Abstract
Following exposure to cytotoxic agents, acute myeloid leukemia (AML) blasts elevate cellular cholesterol in a defensive adaptation that increases chemoresistance, but blockade of HMG-CoA reductase with statins restores chemosensitivity in vitro. This phase 1 study evaluated adding pravastatin (PV) (40-1680 mg/day, days 1-8) to idarubicin (Ida) ([12 mg/(M2 · day), days 4-6]) + high-dose cytarabine (Ara-C; HDAC) [1.5 g/(M2 · day) by CI, days 4-7] in 15 newly diagnosed and 22 salvage patients with unfavorable (n = 26) or intermediate (n = 10) prognosis cytogenetics. Compared with historical experience with Ida-HDAC, the duration of neutropenia and throbmbocytopenia and the toxicity profile were unaffected by the addition of PV. During PV loading (day 0-4) serum triglyceride and total and LDL cholesterol levels decreased in nearly all patients. Pharmacokinetic studies demonstrated higher and more sustained serum PV levels with PV doses above 1280 mg/day. CR/CRp was obtained in 11 of 15 new patients, including 8 of 10 with unfavorable cytogenetics, and 9 of 22 salvage patients. An MTD for PV + Ida-HDAC was not reached. Addition of PV to Ida-HDAC was safe, and the encouraging response rates support conducting further trials evaluating the effect of cholesterol modulation on response in AML.
Introduction
In normal cells, cholesterol is essential to membrane structure and the function of many membrane-bound proteins. Cholesterol homeostasis is a tightly regulated process in which a complex feedback loop determines the cellular cholesterol content.1 This feedback loop involves lipoprotein receptors (eg, low-density lipoprotein receptor or LDLR) and key regulatory enzymes of the mevalonate pathway, including 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG-CoAR) and squalene synthase. Compared with normal cells, cultured acute myeloid leukemia (AML) cells frequently overexpress the genes for LDLR and HMG-CoAR, synthesize cholesterol at a higher level, and import more cholesterol. A manifestation of these findings is that serum hypocholesterolemia is common in de novo patients with AML but rarely seen when the same patients achieve remissions.2
We discovered that cholesterol levels further increase in many AML samples treated with γ-irradiation, daunorubicin (DNR), or cytarabine (Ara-C), and that LDLR and HMG-CoAR mRNAs are commonly increased in AML samples treated in vitro with Ara-C and/or DNR.3 In addition, we found that inhibiting cholesterol synthesis at the first committed step of the mevalonate pathway with mevastatin, an HMG-CoAR inhibitor, sensitizes AML cells to these cytotoxic treatments,4,5 but normal bone marrow cells are generally not chemosensitized by mevastatin. Others have shown that normal cells are relatively insensitive to lovastatin, simvastatin, and pravastatin.6-8 These data led us to hypothesize that statin cotreatment could be used to selectively block adaptive cholesterol responses in AML cells to improve the efficacy of standard induction regimens for patients with AML.
We therefore designed a phase 1 clinical trial using escalating doses of pravastatin (PV) coadministered with a standard idarubicin/high-dose Ara-C (Ida-HDAC) regimen to address this question.9 Although administration of a single conventional dose of pravastatin can substantially reduce cholesterol synthesis in circulating myeloid cells,10 our in vitro data suggest that improved clinical responses in AML regimens are most likely to occur after relatively high statin dosing so that both de novo cholesterol synthesis and LDL import, due to induction of LDL-R synthesis, are significantly reduced in AML cells. In the absence of concomitant chemotherapy, such dosing can apparently be safely achieved. In a phase 1 dose-finding study of 88 patients with cancer, approximately 25-fold conventional lovastatin dosing caused no significant toxicity.11 In our study a continuous reassessment model was used to implement dose escalation and estimate the maximally tolerated dose (MTD) for PV administered to patients with AML with the Ida-HDAC induction regimen.
We chose pravastatin for this study for several reasons. First, pravastatin has a higher bioavailablity than many of the other statins.10,12 Approximately 50% of circulating pravastatin is bound to plasma proteins after a single oral dose, and its elimination half-life in humans is 72 hours, providing a reasonable window of bioavailability for possible antitumor effects. Further, unlike all other commercially available statins, pravastatin is not substantially metabolized by cytochrome p450s (CYP). Because many antitumor agents and commonly used supportive medications (notably antifungals in the azoles class) are themselves CYP substrates, avoiding CYP modulation seems prudent in statin-chemosensitizing strategies.
Patients, materials, and methods
Patient population
A total of 37 patients were treated on this protocol, approved by the MDACC IRB (protocol no. 2004-0185), and informed consent was obtained in accordance with the convention of Helsinki. Patients were eligible for inclusion if they had a diagnosis of AML and had not yet been treated, or had been deemed primary refractory, or in first or second relapse. To more cleanly assess the effect of cholesterol blockade in AML samples that were naive to cholesterol modification, patients were not eligible if they were receiving any cholesterol-lowering agent, including any HMG-CoAR inhibitor, fibric acid agents, bile acid sequestration agents, or niacin. Eligibility requirements included a Zubrod performance status of no more than 2, a cardiac ejection fraction at least 45% by echocardiography or multiple gated acquisition (MUGA) scan, a serum bilirubin level less than 34.2 μM (2.0 mg/dL), and a serum creatinine level less than 1.5 × normal unless felt to be secondary to the leukemia. Patients could not have any uncontrolled life-threatening infection, be pregnant, be HIV positive, or have evidence of acute or chronic hepatic impairment. Characteristics of the patients enrolled on this study are shown in Table 1. Outcome results were compared with those from a historical control group consisting of 178 initial induction patients and 18 salvage patients, aged 18 to 72 treated with the same Ida-HDAC regimen. The historical complete response rate for initial induction patients was 74% among the 82 patients with intermediate cytogenetics (diploid, −Y, insufficient metaphases) and 39% among the 96 patients with unfavorable cytogenetics (−5, −7, +8, 11q23, Ph1, miscellaneous and complex karyotypes).13,14 The historical control response rate in the salvage setting was 39% for this limited population. A broader historical cohort, consisting of patents treated on other anthracycline-HDAC combinations at MDACC since January 1, 2000, included 198 first and 152 second relapse patients with CR rates of 24% and 9%, respectively (S.M.K., unpublished data, June 2006).
Treatment plan
PV was administered orally once daily in the morning on days 1 to 8, idarubicin was administered at a dose of 12 mg/(M2/ · day) intravenously over 30 minutes on days 4, 5, and 6. Cytarabine was administered at a dose of 1.5 g/(M2 · day) by continuous infusion on days 4, 5, 6, and 7. The starting dose of PV was 40 mg/day, and patients were treated in cohorts of 3 with escalation to doses of 80, 160, 320, 480, 680, 880, 1080, 1280, 1480, and 1680 mg/day. Higher PV doses were not planned because higher equivalent lovastatin doses had produced some myotoxicity that necessitated ubiquinone administration.11 Patients achieving remission could receive up to 5 cycles of consolidation therapy with PV days 1 to 6 and idarubicin [12 mg/(M2 · day)] and cytarabine [1.5 g/(M2 · day) by continuous infusion] on days 4 and 5.
Trial statistics
The trial was conducted using a continuous reassessment model (CRM). The CRM approach models the dose-toxicity relationship using a one-parameter curve. A prior distribution was placed on the parameter for acceptable toxicity (.33 for this trial) before the trial. After each set of patients is treated and toxicity observed, the distribution of the parameter is updated, and the next dose level is selected based on the predicted toxicity. The scientific objective of this trial is to obtain the maximum tolerated dose (MTD) for pravastatin in combination with idarubicin-HDAC. The design was to treat a maximum of 36 patients in cohorts of 3, not skipping an untried dose when escalating. Three patient populations were considered: (1) newly diagnosed and previously untreated patients. (2) relapsed patients with a CR duration of longer than 1 year (high responders), (3) relapsed patients with CR durations of less than 1 year (low responders). In the first 2 groups, the expected response rate was 60%, so these were collapsed into one group. In the third group, the expected response rate was 20%.
Supportive care
For supportive care, patients received standard-of-care intravenous hydration, alkalinization, allopurinol (or rasburicase when indicated), Pred-Forte eye drops, antiemetics, antivirals and antibiotics. Fungal prophylaxis with voriconazole was preferred because it is not a CYP substrate, but other antifungals were permitted if the patients did not tolerate voriconazole. Use of G-CSF or GM-CSF was permitted for patients that developed infections while neutropenic if the clinic physician felt that the agents were warranted but were discouraged simply for accelerating the recovery of counts. Use of erythropoietin for the treatment of anemia was permissible with the selection of the agent at the discretion of the treating physician. Ubiquinone (coenzyme Q10) supplementation (60 mg, 4 times per day) was planned for the prevention of myopathy if myalgias had occurred.
Clinical correlates
The effect(s) of pravastatin treatments on levels of total, HDL, and LDL cholesterol and serum triglycerides was determined prior to therapy and on day 4 (after 3 days of PV loading, but before chemotherapy administration), on day 8 (at the completion of PV and chemotherapy), and on day 14 (a week after the completion of therapy). Serum pravastatin levels were also measured across the first chemotherapy cycle to assess for potential significant differences in the pravastatin exposures of AML cells in different patients.
Serum pravastatin analysis
Patient serum was separated from whole blood by centrifugation. All working solutions for the preparation of calibration standards were prepared in methanol. Calibration standards were prepared in blank serum ranging from 1 to 40 ng/mL. A 0.5-mL volume of patient sample, quality assurance samples, or standards were acidified with 0.5 mL of 50 mM, pH 3.0, ammonium formate, followed by the addition of internal standard (atorvastatin at 500 ng). The mixtures were then applied to Bond Elute solid-phase C18 extraction cartridge (Varian Associates, Lake Forest, CA), which was preconditioned with 2 mL methanol, 2 mL deionized water, and 2 mL ammonium formate, 50 mM, pH 3.0. The cartridges were washed with 2 mL deionized water. The compounds of interest were eluted into 12 × 75 glass tubes with 2 mL methanol. Samples were evaporated to dryness under air at 40°C and reconstituted with 50 μL methanol containing 1% acetic acid. Reconstituted extract (10 μL) was injected onto the high-performance liquid chromatography (HPLC) column (Zorbax SB C18, 150 mm × 2.1 mm, 5 μm; Agilent, Palo Alto, CA). The mobile phase consisted of 10 mM, pH 3.0, ammonium formate and 50:50 (vol:vol) methanol to acetonitrile. A gradient was used from 30% organic to 90% over 12 minutes with a flow rate of 0.3 mL/minute. Column temperature was 40°C. Pravastatin peak in the column outflow was monitored using a single quadrapole mass spectrometer (Agilent Series 1100SL G1956B MSD [mass selective detector]; Agilent). The mass spectrometer was operated in the electrospray ionization mode with negative polarity. The m/z 423.3 [M − H]− for pravastatin was monitored and the m/z 557.3 [M − H]− was monitored for atorvastatin. Both the HPLC and the MSD were controlled using Agilent Chemstation software (version B.01.01).
Results
A total of 37 patients were treated in 11 cohorts, including 3 patients who had undergone prior matched or mismatched unrelated donor (MUD) allogeneic hematopoietic stem cell transplantation (HCT) and 1 patient who had undergone prior autologous HCT. Several of the newly diagnosed patients had secondary AML, transformed from myelofibrosis, polycythemia vera after autologous transplantation, or Hodgkin disease (HD). The primary refractory cohort included 3 patients transformed from myelodysplastic syndrome to AML after failing to respond to decitabine or clofarabine chemotherapy, and a patient that developed AML after undergoing a MUD HCT for HD that was unresponsive to conventional induction chemotherapy. The distribution of patients by dose cohort, disease status, and response is shown in Table 2. At the 880 mg/m2 dose one patient developed colitis, so when the first patient at the 1080 mg/m2 dose also developed colitis (making this 2 patients in 5), concern that this could be a dose-limiting toxicity led us to increase the cohort size of the 1080 mg/m2 to 6. However, no further cases of colitis/typhlitis were observed. Among those achieving remission, 1, 2, 3, 4, or 5 cycles of consolidation chemotherapy were administered to 15, 1, 4, 1, and 1 patients each, respectively.
Delaying chemotherapy initiation to permit prechemotherapy PV loading is safe
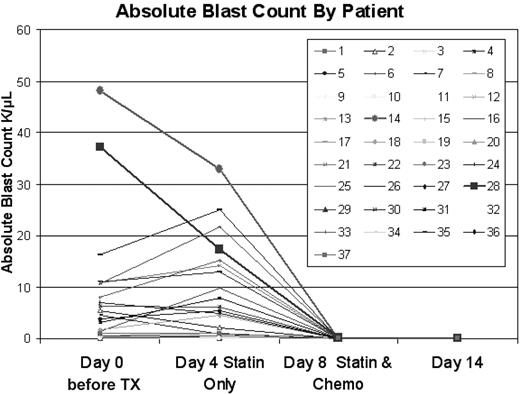
In designing the trial, we were concerned that delaying therapy to load PV prechemotherapy could permit rapid disease progression. Among the 16 patients with a pretreatment white blood cell (WBC) count of greater than 5.0 × 109/L,3 the WBC count increased by greater than 20% by day 4 in 4 patients (5.0→10.3, 6.8→15.8, 18.8→26.7, and 20.8→41.8 × 109/L [5→10.3, 6.8→15.8, 18.8→26.7, and 20.8→41.8 K/μL]) all without clinical consequences. The WBC count decreased by greater than 25% in 5 cases, presumably because of direct cytotoxic effects of PV on AML cells, as previously reported for in vitro treatment with statins.3-7 No patient with a starting WBC count less than 5.0 × 109/L had an increase in WBC count to greater than 5.0 × 109/L by day 4. The absolute blast count rose by greater than 20% between day 0 and 4 in 8 cases [with starting absolute blast count (ABC) of > 0.5 × 109/L, but fell by greater than 25% in 12 cases, as shown in Figure 1. In historical comparison, among 41 patients with identical eligibility to those on this trial (1995-2005) who experienced a similar 3-day delay after initial presentation to await cytogenetic findings before the initiation of therapy with Ida-HDAC, the WBC count and ABC increased by greater than 20% in 6 of 22 with a starting WBC count greater than 5.0 × 109/L and 8 of 23 cases with a starting ABC of greater than 0.5 × 109/L, respectively. Thus, the frequency of a greater than 20% increase, for those starting with a WBC count greater than 5.0 × 109/L or ABC greater than 0.5 × 109/L, was no different among those on this trial compared with the historical controls (P = .87 and .53 for WBC count and ABC comparisons, respectively). The maximum decrease in the ABC in the historical population was 2.12 × 109/L but decreases in the ABC of 2.12, 3.33, 3.67, 15.27, and 19.81 × 109/L were seen in patients on this trial. Among those starting with an ABC greater than 2.0 × 109/L the ABC decreased by greater than 2.0 × 109/L in 5 of 13 trial patients and 1 of 15 historical cases (P = .04) In summary, there were no complications associated with delaying therapy to permit loading of PV, and an apparent antileukemic effect of PV alone was frequently observed.
Effect of pravastatin loading on absolute blast count. Time points shown are pretreatment, on day 4, after 3 days of pravastatin loading, on day 8 at the completion of the PV + Ida-HDAC therapy, and on day 14, a week after the completion of therapy.
Effect of pravastatin loading on absolute blast count. Time points shown are pretreatment, on day 4, after 3 days of pravastatin loading, on day 8 at the completion of the PV + Ida-HDAC therapy, and on day 14, a week after the completion of therapy.
Toxicity
The addition of PV did not result in any identifiable toxicity occurring at a frequency in excess of that expected with the standard Ida-HDAC regimen, and no toxicity occurred at increasing frequency or severity in association with PV dose escalation (Table 3). A detailed breakdown of toxicity by grade and PV dose is available (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). As expected, most patients developed neutropenia, thrombocytopenia, and anemia. Only 1 patient developed grade 3 nausea. As prophylactic antiemetics were used, severe vomiting was uncommon; only 4 patients had grade 3 symptoms and 1 had grade 4 symptoms. Diarrhea of grade 2, 3, or 4 occurred in 19, 6, and 2 cases, respectively, similar to our historical experience with standard Ida-HDAC therapy. A single patient developed grade 3 mucositis. Neutropenic fever was common with 24 grade 3, 1 grade 4, and 3 grade 5 episodes, including 3 cases with typhlitis, 15 with bacteremia/septicemia, and 13 with pneumonia. The toxicity profile during the consolidation cycles was similar to that observed during induction.
There was also no evidence in this study of any toxicity specifically associated with PV administered alone. Myalgias were observed in 6 patients, including 5 grade 1 and 1 grade 2, but all of these began after the administration of PV was completed (beyond day 8) and were not more frequent at higher PV doses. Creatine phosphokinase (CPK) elevation was rare. One patient developed grade 2 CPK elevation by day 4, but his CPK normalized by day 8, despite the continuation of PV. Another patient, with normal CPK on days 0, 4, and 8, not taking prescribed fluconazole, presented on day 17 with a candida fungemia that was ultimately fatal. The CPK was markedly elevated at that time associated with other stigmata of overwhelming infection, suggesting that the CPK rise was due to the infection rather than PV treatment. Hepatic toxicity, when present, was mild and typical of that seen with standard Ida-HDAC. Grade 3 or higher elevations in ALT (2 cases), AST (5 cases), γ-glutamyltransferase (1 case), and bilirubin (9 cases) were observed during the first cycle, but the majority of these were associated with infections later in the cycle and did not develop during the 8 days of PV loading and coadministration. Alkaline phosphatase elevations were not observed. Renal insufficiency developed during the period of PV administration in one patient with a prior history of insulin-dependent diabetes mellitus and hypertension but returned to baseline by day 15. This same patient developed sepsis shortly thereafter with associated renal failure that required dialysis.
The addition of PV to Ida-HDAC did not appear to have any influence on the time to recovery of neutrophils to greater than 0.5 or 1.0 × 109/L or platelets to greater than 20, 50, or 100 × 109/L among patients that achieved CR (Table 2), as compared with our historical experience with newly diagnosed patients treated with Ida-HDAC alone (P = .24 for time to ANC > 1.0 × 109/L and P = .91 for time to platelets > 100 × 109/L). One patient, with a history of myelofibrosis prior to progression to AML, was treated at the 40 mg/day PV dose and developed prolonged aplasia before developing recurrent disease 95 days after the start of PV plus Ida-HDAC therapy. The time to recovery of counts after the consolidation cycles was similar across cycles in with the exception of one patient that was taken off study after demonstrating progressively prolonged recovery after cycles 2 and 3.
Clinical responses
Peripheral blood blasts were cleared by day 8 in 29 of 37 patients and by day 14 in all 37 patients. All but 5 patients cleared their marrow by day 14. Among the newly diagnosed patients, there were 9 CRs and 2 CRps (Table 4) including 6 CRs and 2 CRps among the 10 patients with unfavorable prognosis cytogenetics. Two patients with unfavorable cytogenetics died of complications of infections 3 and 8 weeks after achieving CRp (both with normal marrow differentials and cytogenetics at time of CR). One patient declined further therapy and relapsed at 5 weeks, another was noncompliant with therapy and relapsed at 8 weeks, and 4 others continued on therapy but relapsed at 14, 15, 17, and 51 weeks. One patient with t(6:9) received a transplant in first CR after 2 consolidation cycles. Two others remain in CR at 20 and 57 weeks. The small number of patients treated precludes a formal analysis of whether this combination is beneficial. The rate of CR among patients with intermediate cytogenetics (3 of 5) mirrored our historical experience with Ida-HDAC (72%), whereas the CR rate among patients with unfavorable cytogenetics (8 of 10) was double that of our historical experience with standard Ida-HDAC (40%). In the cohort of 11 patients treated in first relapse, 5 achieved CR and 2 achieved CRp. This 66% CR rate compares favorably with our historical 40% CR experience with standard Ida-HDAC, or 24% CR rate seen in 198 patients treated with anthracycline + HDAC combinations for first relapse at MDACC since 2000.15 Three of these received a transplant in CR2 with remission durations of 7, 10, and 28 weeks. One declined further therapy (she felt that there were too many pills to take) but remains in CR at 16+ weeks, another relapsed at 10 weeks, and 2 remain in CR at 17 and 41 weeks.
Laboratory correlates
Effect of PV on serum cholesterol, HDL and LDL, and triglyceride levels.
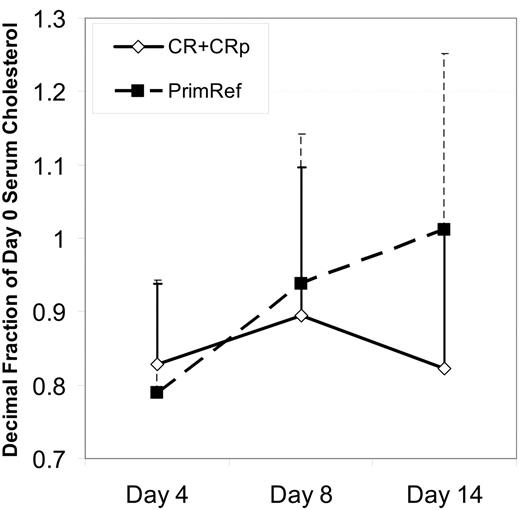
Triglyceride levels decreased by greater than 20% by day 4 and day 8 in 17 and 28 patients, respectively, and increased by greater than 20% by day 4 and day 8 in 4 (only 1 was > normal range) and 0 cases, respectively. The serum LDL/cholesterol level fell between day 0 and day 4 in all but 1 patient during the period of PV loading, but there was not a clear dose response relationship. Between day 4 and 8, during the PV plus Ida-HDAC treatment period, 2 patterns were observed: in one, cholesterol levels stayed near the day 4 level (n = 11 with a > 10% decease at day 4 and no increase between day 4 and day 8); in the second, cholesterol levels showed a “rebound” back toward pretreatment or higher LDL/cholesterol levels (n = 15 with > 10% decrease by day 4 and > 10% increase from day 4 to day 8) despite continued PV administration. The other patients had day 8 levels similar to or less than 10% higher than the day 4 level. HDL/cholesterol levels were generally unaffected (data not shown). Among the 15 newly diagnosed patients, LDL/cholesterol rebounds between day 4 and days 8 and 14 were associated with resistance to therapy, whereas patients achieving CR or CRp tended to have day 8 and day 14 cholesterol levels similar to day 4 levels (P = .06, 2-tailed; P = .03, 1-tailed), as shown in Figure 2. For all patients treated, lack of rebound between day 4 and day 14 was associated with remission attainment for either LDL (13 of 16 without rebound versus 3 of 10 with rebound, P = .003) or total cholesterol (13 of 15 without rebound versus 6 of 14 with rebound, P = .01). The small patient numbers preclude a full statistical analysis of the effect of PV dose and rebound. The patients were divided into 3 groups based on PV dose, no more than 320 mg/day, 480 to 880 mg/day, and at least 1080 mg/day. There was more rebound among the nonresponders than the responders within each dose groups. The degree of rebound was not as great among nonresponders at the highest dose levels.
Rebound in cholesterol level correlates with response to therapy. Mean cholesterol levels ± SD, as a proportion of the day 0 level is shown for 15 newly diagnosed patients, grouped on the basis of response. Time points shown are before treatment, on day 4, after 3 days of pravastatin loading, on day 8 at the completion of the PV + Ida-HDAC therapy, and on day 14, a week after the completion of therapy. All 15 patients were measured at all 3 time points. Patients achieving CR had lower cholesterol levels at day 14 compared with those that were resistant (P = .06, 2-tailed; P = .03, 1-tailed).
Rebound in cholesterol level correlates with response to therapy. Mean cholesterol levels ± SD, as a proportion of the day 0 level is shown for 15 newly diagnosed patients, grouped on the basis of response. Time points shown are before treatment, on day 4, after 3 days of pravastatin loading, on day 8 at the completion of the PV + Ida-HDAC therapy, and on day 14, a week after the completion of therapy. All 15 patients were measured at all 3 time points. Patients achieving CR had lower cholesterol levels at day 14 compared with those that were resistant (P = .06, 2-tailed; P = .03, 1-tailed).
Pravastatin pharmacokinetics.
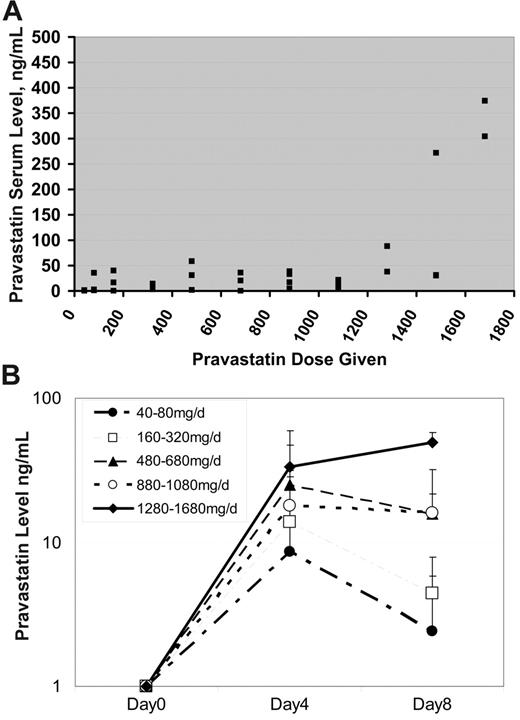
Blood was obtained from subjects to measure the pravastatin trough prior to the fourth dose of pravastatin and starting induction chemotherapy. Pravastatin troughs remained relatively constant until subjects were given a dose at least 1280 mg (Figure 3A). Above this dose, there was a trend toward increased trough levels, although a significant amount of interindividual variation persisted. The overall Pearson correlation coefficient for PV troughs with administered PV doses was 0.58. Serum PV levels at day 8 were similar to levels present on day 4 at doses of 480 mg/day or higher (Figure 3B), suggesting that rebound in cholesterol levels was not the result of decreased serum PV levels.
Serum pravastatin levels. (A) Serum pravastatin troughs (x-axis, ng/mL) were measured in patients prior to receiving their first dose of chemotherapy. As demonstrated in the figure, the pravastatin troughs remained relatively constant until a dose of at least 1280 mg pravastatin was given. Overall, there was an association between increasing pravastatin dose and serum level (Pearson correlation coefficient, R = 0.58), which was primarily due to a relationship between dose and serum levels of at least 1280 mg (Pearson correlation coefficient, R = 0.76). (B) Serum pravastatin trough levels by dose level and day of administration. Doses are grouped as shown in the legend and the mean level + SD at day 4 and 8 is plotted.
Serum pravastatin levels. (A) Serum pravastatin troughs (x-axis, ng/mL) were measured in patients prior to receiving their first dose of chemotherapy. As demonstrated in the figure, the pravastatin troughs remained relatively constant until a dose of at least 1280 mg pravastatin was given. Overall, there was an association between increasing pravastatin dose and serum level (Pearson correlation coefficient, R = 0.58), which was primarily due to a relationship between dose and serum levels of at least 1280 mg (Pearson correlation coefficient, R = 0.76). (B) Serum pravastatin trough levels by dose level and day of administration. Doses are grouped as shown in the legend and the mean level + SD at day 4 and 8 is plotted.
Discussion
We had previously shown that AML cells exposed to cytotoxic agents increase cellular cholesterol content, that preventing this increase sensitizes these cells to chemotherapy, and that this phenomenon is not shared by normal bone marrow cells. The early-phase clinical study reported here represents the first clinical attempt to block these adaptive responses to improve outcomes for patients with AML treated with standard chemotherapy regimens. Our results demonstrate that the addition of PV to the traditional combination chemotherapy regimen of Ida/HDAC is both feasible and safe. By traditional criteria, we were unable to estimate an MTD for PV because we found neither a PV-specific increase in toxicities typically associated with the Ida-HDAC regimen nor any toxicity directly attributable to PV loading periods. Dose escalation was stopped at 1680 mg/day, despite the lack of toxicity, to avoid the myotoxicity observed with higher equivalent lovastatin doses.11 Compliance with therapy did require taking a large number of pills (21 × 80 mg at the highest dose of 1680 mg/day), and one patient cited this reason for withdrawing from therapy (after achieving a CR). However, we felt that further dose escalation would be difficult to achieve because it would require patients to ingest an even higher number of tablets at a single time. Importantly, there was no adverse effect of delaying the initiation of Ida-HDAC therapy for 3 days to permit PV loading. In fact, leukemia blast counts frequently fell during this period, a phenomenon not observed with nearly the same frequency or degree in historical controls. Although this theoretically could reflect an impact of pravastatin on margination of leukemia blasts, it seems more likely that this represents a direct antileukemic effect of pravastatin. Although this was a phase 1 study, and the sample size precluded a formal analysis of response rates to the historical experience, the clinical response rate is encouraging and supports further clinical trials. This was most notable among newly diagnosed patients with unfavorable prognosis cytogenetics in which the observed 80% CR/CRp rate exceeded the historical 40% rate for similar patients treated with the standard Ida-HDAC regimen at MDACC. There was also a higher than expected CR/CRp rate among patients in first relapse (66% versus 40% for Ida-HDAC or 24% for other anthracycline-HDAC regimens).
PV administration led to decreases in total serum cholesterol, serum LDL cholesterol, and triglycerides in nearly all patients between days 0 and 4. Paradoxical increases in one or both of these serum cholesterol levels occurred in many (17 of 37) patients between days 4 and 8, after the PV loading period, despite continued PV administration. Cholesterol measures at day 14 remained lower than baseline (n = 19 of 33), and at least 10% lower than on day 4 (n = 12), after PV administration was discontinued on day 8. Serum cholesterol levels have not been reported to show rebounds between day 4 and 8 of routine statin administration when indicated for hypercholesterolemia.16,17 The cholesterol rebounds observed in our study might reflect (1) unique effects on cholesterol homeostasis of the Ida-HDAC chemotherapy, (2) decreased uptake of cholesterol because of the elimination of the cholesterol-avid leukemic blasts, or (3) the release back into the serum of previously imported cholesterol by the leukemia cells which were killed by the Ida-HDAC chemotherapy. Our previous laboratory studies suggest that the cholesterol levels needed by leukemia cells for survival are quite variable, as is their relative dependence on new cholesterol synthesis versus import. Whether all cells are affected equally or whether the proliferation rate is associated with sensitivity to cholesterol modulation is unknown. The apparent correlation between clinical response and lack of cholesterol level rebound raises the question of whether more complete and prolonged blockade of adaptive cholesterol responses might have a greater therapeutic benefit. The mechanisms responsible for the rebound phenomenon in nonresponders versus responders require further investigation.
Overall, the safety data and clinical response data collected on our phase 1 trial are encouraging and support the design of a phase 3 study to formally test the clinical efficacy of the addition of PV to a standard Ida-HDAC regimen. Because a PV MTD cannot be estimated from our data, other criteria must inform the choice of dose for further study. First, clinical responses and serum cholesterol rebounds were apparently inversely correlated (Figure 2), and rebounds were less frequently noted at higher PV doses. Second, the highest PV serum bioavailability was obtained in patients treated with more than 1280 mg/day PV. Higher bioavailability might also be obtained by dividing the dose and changing to twice daily administration. Consequently we believe that the correlative data suggest that a dose of 1280 mg/day or higher should be selected for the phase 3 study.
Another question is how long the PV loading period should be. Thibault et al11 suggested that maximal effects of statin blockade were seen after 7 days of lovastatin administration. In designing this phase 1 study, we were concerned that delaying therapy for a week to reach cholesterol nadirs might be detrimental to patients with high or rapidly rising blast counts. However, our data clearly demonstrate that no adverse effects were associated with delaying therapy for 3 days to permit PV loading before chemotherapy initiation. Furthermore, in several cases there was evidence of antileukemic efficacy of the PV alone. Likely, a loading period of 4 to 5 days could be tolerated without difficulty.
In summary, the addition of pravastatin to conventional chemotherapy in an attempt to block the adaptive and chemoprotective increase in cellular cholesterol in AML blasts is feasible and apparently safe. Because statin sensitivity has also been observed for other cancers,18-20 this strategy, if effective, may have broad applicability.
Authorship
Contribution: S.M.K. was the study's principal investigator and was responsible for idea creation, patient care, data analysis, and manuscript preparation; D.E.B. was responsible for idea creation, correlative laboratory studies, data analysis, and manuscript preparation; D. Stirewalt and D. Shen were responsible for correlative laboratory studies, data analysis, and manuscript preparation; E.L. was responsible for sample processing for correlative studies, flow cytometry, and data analysis; S.V., Z.E., S.F., J.C., and M.B. were responsible for patient care and manuscript review; C.E.J. was the protocol research nurse and was responsible for data collection and analysis and manuscript review; W.C. was the protocol data manager and was responsible for data collection and data analysis and manuscript review; and E.E. and F.R.A. were responsible for idea creation and manuscript review.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Steven M. Kornblau, Section of Molecular Hematology and Therapy, Unit 448, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030-4009; e-mail: skornbla@mdanderson.org.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) R21 (grant CA-115044). Bristol-Meyers Squibb provided the study drug for the trial.