Abstract
In vitro manipulation of hematopoietic stem cells (HSCs) is a key issue in both transplantation therapy and regenerative medicine, and thus new methods are required to achieve HSC expansion with self-renewal. GATA2 is a transcription factor controlling pool size of HSCs. Of interest, continuous overexpression of GATA2 does not induce HSC proliferation. In this report, we demonstrate that GATA2 expression, in leukemic and normal hematopoietic cells, oscillates during the cell cycle, such that expression is high in S phase but low in G1/S and M phase. GATA2 binding to target Bcl-X gene also oscillates in accordance with GATA2 expression. Using a green fluorescent protein (GFP)–GATA2 fusion protein, we demonstrate cell-cycle–specific activity of proteasome-dependent degradation of GATA2. Immunoprecipitation/immunoblotting analysis demonstrated phosphorylation of GATA2 at cyclin-dependent kinase (Cdk)–consensus motifs, S/T0P+1, and interaction of GATA2 with Cdk2/cyclin A2–, Cdk2/cyclin A2–, and Cdk4/cyclin D1–phosphorylated GATA2 in vitro. Mutants in phosphorylation motifs exhibited altered expression profiles of GFP-GATA2 domain fusion proteins. These results indicate that GATA2 phosphorylation by Cdk/cyclin systems is responsible for the cell-cycle–dependent regulation of GATA2 expression, and suggest the possibility that a cell-cycle–specific “on-off” response of GATA2 expression may control hematopoietic-cell proliferation and survival.
Introduction
Hematopoietic stem cells (HSCs) give rise to the immunohematopoietic system throughout the lifetime of host animals.1 HSCs have distinctive systems regulating their proliferation and differentiation. These systems maintain steady-state hematopoiesis, and respond to stresses, including infection, hypoxia, and blood loss.1,2 Two types of cell division occur in HSCs: one type produces differentiated daughter cells to supply mature blood cells, while the other type reproduces HSCs (self-renewal division) to maintain life-long hematopoiesis.1,2 Of interest, in the bone marrow reservoir of HSCs, more than 70% of bone marrow HSCs are quiescent (G0 phase), maintaining high proliferative capacity and pluripotency.3 In contrast, their quiescent mature progeny generally lack the capacity for growth and division.
Directing the expansion of HSCs in vitro is an urgent problem that must be solved to meet the needs of transplantation therapy and to fulfill the promise of regenerative medicine.4 HSC self-renewal and maturation divisions appear to be controlled, at least in part, by transcription factors (MEL/ELF4,5 Gfi16,7 ), and also by cell-cycle regulatory factors (cyclin-dependent kinase (Cdk) inhibitor, p21Cip1/Waf1,8 p18INK4C9 phosphatase and tensin homologue (PTEN),10 c-Myc,11 and Myc antagonist, Mad 112 ). Analysis of mice deficient in Gfi1,6,7 p21Cip1/Waf1,8 or PTEN10 revealed increased marrow HSC cycling and subsequent exhaustion of HSCs, and suggest possible cooperation between transcription factors and cell-cycle regulators.
GATA2 is a member of the GATA family of transcription factors, and is indispensable for the development of hematopoietic, neural, and urogenital systems.13–17 GATA2 expression is essential for hematopoietic-cell production from embryonic hemangioblasts,18 and GATA2-null mice are severely anemic and do not survive embryonic development.13 Chimeric mouse studies showed that GATA2-null embryonic stem cells did not contribute to bone marrow hematopoiesis, indicating essential roles of GATA2 in production and maintenance of adult-type HSCs.13 HSCs in heterozygous (Gata2+/−) mice express GATA2 at reduced levels. Their ability to maintain HSC pool size in adult bone marrow is impaired,19,20 and their capacity to produce embryonic HSCs is reduced.18 Greater numbers of quiescent HSCs and apoptotic HSCs are found in Gata2+/− bone marrow.19 Therefore, the level of GATA2 expression appears to determine HSC pool size through the regulation of HSC differentiation, self-renewal, and survival.
Abundant Gata2 mRNA expression in myelogenous leukemic blasts also suggests that endogenous GATA2 expression enhances cell proliferation.21 However, forced overexpression of GATA2 in bone marrow hematopoietic stem/progenitor cells does not enhance proliferation, but instead induces differentiation into monocytic/granulocytic lineages, or into eosinophils.22–24 Overexpression of GATA2 also inhibits the proliferation of neuroepithelial and neuroblastoma cells,25 and exogenous expression of GATA2/estrogen-receptor chimeric protein stops the proliferation of Ba/F3 cells in G1 phase, accompanied by the accumulation of p21Cip1/Waf1 and p27Kip1.26 On the other hand, wild-type GATA2 (but not GATA2/estrogen-receptor chimeric protein) induces cell proliferation, when expressed in hematopoietic progenitor cells derived from embryonic stem cells.27 GATA2 is expressed both in quiescent and proliferating hematopoietic stem/progenitor cells28–30 and is rapidly degraded through ubiquitination-proteasome–dependent systems.31 We found marked variation in GATA2 staining among cultured cells,31 suggesting cell-to-cell fluctuations in GATA2 expression. Based on these considerations, we hypothesized that proliferation of hematopoietic cells may be under control of cell-cycle–mediated regulation of GATA2.
Cdks phosphorylate cell-cycle regulators, inducing their proteasome-dependent degradation.32–34 Cyclins bind to Cdks and enhance their kinase activities,32–34 while p21Cip1/Waf1, p27Kip1, and p18INK4C inhibit Cdk activities.2 The optimal phosphorylation site for cdc2(Cdk1)/cyclin B, Cdk2/cyclin A, and Cdk2/cyclin E is reported to be X−1(S/T0)P+1X+2(K/R+3),35 and the optimal site for Cdk4/cyclin D is P−2X−1(S/T0)P+1 or P−3X−2X−1(S/T0)P+1.36 Molecules having cyclin-binding motifs, RXL, interact with Cdk/cyclin complexes and are easily phosphorylated at nonconsensus S/T0P+1 motifs by activated Cdk.35,37 Recent studies revealed that Cdk/cyclin systems also phosphorylate and regulate transcription factors Runx1,38 Runx2,39,40 MEF/ELF4,41,42 B-Myb,43 and NF-Y.44
In the present study, we demonstrate cell-cycle–dependent regulation of GATA2 expression in synchronized leukemic cells, in cultured cord blood CD34+ cells, and in mouse bone marrow lineage marker–negative (Lin−) cells. Analysis of green fluorescent protein (GFP)–GATA2 fusion protein expression and use of immunoprecipitation/immunoblotting reveal the interaction of cyclin A2, the phosphorylation of GATA2 by Cdk2/cyclin A2 and Cdk4/cyclin D1, and the regulation of GATA2 degradation through Cdk-dependent phosphorylation of this factor. These results suggest that hematopoietic-cell proliferation may be modulated by expression of GATA2 in tandem with cell-cycle regulators.
Materials and methods
Cell culture and transfection
The mouse mast-cell leukemia line P815 was cultured in RPMI1640 medium (Sigma-Aldrich, St Louis, MO) containing 10% fetal bovine serum (FBS; BioWest, Nuaillé, France). The transformed human renal epithelial cell line 293T was cultured in DMEM medium (Sigma-Aldrich) containing 10% FBS, and transfected with FuGENE 6 Transfection Reagent (Roche Diagnostics, Indianapolis, IN) or FuGENE HD Transfection Reagent (Roche Diagnostics). MG132 (Z-Leu-Leu-Leu-H; 10 μM; Peptide Institute, Osaka, Japan), roscovitine (20 μM; Calbiochem, Merck, Darmstadt, Germany), and SB203580 (1 μM, Calbiochem, Merck) were used as indicated.
Cord blood samples were obtained from Miyagi Cord Blood Bank, with informed consent obtained from donors in accordance with the Declaration of Helsinki, and approval of the ethics committees of Tohoku University and of Miyagi Cord Blood Bank. We isolated cord blood CD34+ cell fractions and mouse bone marrow Lin− cell fractions using autoMACS (Miltenyi Biotech, Auburn, CA) with CD34 Progenitor Cell Isolation Kit (Miltenyi Biotech) and Lineage Cell Depletion Kit (Miltenyi Biotech), respectively. Cells were subsequently cultured in StemPro34 medium (Invitrogen, Carlsbad, CA). Cytokines used in culture were human or mouse stem-cell factor (SCF, 100 ng/mL; PeproTech, Rocky Hill, NJ), human Flt-3L (100 ng/mL; PeproTech), and human erythropoietin (10 ng/mL; Calbiochem, Merck). Recombinant rat Jagged 1/Fc chimera (1.5 μg/mL; R&D Systems Minneapolis, MN) conjugated to ImmunoPure Immobilized Protein G agarose beads (1.0%; PIERCE, Rockford, IL) was used to induce Notch signals. Cell synchronization was achieved by incubation in medium containing 2 mM thymidine (Sigma-Aldrich). Cells were washed twice and resuspended in fresh medium with/without nocodazole (0.05 μg/mL; Sigma-Aldrich).
Plasmids
Expression plasmids, pcDNA3.0Flag-GATA2, and pcDNA3.0FlagΔNTGATA2 (lacking 1-74 amino acid residues) were described previously.31 We purchased pcDNA3.1/GS His–human cyclin A2 (RG001553) from Invitrogen. A eukaryotic GFP expression vector, pCMX-SAH/Y145F, was a gift from Dr K. Umesono.45 The pCMX-GFP-GATA2 plasmid was generated by serial insertion of a BamHI-BamHI fragment (153-1329th residue of GATA2 cDNA) and EagI-XbaI fragment (1142-1600th) of pcDNA3.0Flag GATA2 into the BamHI (pCMX-SAH/Y145F) and EagI (1142nd of GATA2 cDNA)–NheI (pCMX-SAH/Y145F) sites, respectively. The pCMX-GATA2-GFP plasmid was generated by serial insertion of polymerase chain reaction (PCR) products and the BamHI-EagI fragment of pcDNA 3.0FlagGATA2 into the blunt-ended HindIII-KpnI sites of pCMX-SAH/Y145F. Plasmids for domain analysis were generated with the cloning of PCR products into pCMX-SAH/Y145F. QuikChange II Site-Directed Mutagenesis Kits (STRATAGENE, Cedar Creek, TX) were used to generate mutations.
Immunostaining, flow cytometry, and chromatin immunoprecipitation
Cells were fixed with 4% PFA in PBS on ice for 15 minutes, and permeabilized with 5% DMSO, 0.25% Triton X100 for the last 5 minutes of fixation. SuperBlock Blocking Buffer in PBS (PIERCE) and Blocking Reagent for ELISA (Roche Diagnostics) with 5% FBS and 10% goat serum were used for the blocking and antibody dilution. Immunostaining of transfected 293T cells with anti–cyclin B1 antibody (sc-752; SantaCruz Biotechnology, Santa Cruz, CA) and AlexaFluor 546 F(ab)2 fragment of goat anti–rabbit IgG (H+L) (Molecular Probes, Eugene, OR) was performed as described previously.31 We used a confocal laser scanning microscope (LSM 5 Pascal version 3.2 SP2 with Axiovert zoom inverted microscope; Carl Zeiss, Oberkochen, Germany) with an objective lens (63 ×/1.4). For flow cytometric analysis, staining with anti-CD34 antibody (BD Bioscience, San Jose, CA) was performed before fixation. Fixed cells were stained overnight with first antibody: anti-GATA2 (sc9008; SantaCruz Biotechnology), anti-GATA1 (N6; SantaCruz Biotechnology), anti–cyclin D1 (DCS6; Cell Signaling Technology, Beverly, MA). Subsequently, cells were treated with fluorescein isothiocyanate (FITC)–conjugated anti–rabbit immunoglobulin (Zymed, San Francisco, CA) and phycoerythrin-conjugated anti–rat or anti–mouse immunoglobulin (BD Bioscience). TOPRO3 (Molecular Probes) was used for quantitation of DNA content in GFP- or FITC-expressing cells. For DNA content analysis, P815 cells were fixed in 4% paraformaldehyde (PFA) and stained with propidium iodide (Sigma-Aldrich). Stained cells were analyzed with a FACSCalibur (BD Bioscience) flow cytometer. Results from at least 3 independent experiments were subjected to Student t test to determine the statistical significance.
Chromatin immunoprecipitation was performed following the previously described methods.46 Primers used for quantitative PCR (LightCycler ST3000; Roche Diagnostics) were Bcl-X PF, 5′-TTATCTTGGCTTTGGATCCTGG-3′; Bcl-X PR, 5′-GGGTCTGCTCTGTGTTTAGCG-3′; Myogenin-F, 5′-TTACGTCCATCGTGGACAGC-3′; and Myogenin-R, 5′-TGGGCTGGGTGTTAGTCTTA-3′.
Immunoprecipitation, immunoblot, and kinase analysis
P815 and normal hematopoietic cells were directly suspended into Laemmli sample buffer for immunoblot analysis. Immunoprecipitation and nickel-resin experiments were carried out with Anti-Flag M2 Agarose (Sigma-Aldrich) and EZview RED His–Select HC Nickel Affinity Gel (Sigma-Aldrich) according to previously described methods with some modifications.31 To avoid nonspecific binding to Flag agarose and nickel affinity resin, purified proteins were eluted by incubation in TBS containing 3 × FLAG peptide (Sigma-Aldrich) and in TBS containing 250 mM imidazol and 500 mM NaCl, respectively. Complete (Roche Diagnostics) and Phosphatase Inhibitor Cocktail 1 (Sigma-Aldrich) were used to inhibit protease and phosphatase activities, respectively. To analyze Cdk-dependent phosphorylation of GATA2, Flag-GATA2 binding M2 agarose resin was divided into 4 fractions and incubated for 1 hour at 30°C in the Cdk2 reaction buffer (New England BioLabs, Beverly, MA) containing 3 × FLAG peptide, ATP, with/without the following recombinant Cdk/cyclin complexes: Cdk2/cyclin A2 (CDK2-cyclin A, 0.015 μg, 150 U; New England BioLabs), cdc2/cyclin B1 (Cdc2 Protein Kinase, 0.015 μg, 15 U; New England BioLabs), and Cdk4/cyclin D1 (CDK4/CycD1, 0.4 μg; Biaffin, Kassel, Germany).
SuperSignal West Pico Chemiluminescent Substrate (PIERCE), SuperSignal West Dura Chemiluminescent Substrate (PIERCE), or SuperSignal West Femto Maximum Sensitivity Substrate (PIERCE) was used to detect immunoblot signals. The following antibodies were used: anti-GATA2 rat monoclonal antibody31 ; anti–cyclin A (sc-751, SantaCruz Biotechnology); anti–actin goat polyclonal antibody (sc-1616; SantaCruz Biotechnology); anti–Cdk2 and anti-Cdk4 from BD Transduction Laboratories Cell Cycle Sampler Kit (BD Bioscience); anti–phospho-(Thr) MAPK/CDK substrate mouse monoclonal antibody (anti-pTP; Cell Signaling Technology); antiphosphoserine mouse monoclonal antibody (anti-pSP, 16B4; BIOMOL Research Laboratories, Plymouth Meeting, PA); PhosphoPlus(R) RB (Ser807/811) antibody (Cell Signaling Technology); and horseradish peroxidase–conjugated ZyMax goat antirabbit, antirat, and antimouse antibodies (Zymed).
RNA blot analysis
ISOGEN (Nippon Gene, Toyama, Japan) was used to extract RNA from P815 cells. After electrophoresis, RNA was transferred to a Zeta-Probe GT membrane (Bio-Rad Laboratories, Hercules, CA). Labeling and detection were performed with AlkPhos Direct Labeling and Detection System (Amersham Bioscience, Buckinghamshire, United Kingdom) and CDP-Star Detection Reagent (Amersham Bioscience), and SacI fragments of the mouse Gata2 cDNA were used as probes.
Results
Increased GATA2 expression in S phase
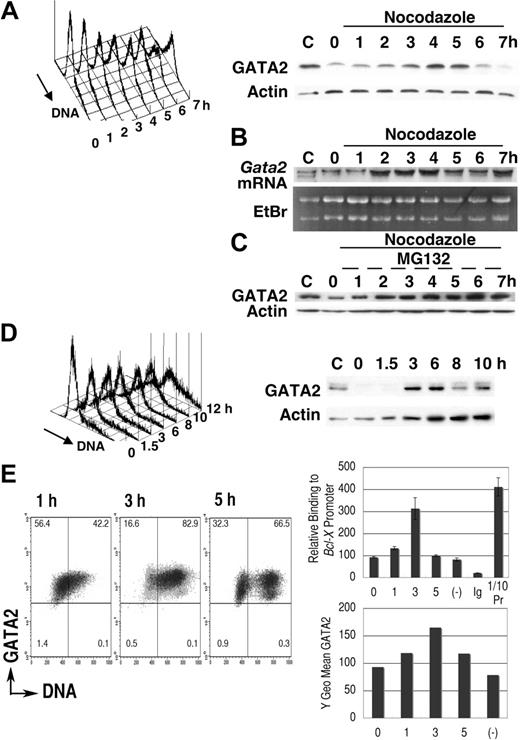
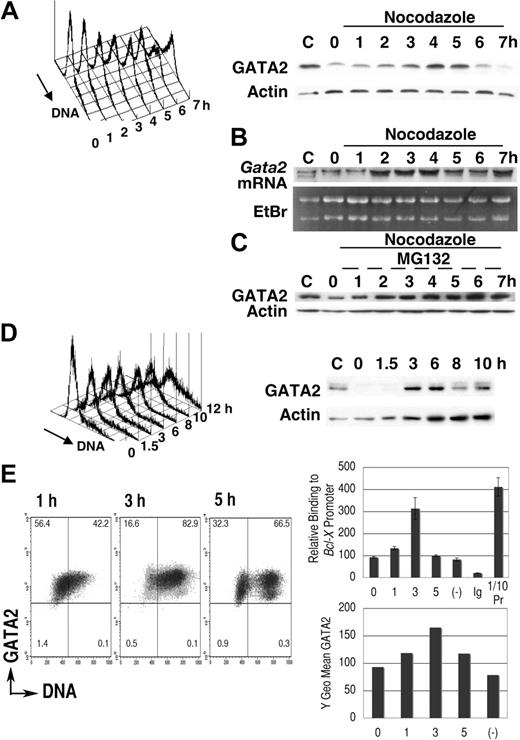
We first examined GATA2 expression in synchronized leukemic P815 cells (Figure 1). After 16-hour cultivation in medium containing excess thymidine, proliferation of P815 cells was arrested in early S phase (Figure 1A). Transfer into medium lacking excess thymidine but containing nocodazole permitted progression into S phase followed by the accumulation of cells containing 4N DNA.40,47 DNA content analysis demonstrated a single peak in M phase, 7 hours after thymidine release. Immunoblot analysis demonstrated that after a 2-hour lag, GATA2 expression increased with S phase progression (3-5 hours) followed by a decrease of GATA2 expression (6-7 hours). Expression of actin was not significantly altered (Figure 1A).
Cell-cycle–dependent oscillation of endogenous GATA2 expression in P815 mast-cell line. (A-C) After culture in medium containing thymidine for 16 hours, P815 cells were washed twice and resuspended at 2 × 105 /mL in fresh medium containing nocodazole. Following a final 1-hour incubation with (C) or without (A,B) MG132, cells were harvested at each time point and divided into 3 portions for DNA content analysis, for immunoblot analysis, and for RNA extraction. Representative results of 3 independent experiments were shown. (B) RNA blot analysis of the thymidine-nocodazole experiment. (C) Immunoblot analysis after incubation with MG132 for last 1 hour. (D) Cells in confluent culture at 40 hours after passage were diluted with fresh medium to 2 × 105/mL and cultured for an additional 10 hours. (A; D, left panel) DNA content histogram. (A; D, right panel) Immunoblot analysis. (E) Chromatin immunoprecipitation experiments of P815 cells released from thymidine treatment into fresh medium lacking nocodazole. A representative result of 3 independent experiments is shown. (Left panel) Density plot analysis of GATA2 expression and DNA content. Horizontal and vertical lines represent borders of control IgG fluorescence and borders of 2N DNA, respectively. Numbers indicate the percentage of cells in each quadrant. (Right upper panel) Results of quantitative PCR analysis of GATA2 binding to Bcl-X promoter GATA sequences. Mean ± standard deviation of triplicate PCR experiments. Ig indicates results of immunoprecipitation using pre–immune rabbit IgG; (−), results of the culture without thymidine treatment; and 1/10Pr, DNA extracted from 1/10 input cell lysates. In negative control experiments, we did not detect significant levels of myogenin gene in immunoprecipitates with GATA2 antibody (data not shown). (Right lower panel) GATA2 expression demonstrated as Y geometric mean channel in density plot analysis.
Cell-cycle–dependent oscillation of endogenous GATA2 expression in P815 mast-cell line. (A-C) After culture in medium containing thymidine for 16 hours, P815 cells were washed twice and resuspended at 2 × 105 /mL in fresh medium containing nocodazole. Following a final 1-hour incubation with (C) or without (A,B) MG132, cells were harvested at each time point and divided into 3 portions for DNA content analysis, for immunoblot analysis, and for RNA extraction. Representative results of 3 independent experiments were shown. (B) RNA blot analysis of the thymidine-nocodazole experiment. (C) Immunoblot analysis after incubation with MG132 for last 1 hour. (D) Cells in confluent culture at 40 hours after passage were diluted with fresh medium to 2 × 105/mL and cultured for an additional 10 hours. (A; D, left panel) DNA content histogram. (A; D, right panel) Immunoblot analysis. (E) Chromatin immunoprecipitation experiments of P815 cells released from thymidine treatment into fresh medium lacking nocodazole. A representative result of 3 independent experiments is shown. (Left panel) Density plot analysis of GATA2 expression and DNA content. Horizontal and vertical lines represent borders of control IgG fluorescence and borders of 2N DNA, respectively. Numbers indicate the percentage of cells in each quadrant. (Right upper panel) Results of quantitative PCR analysis of GATA2 binding to Bcl-X promoter GATA sequences. Mean ± standard deviation of triplicate PCR experiments. Ig indicates results of immunoprecipitation using pre–immune rabbit IgG; (−), results of the culture without thymidine treatment; and 1/10Pr, DNA extracted from 1/10 input cell lysates. In negative control experiments, we did not detect significant levels of myogenin gene in immunoprecipitates with GATA2 antibody (data not shown). (Right lower panel) GATA2 expression demonstrated as Y geometric mean channel in density plot analysis.
P815 cells were taken from confluent cultures (40 hours after passage), at which point DNA content analysis showed a single peak at G1 phase (Figure 1D, 0 hour). After passage, DNA content increased and immunoblot analysis showed an increase of GATA2 expression at 3 to 6 hours, but a subsequent decrease at 8 to 10 hours. These results suggested that GATA2 expression oscillated in synchronized P815 cells, with increases coinciding with S phase and decreases occurring in G2/M phase.
We performed RNA blot analyses (Figure 1B) in the thymidine-nocodazole experiments shown in Figure 1A. Increased Gata2 mRNA expression was noted 2 to 4 hours after transfer into fresh medium, suggesting activation of Gata2 gene transcription in early to middle S phase. Gata2 mRNA expression at 7 hours was still high (Figure 1B), while GATA2 protein expression was diminished at that time (Figure 1A). Incubation with MG132, a proteasome inhibitor, restored GATA2 protein level in G2/M phase (Figure 1C), but did not enhance the expression in S phase. Therefore, transcriptional regulation was responsible for the elevation of GATA2 expression in S phase. In M phase, proteasome-dependent degradation of GATA2 led to the decrease of GATA2 protein.
Flow cytometric analysis of endogenous GATA2 expression in synchronized P815 cells confirmed the increase of GATA2 expression in S phase cells 3 hours after thymidine release. At 5 hours, we detected lower GATA2 fluorescence in cells with 4N and 2N DNA content, which are probably M phase cells and daughter cells (Figure 1E left panel). We next performed chromatin-immunoprecipitation experiments. Expression of Bcl-XL is reported to be low in Sca1+ckit+Lin− bone marrow cells of Gata2+/− mice.19 In addition, GATA4 can bind to the GATA site within the Bcl-X (Bcl2l1) gene promoter.48 Therefore, we analyzed GATA2 binding to Bcl-X gene promoter. Parallel to the increased GATA2 expression in S phase, we detected increased GATA2 binding to the Bcl-X gene promoter in S phase. These results indicated that GATA2 was highly expressed in S phase, that it bound to target Bcl-X genes, and that it might be involved in the regulation of cell survival and proliferation.
GATA2 expression in normal hematopoietic cells
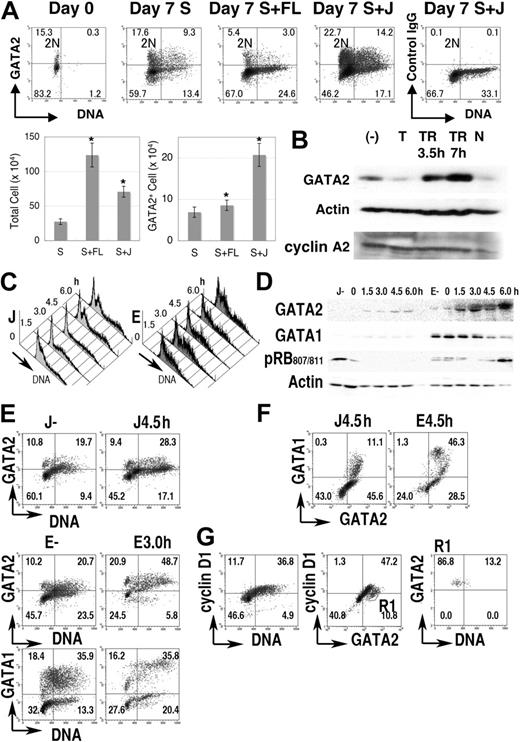
Next, we analyzed GATA2 expression in normal hematopoietic cells. Immediately after fractionation, GATA2 was expressed in 10% to 20% of human cells enriched for CD34 expression (Figure 2A, a representative result). Practically all the GATA2+ cells contained 2N DNA, suggesting that GATA2 was expressed in quiescent (G0) human hematopoietic progenitor cells in cord blood.
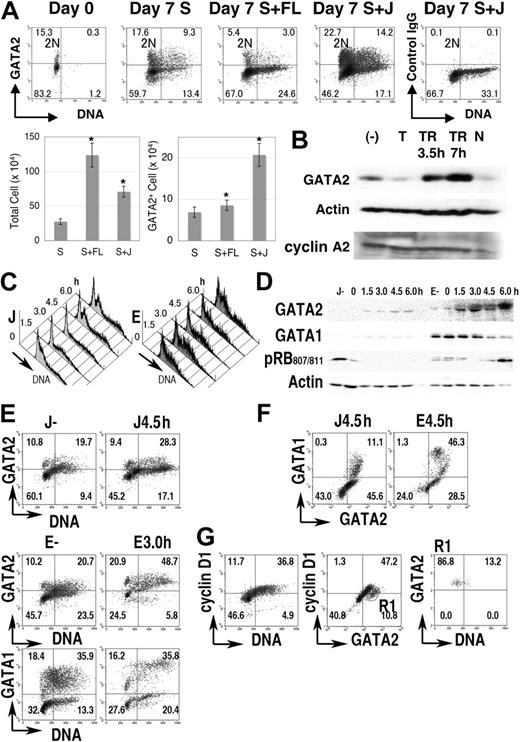
GATA2 expression in normal progenitor-cell fractions. (A) Cord blood CD34+ cells (1 × 105 cells) were cultured for 1 week in medium containing SCF (S), with/without Flt-3L (FL) or Jagged 1/Fc-Protein G-agarose beads (J). Density plot analysis of GATA2 expression and DNA content in the cord blood CD34+ cell fraction. Vertical lines represent borders of 2N population. Bar graphs represent number of total cells (left) and of GATA2+ cells (right) after 1 week's culture. Mean ± standard error of the mean; n = 4. *P < .05 compared with the culture containing SCF. (B) Immunoblot analysis of the cultured cord blood CD34+ fraction. (−) indicates no thymidine addition; T, treated with thymidine for 24 hours; TR, after release from thymidine treatment; and N, treated with nocodazole for 24 hours. (C-G) Lin− fractions of mouse bone marrow cells were cultured with SCF and Jagged 1 (J) or SCF and erythropoietin (E) for 4 days, treated with excess thymidine for additional 24 hours, then released into fresh medium. (C) DNA content histogram. (D) Immunoblot analysis. (E-F) Density plot analysis of GATA1, GATA2, and DNA content. (G) Cyclin D1 expression in cells in J culture without thymidine treatment. R1 in panel G represents GATA2+ cyclin D1− region. Numbers in A and E-G indicate the percentage of cells in each quadrant.
GATA2 expression in normal progenitor-cell fractions. (A) Cord blood CD34+ cells (1 × 105 cells) were cultured for 1 week in medium containing SCF (S), with/without Flt-3L (FL) or Jagged 1/Fc-Protein G-agarose beads (J). Density plot analysis of GATA2 expression and DNA content in the cord blood CD34+ cell fraction. Vertical lines represent borders of 2N population. Bar graphs represent number of total cells (left) and of GATA2+ cells (right) after 1 week's culture. Mean ± standard error of the mean; n = 4. *P < .05 compared with the culture containing SCF. (B) Immunoblot analysis of the cultured cord blood CD34+ fraction. (−) indicates no thymidine addition; T, treated with thymidine for 24 hours; TR, after release from thymidine treatment; and N, treated with nocodazole for 24 hours. (C-G) Lin− fractions of mouse bone marrow cells were cultured with SCF and Jagged 1 (J) or SCF and erythropoietin (E) for 4 days, treated with excess thymidine for additional 24 hours, then released into fresh medium. (C) DNA content histogram. (D) Immunoblot analysis. (E-F) Density plot analysis of GATA1, GATA2, and DNA content. (G) Cyclin D1 expression in cells in J culture without thymidine treatment. R1 in panel G represents GATA2+ cyclin D1− region. Numbers in A and E-G indicate the percentage of cells in each quadrant.
After 7 days of culture of 1 × 105 CD34+ cells with SCF, or SCF + Flt-3L, or SCF + Jagged 1/Fc-protein G-agarose beads, average cell counts reached 2.8 × 105, 12.4 × 105, and 7.1 × 105, respectively, while GATA2+ cells were 6.9 × 104, 8.6 × 104, and 20.0 × 104, respectively (Figure 2A bar graph). Therefore, as in the case with cell lines and developmental systems,49,50 Jagged1-Notch signals had positive effects on the proliferation and GATA2 expression of the CD34+ hematopoietic stem/progenitor cells.
When CD34+ cord blood cells were cultured in the presence of SCF and Jagged1, flow cytometric analyses showed that GATA2 expression varied with DNA content. That is, high expression was seen in cells with 2N DNA content and in mid S phase cells, while lower expression was observed in cells with 4N DNA content and in early S phase cells that contained nearly 2N DNA (Figure 2A). Immunoblot analysis demonstrated a decrease of GATA2 expression in thymidine-blocked cells but an increase of GATA2 expression in cells 3.5 to 7 hours after thymidine release (Figure 2B). After 24 hours of nocodazole treatment, GATA2 expression decreased concomitantly with the decrease of cyclin A2 expression.
We then analyzed mouse bone marrow cells. Lin− cells were isolated and cultured in medium containing either SCF + Jagged1/Fc-protein G-agarose beads (J) or SCF + erythropoietin for 4 days (E), then treated with excess thymidine for 24 hours. After release from the thymidine block, cell-cycle progression was partially synchronized (Figure 2C). Immunoblot analysis demonstrated the increased GATA2 expression 4.5 and 6.0 hours after release of culture J and 1.5, 3.0, and 6.0 hours after release of culture E (Figure 2D). At these time points, the number of S phase cells had increased (Figure 2C). Immunoblot analysis with antiphosphorylated RB at serine 807/811 antibody indicated the entry into the next G1 to S phase at 6.0 hours in culture E (Figure 2D). Apparently different cell populations, such as GATA1++GATA2+, GATA1+GATA2++, GATA1−GATA2+, and GATA1−GATA2− cells were contained in these culture conditions (Figure 2E-F). Each population demonstrated increased GATA2 expression in S phase (DNA > 2N). In contrast, GATA1 expression in culture E was high at 0, 1.5, and 3.0 hours after thymidine release (Figure 2D-E). In addition, GATA2+ cyclin D1− (DNA = 2N) cells in culture J (Figure 2G) exhibited GATA2 expression in G0 (to early G1) hematopoietic cells.
These results indicated that the expression of GATA2, but not GATA1, was high during S phase in normal human and mouse hematopoietic cells.
GFP-GATA2 fusion protein system
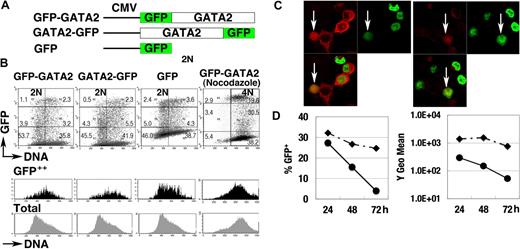
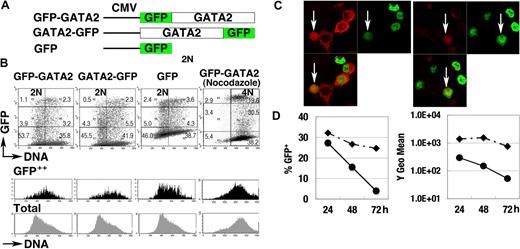
To elucidate the control of GATA2 degradation, we analyzed the expression of GFP-GATA2 and GATA2-GFP fusion proteins, both of which were driven by constitutive promoter activities (Figure 3A). At 24 hours after transfection of 293T cells, we observed strong fluorescence of the fusion proteins. Flow cytometric analyses demonstrated weak and variable fluorescence of GFP-GATA2 or GATA2-GFP fusion proteins in the population with 2N DNA (2N population). In contrast, strong fluorescent signals were observed in the S phase population (Figure 3B black histogram). Nocodazole-treated cells with 4N DNA (4N population), enriched for late S, G2, and early M phase cells, expressed lower fluorescence than did S phase cells (Figure 3B). Immunofluorescent analysis (Figure 3C) demonstrated strong expression of GFP-GATA2 fusion protein in cells coexpressing cytoplasmic cyclin B1 (S to G2 phase), and weak expression in cells with nuclear-envelope breakdown with cyclin B1 expression (M phase),51 suggesting the degradation of GFP-GATA2 fusion proteins in M phase. In addition, the fluorescence intensity and percentage of cells expressing GFP-GATA2 fusion proteins rapidly decreased 48 hours after transfection (Figure 3D), suggesting toxic effects of GFP-GATA2 fusion protein on cell-cycle control and cell proliferation.
GFP-GATA2 fusion protein expression. (A) Schematic diagram of GFP-GATA2, GATA2-GFP, and GFP expression constructs. (B) Expression of GFP fusion proteins and DNA content of 293T cells at 24 hours after transfection. Black and gray histograms represent DNA contents of GFP++ cells and total cells, respectively. Numbers indicate the percentage of cells in 6 rectangle regions. (C) 293T cells transfected with GFP-GATA2 fusion expression plasmids were stained with anti–cyclin B antibody (red). Cyclin B1 accumulates in the cytoplasm in late S phase, and later enters nuclei in M phase. GFP-GATA2 fusion proteins are expressed in cells in which the cytoplasmic stains for cyclin B1 and in cells expressing cyclin B1 with nuclear-envelope breakdown (white arrow). Thus, the fusion protein is expressed in late S phase and M phase. Original magnification × 630. (D) Cells transfected with GFP-GATA2–expressing plasmids (•) and with GFP-expressing plasmids (♦) were replated in separate dishes at 8 hours, and then the percentage of GFP-positive cells and GFP fluorescence intensity (Y geometric mean channel) were analyzed at 24, 48, and 72 hours after transfection.
GFP-GATA2 fusion protein expression. (A) Schematic diagram of GFP-GATA2, GATA2-GFP, and GFP expression constructs. (B) Expression of GFP fusion proteins and DNA content of 293T cells at 24 hours after transfection. Black and gray histograms represent DNA contents of GFP++ cells and total cells, respectively. Numbers indicate the percentage of cells in 6 rectangle regions. (C) 293T cells transfected with GFP-GATA2 fusion expression plasmids were stained with anti–cyclin B antibody (red). Cyclin B1 accumulates in the cytoplasm in late S phase, and later enters nuclei in M phase. GFP-GATA2 fusion proteins are expressed in cells in which the cytoplasmic stains for cyclin B1 and in cells expressing cyclin B1 with nuclear-envelope breakdown (white arrow). Thus, the fusion protein is expressed in late S phase and M phase. Original magnification × 630. (D) Cells transfected with GFP-GATA2–expressing plasmids (•) and with GFP-expressing plasmids (♦) were replated in separate dishes at 8 hours, and then the percentage of GFP-positive cells and GFP fluorescence intensity (Y geometric mean channel) were analyzed at 24, 48, and 72 hours after transfection.
Nonetheless, at 24 hours after transfection, expression of GFP-fusion protein recapitulated the cell-cycle stage–specific control of GATA2 (ie, high in S phase but low in G1/S and M phases).
Cdk-dependent protein stability control
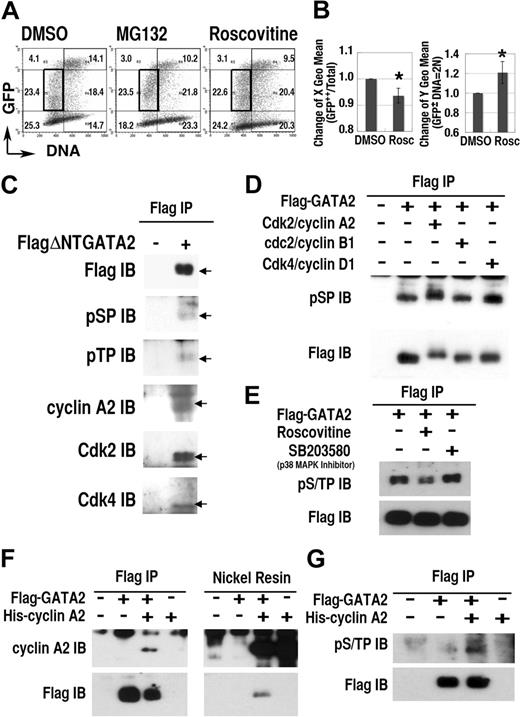
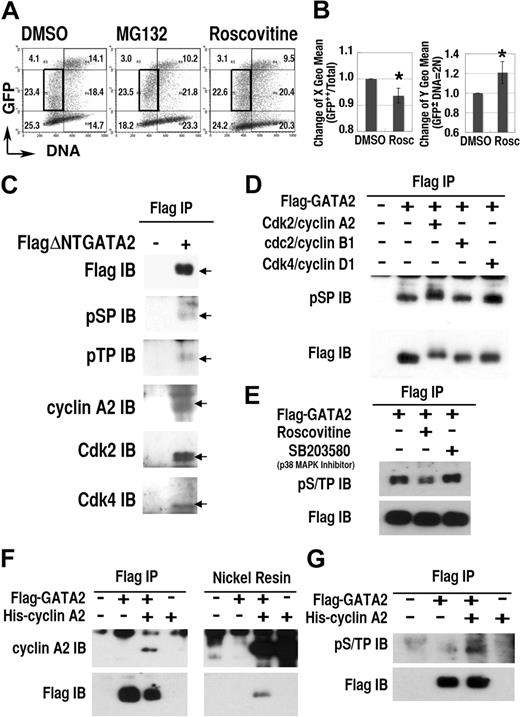
To study the role of Cdk on the regulation of GATA2 expression, we examined the effect of roscovitine, a selective inhibitor of cdc2/Cdk1, Cdk2, and Cdk5, but not of Cdk4.52 Roscovitine treatment induced significant changes in the expression profile of the GFP-GATA2 fusion protein (Figure 4A-B). Specifically, after 12-hour roscovitine treatment, the DNA content (X geometric mean) of GFP++ cells decreased, and the fluorescence intensity (Y geometric mean) in the 2N population increased (Figure 4B). Treatment with MG132 also increased the fluorescence intensity in 2N cells, but not in S phase cells (Figure 4A). These data suggest that certain Cdks activated proteasome-dependent degradation of GFP-GATA2 fusion protein in G1/S and G2/M phase.
Cdks regulate GATA2 stability. (A) Density plot analysis of 293T cells transfected with GFP-GATA2 expression plasmid after incubation with roscovitine or MG132 for 12 hours. Numbers indicate percentages of cells in 6 regions outlined with thin lines. (B, left panel) decrease of DNA content (X geometric mean channel) of GFP++ (channel > 103) cells. (B, right panel) Change of fluorescence intensity (Y geometric mean channel) of cells containing 2N DNA with lower fluorescence expression (in the region outlined with bold rectangles in A) (mean ± standard deviation, *P < .01, n = 6). (C-G) Interaction and phosphorylation of S/T0P+1 motifs of GATA2 with Cdk/cyclin systems. Total cell lysates of 293T cells, expressing FlagΔNTGATA2 (C) or Flag-GATA2 (D-E) were immunoprecipitated (IP) with anti-Flag antibody, then immunoblotted (IB) with antiphosphoS0P+1 (pSP), antiphosphoT0P+1 (pTP), anti–cyclin A2, anti-Cdk2, and anti-Cdk4 antibodies. (D) In vitro phosphorylation analysis of immunoprecipitated Flag-GATA2 by recombinant Cdk/cyclin proteins. (E) Effect of roscovitine or SB203580 treatment for 12 hours on phosphorylation of GATA2. (F-G) Immunoprecipitation and nickel resin purification of cell lysates expressing Flag-GATA2 and His–cyclin A2. (G) Induction of GATA2 phosphorylation with cotransfected His–cyclin A2.
Cdks regulate GATA2 stability. (A) Density plot analysis of 293T cells transfected with GFP-GATA2 expression plasmid after incubation with roscovitine or MG132 for 12 hours. Numbers indicate percentages of cells in 6 regions outlined with thin lines. (B, left panel) decrease of DNA content (X geometric mean channel) of GFP++ (channel > 103) cells. (B, right panel) Change of fluorescence intensity (Y geometric mean channel) of cells containing 2N DNA with lower fluorescence expression (in the region outlined with bold rectangles in A) (mean ± standard deviation, *P < .01, n = 6). (C-G) Interaction and phosphorylation of S/T0P+1 motifs of GATA2 with Cdk/cyclin systems. Total cell lysates of 293T cells, expressing FlagΔNTGATA2 (C) or Flag-GATA2 (D-E) were immunoprecipitated (IP) with anti-Flag antibody, then immunoblotted (IB) with antiphosphoS0P+1 (pSP), antiphosphoT0P+1 (pTP), anti–cyclin A2, anti-Cdk2, and anti-Cdk4 antibodies. (D) In vitro phosphorylation analysis of immunoprecipitated Flag-GATA2 by recombinant Cdk/cyclin proteins. (E) Effect of roscovitine or SB203580 treatment for 12 hours on phosphorylation of GATA2. (F-G) Immunoprecipitation and nickel resin purification of cell lysates expressing Flag-GATA2 and His–cyclin A2. (G) Induction of GATA2 phosphorylation with cotransfected His–cyclin A2.
Phosphorylation and interaction with Cdk/cyclin
To elucidate Cdk-dependent phosphorylation of GATA2, we performed immunoprecipitation/immunoblot analyses (Figure 4C-G). Because the molecular weight of wild-type GATA2 was close to that of immunoglobulin heavy chains, we avoided the influence of contaminating immunoglobulin, by using a deletion mutant, FlagΔNTGATA2 (lacking the first 74 amino acid residues), and extended period of electrophoresis. Immunoblot analysis of immunoprecipitates from FlagΔNTGATA2 transfectants, but not from mock-treated cells, revealed bands at the molecular weight equal to FlagΔNTGATA2, reacting with antibodies specific to the phosphorylated forms of S0P+1 (pSP) or T0P+1 (pTP) motifs. Furthermore, immunoprecipitation/immunoblot analysis demonstrated the interaction of cyclin A2, Cdk2, and Cdk4 with FlagΔNTGATA2. Because S0P+1 and T0P+1 motifs are likely targets of Cdks or MAP kinases,35 but not of the other kinases,53 these results suggested Cdk/cyclin-dependent phosphorylation of FlagΔNTGATA2 at S0P+1 or T0P+1 motifs.
To elucidate the direct phosphorylation of Flag-GATA2, immunoprecipitated Flag-GATA2 proteins were divided into 4 fractions and incubated without Cdk/cyclin or with recombinant Cdk2/cyclin A2, cdc2(Cdk1)/cyclin B1, or Cdk4/cyclin D1 for 1 hour. Immunoblotting analysis with anti-pSP antibody revealed intense bands with higher molecular weight in the Cdk2/cyclin A2–treated lane (Figure 4D). In addition, Cdk4/cyclinD1 treatment also enhanced the intensity of phosphorylated GATA2 at S0P+1 motifs. Therefore, Cdk2/cyclin A2 and also Cdk4/cyclin D1 directly phosphorylated S0P+1 motifs of GATA2. While cdc2 (Cdk1)/cyclin B1 exhibited no apparent effect on GATA2 phosphorylation, we could not exclude the possibility that higher concentration of cdc2 (Cdk1)/cyclin B1 could phosphorylate GATA2. Roscovitine but not SB203580 (a p38 MAP kinase inhibitor) treatment reduced phosphorylation of GATA2 at S/T0P+1 motifs in transfected cells (Figure 4E). Furthermore, reciprocal immunoprecipitation–nickel affinity resin experiments revealed the interaction between Flag-GATA2 and His–cyclin A2 (Figure 4F) and the induction of phosphorylation of Flag-GATA2 at S/T0P+1 motifs caused by coexpressed His–cyclin A2 (Figure 4G). Therefore, Cdk2/cyclin A2 interacted with and phosphorylated S/T0P+1 motifs of GATA2.
Multiple systems regulating cell-cycle dependency
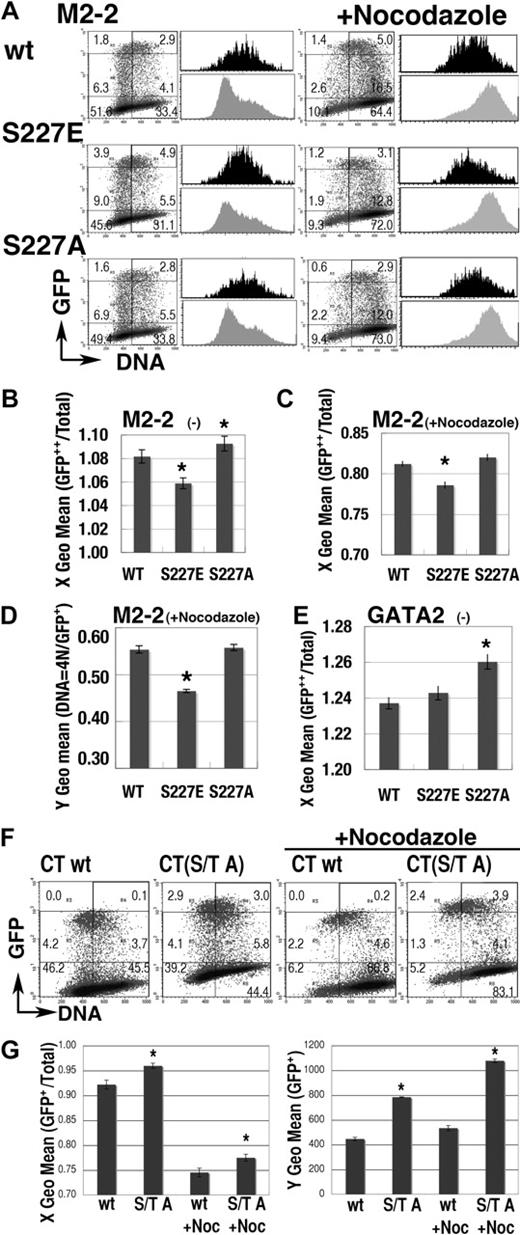
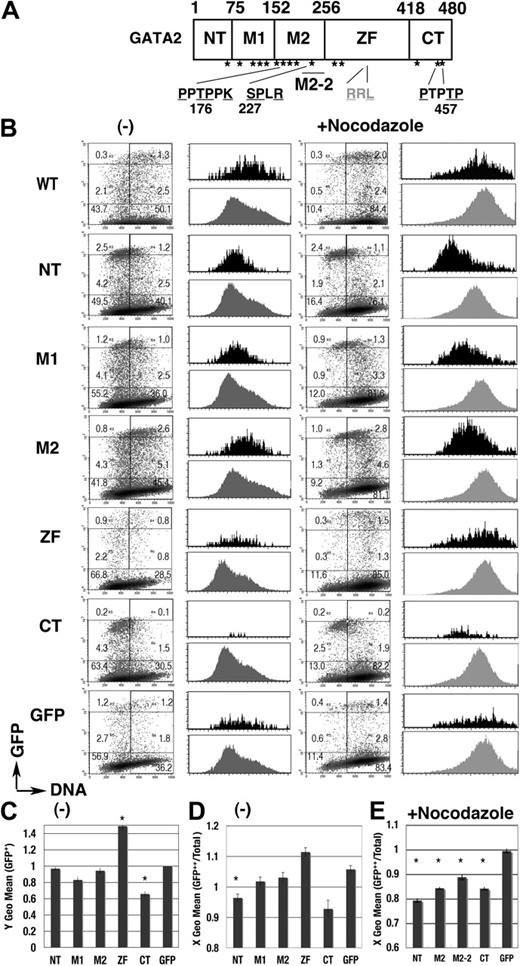
We next performed a domain analysis (Figure 5). Expression profiles of each GFP-GATA2 domain fusion protein were apparently different from the profile of GFP (Figure 5B). Fluorescence intensity was significantly lower in GFP–C-terminal (CT) than in GFP, while intensity was significantly higher in GFP–zinc finger (ZF) (Figure 5C). GFP-CT was expressed in cells in G1 phase but not in late S phase (Figure 5B,D), while the expression of GFP-M1 and GFP-M2 fusion proteins was observed in S phase cells (Figure 5B). After nocodazole treatment, active degradation of GFP–N-terminal (NT), –middle (M)2, and -CT in the 4N population was exhibited (Figure 5B black histogram, 5E). Degradation activity was also identified in the short element of M2 (M2-2, 205-255; Figures 5E, 6A). These results indicated complex systems controlling GATA2 degradation.
cell-cycle–dependent expressions of the GFP-GATA2 domain fusion proteins. (A) Scheme of GATA2 structure. *S/T0P+1 motif. (B) Density plot analysis of DNA content and GFP intensity of 293T cells, expressing GFP-GATA2 domain fusion proteins, at 24 hours after transfection with (right) or without (left) nocodazole treatment for the final 14 hours. Percentage of cells in each rectangle region is shown. Histograms demonstrate DNA content of GFP++ cells (in cells with channel > 103, black) and total cells in the same dish (gray). (C) Fluorescent intensity (Y geometric mean channel) of GFP+ (channel > 101) cells. (D-E) DNA content (X geometric mean channel) of GFP++ (channel > 103) cells with (E) or without (D) nocodazole treatment. (C-E)*Significant difference (P < .05) compared with GFP results. Data are shown as means ± standard error of the mean.
cell-cycle–dependent expressions of the GFP-GATA2 domain fusion proteins. (A) Scheme of GATA2 structure. *S/T0P+1 motif. (B) Density plot analysis of DNA content and GFP intensity of 293T cells, expressing GFP-GATA2 domain fusion proteins, at 24 hours after transfection with (right) or without (left) nocodazole treatment for the final 14 hours. Percentage of cells in each rectangle region is shown. Histograms demonstrate DNA content of GFP++ cells (in cells with channel > 103, black) and total cells in the same dish (gray). (C) Fluorescent intensity (Y geometric mean channel) of GFP+ (channel > 101) cells. (D-E) DNA content (X geometric mean channel) of GFP++ (channel > 103) cells with (E) or without (D) nocodazole treatment. (C-E)*Significant difference (P < .05) compared with GFP results. Data are shown as means ± standard error of the mean.
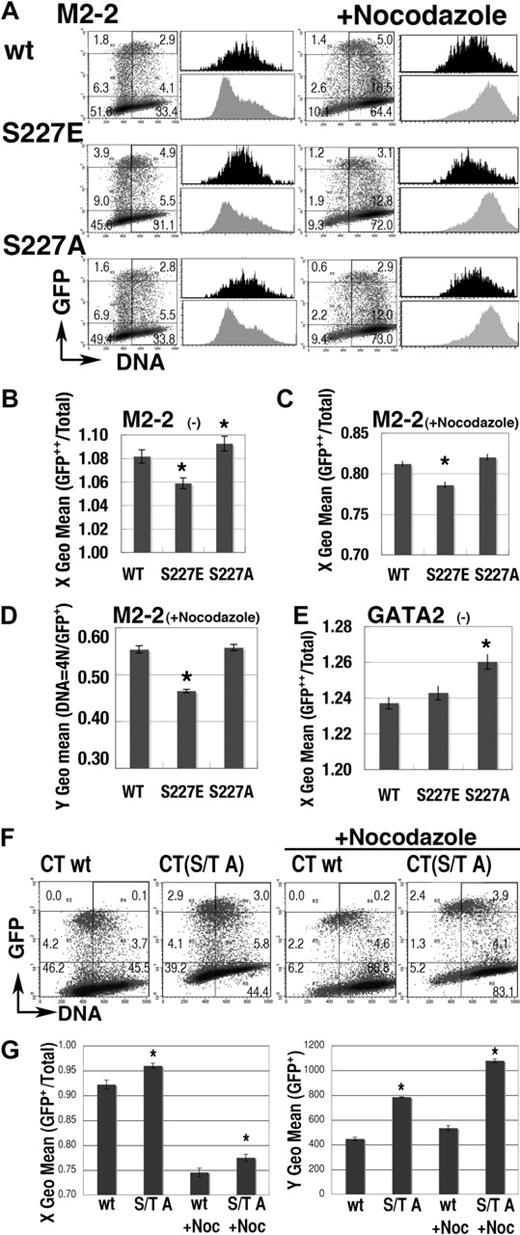
Effect of mutations at S/T0P+1 residues. (A) Representative results of mutations at S227 residue on the expression profile of GFP-M2-2 fusion proteins. Black and gray histograms represent the DNA content of GFP++ (channel > 103) and total cells in the same dishes, respectively. (B-C) S phase–specific expression of GFP-M2-2 fusion proteins was represented as the shift of X geometric mean channel (DNA content) of GFP++ cells, from that of total cells in the experiments with (C) or without (B) nocodazole treatment. A value of 1.00 means that the DNA content of GFP++ cells is equal to that of total cells. (D) Fluorescence intensity (Y geometric mean channel) of cells expressing GFP-M2-2 fusion protein (channel > 101) or mutants in 4N population after nocodazole treatment. (E) Effect of the mutation at S227 residue in GFP-GATA2 fusion protein on the DNA content (X geometric mean) of GFP++ cells. (F-G) Effect of S/T to A mutations on the expression of GFP-CT fusion proteins. Representative results are shown in panel F. Left panel in G shows the change of X geometric mean channel (DNA content) of GFP+ cells (channel > 102). Right panel shows the fluorescent intensity (Y geometric mean channel) of GFP+ cells (channel > 101). (B-E,G) Mean ± standard error of the mean; *P < .05, compared with wild-type results. For panels A and F, numbers indicate the percentage of cells in each rectangle region.
Effect of mutations at S/T0P+1 residues. (A) Representative results of mutations at S227 residue on the expression profile of GFP-M2-2 fusion proteins. Black and gray histograms represent the DNA content of GFP++ (channel > 103) and total cells in the same dishes, respectively. (B-C) S phase–specific expression of GFP-M2-2 fusion proteins was represented as the shift of X geometric mean channel (DNA content) of GFP++ cells, from that of total cells in the experiments with (C) or without (B) nocodazole treatment. A value of 1.00 means that the DNA content of GFP++ cells is equal to that of total cells. (D) Fluorescence intensity (Y geometric mean channel) of cells expressing GFP-M2-2 fusion protein (channel > 101) or mutants in 4N population after nocodazole treatment. (E) Effect of the mutation at S227 residue in GFP-GATA2 fusion protein on the DNA content (X geometric mean) of GFP++ cells. (F-G) Effect of S/T to A mutations on the expression of GFP-CT fusion proteins. Representative results are shown in panel F. Left panel in G shows the change of X geometric mean channel (DNA content) of GFP+ cells (channel > 102). Right panel shows the fluorescent intensity (Y geometric mean channel) of GFP+ cells (channel > 101). (B-E,G) Mean ± standard error of the mean; *P < .05, compared with wild-type results. For panels A and F, numbers indicate the percentage of cells in each rectangle region.
Phosphorylation dependency of GATA2 expression
The mouse GATA2 molecule contains a possible cyclin-binding motif, RXL, and 15 S/T0P+1 motifs (Figure 5A). T176 and S227 are consensus for cdc2/Cdk1 and Cdk2,35 while T176 and T457 are consensus for Cdk4.36 These motifs in GATA2 are conserved among species.
The M2-2 (205-255) region has a single S0P+1 motif (S227), consensus for cdc2/Cdk1 and Cdk235 (Figure 5A). The GFP-M2-2 fusion protein was highly expressed in the S phase population but not in the 4N population (Figures 5E, 6A). We introduced an S to E (M2-2(S227E)) or an S to A mutation (M2-2(S227A)) into GFP-M2-2 fusion proteins to analyze the effect of constitutive phosphorylation or blocked phosphorylation, respectively. Expression profiles of the M2-2(S227E) mutant (Figure 6A) exhibited a significantly lower DNA content in GFP++ cells both with and without nocodazole treatment (Figure 6B-C). The data also revealed significant decreases of GFP fluorescence intensity in the nocodazole-treated 4N population (Figure 6D). Therefore, M2-2(S227E) had a tendency toward stable expression in early S phase, while being rapidly degraded in G2/M phase. In contrast, the expression profile of the M2-2(S227A) mutant (Figure 6A-B), and also of the S227A mutant of full-length GFP-GATA2 fusion protein (GFP-GATA2(S227A); Figure 6E), exhibited a significantly higher DNA content in GFP++ cells. Therefore, the S227A mutation appeared to induce unstable expression in early S phase and/or slow degradation in G2/M phase.
We also introduced T455A, T457A, and S462A mutations into GFP-CT fusion proteins (CT(S/T A)). T457 residue is in the consensus motif for Cdk4-dependent phosphorylation, P−3X−2X−1T0P+1.36 Expression profiles of the CT(S/T A) mutant with or without nocodazole treatment exhibited greater DNA content and significantly intensified fluorescence in the GFP+ (channel > 102) population, indicating that this motif is essential to the low and G1/S-specific expression of GFP-CT fusion proteins (Figure 6F-G). Therefore, analyses of these mutants demonstrated the significance of phosphorylation, in cell-cycle–dependent oscillation of GATA2 expression.
Discussion
This study clearly demonstrated that the level of GATA2 expression is cell-cycle dependent: expression is high in S phase but low in G1/S and M phase. Oscillatory expression was modulated by proteasome-dependent degradation systems, under the control of Cdk/cyclin systems. These results indicate cooperative interaction between GATA2 and cell-cycle machinery and further suggest that stage-specific activity of GATA2 may regulate cell division and cell survival.
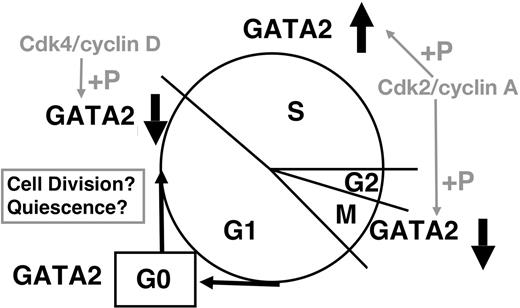
Although control of GATA2 expression remains to be clarified, important conclusions can be derived from our results (Figure 7). Cdk2/cyclin A and Cdk4/cyclin D phosphorylate GATA2; while we have no direct evidence regarding either Cdk2/cyclin E or cdc2 (Cdk1)/cyclin B1, consensus site analysis suggests the possibility that these complexes may also phosphorylate GATA2.35 Considering their cell-cycle–specific activities, Cdk4/cyclin D (and possibly Cdk2/cyclin E) are likely responsible for decreased GATA2 expression in G1/S phase. The expression profiles of GFP-CT and its mutant (Figures 5–6) are consistent with this hypothesis. Cdk2/cyclin A interacts with and phophorylates GATA2 in S phase, and may induce the proteasome-dependent degradation of GATA2 in M phase. Because cyclin A is a typical substrate of anaphase-promoting complex/cyclosome (APC/C),47,54 APC/C might degrade GATA2, which interacts with cyclin A2 in M phase. Low APC/C activity in S phase might contribute to the stable GATA2 expression in S phase. Low Cdk activities in G0 phase55 might spare GATA2 from proteasome-dependent degradation. Our results indicate that GATA2 was phosphorylated by Cdk2/cyclin A2 and bound to Bcl-X gene in S phase. Thus, GATA2 may play a role in the G2/M checkpoint regulation of cell survival. More detailed analysis is required to identify the molecules controlling GATA2 expression, and to identify the target genes of GATA2 during cell-cycle progression.
Schematic representation of cell-cycle–dependent regulation of GATA2 expression.
Schematic representation of cell-cycle–dependent regulation of GATA2 expression.
In addition, we demonstrated that the activation of Gata2 gene transcription contributed to cell-cycle–dependent GATA2 expression. CCAAT boxes in the IG promoter of the Gata2 gene28 likely activate the transcription of the Gata2 gene as in the case with S-G2/M–specific genes,56,57 while the IS promoter activates the transcription of the Gata2 gene in G0 phase hematopoietic stem cells.28–30
GATA2 and GATA1 compete for occupancy of the same GATA-binding motifs in the Gata2 gene, and GATA2 enhances, but GATA1 represses, its transcription.46,58,59 We previously demonstrated stable GATA1 expression (half-life > 6 hours) in erythroid leukemia cells,31 in contrast to the rapid degradation of GATA2. GATA1 has neither an optimal motif for Cdk2 or Cdk4, nor a possible cyclin boxlike structure.60 Furthermore, it is differently regulated during cell-cycle progression (Figure 2). In quiescent HSCs, GATA2 stably occupies GATA-binding motifs in the Gata2 gene, activating transcription to maintain its own stable expression, and thereby maintaining properties of HSCs.46,58,59 Of interest, it is possible that GATA1 could replace GATA2 during the M to G1 phase transition, when GATA2 (but not GATA1) is undergoing rapid degradation, and thereby repress the transcription of the Gata2 gene. As a result, repeated cell cycles could lead to shut down of GATA2 expression, and enhance differentiation into mature erythroid cells. Therefore, cell-cycle–dependent regulation of GATA2 expression might contribute to the mechanisms maintaining stem-cell properties in quiescent HSCs, and mediate cell-division–induced differentiation of progeny. In addition, the induction of GATA2 expression in cord blood CD34+ cells cultured with Jagged 1/Fc chimera (Figure 2) suggests a possible role for GATA2 expression in maintaining self-renewal and pluripotency by Notch-mediated signals.61,62
The Cdk/cyclin A complex phosphorylates transcription factor MEF/ELF4, represses its DNA binding, and restricts its transcriptional activity to the G1 phase of the cell cycle.41 MEF/ELF4-null mice have a larger pool of bone marrow HSCs.5 In contrast, the pool is smaller in GATA2+/− mice, with greater numbers of quiescent HSCs and apoptotic HSCs.19,20 Our results raise the possibility that the collaboration of Cdk/cyclin systems and transcription factors may regulate HSC quiescence, self-renewal, and differentiation, and eventually determine the HSC pool size.
In this study, we demonstrated cell-cycle–dependent regulation of GATA2 expression and control of GATA2 degradation, through its phosphorylation by Cdk/cyclin systems. Although details of these mechanisms remain to be clarified, analysis of cross talk between GATA2 and cell-cycle machinery may shed light on the molecular mechanisms controlling self-renewal divisions and differentiating divisions of HSCs.
Authorship
Contribution: S.K., N.Y., T.A., and N.M. performed and analyzed experiments; M.M. and S.T. contributed to the analysis of cord blood cells; M.Y. contributed to data analysis and interpretation; N.M. designed the research and wrote the paper; all authors checked the final version of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Reprints: Naoko Minegishi, Tohoku University Biomedical Research Organization, Tohoku University, Seiryo-machi, 2-1, Sendai, 980-8575, Japan; e-mail: nmine@tubero.tohoku.ac.jp.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Supported by grants-in-aids from the Ministry of Education, Culture, Sports, Science and Technology (N.M.) and by the Special Coordination Funds for Promoting Science and Technology (N.M.).
S.K. is a PhD candidate at Tohoku University and this work is submitted in partial fulfillment of the requirement for the PhD.
We thank K. Igarashi and A. Muto for support for the flow cytometry, and L. C. Yeng for help in preparation of the paper. We are grateful to M. Obinata for helpful advice throughout the work.