Abstract
Anemia is more common among older blacks than older whites. However, it is unclear whether anemia predicts adverse events similarly in both races. Data on 1018 black and 1583 white adults aged 71 to 82 years were analyzed. Anemia, as defined by World Health Organization (WHO) criteria, was used to predict mortality over 6 years and incidence of mobility disability over 4 years. In proportional hazards models of mortality in whites, the age-adjusted hazard ratio (HR) for anemia in men was 1.96 (95% confidence interval [CI]: 1.35, 2.83) and in women was 2.86 (95% CI: 1.69, 4.82). In contrast, anemia was not associated with mortality in black men (HR = 1.15 [95% CI: 0.77, 1.72]) or women (HR = 1.39 [95% CI: 0.91, 2.14]). Higher mortality rate was observed only in black men with hemoglobin values more than 20 g/L (2.0 g/dL) below the WHO cutoff, whereas mortality rates were elevated in white men with hemoglobin values 1 to 10, 11 to 20, and more than 20 g/L below the WHO cutoff. In conclusion, anemia was significantly associated with increased risk of death and mobility disability in community-dwelling older whites. Conversely, older blacks classified as anemic by WHO criteria were not at risk for adverse events, indicating that alternative criteria are warranted.
Introduction
Anemia is a common hematologic condition among older adults, with prevalence estimates increasing as a function of age after the fifth decade of life.1 Typically, anemia is defined by using World Health Organization (WHO) criteria of hemoglobin concentration lower than 120 g/L (12.0 g/dL) in women and 130 g/L in men.2 Results from the 1988-1994 United States National Health and Nutrition Examination Survey (NHANES III) indicate that fewer than 3% of adults aged 65 and older have hemoglobin levels below 110 g/L, and therefore most anemia cases among community-dwelling older adults are mild.1 Nonetheless, recent evidence indicates that even mild anemia is independently associated with increased risk of recurrent falls, poorer physical function, hospitalization, and mortality in the older population.3–9
While a number of studies have reported differential distributions of anemia by age and sex, less attention has been devoted to disparities in anemia by race. According to NHANES III estimates, older non-Hispanic blacks were 3 times more likely to have anemia compared to older non-Hispanic whites (27.8% vs 9.0%).1 Similar disparities in anemia prevalence have been observed in other population-based studies of older blacks and whites.9,10 This differential has been reported previously in younger adults and is unexplained even after adjusting for multiple diseases, nutritional status, and health behavior.10–12 Indeed, there is evidence that the distribution of hemoglobin values among blacks is shifted toward lower values, even in young healthy blacks, compared to whites.13 These observations have led some to consider race-specific criteria for defining anemia.14 However, before establishing alternative definitions based on distributions alone, research is needed to determine whether the hemoglobin cutoff value below which adverse events occur is lower in blacks than in whites. In view of the adverse outcomes associated with anemia and the large racial disparity in the prevalence of this frequently encountered problem in geriatric practice, the authors examined whether longitudinal effects of anemia vary by race in a biracial cohort of well-functioning older adults.
Patients, materials, and methods
Study population
Between March 1997 and April 1998, a total of 3075 community-dwelling adults aged 70 to 79 years who resided in Memphis, TN, and Pittsburgh, PA, were recruited to participate in the Health, Aging, and Body Composition (Health ABC) Study. The baseline study sample comprised 52% women (n = 1584) and 42% self-identified blacks (n = 1281). Medicare enrollment files were used to sample beneficiaries living in designated zip codes of Memphis and Pittsburgh as potential study participants. White beneficiaries were randomly selected, whereas all black beneficiaries were recruited. Eligibility criteria included self-reporting no difficulty with walking one-quarter of a mile, climbing a flight of stairs without resting, and performing basic activities of daily living (ADLs). Potential study participants were excluded if they planned to move from the area over the next 3 years, required an assistive walking device, reported being actively treated for cancer, or were participating in a clinical trial. Written informed consent was obtained from all study participants, in accordance with the Declaration of Helsinki, during the initial home interview that was followed by a clinic examination and physical function testing. Study protocols were approved by the institutional review boards of both field centers (University of Pittsburgh and University of Tennessee, Memphis) as well as by the coordinating center (University of California, San Francisco, and participants provided informed consent in accordance with the Declaration of Helsinki.).
At the second year of follow-up (1999-2000), which is the baseline for the current study, hemoglobin values were assessed in 2601 participants. Among those participants missing hemoglobin values (n = 474), 126 (27%) had died prior to the second year follow-up visit, 234 (49%) were interviewed by telephone, 56 (12%) were interviewed in the home without blood collection, 6 (1%) were interviewed with a proxy respondent, 5 (1%) withdrew from the study, 23 (5%) were lost to follow-up, and 24 (5%) were missing laboratory values because of technical problems. Participants missing hemoglobin data were significantly older at baseline (73.9 vs 73.6 years, P = .03), more likely to self-identify as black (55.5% vs 39.1%, P < .01), and had more medical conditions at baseline (1.8 vs 1.6 conditions, P < .01) compared to those with hemoglobin data. There were no differences by sex (51.8% vs 50.0% were women, P = .47).
Measures
Race and hemoglobin concentration were the key predictors of interest. Participants were asked to self-identify their race at the baseline interview. Hemoglobin level was measured with automated counters at a laboratory at each field center. In accordance with WHO criteria, anemia was defined as hemoglobin level lower than 120 g/L in women and lower than 130 g/L in men. To evaluate whether risk for adverse outcomes occurred at different hemoglobin levels in older blacks and whites, hemoglobin level was categorized into 5 levels (< 110, 110-119, 120-129, 130-139, 140-149 and ≥ 150 g/L) in women and 6 levels (< 110, 110-119, 120-129, 130-139, 140-149, 150-159 and ≥ 160 g/L) in men. Those with hemoglobin 0-9 g/L above the WHO cutoff (ie, 120-129 in women and 130-139 in men) were in the reference category for regression analyses evaluating alternative thresholds of risk.
Mortality was the primary outcome examined, while incidence of mobility disability was a secondary outcome. Through August 2005, a total of 502 deaths (19.3%) were systematically identified via telephone contact every 6 months and confirmed with death certificates. Survival time was calculated from the date of baseline clinic visit to the date of death or date of last contact (median survival time was 5.3 years). Mobility disability was defined as 2 consecutive reports (6 months apart) of having a lot of difficulty or not being able to walk a quarter of a mile or climb up 10 steps without resting. If a participant reported a lot of difficulty with either task at one contact and died before the next 6-month contact, then the difficulty was presumed to have persisted until death, and the participant was classified with mobility disability. This outcome reflects persistent and severe limitation in lower extremity function, which among older adults is prognostic of future disability in self-care tasks, nursing home admission, and death.15–17 Those with mobility disability at baseline (n = 225, 8.7%) were excluded from time to event analysis for this outcome. There were 345 cases of incident mobility disability (14.5%) over a median follow-up of 3.9 years, which was calculated from the date of the baseline clinic visit to the date of event or date of last contact.
Potential confounding factors specified in the data analysis include age, sex, level of education (< high school, high school graduate, or > high school), and study site (Memphis or Pittsburgh). Body mass index (BMI) was calculated from measured height and weight (BMI = weight in kilograms divided by height in meters squared). Survey questions were used to classify participants as never, former, or current smokers. Hospitalization that required an overnight stay within the past 6 months also was assessed through questionnaire. Serum albumin, creatinine, and cystatin C were determined through blood collection at the 1997-1998 initial clinical visit. Cystatin C is a novel measure of renal function that is not affected by muscle mass and has been shown to predict glomerular filtration rate better than creatinine-based estimates.18–21 For comparative purpose, the abbreviated Modification of Diet in Renal Disease formula that requires age, sex, race, and creatinine was used to estimate glomerular filtration rate (eGFR).22 In addition, medical conditions were assessed through self-report, medications, and/or clinical/laboratory investigation, including cancer, cerebrovascular disease, congestive heart failure, coronary heart disease, diabetes, gastrointestinal bleed/ulcer, hypertension, peripheral arterial disease, and pulmonary disease. Participants were classified with cardiovascular disease if they had cerebrovascular disease, congestive heart failure, coronary heart disease, or peripheral arterial disease. Each medical condition was updated through the baseline of the current study (1999-2000) except for gastrointestinal bleed/ulcer, peripheral arterial disease, and pulmonary disease, which were assessed only at the initial 1997-1998 clinical visit.
Data analysis
The distribution of participant characteristics among men and women were compared by race using Chi square, t test, and Wilcoxon rank sum test statistics. Kaplan-Meier survival curves and cumulative incidence curves of mobility disability were plotted by WHO anemia status for each race-sex subgroup. The log rank test was used in each Kaplan-Meier plot to determine whether event-rates were significantly different in anemic versus nonanemic participants. Cox proportional hazard models were used to assess associations of WHO anemia status and hemoglobin levels with mortality and incidence of mobility disability. All models were estimated separately for each race and sex combination because of statistically significant (P < .05) race by anemia interactions in both outcomes among men and women. Schoenfeld residuals were used to confirm the proportional hazards assumption in each race-sex subgroup. Two sets of models were used to test the association of WHO anemia status with each outcome (Table 2). The first model adjusted for age and study site, while the second model adjusted for all potential confounding factors. The same model specifications also were applied when testing the association of hemoglobin level with each outcome (Table 3). To evaluate whether the comorbid effect of anemia on mortality varies by race, proportional hazard models adjusted for age, sex, and study site were stratified by an index condition (diabetes, eGFR < 60 mL/min/1.73m,2 and cardiovascular disease) and race (Table 4). All analyses were completed with Stata SE software version 9.0 (Stata Corp, College Station, TX).
Results
The baseline prevalence of WHO-defined anemia was significantly higher in older blacks compared to older whites. Table 1 shows that 21% of black women and 26% of black men were anemic at baseline, whereas 7% and 14% of white women and men, respectively, had anemia. The distribution of hemoglobin was shifted significantly toward lower values in older blacks than in whites. On average, the racial difference in hemoglobin values was 7 and 8 g/L in men and women, respectively. Mean corpuscular volume also was significantly lower in blacks than in whites. In addition, older blacks had significantly lower levels of education, were more likely to currently smoke cigarettes, and had higher prevalence of diabetes and hypertension compared to whites (Table 1). In contrast, a history of cancer was significantly more common in older whites than in blacks, and renal function was significantly more impaired (assessed by cystatin C and eGFR) in whites compared to blacks. There were no significant racial differences in age or the prevalence of cerebrovascular disease, congestive heart failure, and pulmonary disease. Both mortality and incidence of mobility disability were significantly higher in blacks relative to whites.
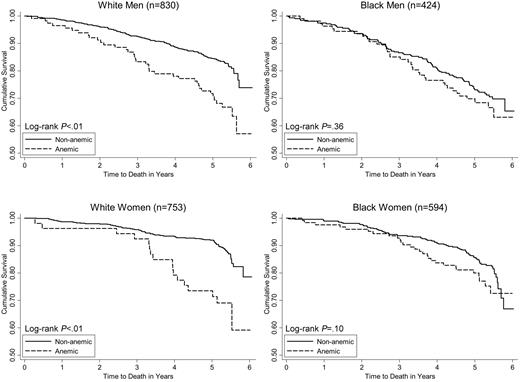
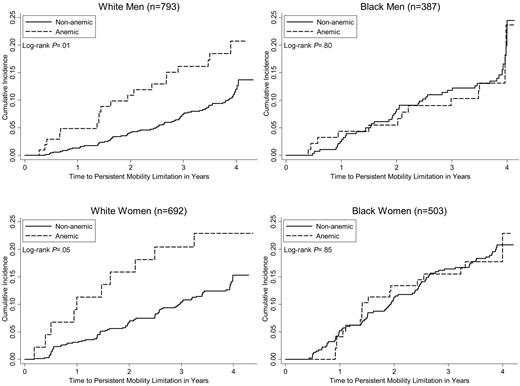
The racial difference in the association of anemia with mortality is displayed in Figure 1. Among white men and women, there was a clear separation of the survival curves, with significantly worse survival in those who were anemic at baseline according to WHO criteria. In contrast, there was no significant difference in survival by WHO anemia status in older blacks, although the survival curves in black women began to separate at 3 years of follow-up and then converged after the fifth year. The risk of death after adjusting for age was 2 to 3 times higher in whites with than without anemia (Table 2), while in blacks there was no increased mortality risk associated with anemia. Further adjustment for other potential confounding factors did not substantively change the differential patterns of effect, as shown in Table 2. Similarly, the incidence of mobility disability was significantly associated with WHO-defined anemia status in older whites, but not in older blacks (Figure 2). Although black men and women experienced higher overall rates of mobility disability than whites (Table 1), mobility disability in older blacks was not was not associated with anemia (Table 2). However, older white men and women with anemia experienced a 2-fold higher risk of mobility disability relative to those without anemia (Table 2). Adjustment for other risk factors reduced the effect of anemia on the risk of mobility disability in white men (P = .09), but not in white women (Table 2).
Kaplan-Meier survival curves by WHO-defined anemia status among race-sex subgroups of older adults.
Kaplan-Meier survival curves by WHO-defined anemia status among race-sex subgroups of older adults.
Cumulative incidence of mobility disability by WHO-defined anemia status among race-sex subgroups of older adults.
Cumulative incidence of mobility disability by WHO-defined anemia status among race-sex subgroups of older adults.
In addition to examining the effects of WHO-defined anemia, death rates were stratified by hemoglobin levels in Table 3 in order to evaluate whether the hemoglobin cutoff below which mortality rates increase is lower in older blacks than in whites. Mortality rates in white women were more than 5 times higher in those with a baseline hemoglobin level lower than 110 g/L relative to those with 120-129 g/L of hemoglobin (ie, 0-9 g/L above the WHO anemia cutoff). Surprisingly, there was practically no difference in mortality when comparing black women with less than 110 g/L to those who had 120-129 g/L of hemoglobin. So while there was a clear stepwise increased risk for mortality among white women with hemoglobin level lower than 110 and 110-119 g/L compared to those with 120-129 g/L, there was no evidence of a lower hemoglobin threshold below which mortality increased in black women. However, in black men mortality risk was significantly elevated only in those with hemoglobin concentration more than 20 g/L below the anemia cutoff (ie, < 110 g/L) compared to those 0-9 g/L above the anemia cutoff. As with white women, mortality risk was more graded in white men with hemoglobin concentration below the WHO anemia cutoff.
Finally, Table 4 presents mortality rates and risk estimates stratified by disease status groups, race, and WHO-defined anemia status. Among all participants (first stratum of Table 4), mortality rates were lowest in nonanemic whites and highest in anemic whites, but intermediate in anemic and nonanemic blacks. In a model combining all participants, there was a significant race by anemia interaction indicating an elevated mortality risk only in whites (P < .01; model not shown). In participants with diabetes, however, mortality was significantly elevated in both anemic blacks and whites compared to nonanemic blacks and whites, respectively. Similarly, anemia was significantly associated with mortality in blacks and whites with moderate renal impairment (eGFR < 60 mL/min/1.73m2). Anemia was not associated with increased risk of death in blacks with cardiovascular disease or in blacks without major diseases; whereas in whites, anemia increased mortality risk in those with cardiovascular disease and even in those without major diseases. Interestingly, mortality rates were higher in anemic whites than anemic blacks in each of the subpopulations shown in Table 4, except in those with moderate renal impairment.
Discussion
In a biracial cohort of well-functioning older adults, the current study finds that WHO-defined anemia was significantly associated with an increased risk of mortality and mobility disability in whites, but not in blacks. The large racial disparity in anemia prevalence observed in the Health ABC Study, as well as in other cohorts of older adults, does not appear to contribute to racial disparities in mortality and mobility disability. In fact, in older black men mortality risk was significantly elevated only in those with hemoglobin concentration more than 20 g/L below the WHO anemia cutoff, although no clear threshold or gradient of risk was observed in older black women. To our knowledge, this is the first study to document that the association of anemia with adverse outcomes varies by race among older adults and provides initial outcomes-based evidence that the hemoglobin “set point” is lower in blacks than in whites.
Previous studies evaluating the adverse effects of anemia in older adults have typically adjusted for race in data analysis, although 2 studies have recently examined race more closely. For instance, Zakai and colleagues adjusted for race in their analyses of the Cardiovascular Health Study (CHS) that showed anemia significantly increased risk of death among older adults, but they also reported that there was no significant statistical interaction between race and hemoglobin in predicting mortality (P = .30).9 More recently, Denney and colleagues analyzed data from the North Carolina Established Population for the Epidemiologic Studies of the Elderly (EPESE) and reported that mortality was significantly elevated in older blacks with WHO-defined anemia compared to blacks without anemia.5 However, both the CHS and North Carolina EPESE differ from the Health ABC in 2 important ways. First, the Health ABC is a relatively healthier cohort of older adults aged 70 to 79 years who were well functioning at baseline, whereas the CHS and North Carolina EPESE study samples had wider age ranges and spectrum of disease burden. Second, the Health ABC cohort has a larger sample of older blacks than either the CHS or North Caroline EPESE, which permitted stratification by race and sex. Given these differences in study design and composition, it is possible that the CHS lacked sufficient statistical power to detect a significant race by hemoglobin concentration interaction, and the significant effect of anemia on mortality in blacks in the North Carolina EPESE might be confounded by unmeasured disease burden.
The striking racial difference in the effect of anemia shown in Figures 1 and 2, as well as the large racial disparity in anemia prevalence, indicate that the WHO definition of anemia is inappropriate for older blacks. Anemia was associated with mortality only in older blacks when it co-occurred with diabetes or moderate renal impairment, whereas whites with anemia experienced high mortality regardless of disease status. Among blacks with diabetes or renal impairment, it is possible that anemia is simply a marker of disease severity, given that anemia is a major complication of renal failure and anemia occurs at earlier stages of chronic kidney disease in diabetic than nondiabetic persons.23 Alternatively, considering that vascular complications associated with diabetes and renal impairment are, in part, ischemic in nature, anemia might act synergistically with these conditions to increase risk of mortality in blacks and whites. Indeed, Astor and colleagues recently reported that anemia combined with moderate renal impairment was associated with increased risk for coronary heart disease events and mortality after adjusting for race and potential confounders in the Atherosclerosis Risk in Communities Study.24 Further, erythropoietin response to anemia has been shown to be impaired in diabetic patients with and without renal impairment,25 and general increases in erythropoietin secretion associated with advancing age were shown to be less dramatic in those with diabetes or hypertension.26 Finally, it is possible that the underlying causes of anemia might vary by race, with some causes having greater functional consequences than others. While any of these potentialities might explain why anemia was associated with death only in black participants with diabetes or renal impairment, the overall pattern suggests that the WHO criteria generally do not identify older blacks at increased risk of death or mobility disability.
Racial differences in hemoglobin concentration among children and adults have long been observed in the United States.13 The shifted distribution of hemoglobin levels in people of African versus European descent is suspected to partially result from gene mutations selected by pressure from endemic malaria in parts of Africa. For example, α-thalassemias are known to protect against severe malaria infection, are associated with lower hemoglobin concentration, and occur more frequently in blacks than in whites in the United States.27–29 However, Beutler and West30 have shown that racial differences in hemoglobin concentration persist after accounting for sickle cell trait, α-thalassemia, and iron deficiency. It is conceivable that other adaptive mutations from environmental demands other than malaria occurring inside and outside of the African continent might contribute to racial variation in hematologic parameters.
In contrast to the results in older blacks, the WHO criteria were clearly prognostic of mortality and mobility disability in older whites. Although previous studies have suggested that a lower threshold (ie, a higher hemoglobin cutoff) for defining anemia in older adults should be considered,3,4,7,9 the age-adjusted hazard ratios in Table 3 do not support a higher hemoglobin cutoff in either older blacks or whites. Caution is warranted when evaluating alternative cut points, as even a 10-g/L increase in the WHO definition of anemia would increase anemia prevalence in the US from 10.6% to 31.5% in older adults (weighted estimates based on NHANES III data), which would likely increase the false positive rate in identifying those truly at risk for adverse events and inefficiently expend health care resources. Identifying the optimal concentration of hemoglobin in community-dwelling older adults is challenging, as there is substantial heterogeneity in the age, sex, racial/ethnic, and health status composition of studies in older adults.
As with all observational studies, there are some limitations to the current study findings. First, hemoglobin was assessed 2 years after the start of the Health ABC Study, and therefore 15% of the original cohort members were missing data. While missing data might cause underestimation of anemia prevalence, it is unlikely to substantially bias the reported associations. In addition, renal function, as measured by cystatin C, was collected 2 years prior to hemoglobin assessment, although unmeasured change in renal function is also unlikely to account for the observed results. Finally, the generalizability of this study's findings is limited to community-dwelling black and white adults aged 71 to 82 years who were generally well functioning. The limited age range and relatively better physical function of the Health ABC cohort are study strengths, as subclinical disease burden and unmeasured disease severity are less likely to confound the association of anemia with mortality and mobility disability. Adjustment for a variety of potential confounding factors provides further evidence that WHO-defined anemia is a risk factor for adverse health in older whites.
In conclusion, the findings from the current study, in combination with evidence of racial differences in hemoglobin concentration from numerous other studies, indicate an alternative definition of anemia in older blacks is warranted. While WHO-defined anemia was associated with a 2- to 3-fold increased risk of mortality and mobility disability in older white participants, applying the WHO criteria in older blacks did not define a group at increased risk of adverse events. Further outcomes-based research is required to determine hemoglobin thresholds for defining anemia in major racial/ethnic subpopulations. This research is needed to improve not only clinical decision making, but also the design of clinical trials evaluating treatment regimens of anemia.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported in part by the Intramural Research Program of the National Institutes of Health, National Institute on Aging, and contracts N01-AG-6-2101, N01-AG-6-2103, and N01-AG-6-2106.
National Institutes of Health
Authorship
Contribution: K.V.P. and J.M.G. conceived and designed the study, analyzed the data, and drafted the manuscript; T.B.H. and A.B.N. acquired the data and are principal investigators of the Health ABC Study; and T.B.H., M.F., S.B.A., S.C., D.C.B., L.H.K., and A.B.N. provided critical intellectual content in drafting the manuscript.
Conflict-of-interest disclosure: The authors declare no financial conflict of interests in publishing the study results.
The complete list of members of the Health ABC Study is available on the Blood website; see the Supplemental Materials link at the top of the outline article.
Correspondence: Kushang V. Patel, 7201 Wisconsin Ave, Gateway Bldg, Suite 3C309, Bethesda, MD 20892-9205; e-mail:patelku@mail.nih.gov.