Abstract
Fetal hematopoietic stem cells (HSCs) regenerate daughter HSCs in irradiated recipients more rapidly than do adult HSCs. However, both types of HSCs divide in vitro with the same cell-cycle transit times, suggesting different intrinsically determined self-renewal activities. To investigate the mechanism(s) underlying these differences, we compared fetal and adult HSC responses to Steel factor (SF) stimulation in vitro and in vivo. These experiments were undertaken with both wild-type cells and W41/W41 cells, which have a functionally deficient c-kit kinase. In vitro, fetal HSC self-renewal divisions, like those of adult HSCs, were found to be strongly dependent on c-kit activation, but the fetal HSCs responded to much lower SF concentrations in spite of indistinguishable levels of c-kit expression. Fetal W41/W41 HSCs also mimicked adult wild-type HSCs in showing the same reduced rate of amplification in irradiated adult hosts (relative to fetal wild-type HSCs). Assessment of various proliferation and signaling gene transcripts in fetal and adult HSCs self-renewing in vitro revealed a singular difference in Ink4c expression. We conclude that the ability of fetal HSCs to execute symmetric self-renewal divisions more efficiently than adult HSCs in vivo may be dependent on specific developmentally regulated signals that act downstream of the c-kit kinase.
Introduction
Hematopoietic stem cells (HSCs) possess the unique property of being able to activate multiple lineage-specific differentiation programs, as well as mechanisms that can either initiate these very rapidly or maintain them through thousands of so-called “self-renewal” divisions.1,2 Recent studies indicate that the mechanisms that discriminate between these outcomes are subject to modulation by a developmentally regulated intrinsic switch.3 Differences in the properties of primitive fetal and adult hematopoietic cells and the differentiation programs they activate have been noted for many decades.4 New evidence now shows that between 3 and 4 weeks after birth HSCs change abruptly from a fully cycling population to a largely quiescent one,5 and this is accompanied by changes in their phenotype from one typical of activated HSCs to one characteristic of quiescent (G0) HSCs.6–9 During the same 1-week interval, the HSCs present in the bone marrow of neonatal mice also abruptly change the pace at which they regenerate daughter HSCs in irradiated adult hosts that have undergone transplantation with these cells. Concurrently, the numbers of mature lymphoid and myeloid cells they produce change from a “fetal” to an “adult” HSC program.3 Interestingly, these biological changes also correlate with a consistent change in HSC gene expression.3
Assessing the responses of purified fetal and adult HSCs to defined growth factor conditions in vitro offers a powerful approach to elucidating the mechanisms that force HSCs to adopt different biologic fates. In the past decade, considerable progress has been made in delineating the growth factor control of adult bone marrow HSC self-renewal divisions in vitro.10–20 Although analogous studies of fetal liver HSCs have been more limited, these have shown that fetal and adult HSCs respond differently to the same growth factors.21 Paradoxically, fetal HSCs have proven more difficult to maintain in vitro than adult HSCs even though fetal HSCs display a greater self-renewal activity when stimulated to proliferate in vivo.6,22–24
The full spectrum of factors that may be involved in stimulating HSC expansion in vivo, either during development or in irradiated mice, is not known. However, studies of HSCs from mice with defective or absent growth factors, or their cognate receptors, have implicated both Steel factor (SF)–c-kit (W) and thrombopoietin (TPO)–c-mpl as important.25–27 The W41 mutation encodes a single amino acid change in the tyrosine kinase domain of c-kit and this attenuates c-kit–activated signaling following SF binding.28 Mice homozygous for the W41 allele also show a deficiency in HSC numbers that is more striking in the adult than in the fetus.29 Based on these findings, it seemed possible that the developmental change in HSC self-renewal potential and cycling activity in vivo might involve c-kit signaling or a key downstream event. The studies described here were designed to test this hypothesis.
Materials and methods
Mice
C57Bl/6J-PtprcaPep3b/BoyJ (Peb3b) and congenic, homozygous W41 (Ly5.2) mice were used as donors and recipients, respectively, or vice versa, as indicated.
Cell isolation, HSC purification, and c-kit staining
Cell suspensions were prepared from embryonic day (E) 14.5 fetal liver and the bone marrow of 10-week-old adult mice. The lin−(no Mac1)Sca1+CD43+Mac1+ and lin−Rhodamine-123(Rho)−Hoechst 33342− (side population [SP]) fractions of each that are highly enriched in HSCs (20%-40% pure), respectively, were isolated by fluorescence-activated cell sorting (FACS), as previously described.3,30 Cell-surface c-kit expression was assessed on these cells by FACS using an allophycocyanin (APC)–labeled anti–c-kit antibody from Becton Dickinson (Franklin Lakes, NJ) and the geometric mean of the modal c-kit fluorescence intensities measured were then determined.
HSC quantification
HSCs were quantified using the limiting dilution competitive repopulating unit (CRU) assay5,31 with minor modifications. When Pep3b mice were used as recipients, they were irradiated with 2 doses of 400 cGy x-rays, separated by 4 hours, whereas W41 recipients were irradiated with a single, sublethal dose of 360 cGy x-rays. All cells were injected intravenously. Sixteen weeks later, the peripheral blood of mice that had undergone transplantation was analyzed for the presence of donor-derived granulocytes and monocytes (Ly6g+ and/or Mac1+ cells), B (B220+) cells, and T (Ly1+) cells. CRU frequencies were calculated using L-Calc software (StemCell Technologies, Vancouver, BC, Canada) as described.5 To measure rates of CRU expansion in vivo, 105 unfractionated fresh W41/W41 fetal liver (FL) cells, containing an estimated 10 CRUs,29 were injected into irradiated primary Pep3b hosts and then 1, 2, 3 and 4 weeks later, groups of these were killed and the bone marrow cells from both femurs and tibias of each of these primary recipients were harvested. Single cell suspensions were prepared and tested for regenerated W41/W41 CRU content by limiting dilution assays performed in secondary irradiated Pep3b recipients.
HSC cultures
Fetal liver cells (E14.5) depleted of Ter119+ cells and adult bone marrow cells depleted of lin+ cells (EasySep; StemCell Technologies) were cultured for 48 hours at 106 cells/mL in serum-free medium5 with various growth factors (from either StemCell Technologies or Genetics Institute, Cambridge, MA), as indicated. At the end of this period, the cells were harvested and CRU assays were performed.
Quantitative real-time (Q-RT) polymerase chain reaction (PCR) analysis
A PicoPure kit (Arcturus Bioscience, Mountain View, CA) was used to extract RNA from 48-hour-culture cells initiated with lin−Sca-1+CD43+Mac1+ E14.5 fetal liver cells and lin−Rho−SP adult bone marrow cells. These cultures were prepared by sorting 100 to 500 cells directly into 100 μL serum-free medium and then adding 100 μL of a 2X cytokine mixture to bring the final growth factor concentrations to 50 ng/mL SF for the fetal liver cell cultures and 300 ng/mL SF and 20 ng/mL IL-11 for the adult bone marrow cell cultures. The extracted RNA was reverse transcribed into cDNA using the SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen, Burlington, ON, Canada) and then Q-RT-PCR analyses were performed using Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA).5 Primers were as follows: c-kit (NM_021099.2) forward: ACAAGAGGAGATCCGCAAGA and reverse: GAAGCTCAGCAAATCATCCAG; Gapdh (NM_008084) forward: AACTTTGGCATTGTGGAAGG and reverse: ATGCAGGGATGATGTTCTGG; Ccnd1 (NM_007631) forward: CGCCCTCCGTATCTTACTTCA and reverse: TCGCACTTCTGCTCCTCACAG; Ccnd2 (NM_009829) forward: GGCCAAGATCACCCACACT and reverse: ATGCTGCTCTTGACGGAACT; Ccnd3 (NM_007632) forward: CGAAACCACGCCCCTGAC and reverse: GACCAGCACCTCCCACTCC; Ink4c (NM_007671) forward: GAACTGCGCTGCAGGTTAT and reverse: TCAAATTGGGATTAGCACCTC; p21Cip1 (NM_007669) forward: GTACTTCCTCTGCCCTGCTG and reverse: TCTGCGCTTGGAGTGATAGA; p27Kip1 (NM_009875) forward: GGTTAGCGGAGCAGTGTCCA and reverse: GGCCCTTTTGTTTTGCGAAG; Jak2 (NM_008413) forward: TGGGAATGTGTGTGCTAAAAA and reverse: TTTGATGAAAGGTGGGTTCC; Stat3 (NM_213659) forward: GGCACCTTGGATTGAGAGTC and reverse: ACTCTTGCAGGAATCGGCTA; and Stat5a (NM_011488) forward: GTTCGCGAAGCCAACAAT and reverse: TTCTCCGTGTCCTGTGTGAT.
Cell-cycle analysis
Lin−Sca1+CD43+Mac1+ and Ter119− fetal liver cells were costained with Hoechst 33342 (Invitrogen) and the proportions of G0/G1 and S/G2/M subsets assessed using gates to distinguish between cells with 2n versus more than 2n DNA, respectively, as described.5
Statistical analysis
Comparisons were made using the Wald test except for the transcript comparisons, for which the Student t test was used. P values below .05 were considered to represent a significant difference.
Results
SF alone, and at low concentrations, optimally sustains fetal HSCs in vitro
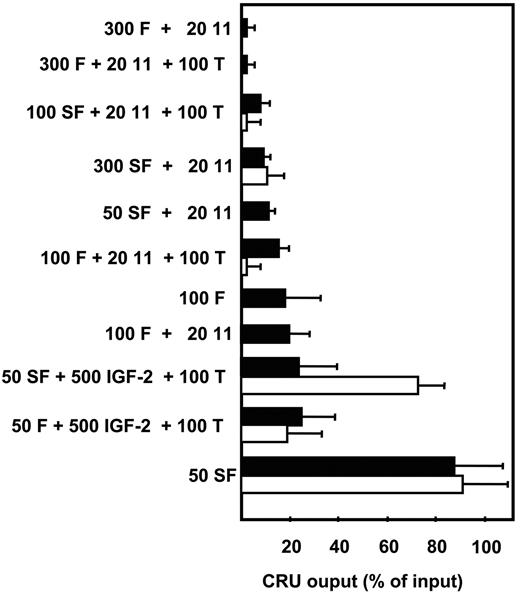
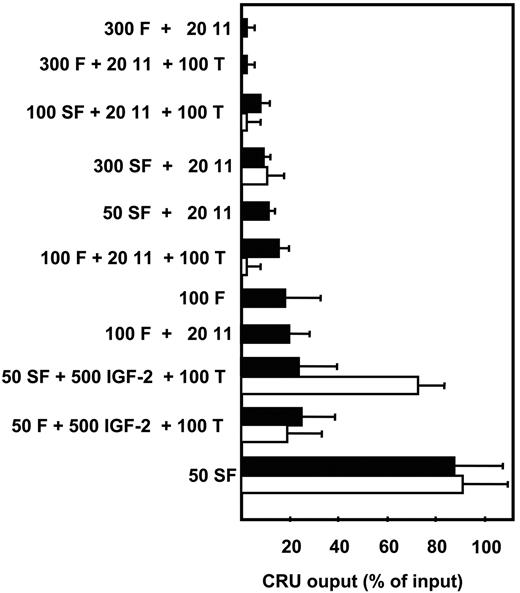
In a first series of experiments, we investigated the growth factors required to sustain fetal HSC activity in vitro for 2 to 7 days. Serum-free suspension cultures were initiated with HSC-enriched (∼10x by removal of Ter-119− cells5 ) E14.5 mouse fetal liver cells. Changes in HSC numbers were determined using the CRU assay to measure input and output HSC values. Consistent with previous reports, CRU numbers rapidly declined (within the first 48 hours) under most of the conditions tested (Figure 1). These included a growth factor cocktail that stimulates adult mouse CRUs to amplify approximately 2- to 4-fold within 10 days13,18 and also one reported to amplify fetal HSCs.24 However, we did find that all of the input CRU activity was retained for at least 7 days when the cells were cultured with 50 ng/mL SF alone. Interestingly, the further addition of 20 ng/mL IL-11, which, in combination with SF, is absolutely required to retain adult bone marrow CRU activity in vitro, negated the ability of 50 ng/mL SF to sustain fetal CRUs. Additional experiments were then undertaken to monitor the cell division kinetics of highly purified CRUs from E14.5 fetal liver (∼20% purity3 ) in single cell cultures. The results of these experiments showed that all of these cells completed one division within 48 hours in the presence of 50 ng/mL SF and most had completed 2 divisions by that time.3 The concomitant retention of CRU numbers over the first 48 hours indicates that some of the initial fetal liver CRUs are stimulated to execute self-renewal divisions under these conditions.
Comparison of the effects of different growth factor cocktails on FL CRU self-maintenance in vitro. The number of CRUs recovered from each culture after 2 days (■) and 7 days (□) is expressed as a percent of the input number. CRU numbers were determined by 16-week limiting dilution transplantation assays as described in “Materials and methods.” Values shown are the mean plus or minus the standard error of the mean (SEM) of results pooled from 2 to 5 experiments. Growth factor concentrations are in ng/mL. F indicates FLT-3; 11, IL-11; T, TPO; IGF-2, insulin-like growth factor-2.
Comparison of the effects of different growth factor cocktails on FL CRU self-maintenance in vitro. The number of CRUs recovered from each culture after 2 days (■) and 7 days (□) is expressed as a percent of the input number. CRU numbers were determined by 16-week limiting dilution transplantation assays as described in “Materials and methods.” Values shown are the mean plus or minus the standard error of the mean (SEM) of results pooled from 2 to 5 experiments. Growth factor concentrations are in ng/mL. F indicates FLT-3; 11, IL-11; T, TPO; IGF-2, insulin-like growth factor-2.
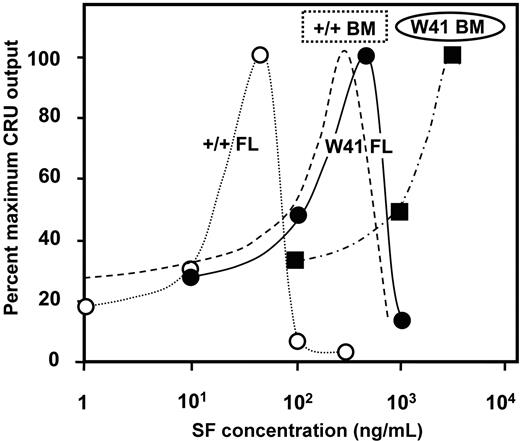
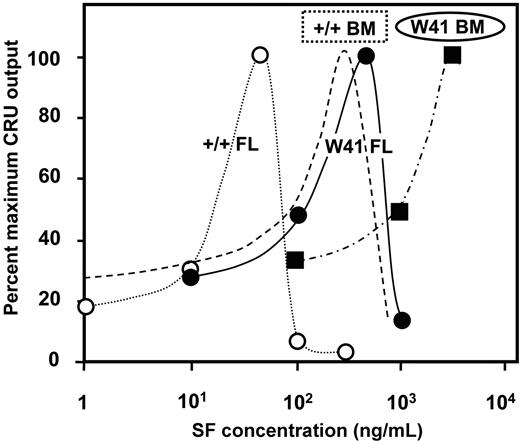
We next undertook a more complete dose-response analysis of the SF sensitivity of fetal liver CRUs in terms of their ability to execute self-renewal divisions in the same type of 48-hour serum-free suspension culture experiment. The results showed that the number of fetal CRUs recovered was significantly reduced below the value obtained with 50 ng/mL SF when the SF concentration was either increased or decreased (Figure 2). For comparison, SF dose-response data for adult bone marrow CRU outputs assessed after 10 days in similar cultures with variable concentrations of SF (plus 20 ng/mL IL-11 and 1 ng/mL flt3-ligand)13 are shown as a dotted line in Figure 2. This result suggests an approximately 10-fold-lower SF self-renewal requirement of fetal CRUs. Taken together, these studies demonstrate that both fetal and adult CRUs depend on c-kit activation to maintain their stem cell properties when stimulated to divide, but fetal CRUs appear much more sensitive to SF than their adult counterparts.
SF dose-response curves for the in vitro self-renewal of wild-type and W41 CRUs from FL and adult BM. Data shown for each source of CRUs are expressed as a percent of the number of CRUs in the cultures from which the maximum number of CRUs were recovered. CRUs analyzed were from cultured +/+ fetal liver (FL, open circles), W41/W41 fetal liver (W41 FL, solid circles), and W41/W41 bone marrow (W41 BM, solid squares) cells. Data for W41/W41 was derived from experiments in which the culture medium also contained 20 ng/mL IL-11. Results for wild-type (+/+) bone marrow (dashed line) is redrawn from Audet et al13 and reflects conditions that also included varying concentrations of IL-11 and FLT3-ligand in addition to varying concentrations of SF, and cultures were maintained for 10 rather than 2 days.
SF dose-response curves for the in vitro self-renewal of wild-type and W41 CRUs from FL and adult BM. Data shown for each source of CRUs are expressed as a percent of the number of CRUs in the cultures from which the maximum number of CRUs were recovered. CRUs analyzed were from cultured +/+ fetal liver (FL, open circles), W41/W41 fetal liver (W41 FL, solid circles), and W41/W41 bone marrow (W41 BM, solid squares) cells. Data for W41/W41 was derived from experiments in which the culture medium also contained 20 ng/mL IL-11. Results for wild-type (+/+) bone marrow (dashed line) is redrawn from Audet et al13 and reflects conditions that also included varying concentrations of IL-11 and FLT3-ligand in addition to varying concentrations of SF, and cultures were maintained for 10 rather than 2 days.
Fetal and adult HSCs express the same levels of c-kit but when activated by SF show different levels of Ink4c gene expression
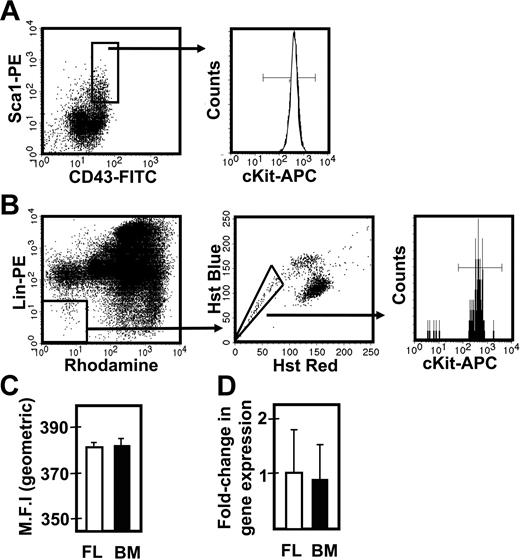
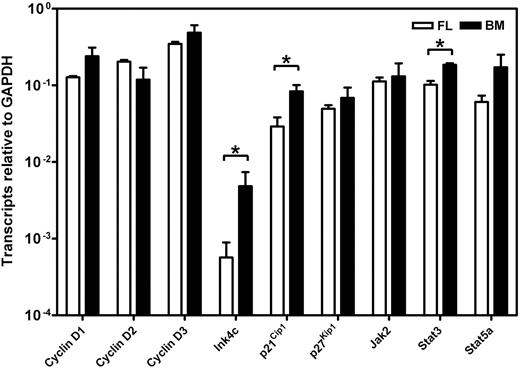
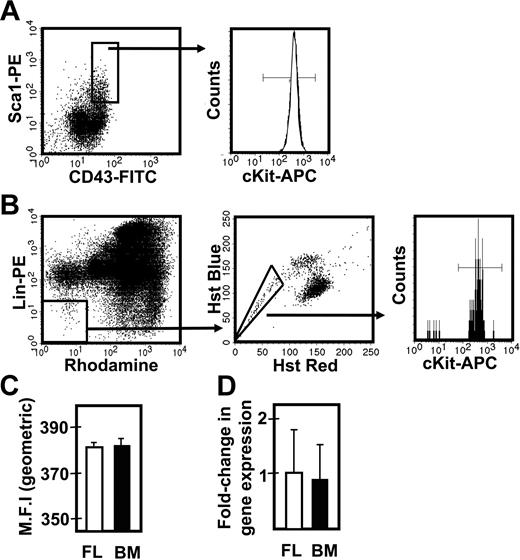
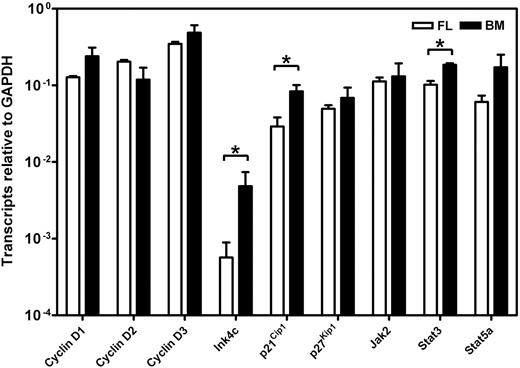
To determine whether the different responses exhibited by fetal and adult HSCs were attributable to differences in their expression of c-kit, we isolated the lin−Sca1+CD43+Mac1+ subpopulation of E14.5 fetal liver and the lin−Rho−SP subset of adult bone marrow cells (∼20%3 and ∼30%30 pure HSCs, respectively) and compared the levels of c-kit mRNA and cell-surface protein they each express (Figure 3). Q-RT-PCR measurements showed no difference in c-kit transcripts between the 2 highly enriched HSC populations. Similarly, FACS analysis showed indistinguishable levels of c-kit protein on the surface of these 2 highly HSC-enriched cell populations, extending previous data of this type.32 We then compared the expression of 9 signaling and proliferation control genes in the immediate progeny of these cells generated within 48 hours in culture under conditions that maximize the self-renewal responses of fetal and adult HSCs (ie, 50 ng/mL SF, Figure 1; and 300 ng/mL plus 20 ng/mL IL-11,13 respectively). After 48 hours, previous time course studies have shown that none of the input cells have died and all have completed one but not more than 4 divisions.3,30 The results of the Q-RT-PCR analyses performed on extracts of these cultured cells are shown in Figure 4. These showed the transcript levels for 8 of the 9 genes studied to be very similar in both the proliferating fetal and adult HSCs. Strikingly, Ink4c transcripts were 20-fold lower in the fetal cells (P < .05).
Comparison of c-kit expression on fetal liver and adult bone marrow cell populations that are highly enriched in their CRU content. (A) Representative profile of E14.5 viable, lin− fetal liver (FL) cells assessed for Sca1 and CD43 expression (left panel), further analyzed for the level of surface c-kit protein expression (right panel). (B) Representative profile of viable lin−Rho−SP adult bone marrow (BM) cells (left and middle panels) assessed for the level of surface c-kit protein expression (right panel). (C) Comparison of the geometric mean fluorescence intensity (MFI ± SEM, n = 2) of surface c-kit protein expression in the lin−Sca1+CD43+ fetal liver cells (shown in panel A) and lin−Rho−SP adult bone marrow cells (shown in panel B). (D) Comparison of the average fold-change (± SEM, n = 3) in c-kit gene expression relative to Gapdh between the lin−Sca1+CD43+ fetal liver cells (shown in panel A) and lin−Rho−SP adult bone marrow cells (shown in panel B).
Comparison of c-kit expression on fetal liver and adult bone marrow cell populations that are highly enriched in their CRU content. (A) Representative profile of E14.5 viable, lin− fetal liver (FL) cells assessed for Sca1 and CD43 expression (left panel), further analyzed for the level of surface c-kit protein expression (right panel). (B) Representative profile of viable lin−Rho−SP adult bone marrow (BM) cells (left and middle panels) assessed for the level of surface c-kit protein expression (right panel). (C) Comparison of the geometric mean fluorescence intensity (MFI ± SEM, n = 2) of surface c-kit protein expression in the lin−Sca1+CD43+ fetal liver cells (shown in panel A) and lin−Rho−SP adult bone marrow cells (shown in panel B). (D) Comparison of the average fold-change (± SEM, n = 3) in c-kit gene expression relative to Gapdh between the lin−Sca1+CD43+ fetal liver cells (shown in panel A) and lin−Rho−SP adult bone marrow cells (shown in panel B).
Comparison of gene expression in cultured fetal and adult HSCs. Transcript levels were determined by Q-RT-PCR for each gene relative to Gapdh on the 48-hour progeny of highly purified suspensions of HSCs from E14.5 fetal liver (FL, □) and adult bone marrow (BM, ■) cultured under serum-free conditions with growth factors that optimize HSC maintenance (50 ng/mL SF for the fetal liver cells and 300 ng/mL SF plus 20 ng/mL IL-11 for the adult bone marrow cells). Lin−Sca1+CD43+Mac1+ cells were isolated from E14.5 fetal liver cells and lin−Rho−SP cells were isolated from adult bone marrow as described in “Materials and methods.” Results are the mean plus or minus SEM of data from 2 to 3 experiments, in each of which Q-RT-PCR measurements were performed in triplicate. Significant differences between fetal liver and adult bone marrow values (P < .05) were determined by a Student t test and are indicated by an asterisk.
Comparison of gene expression in cultured fetal and adult HSCs. Transcript levels were determined by Q-RT-PCR for each gene relative to Gapdh on the 48-hour progeny of highly purified suspensions of HSCs from E14.5 fetal liver (FL, □) and adult bone marrow (BM, ■) cultured under serum-free conditions with growth factors that optimize HSC maintenance (50 ng/mL SF for the fetal liver cells and 300 ng/mL SF plus 20 ng/mL IL-11 for the adult bone marrow cells). Lin−Sca1+CD43+Mac1+ cells were isolated from E14.5 fetal liver cells and lin−Rho−SP cells were isolated from adult bone marrow as described in “Materials and methods.” Results are the mean plus or minus SEM of data from 2 to 3 experiments, in each of which Q-RT-PCR measurements were performed in triplicate. Significant differences between fetal liver and adult bone marrow values (P < .05) were determined by a Student t test and are indicated by an asterisk.
HSCs from fetal W41/W41 mice display the same SF sensitivity as HSCs from wild-type adult mice
HSCs from W41/W41 mice would be expected to display an increased SF requirement in vitro due to their decreased c-kit signaling activity. Any resultant biologic phenotype in vivo might then be inferred to indicate where SF levels were limiting. Preliminary experiments confirmed previous data that E14.5 fetal liver cells from W41/W41 and wild-type mice contain similar frequencies of CRUs (1/4120 ± 910 vs 1/4940 ± 830 respectively).29 To evaluate the effect of the W41 mutation on fetal and adult HSC self-renewal in vitro, we used the same type of experiment described in Figure 1, in which CRU assays were performed on cells recovered from short-term suspension cultures initiated with either E14.5 fetal liver or adult bone marrow cells from W41/W41 mice. The results showed that the SF dose-response curve for W41/W41 fetal CRU recovery after 2 days is very similar to that of wild-type adult CRUs after 10 days, with a peak self-maintenance response requiring 500 ng/mL SF (Figure 2). In addition, the CRUs from adult W41/W41 mice showed a further (> 10-fold) reduction in SF sensitivity. Thus, both fetal and adult CRUs from W41/W41 mice show a significantly decreased sensitivity to SF relative to their wild-type counterparts, but with the same magnitude of developmental change in this parameter.
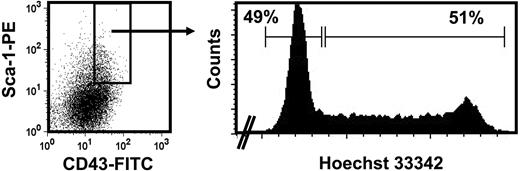
In the normal (wild-type) adult, the decreased sensitivity to SF that HSCs acquire during ontogeny is associated with changes in several other properties.3,5 One of these is a change in their proliferative activity in vivo from a fully cycling to a largely quiescent population. We therefore asked whether the reduced SF sensitivity of the CRUs in fetal W41/W41 mice might be sufficient to reduce their proliferative activity in vivo. To address this question, we isolated the lin−Sca1+CD43+Mac1+ fraction of cells from the fetal liver of W41/W41 mice and then used Hoechst 33342 staining to determine the G0/G1 versus S/G2/M distribution of these cells. As shown in Figure 5, the results of the Hoechst staining revealed an approximately equal distribution of cells between the G0/G1 (2n DNA) and S/G2/M (> 2n DNA) fractions. These results indicate that the HSCs in fetal W41/W41 mice are cycling despite their lower-than-normal (adultlike) SF responsiveness.
Cell-cycle analysis of the HSC-enriched fraction of W41/W41 fetal liver cells. Isolation of viable, lin−Sca1+CD43+E14.5 fetal liver cells from W41/W41 mice (left panel) that were then further enriched for HSCs by isolation of the Mac1+ subset and their distribution in G0/G1 versus S/G2/M assessed by staining with Hoechst 33 342 (right panel). Data are representative of 2 independent experiments.
Cell-cycle analysis of the HSC-enriched fraction of W41/W41 fetal liver cells. Isolation of viable, lin−Sca1+CD43+E14.5 fetal liver cells from W41/W41 mice (left panel) that were then further enriched for HSCs by isolation of the Mac1+ subset and their distribution in G0/G1 versus S/G2/M assessed by staining with Hoechst 33 342 (right panel). Data are representative of 2 independent experiments.
CRUs from fetal W41/W41 mice display a reduced self-regenerative activity after transplantation into irradiated hosts
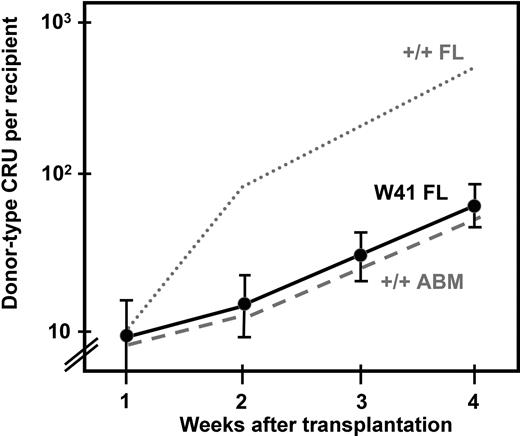
In view of the reduced sensitivity of the CRUs from W41/W41 fetal mice as compared with CRUs from wild-type fetal mice and the similar behavior of the CRUs from W41/W41 fetal mice in vitro to CRUs from wild-type adult mice, it was of interest to investigate whether the W41/W41 genotype would similarly affect the in vivo self-regenerative activity of fetal CRUs. For this we used a double transplant design. First, we transplanted 10 Ly5-congenic W41/W41 CRUs into lethally irradiated Pep3b recipients. Then, at weekly intervals up to 4 weeks later, the bone marrow of the primary transplanted mice was harvested and assayed for donor-type CRUs in secondary lethally irradiated hosts (same strain as the primary hosts). From the CRU frequencies thus measured, we calculated the number of W41/W41 CRUs that had been amplified from the original transplant injected into the primary mice. The results, shown in Figure 6, indicate that the initial rate of expansion of W41/W41 fetal CRUs in irradiated adult recipients was different from a previously determined rate of expansion of wild-type fetal CRUs in the same type of hosts and was the same as previously measured for adult wild-type CRUs.3
Rate of expansion of CRUs from W41/W41 fetal liver after their transplantation into irradiated adult recipients. Results shown are the mean (± SEM, data pooled from 4 experiments) number of donor CRUs per recipient, as determined by secondary limiting dilution CRU assays of cells regenerated in irradiated Ly5 congenic primary recipients of 10 CRUs from E14.5 W41/W41 fetal livers. For comparison, the results from similarly treated recipients of 10 CRUs from wild-type fetal livers (wild-type FL, dotted line) and +/+ adult bone marrow (ABM, dashed line) as described in Bowie et al3 are also shown.
Rate of expansion of CRUs from W41/W41 fetal liver after their transplantation into irradiated adult recipients. Results shown are the mean (± SEM, data pooled from 4 experiments) number of donor CRUs per recipient, as determined by secondary limiting dilution CRU assays of cells regenerated in irradiated Ly5 congenic primary recipients of 10 CRUs from E14.5 W41/W41 fetal livers. For comparison, the results from similarly treated recipients of 10 CRUs from wild-type fetal livers (wild-type FL, dotted line) and +/+ adult bone marrow (ABM, dashed line) as described in Bowie et al3 are also shown.
Discussion
The overall objective of this study was to determine whether the c-kit signaling pathway contributes to the different self-renewal activity displayed by fetal and adult HSCs stimulated to proliferate under the same conditions in vivo.3 For this purpose, we used the CRU assay to define HSCs functionally and to quantify them using limiting dilution analysis.31 Two lines of evidence were obtained to support the conclusion that the same exposure of fetal and adult HSCs to SF can produce different outcomes in these cells. First, we found that c-kit activation promotes the execution of self-renewal divisions in vitro by fetal HSCs, as has previously been demonstrated for adult HSCs13,33 but, for fetal HSCs, optimization of this response is achieved using an approximately 10-fold-lower concentration of soluble SF than is necessary to optimize the self-renewal of adult HSCs cultured under otherwise similar conditions except for the requisite additional presence of IL-11. Notably, the requirement of adult HSCs for coincident activation of gp13012 was not shared by fetal HSCs. In fact, additional activation of gp130 (via addition of IL-11) had an inhibitory effect on the self-renewal of fetal HSCs. A similar inhibitory effect was seen when fetal HSCs were exposed to an excessive concentration of SF.
Interestingly, c-kit expression was indistinguishable on fetal and adult cells that were highly enriched in their HSC content. Thus it would appear that the critical discriminating events in fetal and adult HSCs are most likely to be related to mechanisms that regulate the surface organization of c-kit or that affect cellular intermediates downstream of the c-kit kinase. A comparison of the level of expression of several candidates at the RNA level showed most of these to be remarkably similar in the earliest progeny generated in 2-day cultures of purified HSCs maintained under conditions that sustain HSC function. The exceptions to this were Ink4c, a gene encoding an early G1 phase inhibitor of the cyclin D–dependent kinases,34 p21Cip1, and Stat3. Ink4c was expressed at a 20-fold-lower level in the fetal cells and is of interest in view of recent data indicating that deletion of Ink4c enhanced the self-renewal activity of adult HSCs when these were assessed in a competition experiment with cotransplanted wild-type adult HSCs.35
The second line of evidence pointing to c-kit signaling as a trigger of events that cause fetal and adult HSCs to display different biologic properties was provided by experiments with HSCs from W41/W41 mice. These studies showed that decreased c-kit signaling (caused by activation of cells expressing a defective c-kit kinase) was sufficient to convert a fetal HSC self-renewal response either in vitro or in vivo to one typical of adult HSCs. The finding of Iscove and Nawa2 that the self-renewal of adult HSCs from wild-type mice can be enhanced by in vivo administration of SF (and IL-11) is consistent with the hypothesis that SF signaling is a limiting parameter in regulating adult HSC amplification in vivo. Nevertheless, it is notable that the decreased responsiveness of fetal HSCs from W41/W41 mice did not reduce their cycling activity in the embryo. Thus it would appear that the levels of SF available at early stages of development (or the levels of other factors with redundant activities) are sufficient to maximize HSC cycling activity. Perhaps this is related to previous observations that membrane-bound SF exerts a more potent effect on HSCs than soluble SF36 but its absence does not compromise HSC expansion until later stages of development.25
Recently we identified an abrupt switch in HSC properties that occurs in mice between 3 and 4 weeks after birth and affects their self-renewal and cycling control, in addition to their outputs of mature myeloid and lymphoid cells.3,5 The present results indicate that this switch must target part of the mechanism by which activated c-kit promotes HSC self-renewal and suggest that this may involve changes in Ink4c expression. They also help to explain why HSCs from W-mutant mice are typically more affected in the adult mouse than during fetal development.29,32 In humans, the role of SF activation of c-kit in regulating HSC self-renewal divisions in the mouse appears to be largely supplanted by flt3-ligand activation of the cognate flt3 receptor.17 It will therefore be interesting to determine whether these 2 receptors activate downstream intermediates whose function is blocked by a developmentally regulated change in expression of Ink4c.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Flow Cytometry Facility of the Terry Fox Laboratory and the Animal Resource Centre of the BC Cancer Research Centre for expert technical support, and Debra Wytrykush for secretarial assistance. This work was supported by grants from the National Cancer Institute of Canada (NCIC, with funds from the Terry Fox Foundation), the Stem Cell Network, and P01 HL-55 435 from the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health (NIH). M.B. and D.K. were recipients of studentships from the Stem Cell Network, the Canadian Institutes of Health Research, and the Michael Smith Foundation for Health Research.
Authorship
Contribution: M.B. and C.E. shared in the overall design of the experiments; M.B. undertook the majority of the experimental work and data analysis; D.K. and M.C. performed most of the quantitative RT-PCR data collection and analysis; all authors contributed to the preparation of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Connie J. Eaves, Terry Fox Laboratory, 675 West 10th Ave, Vancouver, BC, V5Z 1L3, Canada; e-mail: ceaves@bccrc.ca.