML-7 and Y27632 (20-μM each). (D) Myosin II phosphorylation requires Rac1 activity in mouse neutrophils. Quantitation of immunoblots of phospho-myosin II regulatory light chain (MLC) in bone marrow neutrophils exposed to fMLP (1 μM). FMLP-mediated phospho-MLC level increases in a similar pattern in wild-type and Rac2-null neutrophils over 120 seconds, but is severely perturbed in Rac1-null neutrophils.
Chemoattractant-mediated phosphorylation of myosin light chain requires Rac1. (A) Effect of GTPase mutants on phosphorylation of myosin light chain in human neutrophils. Human neutrophils were transduced with the indicated GTPases using BioPorter reagent, globally stimulated with 1 μM fMLP, fixed, and simultaneously stained with antibodies to myosin heavy chain (green) and phosphorylated MLC (red). There is a significant reduction in myosin phosphorylation upon treatment with Rac1T17N compared with control. In contrast, phosphorylation is significantly restored by RhoAG14V. Inhibition of RhoA by RhoAT19N also suppresses MLC phosphorylation. Cells shown are representative of more than 20 cells screened per condition. (B) Suppression of myosin light chain phosphorylation by Rac1T17N. Human neutrophils transduced (using BioPorter reagent) with the dominant-negative Rac1T17N mutant (3 μg/mL), with dominant-negative RhoA (9 μg/mL), with Rac1T17N (3 μg/mL) plus constitutively active RhoAG14V (12 μg/mL), or with a combination of the drugs ML-7 and Y27632 (at 20 μM each) to simultaneously inactivate MLCK and Rho kinase, respectively, were stimulated with fMLP and stained with antibodies to MHC and pMLC. After normalization for total myosin, the relative intensity of pMLC, as determined from the immunofluorescent images, was compared with untreated control in the bar graph shown. Values represent an average of at least 100 cells. (C) Biochemical analysis of MLC Ser19 phosphorylation. In addition to single-cell measurements, overall changes in myosin phosphorylation were also measured by immunostaining of blots made from cell lysates. Lane a represents controls; b and c, cells treated with Rac1T17N at 3 and 30 μg/mL, respectively; and d, cells treated with a combination of ML-7 and Y27632 (20-μM each). (D) Myosin II phosphorylation requires Rac1 activity in mouse neutrophils. Quantitation of immunoblots of phospho-myosin II regulatory light chain (MLC) in bone marrow neutrophils exposed to fMLP (1 μM). FMLP-mediated phospho-MLC level increases in a similar pattern in wild-type and Rac2-null neutrophils over 120 seconds, but is severely perturbed in Rac1-null neutrophils.
Transduction of active RhoA restores tail retraction and chemotaxis to Rac1T17N-inhibited neutrophils
Consistent with previous findings that Rho modulates myosin contractility and tail retraction in neutrophils, we observed a similar decrease in uropod levels of Ser19-phosphorylated MLC upon expression of dominant-negative RhoAT19N in the human neutrophils (Figure 3A). Since we have noted previously in human cells15 that low-level expression of Rac1T17N selectively induces a tail retraction defect without a major effect on leading edge formation, we wondered whether Rho activation could act to reverse these inhibitory effects of low-level dominant-negative Rac1 on tail retraction and thereby restore normal chemotaxis. As shown in Figure 1A, we found that the effect of low-level Rac1T17N to inhibit chemotactic responsiveness to fMLP was almost totally reversed by low-level expression of active RhoAG14V. Reconstitution of chemotactic migration in Rac1T17N-expressing cells by RhoAG14V was associated with restoration of normal uropod retraction and a loss of tail adhesion to the matrix (Figure 1B and Video S4, which is available on the Blood website; see the Supplemental Materials link at the top of the online article). We note that expression of RhoAG14V in the absence of the suppression of endogenous Rho activity by Rac1T17N inhibited chemotaxis in association with cell rounding and the formation of multiple, unstable pseudopods. The restoration of tail retraction by RhoAG14V expression appeared to be a result of the partial recovery of phospho-MLC levels in the uropod (Figure 3A-B; see also the Supplemental Materials link). The inability of active Rho to fully restore levels of phospho-MLC suggests that an additional Rac-mediated myosin regulatory mechanism might exist. Alternatively, spatial considerations resulting from the exogenous reconstitution of RhoA GTPase might affect efficient coupling to the endogenous myosin-phosphorylating machinery.
Discussion
Migratory cells execute different cytoskeletal programs at their leading and trailing edges under the control of Rho GTPase signaling.1,11 While Rac localizes to and is necessary for efficient actin polymerization and protrusion of the leading edge, Rho localizes to and is necessary for myosin-based contraction at the trailing edge. The establishment and maintenance of polarity thus requires communication between Rac and Rho, and these GTPases depend on one another for proper localization and regulation of chemotaxis. Previous studies have focused on the antagonistic relationship between these 2 GTPases during neutrophil chemotaxis.
Substantial data support the idea that Rac generally inhibits Rho activity. Global Rac activation has been shown to inhibit Rho activation in a variety of cells,19,20 and disruption of signaling to Rac enables the backness program to act at the leading edge of HL60 cells,11 although the relative contribution of Rac1 versus Rac2 to this effect has not been defined. Conversely, these same studies suggest that Rho locally antagonizes frontness: inhibition of Rho produces HL60 cells with multiple leading edges, and global activation of Rho inhibits actin polymerization, Rac activation, and PIP3 production (another readout of frontness).11,21 A current model for polarity in neutrophils entails a Gαi-mediated activation of Rac to stimulate F-actin assembly and frontness, a Gα12/13-mediated activation of Rho to stimulate myosin-based contractility and backness, and mutual repulsion of these networks to generate a distinct front and back.11 However, other data suggest that the interaction of Rac and Rho might not simply be antagonistic. Rac produces cytoskeletal rearrangements consistent with Rho activation in some cell types,22 and receptor-independent activation of the frontness program in neutrophils also produces cells with a well-organized trailing edge.23 Both of these observations suggest that Rac may also positively regulate Rho.
In the current study, we use primary neutrophils derived from murine transgenic conditional Rac1/Rac2 protein knock outs in combination with expression of active and inactive Rac and Rho GTPases in primary human neutrophils to demonstrate that Rac1 is essential for activation of Rho and myosin at the trailing edge of migrating neutrophils responding to chemoattractant stimulation. The use of primary human neutrophils and the rapid introduction of dominant Rho GTPase mutant proteins into these cells provide certain distinct advantages. These include the fact that we are able to assess Rho GTPase signaling in actual primary human neutrophils, as opposed to in vitro differentiated myeloid cell lines that exhibit differences in both chemotactic behavior and in the intracellular signaling pathways regulating chemotaxis. Also, by acutely introducing dominant Rho GTPase mutant proteins, we are able to evaluate immediate effects on Rho GTPase-mediated signaling events in the absence of compensatory changes that might occur in genetically altered GTPase-null cells. However, there are limitations to this approach. First of all, there is some concern regarding the fact that the dominant inhibitor protein cytosolic concentrations are not known. We have shown previously that the overall level of dominant-negative GTPase expression when introduced at 9 μg/mL was about 3-fold greater than the endogenous Rac2 level. In an experiment to evaluate the actual levels in the cytosol, we find that this approximate ratio appears to hold up, although this number itself may be inaccurate with respect to the level in intact cell cytosol (which may be even higher), as we observe the presence of apparent GTPase protein aggregates in the cytosol that are spun down in the analysis we performed. Additionally, the concentration of Rac1T17N in the membrane itself where inhibition of endogenous GEFs is likely to occur is unknown. Overall, there is no evidence against the idea that the dominant-negative GTPases are acting in any way other than as dominant-negative GTPases. Second, we have found that introduction of dominant-negative Rac1 versus Rac2 proteins into human neutrophils does not allow us to effectively examine the distinct roles of these 2 Rac isoforms in neutrophil chemotactic behavior (data not shown and Glogauer et al24 ). RacT17N dominant-negative mutants are thought to act by binding to and inhibiting endogenous Rac guanine nucleotide exchange factors (GEFs) that regulate Rac1 and Rac2 activation in response to chemoattractant receptor signaling. Most Rac GEFs, including Vav1 and PRex-1, which have been implicated in regulating Rac activity in neutrophils, exhibit selectivity but not absolute specificity for Rac2 versus Rac1 in vitro and in vivo. Consequently, the introduction of Rac1T17N versus Rac2T17N mutants by current transduction methods is too crude to selectively distinguish between Rac1-versus Rac2-mediated signaling events.
Because of these issues, we therefore complemented these analyses in primary human cells with studies of murine neutrophils in which Rac1 or Rac2 function has been genetically ablated, thereby providing a clean and specific means to evaluate the relative contributions of these 2 Rac isoforms. It is striking that we obtained very complementary evidence for the regulation of Rho and myosin activation in the uropod by Rac in both the human and murine systems. While the mouse results indicate that it is Rac1 that selectively regulates Rho activity and myosin function, our data do not definitively rule out a contribution of Rac2 to this regulatory pathway, given the abundance of the Rac2 isoform in human neutrophils.
Although we do not yet know the exact molecular mechanism(s) by which Rac1 activity regulates RhoA activity within the uropod, it is clear from our prior work that Rac activation is induced by chemoattractant stimulation both at the front and the rear of the neutrophil,15 providing a localized source of active Rac within the tail. It is likely that signals generated upon initiation of the chemotactic response are rapidly propagated to modulate Rac activity at both locations, thereby secondarily coordinating Rho activity as well. These data suggest that Rac not only locally inhibits the backness program at the leading edge, but also is globally required for generation of Rho-myosin mediated backness at the trailing edge of motile cells. In mouse neutrophils, Rac1 thus plays a key role in linking front to back signaling during neutrophil chemotaxis. This is in contrast to Rac2, which has been previously shown to be the key Rac isoform responsible for regulation of leading edge actin polymerization during chemotaxis, possibly through activation of Cdc42.9 Differences in the biologic effects of Rac1 versus Rac2 in neutrophils have been previously described, and a number of potential mechanisms to explain the different biologic activities of these highly similar GTPases have been proposed.5 Along with the current description of Rac1-specific activation of Rho, these studies begin to outline how these 2 Rac isoforms differentially regulate key processes during neutrophil chemotaxis.
This dual communication from front to back with Rac1 and/or Rac2 locally inhibiting and Rac1 globally activating Rho-mediated backness facilitates a more robust balance between these essential polarity programs than is possible with a purely antagonistic relationship. Thus, the effect of chemoattractant-mediated Rac1 activation propagated to the tail to stimulate Rho-mediated myosin activation would promote a proportionally stronger uropod to balance the dynamic primary pseudopod. Such mutual crosstalk would provide a means for chemoattractants to maintain stable polarity during the course of migration to infectious sites. It is of interest in this regard that in our earlier analyses of Rac activation,15 we observed that while Rac activity was quite dynamic at the front of the neutrophil, it remained relatively constant within the uropod. This suggests the possibility that Rho activation mediated by Rac within the uropod may be maintained in order to support stable cell polarity, while the local regulation of Rho by Rac at the leading edge may change dynamically as the cell responds to continually changing directional cues during chemotaxis. While expression of active RhoAG14V alone disrupts the formation of a stable leading edge, the ability of RhoAG14V to restore normal uropod retraction and chemotaxis to cells inhibited by dominant-negative Rac1T17N may indicate the relative importance of a properly organized uropod in directed motility. It will be of interest to examine in more detail the exact temporal relationship between Rac and Rho activation at the front versus the back of chemotaxing neutrophils.
Prepublished online as Blood First Edition Paper, June 29, 2006; DOI 10.1182/blood-2006-01-010363.
Supported by USPHS grant GM39434 (G.M.B.) and a CIHR grant (M.G.).
G.M.B. and M.G. contributed equally to this report.
The online version of this article includes a data supplement.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors acknowledge the technical assistance of Bruce Fowler (TSRI).