Abstract
The chicken embryonic β-type globin gene, ρ, is a member of a small group of vertebrate genes whose developmentally regulated expression is mediated by DNA methylation. Previously, we have shown that a methyl cytosine-binding complex binds to the methylated ρ-globin gene in vitro. We have now chromatographically purified and characterized this complex from adult chicken primary erythroid cells. Four components of the MeCP1 transcriptional repression complex were identified: MBD2, RBAP48, HDAC2, and MTA1. These 4 proteins, as well as the zinc-finger protein p66 and the chromatin remodeling factor Mi2, were found to coelute by gel-filtration analysis and pull-down assays. We conclude that these 6 proteins are components of the MeCPC. In adult erythrocytes, significant enrichment for MBD2 is seen at the inactive ρ-globin gene by chromatin immunoprecipitation assay, whereas no enrichment is observed at the active βA-globin gene, demonstrating MBD2 binds to the methylated and transcriptionally silent ρ-globin gene in vivo. Knock-down of MBD2 resulted in up-regulation of a methylated ρ-gene construct in mouse erythroleukemic (MEL)-ρ cells. These results represent the first purification of a MeCP1-like complex from a primary cell source and provide support for a role for MBD2 in developmental gene regulation.
Introduction
Methylation of the 5-position of cytosine residues in DNA has an important role in the regulation of gene expression in higher eukaryotes. The first descriptions of an inverse correlation between DNA methylation and expression of a protein-coding eukaryotic gene were those made for the vertebrate globin genes of the chicken, rabbit, and human.1-4 The tremendous interest in DNA methylation seen within the past decade stems from its important role in the silencing of tumor suppressor genes in cancer.5,6 Despite the prediction of a widespread role for DNA methylation in gene regulation,7 the expression of only a small number of vertebrate genes has been shown to be tissue-restricted or developmentally restricted by DNA methylation.8 Studies showing tissue-restricted or developmentally restricted expression in nontransformed, primary cells are even more scarce and are limited to 4 main examples: the Gallus gallus ρ-globin gene,9,10 the Mus musculus interleukin-4 and interferon-γ genes,11 and the Xenopus laevis mesodermal genes.12
Intense study has been directed toward elucidating the mechanisms through which DNA methylation represses transcription. The discovery of a family of proteins that specifically recognize methylated DNA, the methyl-CpG-binding proteins (MCBPs),13,14 has led to the general observation of these proteins and their associated corepressor complexes as the mediators of DNA methylation-induced gene silencing in a variety of systems.15-19 Biochemical studies have shown that the MCBPs are members of distinct and nonoverlapping transcriptional repression complexes. MBD1 was identified as a critical component of an S phase-specific complex that propagates the DNA methylation signal into a dimethylation of lysine 9 of histone H3 (H3-K9-Me2) signal during DNA replication.17 MBD2 is the methyl-CpG-binding component of the MeCP1 transcriptional repression complex.20 MBD3 is a core component of the NuRD transcriptional repression complex that can be recruited by DNA-binding proteins such as Ikaros and FOG1.21,22 Kaiso has been found to be a component of the N-CoR transcriptional repression complex.23
The embryonic-to-adult β-globin switch in the chicken serves as an informative model of transcriptional silencing. The ρ-globin gene is expressed abundantly in primitive erythrocytes produced in the yolk sac from 36 hours of embryogenesis to day 5, and it is not expressed in the definitive erythrocytes which are produced beginning at day 5.24 The definitive cells do not express ρ-globin because of transcriptional silencing.25 Work in our laboratory has established that every CpG site in the promoter and proximal transcribed region (exon 1 and intron 1) of the ρ-globin gene is methylated in adult erythrocytes but unmethylated in primitive erythrocytes.10,26,27 This observation is functionally significant, because expression of ρ-globin and a concomitant loss of CpG methylation can be induced in adult chickens by treatment with the DNA methylation inhibitor 5-aza-cytidine.9
Our laboratory has also shown that an erythroid methyl cytosine-binding protein complex (MeCPC) forms on the methylated ρ-globin promoter and proximal transcribed region (ρ-PTR) when incubated with chicken erythroid nuclear extracts in vitro.10 This complex exhibits a 2-fold binding preference for the ρ-PTR over the canonical MeCP1 binding substrate M-CG11, in contrast to a 20-fold binding preference exhibited by the MeCP1 complex for M-CG11 over the ρ-PTR.27 In an effort to understand the mechanistic basis for DNA methylation-mediated transcriptional silencing of the ρ-globin gene during normal development in the chicken, we purified the MeCPC from primary erythroid cells and show that it contains 6 components of the MeCP1 complex. Importantly, we find that the chicken homolog of MBD2 is a component of the MeCPC and is bound to the methylated ρ-globin gene in primary adult erythrocytes. cMBD2 associates with the other components of the MeCP1 complex in vivo, and the MeCPC components MBD2 and MTA2 are present at the methylated ρ-globin gene in mouse erythroleukemic (MEL) cells containing a methylated ρ-globin mini-locus. This study therefore presents the first evidence of a direct association of a MeCP1-type complex with a gene regulated by DNA methylation during the course of normal development.
Methods and materials
Additional details and methods are described in Document S1 (available at the Blood website; see the Supplemental Materials link at the top of the online article). All enzymes were from New England Biolabs (Beverly, MA), and all chromatography columns were from Amersham Biosciences (Piscataway, NJ) unless otherwise noted.
Expression and purification of recombinant cMBD2
EST clone pat.pk0057.c7.f was obtained from the UD Chick EST Database28 and sequenced. This sequence has been deposited in GenBank as accession no. AY888006. The insert, encoding chicken MBD2, was amplified by polymerase chain reaction (PCR) and cloned into the vector pGEX-6P. This plasmid was introduced into Rosetta Escherichia coli (Novagen, Madison, WI), and cells were prepared by standard protocols. Recombinant GST/cMBD2 was purified using a 5-mL GSTrap HP column followed by a 1-mL HiTrap SP FF column. For production of polyclonal antibodies, the purified protein was excised from a preparative 12% tris-glycine sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and used to immunize 2 rabbits (Cocalico Biologicals, Reamstown, PA).
Anti-cMBD2 IgG purification and affinity purification
Purification of the antiserum derived from the 2 immunized rabbits (V1 and V2) was performed using a 5-mL HiTrap rProtein A FF column according to the manufacturer's recommendations. Protein containing fractions were pooled and dialyzed against PBS overnight. For affinity purification, purified GST/cMBD2 was bound to 0.4 mL Affi-Gel 10 (Bio-Rad, Hercules, CA) by the manufacturer's protocol. After equilibration with PBS, V1 IgG was passed over the column 4 times. The column was then washed with 15 CV PBS, and then the bound antibodies were eluted with 5 CV 0.1 M glycine (pH 2.8). The antibody-containing fractions were concentrated by spinning with the use of an Ultrafree-4 (Millipore, Bedford, MA).
Electrophoretic mobility shift assay
DNA fragments were methylated as previously described27 and labeled with [α-32P]dCTP (MP Biomedicals, Solon, OH) and Klenow or [γ-32P]ATP and polynucleotide kinase. The assay was performed by adding 4 to 10 μL column fractions or 20 μg nuclear extract to a 21.5-μL binding reaction containing 10 to 20 fmol probe, 2 μg sonicated M lysodeikticos DNA, 20 mM HEPES (pH 7.9), 3 mM MgCl2, 1 mM EDTA, 10 mM β-ME, 0.1% Triton X-100, 4% glycerol. Binding reactions were incubated on ice for 20 minutes and then loaded onto a 2% agarose gel and resolved in 0.5 × TBE buffer. Supershift experiments were performed as previously described27 by using protein A-purified anti-cMBD2 antibodies or anti-mMBD2 antibodies (no. 07-198; Upstate, Charlottesville, VA).
Isolation of chicken erythrocytes and preparation of nuclear extracts
Whole chicken blood was obtained from Pel-Freez Biologicals (Rogers, AR). Erythrocytes were isolated by centrifugation on a 8:3 (vol:vol) cushion of Histopaque 1083 (Sigma, St Louis, MO) by spinning at 3100g for 5 minutes. Nuclear extracts were prepared using the method of Dignam et al.29
Purification of the MeCPC by strategies I and II
Detailed methods are described in Document S1. In strategy I, adult chicken red blood cell (RBC) nuclear extract was desalted using a HiPrep Desalting 16/20 column and loaded onto a 20-mL DEAE Sepharose FF column. The eluted fractions were desalted and injected onto a UnoQ6 column (BioRad). Bound protein was eluted and injected to a Superose 6 10/30 column. Fractions 8 through 13 were concentrated and injected onto a 1-mL HiTrap Heparin Sepharose HP column. Purification strategy II was similar to purification strategy I except for the insertion of a SP Sepharose column step after the initial DEAE column and omission of the Heparin Sepharose HP column. Methods used for protein electrophoresis, mass spectrometry, and peptide mass fingerprint data analysis are described in Document S1.
BirA system and pull-down assays
The cDNA for BirA was subcloned from pGEM-SD2-BirA (kind gift of John Strouboulis, Erasmus University Medical Center, Rotterdam, The Netherlands) by digestion with EcoRI/SpeI followed by incubation with Klenow. This blunt-ended fragment was ligated into pTarget (Promega, Madison, WI) that had been cut with EcoRI and incubated with Klenow. A cDNA encoding the 23 amino acid BirA acceptor peptide (BAP) was commercially synthesized (Blue Heron Biotechnology, Bothell, WA) and cloned into the NheI and EcoRI sites of pTracer/CMV-Bsd (Invitrogen, Carlsbad, CA) to create pTracer/BAP. The cDNA for cMBD2 was amplified using Platinum Pfx polymerase (Invitrogen) and cloned into the EcoRI and EcoRV sites of pTracer/BAP to create BAP-cMBD2. 6C2 cells were transfected with Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Pull-down assays were performed as described,30 except that the beads were washed 6 times with wash buffer containing NaCl (200 mM × 1, 400 mM × 2, 600 mM × 2, 50 mM × 1). Methods used for protein electrophoresis and mass spectrometry are described in detail in Document S1.
Chromatin immunoprecipitation assay
RBCs were collected from 5-day or 18-day chicken embryos and purified by Histopaque as above, washed twice with Leibovitz medium, and crosslinked with formaldehyde (0.5% final). MEL cells were crosslinked in culture medium with formaldehyde (1.0% final). Crosslinking was quenched after 10 minutes with 125 mM glycine. Cells were washed twice with PBS and resuspended in cell lysis buffer31 and incubated on ice for 10 minutes. The resulting nuclei were collected by centrifugation at 850g for 2 minutes and resuspended in nuclei lysis buffer31 without sodium butyrate and incubated on ice for 10 minutes. After supplementation with glass beads, the lysate was sonicated (model W-220F; Misonix, Farmingdale, NY). The rest of the protocol was performed as recommended by Upstate. Antibodies used for chromatin immunoprecipitation (ChIP) were affinity purified anti-cMBD2, anti-H3-K4-Me3 (Abcam, Cambridge, United Kingdom), anti-mMBD2 (Upstate), and anti-MTA2 (Santa Cruz Biotechnology, Santa Cruz, CA). Quantitative PCR was performed on the final precipitated samples using an ABI PRISM 7900 (Applied Biosystems, Foster City, CA) or an iCycler/iQ5 (Bio-Rad). The primer sets used for analysis are listed in Table S1.
Derivation of MEL-ρ cells and RNAi
The ρ-globin mini-locus was assembled as described32 and cloned into the vector pcDNA4/TO/delP using PmeI. A 2.5-kb (kilobase) fragment of the ρ-globin gene extending from 248 bp (base pair) upstream to 2.2 kb downstream of the cap site was excised from the vector by digesting with BstBI and NotI, methylated in vitro with SssI methylase and religated. The ligation reaction was digested with ScaI and run on an agarose gel. The correctly ligated target product runs on the gel as a 13-kb linear band. The band was excised, and then the DNA was recovered by digestion with GELase (Epicentre Biotechnologies, Madison, WI). MEL cells were transfected by Lipofectamine 2000 (Invitrogen). MEL-ρ cells were selected with zeocin (Invitrogen) at 500 μg/mL and dilution-cloned. Methods used for knock-down of MBD2 by RNAi can be found in Document S1.
Antibodies, Western blotting, cell culture, and mouse splenocytes
Anti-MENT antibodies were the kind gift of Sergei Grigoryev (Penn State College of Medicine, State College, PA). Anti-RBAP48, anti-HDAC2, anti-p66, and anti-Mi2 antibodies were from Upstate (catalog nos. 05-524, 07-222, 05-814, 07-365, 06-878). Anti-MTA1 antibodies were from Peter O'Connell (Virginia Commonwealth University School of Medicine, Richmond, VA). Anti-MTA2 antibodies were from Santa Cruz Biotechnology (sc-9447).
Western blotting was performed as described previously.27 6C2 cells were grown as described.33 MEL cells were grown as described previously.27 Splenocytes were prepared by first removing spleens from MBD2 wild-type and MBD2-/- mice (kind gift of Adrian Bird, University of Edinburgh, Edinburgh, Scotland), and cells were prepared by the method of Kazazian et al.34 The protocol for obtaining mouse tissues was approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee.
Results
Data from our earlier electrophoretic mobility shift assay (EMSA) supershift experiments suggested that MBD2 is a component of the MeCPC.27 In addition to confirming this finding, we sought to identify and characterize the chicken homolog of MBD2 (cMBD2). At the initiation of these studies, the genomic data resources for the chicken were limited to 2 expressed sequence tag (EST) libraries. Both libraries were searched in silico with the cDNA sequence of mouse MBD2 (mMBD2). We identified an EST sequence, present in both libraries, that was highly similar to mMBD2. The corresponding EST clone was obtained, and the insert was sequenced. The clone encoded an open reading frame of 257 amino acids that was 83% identical at the amino acid level to the short or “B” isoform of human and mouse MBD2 (Figure S1).14 Because of the high degree of sequence similarity between the mammalian and chicken proteins and conservation of amino acid residues known to be required for binding of the MBD to the methyl-CpG,35 this protein was thought likely to be the chicken homolog of MBD2.
To determine whether cMBD2 has methyl-CpG-binding activity in vitro, a recombinant GST/cMBD2 fusion protein was produced for use in gel-shift assays. A 50-bp subfragment of the ρ-globin gene MeCPC binding substrate containing a single CpG (a1CpG) was the parent sequence used as a probe in the gel-shift assays. This sequence was mutated to create additional probes containing 0 (a0CpG), 2 (a2CpG), or 3 CpGs (a3CpG). The sequences of these probes are listed in Figure 1A. The probes were methylated in vitro, end-labeled, then incubated with and without GST/cMBD2 and subjected to EMSA. As can be seen in Figure 1B, GST/cMBD2 binds to the probes containing 1, 2, and 3 methyl-CpGs but not to the mock-methylated probe containing 0 methyl-CpGs. Thus, cMBD2 is a bona fide MCBP and can bind to DNA fragments containing as few as 1 methyl-CpG. This finding is consistent with the results obtained by Fraga et al36 for recombinant mouse MBD2.
The chicken homolog of MBD2 is a bona fide methyl-CpG-binding protein and is a component of the MeCPC. (A) DNA sequences of the 50-bp probes used for EMSA with cMBD2. The sequence is derived from the first exon of the ρ-globin gene. The unmodified sequence contains 1 CpG (a1CpG). The sequence was modified to contain no CpGs (a0CpG), 2 CpGs (a2CpG), and 3 CpGs (a3CpG). All CpGs are indicated in bold. Bases that have been mutated compared with the wild-type ρ-globin gene are underlined. (B) cMBD2 is a bona fide MCBP in vitro. The probes a0CpG, a1CpG, a2CpG, and a3CpG were methylated and incubated with recombinant cMBD2. The binding reactions were subjected to EMSA. cMBD2 forms a complex (indicated by the arrow) with the probes containing methyl-CpGs. (C) Anti-cMBD2 antibodies supershift the MeCPC. Inclusion of V2 anti-cMBD2 IgG, but not control IgG, retarded the mobility of the MeCPC complex (supershift) during EMSA.
The chicken homolog of MBD2 is a bona fide methyl-CpG-binding protein and is a component of the MeCPC. (A) DNA sequences of the 50-bp probes used for EMSA with cMBD2. The sequence is derived from the first exon of the ρ-globin gene. The unmodified sequence contains 1 CpG (a1CpG). The sequence was modified to contain no CpGs (a0CpG), 2 CpGs (a2CpG), and 3 CpGs (a3CpG). All CpGs are indicated in bold. Bases that have been mutated compared with the wild-type ρ-globin gene are underlined. (B) cMBD2 is a bona fide MCBP in vitro. The probes a0CpG, a1CpG, a2CpG, and a3CpG were methylated and incubated with recombinant cMBD2. The binding reactions were subjected to EMSA. cMBD2 forms a complex (indicated by the arrow) with the probes containing methyl-CpGs. (C) Anti-cMBD2 antibodies supershift the MeCPC. Inclusion of V2 anti-cMBD2 IgG, but not control IgG, retarded the mobility of the MeCPC complex (supershift) during EMSA.
For further characterization of cMBD2, polyclonal antibodies were generated by immunizing 2 rabbits with GST/cMBD2. Both anti-cMBD2 antibodies (named V1 and V2) recognized an endogenous protein of 28 kDa in multiple chicken cell types, including primary erythroid cells (see Figure S2). Using the anti-cMBD2 antibodies, we wanted to establish that cMBD2 was a component of the MeCPC complex we observed by EMSA. “Supershift” experiments were performed by adding the V1 or V2 antibodies to the EMSA binding reactions. The addition of V2 antibodies, but not control IgG, to the binding reactions retarded the mobility of the MeCPC complex during EMSA (Figure 1C). These data confirmed that MBD2 is likely a component of the MeCPC.
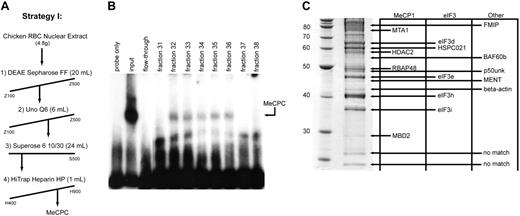
To understand the binding preference of the MeCPC for the methylated ρ-PTR over the canonical MeCP1 binding substrate M-CG11, we sought to determine what the protein components of the complex are, in particular any factors that may be unique to the MeCPC as compared with the MeCP1 complex derived from HeLa cells.20 We developed a chromatography scheme to purify the complex from primary chicken erythroid cell nuclear extracts composed of 4 column steps (Figure 2A). The fractions from the final column of the purification scheme displayed intact MeCPC binding activity in fractions 32 through 36 (Figure 2B), indicating that the complex was stable during purification. The purified sample was run on a 10% SDS-PAGE gel, and the proteins in the sample less than 85 kDa were identified by MALDI-TOF followed by peptide mass fingerprinting (PMF). The SDS-PAGE gel of the final MeCPC sample is shown in Figure 2C (left) with the match for each gel band indicated in Figure 2C (right). Using a novel algorithm that fits PMF data to EST contigs within the ChickEST database28 (Ian M. Overton and Simon J. Hubbard, unpublished observations, August 2004) we were able to match 15 of the 17 bands in the gel analyzed by MALDI-TOF mass spectrometry (see Table S2 for characteristics of these proteins).
Identification of components of the MeCPC purified from primary erythroid cells. (A) Chromatographic scheme (strategy I) used to purify the MeCPC from primary erythroid cells. The fractions eluted from each column were assayed for MeCPC activity by EMSA using the M-ρ248 probe. (B) EMSA on the eluted fractions from the final column of MeCPC purification strategy I (Heparin Sepharose HP) using the M-ρ248 probe. The MeCPC elutes in fractions 32 through 36. After 4 column chromatography steps the complex remains intact. (C, left) Sypro Ruby-stained protein gel containing 15 μg purified MeCPC. The identity of the bands was determined by matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry followed by peptide mass fingerprint data analysis. The molecular mass of markers in kDa is indicated. (Right) Identities of the bands in the gel. Proteins are grouped into columns based on the complex the protein has previously been associated with (MeCP1, eIF3, or Other). Four components of the MeCP1 complex were identified in the purified MeCPC sample: MBD2, RBAP48, HDAC2, and MTA1. Four components of the eIF3 complex as well as an associated protein were identified: eIF3i, eIF3h, eIF3e, eIF3d, and HSPC021. Five additional proteins were identified: β-actin, MENT, p50unk, BAF60b, and FMIP. The identity of the 2 smallest proteins in the gel could not be established.
Identification of components of the MeCPC purified from primary erythroid cells. (A) Chromatographic scheme (strategy I) used to purify the MeCPC from primary erythroid cells. The fractions eluted from each column were assayed for MeCPC activity by EMSA using the M-ρ248 probe. (B) EMSA on the eluted fractions from the final column of MeCPC purification strategy I (Heparin Sepharose HP) using the M-ρ248 probe. The MeCPC elutes in fractions 32 through 36. After 4 column chromatography steps the complex remains intact. (C, left) Sypro Ruby-stained protein gel containing 15 μg purified MeCPC. The identity of the bands was determined by matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry followed by peptide mass fingerprint data analysis. The molecular mass of markers in kDa is indicated. (Right) Identities of the bands in the gel. Proteins are grouped into columns based on the complex the protein has previously been associated with (MeCP1, eIF3, or Other). Four components of the MeCP1 complex were identified in the purified MeCPC sample: MBD2, RBAP48, HDAC2, and MTA1. Four components of the eIF3 complex as well as an associated protein were identified: eIF3i, eIF3h, eIF3e, eIF3d, and HSPC021. Five additional proteins were identified: β-actin, MENT, p50unk, BAF60b, and FMIP. The identity of the 2 smallest proteins in the gel could not be established.
The proteins cMBD2, RBAP48, HDAC2, and MTA1 were identified in the purified MeCPC sample. Given that these 4 proteins were previously identified as components of the MeCP1 complex,20 they were considered putative components of the MeCPC. We identified 4 components of the eukaryotic translation initiation factor 3 (eIF3) complex: eIF3i, eIF3h, eIF3e, and eIF3d,37 as well as HSPC021, a protein that is known to coelute with the eIF3 complex.38 We identified 5 additional proteins: β-actin, MENT, p50unk, BAF60b, and FMIP. Neither the eIF3 complex, FMIP, nor p50unk has been shown to have a role in transcriptional repression or chromatin modulation; therefore, it is likely that these proteins were contaminants in the purified MeCPC sample.
Although β-actin and BAF60b are components of mammalian SWI/SNF-type chromatin remodeling complexes39,40 and β-actin in particular has been shown to have an important role in transcription,41 we did not further investigate this complex because of our failure to identify any other components of this complex (such as BAF53). However, the functional properties of MENT suggested that it might be associated with the MeCPC. MENT is a member of the serine-protease inhibitor family that has been found to be sufficient for higher-order chromatin folding and nucleosome compaction.42,43 It has also been shown to possess DNA-binding and chromatin-modulating activity.43,44 Because MENT is abundantly expressed in terminally differentiated hematopoietic cells such as erythrocytes, we hypothesized that MENT could be a component of the MeCPC and could contribute to the binding preference of the complex.
We repeated the purification of the MeCPC by using a modified scheme (Figure 3A), in which the Superose 6 gel-filtration column was the final step in the procedure. The fractions from the Superose 6 column displayed intact MeCPC binding activity that eluted in fractions 8 through 15, in a molecular weight range of 2000 to 667 kDa (Figure 3B). In addition, a slower-migrating supercomplex elutes in fractions 10 through 13 and a faster-migrating subcomplex elutes in fractions 18 and 19. Both the MeCPC and supercomplex fail to retard an unmethylated ρ-globin probe, whereas the subcomplex is able to form on an unmethylated substrate (Figure 3C). The origin of the supercomplex remains unknown. However, the appearance of the lower molecular weight subcomplex in fractions 18 and 19 coincides with the presence of undersized immunoreactive bands by Western blotting for the MeCPC component proteins Mi2 and p66 (data not shown). Thus, we hypothesize that the subcomplex results from cleavage of MeCPC component proteins during biochemical purification.
Western blotting was performed on the Superose 6 fractions with antibodies against several of the proteins identified by mass spectrometry as well as the other known components of the MeCP1 complex.20,45 Western blots with antibodies against cMBD2, HDAC2, p66, MTA1, and Mi2 show a strikingly similar profile (Figure 3D) that matches the elution profile of the MeCPC activity. Western blotting with antibodies against MENT showed that this protein coeluted with the MeCP1 factors, suggesting that this abundantly expressed protein was not a contaminant in the purified MeCPC fractions. Western blotting with antibodies against RBAP48 showed that this protein also coeluted with the MeCP1 factors, although it elutes over a larger set of fractions. This may be because RBAP48 is a component of multiple transcriptional repression complexes, such as the Sin3A46 and the Rb complexes.47
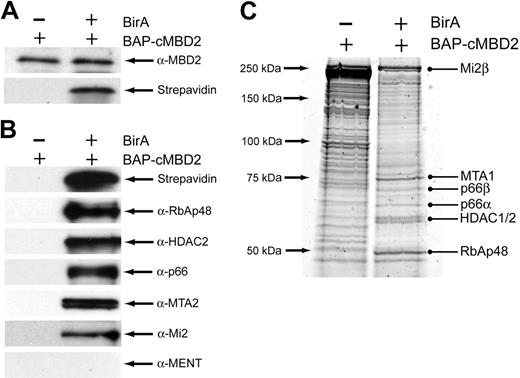
To confirm the biochemical interaction of cMBD2 with the other components of the MeCP1 complex identified in the Superose 6 fractions, we performed pull-down assays using the BirA biotin-tagging system.30 First, we generated BirA+ stable transfectants in the avian pre-erythroid cell line 6C2.33 Next, a cMBD2 expression construct with an N-terminal biotin acceptor peptide (BAP) was introduced into the cells. Expression of cMBD2 was similar in BirA- and BirA+ 6C2 cells containing BAP-cMBD2; however, biotinylation of cMBD2 was only seen in BirA+ cells (Figure 4A). Pull-down assays were then performed by binding nuclear extracts from BirA- and BirA+ BAP-cMBD2 6C2 cells to streptavidin beads. We identified the 5 major components of the MeCP1 complex in the eluate from BirA+ cells, whereas no components were identified in the eluate from BirA- cells (Figure 4B). Interestingly, MENT was not found in the eluate, indicating that this protein does not directly interact with MBD2 in vivo. To further delineate the interaction partners of cMBD2, the eluate from cMBD2 pull-down assays was analyzed by mass spectrometry. Analysis of major bands again revealed the 5 major components of the MeCP1 complex (Figure 4C). Given the chromatographic and affinity copurification of cMBD2, RBAP48, HDAC2, p66, MTA2, and Mi2, we conclude that these 6 factors are components of the MeCPC in chicken erythroid cells.
Putative components of the MeCPC copurify from a Superose 6 gel-filtration column. (A) The second chromatographic scheme (strategy II) was used to purify the MeCPC from primary erythroid cells. The fractions eluted from each column were assayed for MeCPC activity by EMSA using the M-ρ248 probe. (B) EMSA on the high molecular weight fractions from the final column of MeCPC purification strategy II (Superose 6) using the methylated M-ρ248 probe. The MeCPC elutes primarily in fractions 8 through 15 in a molecular weight range of 670 to 2000 kDa. The position of the MeCPC is indicated as “M,” the slower-migrating supercomplex as “Sup,” and the faster-migrating subcomplex as “Sub.” Although the exact composition of the supercomplex is unknown, we hypothesize that the subcomplex results from cleavage of MeCPC component proteins during biochemical purification. (C) EMSA on the high molecular weight fractions from the final column of MeCPC purification strategy II (Superose 6) using the unmethylated ρ248 probe. Both the MeCPC and the supercomplex fail to retard the mobility of an unmethylated probe. In contrast, the subcomplex is able to retard the ρ248 probe (fractions 18 and 19). (D) Western blot analysis of the high molecular weight fractions from the Superose 6 gel-filtration column. The antibody used for each blot is indicated at the right. The putative MeCPC components MBD2, RBAP48, HDAC2, p66, MTA1, and Mi2 copurify from the column. In addition, the erythroid-specific heterochromatin protein MENT also copurifies with the MeCPC factors.
Putative components of the MeCPC copurify from a Superose 6 gel-filtration column. (A) The second chromatographic scheme (strategy II) was used to purify the MeCPC from primary erythroid cells. The fractions eluted from each column were assayed for MeCPC activity by EMSA using the M-ρ248 probe. (B) EMSA on the high molecular weight fractions from the final column of MeCPC purification strategy II (Superose 6) using the methylated M-ρ248 probe. The MeCPC elutes primarily in fractions 8 through 15 in a molecular weight range of 670 to 2000 kDa. The position of the MeCPC is indicated as “M,” the slower-migrating supercomplex as “Sup,” and the faster-migrating subcomplex as “Sub.” Although the exact composition of the supercomplex is unknown, we hypothesize that the subcomplex results from cleavage of MeCPC component proteins during biochemical purification. (C) EMSA on the high molecular weight fractions from the final column of MeCPC purification strategy II (Superose 6) using the unmethylated ρ248 probe. Both the MeCPC and the supercomplex fail to retard the mobility of an unmethylated probe. In contrast, the subcomplex is able to retard the ρ248 probe (fractions 18 and 19). (D) Western blot analysis of the high molecular weight fractions from the Superose 6 gel-filtration column. The antibody used for each blot is indicated at the right. The putative MeCPC components MBD2, RBAP48, HDAC2, p66, MTA1, and Mi2 copurify from the column. In addition, the erythroid-specific heterochromatin protein MENT also copurifies with the MeCPC factors.
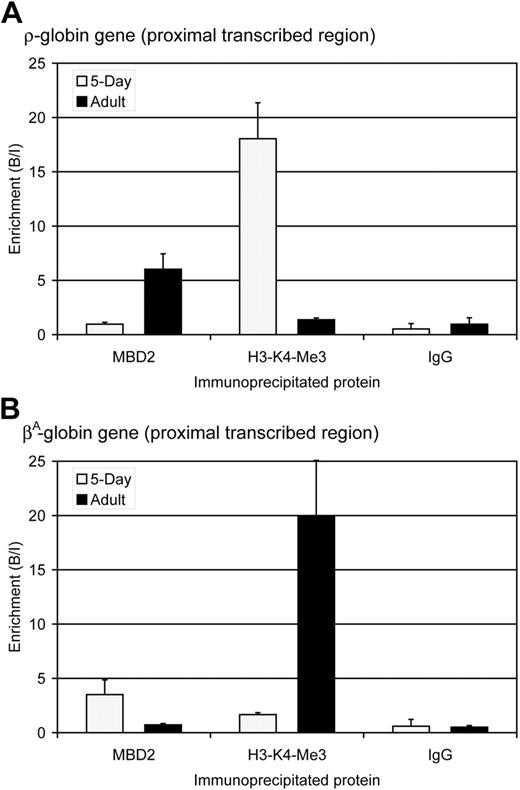
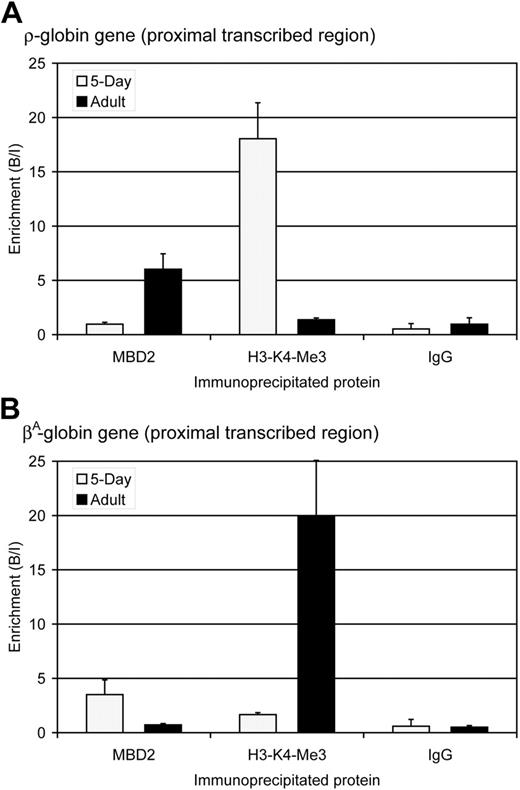
The identification of MBD2 as a component of the MeCPC supports a role for this protein in the transcriptional repression of the ρ-globin gene in adult chicken red blood cells. To determine whether MBD2 is associated with the methylated ρ-globin gene in vivo, ChIP assays were performed using affinity purified anti-cMBD2 antibodies in embryonic day 5 and adult erythrocytes. Antibodies against trimethylated lysine 4 of histone H3 (H3-K4-Me3), a marker of active transcription in chicken erythrocytes,48 were used as a control. The amount of ρ-globin or βA-globin DNA in the total DNA immunoprecipitated with each antibody was determined by quantitative PCR. At the ρ-globin gene, no enrichment for cMBD2 is seen in embryonic day 5 erythrocytes in which the gene is transcriptionally active and highly enriched for H3-K4-Me3 (Figure 5A). In contrast, in adult erythrocytes significant enrichment for cMBD2 is seen at the transcriptionally inactive and methylated ρ-globin gene that is depleted of H3-K4-Me3-modified chromatin. The ChIP assay therefore establishes that cMBD2 binds to the transcriptionally inactive ρ-globin gene in adult chicken erythroid cells in vivo.
The expression and methylation pattern of the βA-globin gene is opposite that of the ρ-globin gene: it is transcriptionally active in adult erythrocytes and transcriptionally inactive and methylated in primitive erythrocytes.26 The observed pattern of cMBD2 and H3-K4-Me3 enrichment around the βA-globin gene mirrors that of the ρ-globin gene (Figure 5B) and thus serves as a reciprocal internal control for the ChIP assays. Significant enrichment for cMBD2 is seen at the transcriptionally inactive βA-globin gene in embryonic day 5 erythrocytes in which H3-K4-Me3 is depleted. In contrast, no enrichment is observed for MBD2 at the transcriptionally active βA-globin gene in which H3-K4-Me3 is strongly enriched.
cMBD2 affinity copurifies the other components of the MeCP1 complex. (A) Western blot analysis of cMBD2 and streptavidin expression in 6C2 cells containing the BAP-cMBD2 construct with or without the biotin ligase BirA. Equal expression of cMBD2 is seen in BirA- and BirA+ BAP-cMBD2 6C2 cells, whereas biotinylation of cMBD2 only occurs in the BirA+ cells. (B) Western blot analysis of the eluate from cMBD2 pull-down experiments in BAP-cMBD2 6C2 cells. Abundant biotinylated cMBD2 as well as the major components of the MeCP1 complex are seen in the eluate from pull-down assays performed using BirA+ BAP-cMBD2 6C2 cells as the input. In contrast, no biotinylated cMBD2 or any MeCP1 components are seen in the eluate from pull-down assays performed using BirA- BAP-cMBD2 6C2 cells as the input. (C) Sypro Ruby-stained protein gel of the eluate from cMBD2 pull-down experiments in BAP-cMBD2 6C2 cells. Interestingly, despite equal amounts of input protein, less protein elutes from streptavidin beads bound to BirA+ BAP-cMBD2 6C2 cell nuclear extract. The identities of bands in the gel were determined by tandem-mass spectrometry on excised and trypsin-digested bands. The identities of the indicated bands are listed on the right. At least 2 experimentally derived peptides were required for protein identification.
cMBD2 affinity copurifies the other components of the MeCP1 complex. (A) Western blot analysis of cMBD2 and streptavidin expression in 6C2 cells containing the BAP-cMBD2 construct with or without the biotin ligase BirA. Equal expression of cMBD2 is seen in BirA- and BirA+ BAP-cMBD2 6C2 cells, whereas biotinylation of cMBD2 only occurs in the BirA+ cells. (B) Western blot analysis of the eluate from cMBD2 pull-down experiments in BAP-cMBD2 6C2 cells. Abundant biotinylated cMBD2 as well as the major components of the MeCP1 complex are seen in the eluate from pull-down assays performed using BirA+ BAP-cMBD2 6C2 cells as the input. In contrast, no biotinylated cMBD2 or any MeCP1 components are seen in the eluate from pull-down assays performed using BirA- BAP-cMBD2 6C2 cells as the input. (C) Sypro Ruby-stained protein gel of the eluate from cMBD2 pull-down experiments in BAP-cMBD2 6C2 cells. Interestingly, despite equal amounts of input protein, less protein elutes from streptavidin beads bound to BirA+ BAP-cMBD2 6C2 cell nuclear extract. The identities of bands in the gel were determined by tandem-mass spectrometry on excised and trypsin-digested bands. The identities of the indicated bands are listed on the right. At least 2 experimentally derived peptides were required for protein identification.
MBD2 occupancy inversely correlates with transcription and histone H3-lysine 4-trimethylation (H3-K4-Me3) at the ρ- and βA-globin genes. (A) Enrichment for MBD2, H3-K4-Me3, and IgG at the ρ-globin gene in 5-day ( ) and adult (▪) erythrocytes as determined by ChIP assay. The data show that MBD2 is depleted from the transcriptionally active ρ-globin gene in 5-day erythrocytes but enriched at the transcriptionally inactive and methylated ρ-globin gene in adult erythrocytes. In contrast, H3-K4-Me3 is enriched at the transcriptionally active ρ-globin gene and depleted at the transcriptionally inactive gene. No enrichment was seen using anti-rabbit IgG in either 5-day or adult erythrocytes, verifying the interaction of these specific proteins with the ρ-globin gene. The data represent the average of 3 independent experiments, with the SD indicated by the bar. (B) Enrichment for MBD2, H3-K4-Me3, and IgG at the βA-globin gene in 5-day (
) and adult (▪) erythrocytes as determined by ChIP assay. The data show that MBD2 is depleted from the transcriptionally active ρ-globin gene in 5-day erythrocytes but enriched at the transcriptionally inactive and methylated ρ-globin gene in adult erythrocytes. In contrast, H3-K4-Me3 is enriched at the transcriptionally active ρ-globin gene and depleted at the transcriptionally inactive gene. No enrichment was seen using anti-rabbit IgG in either 5-day or adult erythrocytes, verifying the interaction of these specific proteins with the ρ-globin gene. The data represent the average of 3 independent experiments, with the SD indicated by the bar. (B) Enrichment for MBD2, H3-K4-Me3, and IgG at the βA-globin gene in 5-day ( ) and adult (▪) erythrocytes as determined by ChIP assay. The data show that MBD2 is enriched at the transcriptionally inactive and methylated βA-globin gene in 5-day erythrocytes but depleted from the transcriptionally active βA-globin gene in adult erythrocytes. Once again, H3-K4-Me3 is depleted from the transcriptionally inactive βA-globin gene and enriched at the transcriptionally active gene. No enrichment was seen using anti-rabbit IgG in either 5-day or adult erythrocytes, verifying the interaction of these specific proteins with the βA-globin gene. The data represent the average of 3 independent experiments, with the SD indicated by the bar.
) and adult (▪) erythrocytes as determined by ChIP assay. The data show that MBD2 is enriched at the transcriptionally inactive and methylated βA-globin gene in 5-day erythrocytes but depleted from the transcriptionally active βA-globin gene in adult erythrocytes. Once again, H3-K4-Me3 is depleted from the transcriptionally inactive βA-globin gene and enriched at the transcriptionally active gene. No enrichment was seen using anti-rabbit IgG in either 5-day or adult erythrocytes, verifying the interaction of these specific proteins with the βA-globin gene. The data represent the average of 3 independent experiments, with the SD indicated by the bar.
MBD2 occupancy inversely correlates with transcription and histone H3-lysine 4-trimethylation (H3-K4-Me3) at the ρ- and βA-globin genes. (A) Enrichment for MBD2, H3-K4-Me3, and IgG at the ρ-globin gene in 5-day ( ) and adult (▪) erythrocytes as determined by ChIP assay. The data show that MBD2 is depleted from the transcriptionally active ρ-globin gene in 5-day erythrocytes but enriched at the transcriptionally inactive and methylated ρ-globin gene in adult erythrocytes. In contrast, H3-K4-Me3 is enriched at the transcriptionally active ρ-globin gene and depleted at the transcriptionally inactive gene. No enrichment was seen using anti-rabbit IgG in either 5-day or adult erythrocytes, verifying the interaction of these specific proteins with the ρ-globin gene. The data represent the average of 3 independent experiments, with the SD indicated by the bar. (B) Enrichment for MBD2, H3-K4-Me3, and IgG at the βA-globin gene in 5-day (
) and adult (▪) erythrocytes as determined by ChIP assay. The data show that MBD2 is depleted from the transcriptionally active ρ-globin gene in 5-day erythrocytes but enriched at the transcriptionally inactive and methylated ρ-globin gene in adult erythrocytes. In contrast, H3-K4-Me3 is enriched at the transcriptionally active ρ-globin gene and depleted at the transcriptionally inactive gene. No enrichment was seen using anti-rabbit IgG in either 5-day or adult erythrocytes, verifying the interaction of these specific proteins with the ρ-globin gene. The data represent the average of 3 independent experiments, with the SD indicated by the bar. (B) Enrichment for MBD2, H3-K4-Me3, and IgG at the βA-globin gene in 5-day ( ) and adult (▪) erythrocytes as determined by ChIP assay. The data show that MBD2 is enriched at the transcriptionally inactive and methylated βA-globin gene in 5-day erythrocytes but depleted from the transcriptionally active βA-globin gene in adult erythrocytes. Once again, H3-K4-Me3 is depleted from the transcriptionally inactive βA-globin gene and enriched at the transcriptionally active gene. No enrichment was seen using anti-rabbit IgG in either 5-day or adult erythrocytes, verifying the interaction of these specific proteins with the βA-globin gene. The data represent the average of 3 independent experiments, with the SD indicated by the bar.
) and adult (▪) erythrocytes as determined by ChIP assay. The data show that MBD2 is enriched at the transcriptionally inactive and methylated βA-globin gene in 5-day erythrocytes but depleted from the transcriptionally active βA-globin gene in adult erythrocytes. Once again, H3-K4-Me3 is depleted from the transcriptionally inactive βA-globin gene and enriched at the transcriptionally active gene. No enrichment was seen using anti-rabbit IgG in either 5-day or adult erythrocytes, verifying the interaction of these specific proteins with the βA-globin gene. The data represent the average of 3 independent experiments, with the SD indicated by the bar.
We sought to extend the ChIP analysis at the ρ-globin gene to the other proteins identified in Figure 4 as components of the MeCPC. Unfortunately, we were not able to enrich for ρ-globin or control sequences in adult chicken RBCs using any available antibodies to MeCPC component proteins. To facilitate these studies we established a stably transfected cell model by introducing a chicken ρ-globin mini-locus into the MEL cell line. The mini-locus contains (1) a 4.5-kb ρ-globin genomic sequence methylated at the same sites as the endogenous gene in chicken adult erythroid cells, (2) chicken locus control region (LCR) enhancer elements, and (3) 5′ and 3′ cHS4 insulator elements49 that surround the gene and enhancer (depicted in Figure 6A).
Preliminary experiments indicated that the MeCPC is present in MEL cells (Figure S3A). Furthermore, the addition of anti-mMBD2 antibodies, but not control IgG, to the binding reactions retarded the mobility of the MeCPC complex during EMSA (Figure S3A), thus confirming that MBD2 is also a component of the MeCPC in murine cells. MEL cells were transfected with the ρ-globin mini-locus that had been methylated to recapitulate the pattern of methylation observed at the endogenous gene in adult chicken RBCs.50 After selection and dilution cloning, DNA slot blotting confirmed that the ρ-globin mini-locus was present in MEL cells, termed MEL-ρ cells (Figure 6D).
To show that other components of the MeCPC associate with the methylated ρ-globin gene in vivo, ChIP assays were performed using antibodies to mMBD2 as well as the MeCPC component MTA2. Binding for each protein at the methylated and transcriptionally silent ρ-globin gene in wild-type mMBD2+ MEL-ρ cells was compared with binding at the transcriptionally active ρ-globin gene in mMBD2- knock-down MEL-ρ cells. As expected, a major enrichment for mMBD2 is seen at the ρ-globin gene in wild-type mMBD2+ MEL-ρ cells as compared with knock-down cells expressing less than 10% as much mMBD2 (Figure S3B). Importantly, there was also a major (25-fold) enrichment for MTA2 at the ρ-globin gene in wild-type mMBD2+ MEL-ρ cells as compared with the mMBD2- knock-down cells (Figure S3B). These data show that other components of the MeCPC bind with MBD2 to the methylated ρ-globin gene in vivo. Furthermore, the loss of MTA2 binding to the ρ-globin gene in mMBD2- knock-down MEL-ρ cells serves as evidence that MTA2 is recruited by MBD2 to the gene.
Given that cMBD2 binds to the methylated ρ-globin gene and is able to pull-down the other major components of the MeCPC in vivo, it is an attractive target for clinical therapies aimed at alleviating methylation-mediated transcriptional silencing by disrupting the effector complexes of methylation. We made use of the MBD2-knockout mouse51 to assess whether MBD2 is required for formation of the MeCPC in vivo. Primary splenic erythroid cells were isolated from both MBD2+/+ and MBD2-/- mice rendered anemic to convert the spleen to a primarily erythropoietic organ.52 Erythroblasts were subjected to nuclear extract preparation. As can be seen in Figure 6B, the MeCPC is present in primary erythroid cells from MBD2+/+ animals and MBD2 is a component of the complex, as indicated by supershift of the MeCPC band by the inclusion of anti-mMBD2 antibodies but not control IgG. In contrast, no MeCPC activity is detectable in primary splenic erythroblasts from MBD2-/- animals, indicating that MBD2 is essential for formation of the MeCPC.
Next, we assessed the necessity of MBD2 for transcriptional repression of the methylated ρ-globin gene by knocking-down mMBD2 by RNAi in the MEL-ρ cell model. MEL-ρ cells were transfected with a vector expressing short hairpin RNA (shRNA) targeting mMBD2. After selection and dilution cloning, both RNase protection assays and Western blotting confirmed that mMBD2 was knocked-down by greater than 90% in MEL cells containing mMBD2 shRNAs as compared with cells containing empty vector (Figure S4). Expression of ρ-globin was then analyzed by using an RNase protection assay. Knock-down of mMBD2 in MEL-ρ cells resulted in an approximately 25-fold increase in ρ-globin expression compared with control cells (Figure 6C), indicating that MBD2 is functionally required for full transcriptional silencing of the methylated ρ-globin gene in this adult erythroid cell model. These data are consistent with the level of repression seen with methylated ρ-globin gene constructs transfected into primary avian erythrocytes.27
MBD2 is a critical component of the MeCPC in primary mouse splenocytes and MEL-ρ cells. (A) Graphic depiction of the ρ-globin mini-locus introduced into MEL cells. The locus contains (1) a 4.5-kb ρ-globin genomic sequence, (2) a 4-kb chicken LCR enhancer element (HSS2 and HSS3), and (3) 5′ and 3′ cHS4 insulator elements that surround the gene and enhancer. A 2.5-kb fragment of the ρ-globin genomic sequence extending from 248 bp upstream to 2.2 kb downstream of the cap site was excised, in vitro methylated, and religated prior to transfection into MEL cells. In this way the ρ-globin gene is methylated at the same sites as the endogenous gene in chicken adult erythroid cells. (B) EMSA performed with 20 μg nuclear extract from primary mouse splenocytes. Extracts derived from spleens of MBD2+/+ mice form the MeCPC and can be supershifted by the addition of anti-mMBD2 IgG but not control IgG. In contrast, extracts derived from the spleens of MBD2-/- mice do not form a complex on the M-ρ248 probe. (C) RNase protection assay analyzing expression of ρ-globin, mMBD2, and 18S RNAs in MEL-ρ cells treated with shRNAs targeting mMBD2. Significant knock-down of mMBD2 expression is seen in MEL-ρ cells containing shRNA-expressing plasmids that target mMBD2, as compared with control cells (lanes 3 and 4 as compared with lanes 1 and 2). No ρ-globin expression is seen in MEL-ρ cells with wild-type MBD2 expression (lane 2). In contrast, robust ρ-globin expression is seen in MEL-ρ cells in which mMBD2 expression has been knocked down by shRNA (lane 4). Loading of RNA for all MEL samples was equal, as shown by equal amounts of 18S RNA present in the samples. This datum indicates that MBD2 is functionally required for full transcriptional silencing of the methylated ρ-globin gene. (D) Slot blots performed with genomic DNA from wild-type MEL or MEL-ρ cells that contain a stably integrated ρ-globin mini-locus. Genomic DNA from MEL-ρ cells contains ρ-globin gene sequences, whereas wild-type MEL cells display only background hybridization. A 2-fold difference in copy number is observed between mMBD2+ MEL-ρ and mMBD2- knock-down MEL-ρ cells. In contrast, a similar amount of endogenous mouse GAPDH gene sequences is seen in all 3 samples, demonstrating equal DNA loading. Chicken genomic DNA was used as a positive control for the ρ-globin probe.
MBD2 is a critical component of the MeCPC in primary mouse splenocytes and MEL-ρ cells. (A) Graphic depiction of the ρ-globin mini-locus introduced into MEL cells. The locus contains (1) a 4.5-kb ρ-globin genomic sequence, (2) a 4-kb chicken LCR enhancer element (HSS2 and HSS3), and (3) 5′ and 3′ cHS4 insulator elements that surround the gene and enhancer. A 2.5-kb fragment of the ρ-globin genomic sequence extending from 248 bp upstream to 2.2 kb downstream of the cap site was excised, in vitro methylated, and religated prior to transfection into MEL cells. In this way the ρ-globin gene is methylated at the same sites as the endogenous gene in chicken adult erythroid cells. (B) EMSA performed with 20 μg nuclear extract from primary mouse splenocytes. Extracts derived from spleens of MBD2+/+ mice form the MeCPC and can be supershifted by the addition of anti-mMBD2 IgG but not control IgG. In contrast, extracts derived from the spleens of MBD2-/- mice do not form a complex on the M-ρ248 probe. (C) RNase protection assay analyzing expression of ρ-globin, mMBD2, and 18S RNAs in MEL-ρ cells treated with shRNAs targeting mMBD2. Significant knock-down of mMBD2 expression is seen in MEL-ρ cells containing shRNA-expressing plasmids that target mMBD2, as compared with control cells (lanes 3 and 4 as compared with lanes 1 and 2). No ρ-globin expression is seen in MEL-ρ cells with wild-type MBD2 expression (lane 2). In contrast, robust ρ-globin expression is seen in MEL-ρ cells in which mMBD2 expression has been knocked down by shRNA (lane 4). Loading of RNA for all MEL samples was equal, as shown by equal amounts of 18S RNA present in the samples. This datum indicates that MBD2 is functionally required for full transcriptional silencing of the methylated ρ-globin gene. (D) Slot blots performed with genomic DNA from wild-type MEL or MEL-ρ cells that contain a stably integrated ρ-globin mini-locus. Genomic DNA from MEL-ρ cells contains ρ-globin gene sequences, whereas wild-type MEL cells display only background hybridization. A 2-fold difference in copy number is observed between mMBD2+ MEL-ρ and mMBD2- knock-down MEL-ρ cells. In contrast, a similar amount of endogenous mouse GAPDH gene sequences is seen in all 3 samples, demonstrating equal DNA loading. Chicken genomic DNA was used as a positive control for the ρ-globin probe.
Discussion
In this study we have characterized the chicken homolog of MBD2 and found that an erythroid methyl cytosine-binding protein complex isolated from primary erythroid cells contains cMBD2 as well as the other major components of the MeCP1 complex: RBAP48, HDAC2, p66, MTA2, and Mi2. Given that the ρ-globin gene is methylated during development27 and that a methylated probe corresponding to a segment of the ρ-globin gene was used to monitor the elution of MeCPC activity from each column, it is likely that our biochemical data are highly relevant to the in vivo environment. Indeed, we have verified that cMBD2 binds to the methylated βA-globin gene in embryonic day 5 erythrocytes and the methylated ρ-globin gene in adult erythrocytes. Furthermore, we have shown that MBD2 is required for formation of the MeCPC in primary splenic erythroid cells and that expression of the methylated ρ-globin gene is increased in MEL-ρ cells lacking mMBD2. Taken together, these data indicate that MBD2 is a critical component of the MeCPC, because transcriptional derepression occurs when its function is lost.
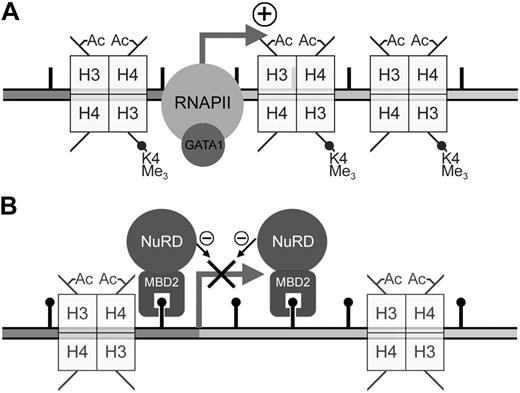
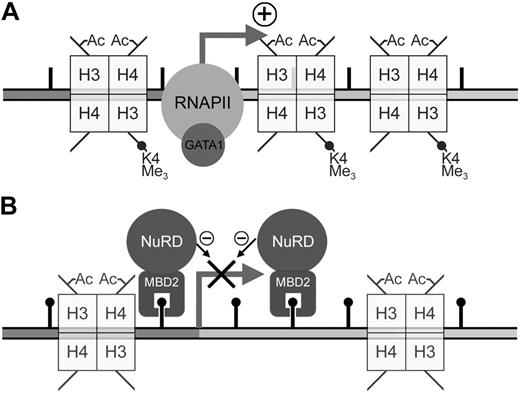
Previous work from our laboratory has shown that the MeCPC is present in both embryonic day 5 and adult chicken erythrocytes.10 We have also shown that the DNA methylation pattern at the ρ-globin gene is dynamic, marked by a total absence of methylation at CpG sites within the promoter, ρ-PTR, and downstream regions in primitive day 4 chicken erythrocytes,50 but dense methylation in definitive day 14 erythrocytes. Taken together with the data presented here, we present a model for the developmental regulation of ρ-globin transcription (Figure 7). In day 4 erythrocytes, the presence of positively acting trans factors such as GATA153 drives high levels of transcription from the unmethylated gene (Figure 7A). Transcription of the gene is already silenced in early (embryonic day 7) definitive erythrocytes,54 before methylation at the ρ-globin is complete. Work presented here demonstrates that in adult erythrocytes where there is complete methylation of the ρ-globin gene, MBD2 binds to the methylated gene and recruits MTA2, and likely the other components of the MeCPC, to the gene (Figure 7B). The complex maintains transcriptional inactivity by remodeling chromatin into a nonpermissive configuration.20
Composition of methyl-DNA-binding protein complexes
Because of their potential fundamental importance in interpreting cytosine methylation to regulate transcription, numerous studies have sought to define the components of methyl cytosine-binding transcriptional complexes.20,23,55,56 Here, we report the purification of a MeCP1-like complex from primary erythroid cells. This represents the first purification of such a complex from a primary cell source. Analysis of protein complexes in primary cells serves as an important adjunct to studies of complexes in cultured cells, because biochemical interactions based on transient signals such as protein posttranslational modification or kinase signaling may be lost or artifactually gained over time in culture. Indeed, we observe the formation of a methylation-dependent supercomplex of unknown composition that has not been previously observed during the isolation of MeCP1-like complexes from immortalized cells.
Model for the developmental regulation of ρ-globin transcription in chicken erythrocytes. (A) In embryonic day 4 primitive chicken erythrocytes, robust expression of the ρ-globin gene is seen. The presence of positively-acting trans factors such as GATA1 recruits RNA polymerase (RNAPII) and drives high levels of transcription from the unmethylated ρ-globin gene. Histones throughout the gene exhibit high levels of transcriptionally active modifications, such as trimethylation of H3-K4 as well as H3 and H4 acetylation. (B) No transcription of the ρ-globin gene is seen in adult definitive chicken erythrocytes. In these cells there is dense methylation at the promoter, ρ-PTR, and downstream regions of the gene. In this work we have shown that cMBD2 binds to the methylated ρ-globin gene in adult cells. Because cMBD2 can affinity copurify the other components of the MeCPC complex in vivo, it is likely that MBD2 recruits these components to the methylated ρ-globin gene. Indeed, we show that MBD2 and MTA2 occupy the methylated gene in MEL-ρ cells. The complex maintains transcriptional inactivity by remodeling chromatin into a nonpermissive configuration. Coincident with this loss of transcriptional activity is the loss of trimethylation of H3-K4.
Model for the developmental regulation of ρ-globin transcription in chicken erythrocytes. (A) In embryonic day 4 primitive chicken erythrocytes, robust expression of the ρ-globin gene is seen. The presence of positively-acting trans factors such as GATA1 recruits RNA polymerase (RNAPII) and drives high levels of transcription from the unmethylated ρ-globin gene. Histones throughout the gene exhibit high levels of transcriptionally active modifications, such as trimethylation of H3-K4 as well as H3 and H4 acetylation. (B) No transcription of the ρ-globin gene is seen in adult definitive chicken erythrocytes. In these cells there is dense methylation at the promoter, ρ-PTR, and downstream regions of the gene. In this work we have shown that cMBD2 binds to the methylated ρ-globin gene in adult cells. Because cMBD2 can affinity copurify the other components of the MeCPC complex in vivo, it is likely that MBD2 recruits these components to the methylated ρ-globin gene. Indeed, we show that MBD2 and MTA2 occupy the methylated gene in MEL-ρ cells. The complex maintains transcriptional inactivity by remodeling chromatin into a nonpermissive configuration. Coincident with this loss of transcriptional activity is the loss of trimethylation of H3-K4.
Our analysis of the MeCPC failed to identify cMBD3 as a component, either by mass spectrometry or Western analysis (data not shown). Recent work by Le Guezennec et al56 supports this finding, showing that, indeed, MBD2 and MBD3 form mutually exclusive complexes with NuRD components. We also failed to identify GATA1 or FOG1 as tissue-specific components of the MeCPC, either by mass spectrometry or Western analysis (data not shown). Recent work by Rodriguez et al57 found that GATA1 pulled down the complete components of the MeCP1 complex, and work by Hong et al22 found that FOG1 pulled down the complete components of the NuRD complex. Our failure to identify either of these tissue-restricted factors likely reflects the known heterogeneity of MeCP1- and NuRD-like complexes.58
Our chromatographic analysis of the composition of the MeCPC identified MENT as a protein that tightly copurified with the other MeCPC components. However, MENT was not pulled down by biotin-tagged cMBD2 in cultured cell extracts, indicating either that it does not biochemically associate with cMBD2 in vivo or that cultured cells differ from primary cells.
RbAp48, which is known to be a component of multiple protein complexes, is pulled down by biotin-tagged MENT (data not shown). This finding provides an explanation for the observation of MENT in the same Superose 6 fractions that contain the MeCPC. Therefore, we favor the conclusion that MENT is not part of the MeCPC.
Role of MBD2 in developmental gene regulation
Despite numerous reports of genes whose transcription is inversely related to the level of DNA methylation at CpG sites in a specific cell-type and the demonstration of a profound effect of DNA methylation during vertebrate embryonic development,59,60 only a limited number of genes have been shown to be developmentally regulated by DNA methylation. A distinct yet important class of genes is that of tumor suppressor genes methylated in cancer cells, whereby the methylation pattern is abnormal (ie, it differs from that of a nontransformed cell of the same type).
Two particularly well-studied examples of normal developmental regulation by DNA methylation are the avian ρ-globin gene and the murine interleukin-4 gene. Previously, our laboratory has shown that every CpG within the promoter- and proximal-transcribed region of the ρ-globin gene is methylated in adult chicken erythroid cells in which the gene is transcriptionally inactive.10,27 DNA methylation has also been shown to be involved in the transcriptional silencing of the baboon and human γ-globin genes.61-63 Clinically, erythroid cells of patients receiving treatment of sickle cell disease and β-thalassemia with the DNA methylation inhibitors 5-aza-cytidine and 5-aza-2′-deoxycytidine have increased transcription of the γ-globin genes.61,64,65
Here, we show that MBD2 binds to the methylated and transcriptionally silent ρ-globin gene in adult erythroid cells, but it does not bind to the same region in embryonic day 5 erythrocytes in which the gene is transcriptionally active. Furthermore, abrogation of MBD2 function is associated with loss of MeCPC formation and derepression of a methylated ρ-globin gene. The data presented here on the composition of the MeCPC and the role of MBD2 in regulating the chicken ρ-globin gene are potentially highly relevant to the human β-globin locus. In support of this, our laboratory has recently determined that the fetal γ-globin gene is ectopically expressed at high levels in adult RBCs from MBD2-/- mice that contain a single copy human β-globin locus YAC.66
Our data parallel those of Hutchins et al,15 who found that MBD2 was bound to 2 methylated regions of the interleuken-4 gene in Th1 cells when the gene was transcriptionally inactive but displaced from the same regions when the transcriptional activator GATA-3 was expressed in the cells. Considering the work presented here and that of Hutchins et al15 together, a role for MBD2 in developmental gene regulation is emerging. Given that MBD2 is not required for normal mammalian embryonic development and confers a minimally abnormal phenotype,51 MBD2 may be an excellent target for therapeutic modulation aimed at altering developmental and neoplastic gene expression patterns.
Prepublished online as Blood First Edition Paper, June 15, 2006; DOI 10.1182/blood-2006-04-016394.
Supported by the National Institutes of Health (grant DK29902) and by institutional funding from the Massey Cancer Center.
E.P.K. designed and performed the research, analyzed the data, and wrote the paper; S.Z.W., S.Z.Z., and T.B.L. performed the research and analyzed the data; J.W.R. contributed reagents; and G.D.G. designed the research and wrote the paper.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Richard Moran for advice and discussions regarding protein characterization and Catharine Tucker for assistance in the preparation of this manuscript.