Abstract
Overexpression of the transcription factor Spi-1/PU.1 in mice leads to acute erythroleukemia characterized by a differentiation block at the proerythroblastic stage. In this study, we made use of a new cellular system allowing us to reach graded expression of Spi-1 in preleukemic cells to dissect mechanisms of Spi-1/PU-1 in erythroleukemogenesis. This system is based on conditional production of 1 or 2 spi-1–interfering RNAs stably inserted into spi-1 transgenic proerythroblasts. We show that Spi-1 knock-down was sufficient to reinstate the erythroid differentiation program. This differentiation process was associated with an exit from the cell cycle. Evidence is provided that in the presence of erythropoietin (Epo), Spi-1 displays an antiapoptotic role that is independent of its function in blocking erythroid differentiation. Apoptosis inhibited by Spi-1 did not involve activation of the Fas/FasL signaling pathway nor a failure to activate Epo receptor (EpoR). Furthermore, we found that reducing the Spi-1 level yields to ERK dephosphorylation and increased phosphorylation of AKT and STAT5, suggesting that Spi-1 may affect major signaling pathways downstream of the EpoR in erythroid cells. These findings reveal 2 distinct roles for Spi-1 during erythroleukemogenesis: Spi-1 blocks the erythroid differentiation program and acts to impair apoptotic death in cooperation with an Epo signaling.
Introduction
The ETS family transcription factor Spi-1/PU.1 plays a crucial role in hematopoiesis by determining cell fate through a temporal and spatial control of its expression and activity. Besides its role in myelopoiesis and B lymphopoiesis,1,2 Spi-1/PU.1 participates to the self-renewal of hematopoietic stem cells.3-6 PU.1 is expressed in erythroid progenitors and is silenced at an early stage of both fetal and adult erythropoiesis.7,8 With regard to its role in erythropoiesis, conflicting hypotheses are reported. Fisher et al9,10 brought argument, suggesting that PU.1 is required for erythropoiesis in adult bone marrow but not in fetal liver. Others showed that PU.1 is involved in the self-renewal of fetal erythroid progenitors,7 whereas its role in erythroid progenitors from the adult seemed to be excluded.5
A dysregulation of PU.1 activity can lead to murine malignant hemopathies. Rosenbauer et al,11 by creating a hypomorphic function, reduced PU.1 expression by 80%, and established an association between PU.1 and the occurrence of acute myeloid leukemia (AML). This report led to the concept that a decrease to a critical threshold level of PU.1 activity, rather than a complete abrogation, contributes to AML pathogenesis. Other publications describing deletion of one PU.1 allele associated with mutations of the other allele leading to reduction in the function of the protein corroborated this paradigm.12,13 However, a recent study in which the function of PU.1 was examined in adult hematopoiesis using conditional gene targeting describes the development of AML in the absence of PU.1 expression.14
In contrast to the myeloid lineage, a high expression of spi-1 is oncogenic in the erythroid lineage.15 In transgenic mice, Spi-1 overexpression leads to the development of severe anemia and hepatosplenomegaly resulting from the proliferation of proerythroblasts blocked in differentiation.16 At the disease onset, survival and growth of spi-1 transgenic proerythroblasts remain under erythropoietin (Epo) control. Later on, spi-1–transgenic proerythroblasts lose their Epo requirement for proliferation and become tumorigenic. This malignant phenotype requires additional genetics events that include activating mutations of Kit, the receptor for the stem cell factor.17
The pathology that develops in spi-1–transgenic mice revealed that high Spi-1 expression is involved in the erythroid differentiation blockage at the basophilic stage. However, the precise mechanisms responsible for this blockage remain to be determined. Notably, it is not known whether other molecular events are required in addition to Spi-1 overexpression to generate undifferentiated preleukemic proerythroblasts. As an approach to understand the mechanism(s) by which Spi-1 blocks erythroid differentiation and participates in leukemogenesis, we developed a cellular model in which Spi-1 expression level was decreased by a conditional production of 1 or 2 spi-1–interfering RNAs stably inserted into preleukemic proerythroblasts derived from spi-1 transgenic mice.
Herein, we report that knock-down of Spi-1–reinstated erythroid differentiation. In addition, we found that Spi-1 protected cells against apoptotic death in the absence of differentiation signals. Moreover, the Fas/FasL pathway controlling apoptosis of immature proerythroblasts in a context of differentiation was not implicated in cellular death associated with Spi-1 depletion. These results argue for an antiapoptotic role of Spi-1 distinct from its function in the differentiation blockage. Our data also suggest that the continuous expansion of proerythroblasts may be due to both the antiapoptotic function and the ability of Spi-1 to push cells into cycle. Spi-1 knock-down was not correlated with an inhibition of Epo receptor (EpoR) activation but modulated downstream signaling pathways known to be involved in proliferation, survival, and differentiation of erythroid cells. Further investigations on the multifaceted function of Spi-1 in preleukemic cells should be facilitated by our cellular model.
Materials and methods
Cell line
Spi-1 transgenic proerythroblasts (663) were cultured in α-modified Dulbecco medium (Invitrogen, Cergy Pontoise, France) supplemented with 5% fetal calf serum (FCS) and 1 U/mL Epo. Doxycycline (dox; 100 ng/mL) was added to the culture medium when indicated. For growth with stem cell factor (SCF), Epo was washed out from the cells and replaced by SCF (100 ng/mL).
Plasmid constructs and generation of proerythroblasts producing anti–spi-1 siRNA
pcDNA6/TR-EF-1α, containing the tetracycline repressor (TetR) and the blasticidin resistance genes, was constructed by replacing the CMV promoter of pcDNA6/TR (Invitrogen) by the human EF-1α promoter excised from pEF-BOS. pmTER was derived from pTER, a kind gift of Dr Clevers,18 by replacing the human H1 promoter by its murine orthologue. The murine H1 promoter was obtained by polymerase chain reaction (PCR) amplification from the 10G6 plasmid provided by Dr de Murcia. The vector pmTER-siA contained an oligonucleotide allowing expression of the siRNA-A (underlined), 5′-GATCCCCGTCCAATGCATGACTACTTTCAAGAGAAGTAGTCATGCATTGGACGTTTTTGGAAA-3′, and the zeocyne resistance gene. The vector pGJ10-siB contained an oligonucleotide allowing expression of the siRNA-B (underlined), 5′-GATCCCCCCGGATGTGCTTCCCTTATTCAAGAGATAAGGGAAGCACATCCGGGTTTTTGGAAA-3′, and the geneticin resistance gene. pGJ10 used to introduce the oligonucleotide siRNA-B was a kind gift from Dr Morlé. 663 cells producing the TetR were obtained by nucleofection of pcDNA6/TR-EF-1α using the Amaxa nucleofector and G16 program, selected, and cloned in the presence of blasticidine (25 μg/mL). These cells are called C11. The pmTER-siA vector was introduced into these cells by nucleofection, and a clonal selection was performed in the presence of zeocyne (300 ng/mL). Clones with the lowest expression of Spi-1 in the presence of dox, as verified by Western blotting, were isolated. Then, the pGJ10-siB vector was introduced into clones producing the siRNA-A. Cells were cloned and selected in the presence of G418 (800 ng/mL). Three types of controls were used; the Tet repressor producers C11, the C11 with pmTER, and the C11pmTER with pGJ10. The 3 cellular controls were used in all experiments. However, because they behaved similarly in the presence of dox, only the results obtained with one representative control (designated C) are shown.
Proliferation, viability, clonogenicity, and differentiation assays
For proliferation assays and measurement of viability, cells were seeded at 104 cells/mL in liquid medium. Numbers of living cells and cell size were measured by trypan blue exclusion using a Vi-Cell analyzer (Beckman Coulter, Villepinte, France). For clonogenic assays, 500 cells were seeded in 1 mL methylcellulose medium (1%) supplemented with FCS (5%) and Epo (1 U/mL), and colonies were counted on day 6. For erythroid differentiation, cell hemoglobinization was detected by the benzidine coloration test (0.2%).
Apoptosis and cell cycle
Cells (104 cells/mL) were plated in medium containing dox (100 ng/mL) or without. Hoechst staining, caspase-3, and PARP cleavage were studied as previously described.19,20 Sub-G1 fraction and cell-cycle distribution of EtOH-fixed cells stained with propidium iodide were determined by flow cytometry analyses using FACSCalibur (Becton Dickinson, Meylan, France). Data were analyzed using CellQuest Pro (Becton Dickinson) and ModfitLT (Verity, Topsham, ME) software.
Antibody staining and flow cytometry
Cells were immunostained for 30 minutes at 4°C in PBS/0.5%BSA in the presence of hamster IgG (BD Biosciences) or 2 μg/mL PE–anti-Fas antibodies (BD Biosciences, le Pont de Claix, France). Cells were analyzed on a FACSCalibur, and data were analyzed with a FlowJo software (TreeStar, San Carlos, CA).
Immunoprecipitation, immunoblotting, and antibodies
Analysis of cell extracts by Western blot and antibodies against Spi-1, ERK, and AKT were as described.16,17 For EpoR immunoprecipitation, cells were deprived in serum and Epo for 4 hours at 37°C and then stimulated for 5 minutes with 5% FCS and 10 U/mL Epo. Immunoprecipitation was performed as described17 with EpoR antibody (N-20; Santa Cruz Biotechnology, Le Perray en Yvelines, France). The anti–phospho-STAT5 Tyr694 (9351) and anti-STAT5 (N-20) were purchased from Cell Signaling Technology (Ozyme, France) and Santa Cruz Biotechnology, respectively.
Epo receptor measurement
The number of EpoRs at the cell surface was determined 30 hours after dox treatment by incubating cells with a saturating concentration (4 U/mL) of iodinated Epo as previously described.21 Unspecific binding of 125I-Epo was determined by using 500 nM unlabeled Epo and was subtracted from total binding. 125I-Epo–specific activity was used to calculate the number of EpoRs per cell. Experiments were performed in agreement with relevant institutional guidelines from the Institut National de la Santé et de la Recherche Médicale.
Results
Conditional extinction of Spi-1 in transgenic proerythroblasts
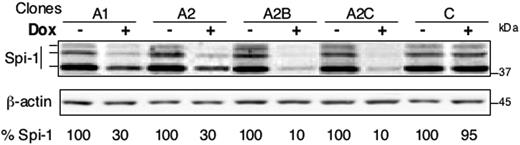
Because the level of Spi-1 expression is a determinant for the development of normal and malignant hematopoietic tissues, we reasoned that generating proerythroblasts with different expression levels of Spi-1 might be informative in understanding Spi-1 functions in erythroleukemic transformation. To this aim, we used the Tet system in which the tetracycline repressor (TetR) would block the transcription of a short hairpin RNA (shRNA) driven from the H1 gene promoter,18 whereas the addition of doxycycline (dox) would activate the shRNA production. The novelty of the cellular system developed here stands from the introduction of either 1 or 2 distinct siRNA against a unique mRNA. Three types of cells were generated derived from preleukemic spi-1 transgenic proerythroblasts (663): the controls that express only the TetR (C); the cells expressing the TetR and the siRNA-A in the presence of dox (clones derived from C and designated A1 and A2); and the cells expressing the TetR, the siRNA-A, and the siRNA-B in the presence of dox (clones derived from A2 and designated A2B and A2C). At each step, independent clones were retained on the basis of the lowest Spi-1 expression level in the presence of dox. As shown in Figure 1, treatment of cells with dox decreased the level of the Spi-1 protein compared with untreated cells up to 70% when only siRNA-A was expressed (clone A1 and A2) and to 90% when both siRNA-A and siRNA-B were produced (clone A2B and A2C). The decrease in Spi-1 was reproducible and maximal from 24 hours to day 5. Dox did not affect Spi-1 expression in the absence of siRNA production as seen with the control (C). Because transfection of siRNA may activate an interferon-like response, we monitored the expression of the interferon-induced oligoadenylate synthase 1 (OAS-1) mRNA by reverse transcriptase (RT)–PCR. No modification could be detected in the presence of dox (data not shown). We also controlled that each of the cellular clones remained dependent on Epo for survival (data not shown). Thus, the strategy with cells expressing a single or 2 spi-1 siRNAs gave access to proerythroblasts with different levels of Spi-1 expression.
Knock-down of Spi-1/PU.1 in spi-1 transgenic proerythroblasts. siRNA expression was induced by a 24-hour treatment with 100 ng/mL dox (+) in cells expressing one siRNA (A1 and A2) or 2 siRNA (A2B and A2C) or the empty vectors (C). Untreated cells were grown without dox (−). Spi-1 expression was analyzed by Western blotting. Three bands corresponding to Spi-1 protein were detected. Equal loading was verified by β-actin. The percentage of Spi-1 expression was calculated relatively to untreated cells by taking into account the 3 bands. C stands for control cells.
Knock-down of Spi-1/PU.1 in spi-1 transgenic proerythroblasts. siRNA expression was induced by a 24-hour treatment with 100 ng/mL dox (+) in cells expressing one siRNA (A1 and A2) or 2 siRNA (A2B and A2C) or the empty vectors (C). Untreated cells were grown without dox (−). Spi-1 expression was analyzed by Western blotting. Three bands corresponding to Spi-1 protein were detected. Equal loading was verified by β-actin. The percentage of Spi-1 expression was calculated relatively to untreated cells by taking into account the 3 bands. C stands for control cells.
Spi-1 is required for expansion of preleukemic proerythroblasts
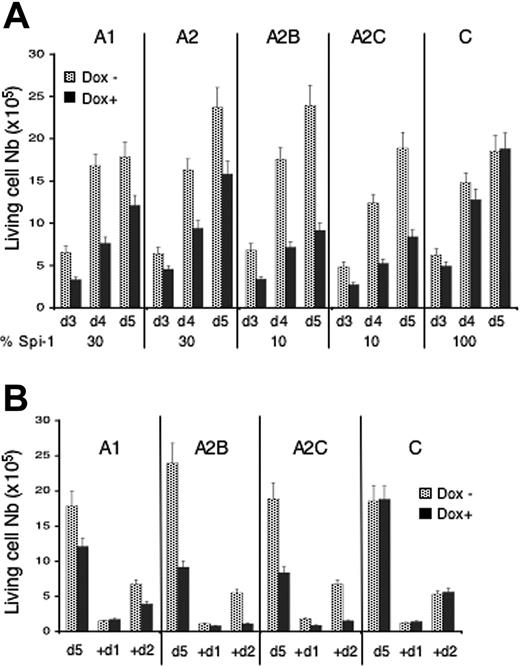
The consequences of Spi-1 knock-down on proliferation were first investigated through clonogenicity in semisolid medium (Table 1). The clonogenic activity (Table 1) of spi-1–interfered cells (dox+) was reduced compared with spi-1–overexpressing cells (dox−). In contrast, dox treatment had no effect on the control cells (C). Cell amplification was then examined in suspension culture as a function of time of dox treatment (Figure 2). Dox had no effect on the proliferation of control cells (Figure 2A). Clones A1 and A2 displaying a 70% decrease in Spi-1 in the presence of dox exhibited a delay in proliferation. Strikingly, cell proliferation was even more reduced in clones A2B and A2C which expressed around 10% of Spi-1 in the presence of dox. When the dox treatment was prolonged by diluting the living cells at day 5 (Figure 2B), the A1 cell population continued to expand at a reduced rate, whereas A2B and A2C cells ultimately arrested their growth. Interestingly, colonies seen in the clonogenic assay derived from 2 siRNAs-expressing cells in the presence of dox (A2B-A2C) displayed a smaller size than those derived from 1 siRNAs-expressing cells (A1, A2) (Figure 3A). The different expansion abilities of cells expressing 1 or 2 siRNAs are most probably responsible for the different sizes of colonies.
Knock-down of Spi-1 arrests proerythroblasts expansion. (A) Cells were seeded at 104 cells/mL in liquid medium with dox (100 ng/mL) or without. Living cells were counted at days (d) indicated using trypan blue exclusion test. Data are mean ± SD of at least 8 experiments. The percentage of Spi-1 expression relative to untreated cells is indicated under the histograms. (B) Five days after dox treatment, cells were diluted to 5 × 104 cell/mL in medium with or without dox, and living cells were counted 24 hours (+d1) and 48 hours (+d2) later by trypan blue exclusion test. Data are the mean ± SD of 3 experiments.
Knock-down of Spi-1 arrests proerythroblasts expansion. (A) Cells were seeded at 104 cells/mL in liquid medium with dox (100 ng/mL) or without. Living cells were counted at days (d) indicated using trypan blue exclusion test. Data are mean ± SD of at least 8 experiments. The percentage of Spi-1 expression relative to untreated cells is indicated under the histograms. (B) Five days after dox treatment, cells were diluted to 5 × 104 cell/mL in medium with or without dox, and living cells were counted 24 hours (+d1) and 48 hours (+d2) later by trypan blue exclusion test. Data are the mean ± SD of 3 experiments.
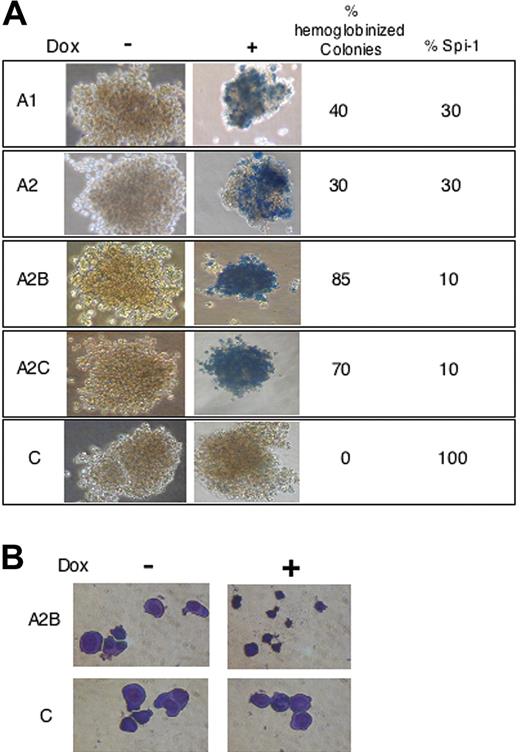
Knock-down of Spi-1 reinitiate erythroid differentiation. (A) Benzidine staining of representative colonies grown in methylcellulose with or without dox for 6 days. The percentage of Spi-1 expression and the percentage of hemoglobinized colonies with dox relative to untreated cells are given. Magnification, × 40. Cells were observed using a Nikon Eclipse TE300 microscope (Nikon, Champigny sur Marne, France) with a 20×/0.45 NA objective lens. Images were acquired with Nikon Coolpix 950 and processed using Adobe Photoshop (Adobe Systems, San Jose, CA) (B) Cytospin smears of A2B and control cells grown during 6 days in methylcellulose with or without dox and stained with May-Grünwald-Giemsa. Images were acquired as in panel A, except a 40×/0.6 NA objective lens was used.
Knock-down of Spi-1 reinitiate erythroid differentiation. (A) Benzidine staining of representative colonies grown in methylcellulose with or without dox for 6 days. The percentage of Spi-1 expression and the percentage of hemoglobinized colonies with dox relative to untreated cells are given. Magnification, × 40. Cells were observed using a Nikon Eclipse TE300 microscope (Nikon, Champigny sur Marne, France) with a 20×/0.45 NA objective lens. Images were acquired with Nikon Coolpix 950 and processed using Adobe Photoshop (Adobe Systems, San Jose, CA) (B) Cytospin smears of A2B and control cells grown during 6 days in methylcellulose with or without dox and stained with May-Grünwald-Giemsa. Images were acquired as in panel A, except a 40×/0.6 NA objective lens was used.
In conclusion, according to the residual Spi-1 expression level, proerythroblasts exhibit either a growth delay or a growth arrest.
Spi-1 reduction is sufficient to reinstate erythroid differentiation program
To determine whether the growth arrest of preleukemic proerythroblasts was associated with a terminal differentiation, hemoglobinization was analyzed in colonies grown in methylcellulose with or without dox. After 6 days of culture with dox, both the numbers of hemoglobinized colonies and hemoglobinized cells inside a colony were increased in correlation with Spi-1 reduction (Figure 3A). When Spi-1 expression was reduced to 30%, around 40% of colonies were hemoglobinized, whereas most of the colonies were hemoglobinized when residual Spi-1 expression was around 10%. In addition to resembling morphologically differentiating erythroid cells, the cell size was greatly reduced in the presence of dox (Figure 3B). Thus, the decrease in Spi-1 expression inhibits cellular expansion and induces cell size reduction and hemoglobinization, demonstrating that inhibition of Spi-1 expression is sufficient to resume the erythroid differentiation program.
Apoptosis and cell-cycle modification
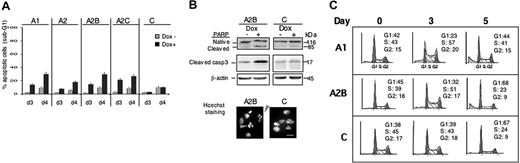
To further investigate the proliferative advantage provided by Spi-1 overexpression in preleukemic proerythroblasts, we examined apoptosis and cell cycle. Apoptosis was analyzed using morphologic and molecular parameters. First, the rate of apoptotic cells was evaluated by determining the percentage of cells with hypodiploid DNA (sub-G1) by flow cytometry analysis. As compared with their respective untreated cells (Figure 4A), the percentage of cells in the sub-G1 fraction was increased when Spi-1 was decreased (dox+). The maximum of apoptotic cells was seen after 4 days of treatment and was of the same order of magnitude for all clones (from 18% to 29%). Other parameters that distinguish apoptotic cells were also examined. Typical figures with condensed and fragmented DNA were seen in the presence of dox after Hoechst staining, as illustrated for A2B cells (Figure 4B). Moreover, PARP was cleaved and caspase 3 was activated as deduced from the increase in cleaved caspase 3 with dox (Figure 4B). None of these features were seen in control cells treated with dox. Notably, no correlation between the level of apoptosis and the extent of Spi-1 decrease was observed, suggesting that a high expression of Spi-1 may be necessary to maintain cells alive. Therefore, when Spi-1 was not sufficiently expressed, a part of the preleukemic proerythroblasts died by apoptosis, whereas a large part of cells survived and differentiated.
Apoptosis and cell cycle are dependent on the Spi-1 expression level. (A) The cells were cultured in the presence (Dox+) or absence of dox (Dox−) for 3 and 4 days (d3, d4). The cells were then fixed and stained with propidium iodide, and the sub-G1 part of the cell population was assessed using a FACS. The figure illustrates the mean ± SD of 4 experiments (B, top) The cleavage of PARP and caspase 3 was detected by Western blot using antibodies against PARP and cleaved caspase 3 in A2B and C cells grown with dox (100 ng/mL) or without dox for 3 days. (Bottom) Representative image of A2B and control cells treated with dox for 3 days and stained by Hoechst. Gray arrows designate apoptotic cells. Scale bar equals 13 μm. (C) Cell-cycle analysis by quantitative flow cytometry. The cells were fixed and stained with propidium iodide after different times in culture with dox, and the DNA content was assessed using a FACS. Each phase was expressed as the percentage of cells in cycle after exclusion of the sub-G1 population. Debris derived from dead cells are shown in gray on the left part of G1 phase. Analysis was performed at least 4 times with similar results.
Apoptosis and cell cycle are dependent on the Spi-1 expression level. (A) The cells were cultured in the presence (Dox+) or absence of dox (Dox−) for 3 and 4 days (d3, d4). The cells were then fixed and stained with propidium iodide, and the sub-G1 part of the cell population was assessed using a FACS. The figure illustrates the mean ± SD of 4 experiments (B, top) The cleavage of PARP and caspase 3 was detected by Western blot using antibodies against PARP and cleaved caspase 3 in A2B and C cells grown with dox (100 ng/mL) or without dox for 3 days. (Bottom) Representative image of A2B and control cells treated with dox for 3 days and stained by Hoechst. Gray arrows designate apoptotic cells. Scale bar equals 13 μm. (C) Cell-cycle analysis by quantitative flow cytometry. The cells were fixed and stained with propidium iodide after different times in culture with dox, and the DNA content was assessed using a FACS. Each phase was expressed as the percentage of cells in cycle after exclusion of the sub-G1 population. Debris derived from dead cells are shown in gray on the left part of G1 phase. Analysis was performed at least 4 times with similar results.
Modification of the cell cycle accordingly to the decrease in Spi-1 level was investigated by fluorescence-activated cell sorting (FACS) analysis of DNA content. Results are shown for clones A1, A2B, and C that are representative for cells expressing 1, 2, or no spi-1 siRNA, respectively (Figure 4C). In the presence of high Spi-1 levels (Control with dox, C), no substantial change in the cell cycle was detected between day 0 and day 3. However, accumulation of cells into G0/G1 phase occurred at day 5 when a plateau of growth was reached (around 2 × 106cells/mL; Figure 2A), suggesting a transitory pause in the cell cycle because of the saturation of the cell culture. In contrast, when A1 and A2B cells were treated with dox, an accumulation of cells into S phase was first seen on day 3 and thereafter into G0/G1 phase on day 5 (Figure 4C). Cells into S phase were still synthesizing DNA as verified by BrdU incorporation (data not shown). The meaning of cell accumulation in S phase is under investigation. Increase of the proportion of Spi-1 knocked-down cells into G0/G1 from day 3 to day 5 probably reflects an exit from cell cycle as it started at day 4 before cell density had reached saturation (data not shown). Consistently, if A1, A2B, and C cells were diluted on day 5 to allow renewal conditions, the control cells cultured in the presence of dox reentered into the cell cycle, whereas A1 cells proliferate at a reduced rate and A2B proliferation was completely arrested (Figure 2B). Interestingly, A2B cells, expressing 10% of Spi-1 with dox, accumulated into G0/G1 more rapidly (+36% of cells between day 3 and 5) than did A1 cells expressing 30% of Spi-1 (+21% of cells between day 3 and 5), indicating that the propensity to exit from the cell cycle was related to the Spi-1 expression level.
In conclusion, the arrest of proliferation in a context of low Spi-1 expression was due to both apoptotic death and cell-cycle arrest. Furthermore, the differences in proliferative response as a function of Spi-1 residual expression most probably reflect the differences in cell-cycle arrest because apoptosis was not significantly different between the clones expressing 1 or 2 spi-1 siRNA. From these results, we hypothesized that a high Spi-1 expression into proerythroblasts would promote renewal, on the one hand by avoiding an exit from the cell cycle and on the other hand by permitting survival.
Spi-1 exhibits an antiapoptotic role per se
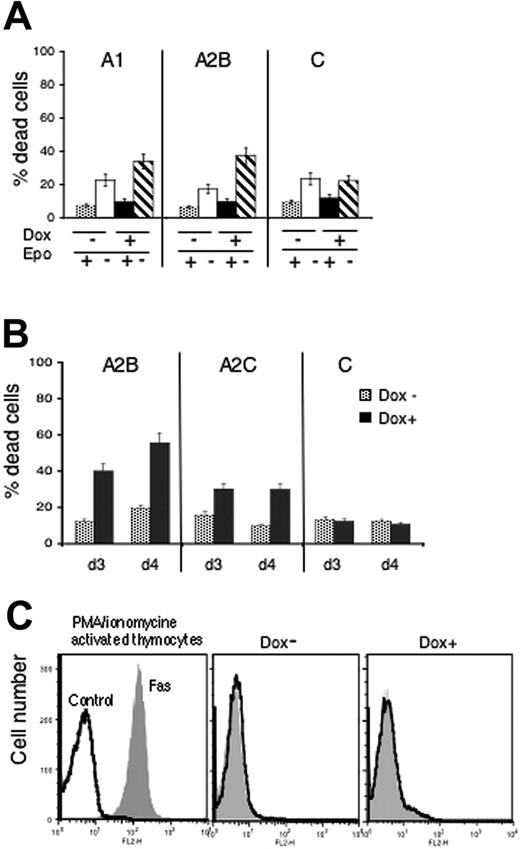
Evaluation of apoptosis provided evidence that Spi-1 overexpression promotes survival of preleukemic proerythroblasts. To understand whether apoptosis was a consequence of a stimulation to differentiation or whether Spi-1 displays an antiapoptotic activity per se, we first investigated whether Spi-1 protects against apoptosis due to Epo withdrawal, a context in which the process of erythroid differentiation is not operative. A1 and A2B cells pretreated for 18 hours with dox or untreated were Epo starved during 12 hours (representing 30 hours of dox treatment). Mortality due to Spi-1 decrease (Epo+ dox+) started 30 hours after dox treatment and cells died 12 hours after Epo withdrawal (Epo− dox−) (Figure 5A). However, the incidence of Epo withdrawal on cell death was increased when Spi-1 expression was reduced (compare the white and hatched bars). Spi-1 thus was able to delay the apoptotic death of proerythroblasts when Epo signaling was not functional, indicating that Spi-1 and Epo signaling may have additive effects on proerythroblast survival. Then, we tested whether Spi-1 was necessary to maintain cell survival when cell expansion was driven by another growth factor than Epo. Stem cell factor (SCF) is able to sustain the growth of transgenic proerythroblasts, although with a poor efficiency.17 SCF is known to be essential for proliferation and survival of immature erythroid cells but is not associated with their differentiation.22-25 Indeed, transgenic proerythroblasts with reduced Spi-1 expression did not differentiate in the presence of SCF (data not shown). However, as shown in Figure 5B, mortality of cells cultured with SCF was increased when Spi-1 was reduced. Altogether, these data indicate that Spi-1 overexpression protects preleukemic cells against apoptosis in response to survival factors such as Epo and SCF.
Antiapoptotic role of Spi-1 in preleukemic proerythroblasts. (A) Correlation between cytotoxic effect of Epo withdrawal and Spi-1 reduction was measured by the trypan blue exclusion test. Cells were grown for 18 hours with dox or without and then seeded for 12 hours (30-hour dox treatment) with or without Epo before being counted. (B) Cytotoxic effects of Spi-1 reduction on cells cultured in the presence of SCF. Cells were incubated with dox (100 ng/mL) for 3 or 4 days in the presence of SCF (100 ng/mL), and the percentage of dead cells was measured by trypan blue exclusion test. Data are the mean ± SD of 3 experiments. (C) Fas/FasL signaling does not participate to the cellular death stimulated by Spi-1 depletion. Cells were grown for 3 days with dox (100 ng/mL) or without. They were labeled with PE-conjugated Fas antibody and examined by flow cytometry. Control cells were labeled with a PE-conjugated isotype control. A representative experiment of 3 performed with either A2B or A2C cells is shown. (Left) Thymocytes stimulated with PMA/ionomycine for 12 hours and labeled with control or PE–anti-Fas; middle panel (dox−) A2B cells without dox; right panel (dox+) A2B cells with dox for 3 days.
Antiapoptotic role of Spi-1 in preleukemic proerythroblasts. (A) Correlation between cytotoxic effect of Epo withdrawal and Spi-1 reduction was measured by the trypan blue exclusion test. Cells were grown for 18 hours with dox or without and then seeded for 12 hours (30-hour dox treatment) with or without Epo before being counted. (B) Cytotoxic effects of Spi-1 reduction on cells cultured in the presence of SCF. Cells were incubated with dox (100 ng/mL) for 3 or 4 days in the presence of SCF (100 ng/mL), and the percentage of dead cells was measured by trypan blue exclusion test. Data are the mean ± SD of 3 experiments. (C) Fas/FasL signaling does not participate to the cellular death stimulated by Spi-1 depletion. Cells were grown for 3 days with dox (100 ng/mL) or without. They were labeled with PE-conjugated Fas antibody and examined by flow cytometry. Control cells were labeled with a PE-conjugated isotype control. A representative experiment of 3 performed with either A2B or A2C cells is shown. (Left) Thymocytes stimulated with PMA/ionomycine for 12 hours and labeled with control or PE–anti-Fas; middle panel (dox−) A2B cells without dox; right panel (dox+) A2B cells with dox for 3 days.
Fas-mediated apoptosis contributes to the negative regulation of physiologic erythropoiesis either through an effect of mature cells, expressing Fas ligand (FasL), on immature erythroblasts,26 or through an interaction between immature erythroblasts coexpressing Fas receptor (Fas) and FasL.27 In both cases, apoptosis of early erythroblasts through Fas signaling is inhibited by high levels of Epo. Thus, we examined the expression of Fas on the membrane of the proerythroblasts overexpressing or not Spi-1. Proerythroblasts cultured with or without dox for 3 days were labeled using Fas fluorescent antibodies and analyzed by flow cytometry (Figure 5C). Murine thymocytes isolated from fresh spleen and activated using PMA and ionomycin were used as a positive control of Fas expression. Spi-1–preleukemic cells did not express Fas and decrease of Spi-1 expression did not initiate Fas expression. These experiments indicate that a Fas-negative feedback related to erythroid maturation does not participate to early apoptosis induced by Spi-1 depletion in the preleukemic proerythroblasts.
Altogether, these findings, showing that Spi-1 protects against apoptosis independently of the differentiation program, suggest that in parallel to block erythroid differentiation Spi-1 exerts an antiapoptotic function per se in the preleukemic proerythroblasts.
Spi-1 does not inhibit EpoR activation but affects mediators of the Epo/EpoR signaling cascade
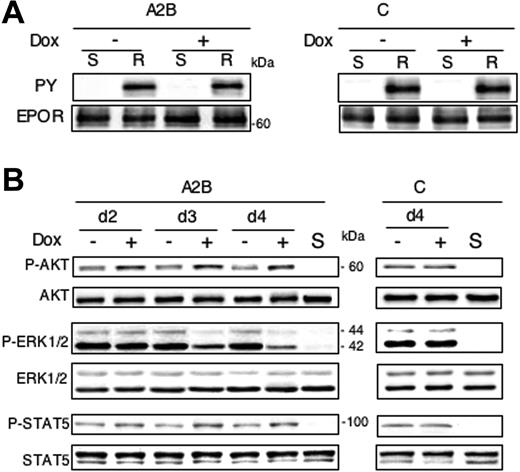
All experiments described were performed in the presence of Epo because the different clones were Epo dependent for survival. Therefore, we checked the possibility that the expansion default of preleukemic proerythroblasts with reduced expression of Spi-1 might result from abnormalities in Epo signaling. The number of functional EpoR at the cell surface was investigated as a function of Spi-1 expression. We found that EpoR numbers were not significantly modified after a 30-hour dox treatment (Table 2), a time when cells started to dye. High apoptosis impeded the accurate determination of the number of EpoRs at a later time. Epo binding to its cognate receptor activates the receptor-associated Janus kinase-2 (Jak2) tyrosine kinase. Thereby, the EpoR is tyrosine phosphorylated and initiates the activation of signaling pathways, including Jak2/STAT5, PI3K/AKT,28 and mitogen-activated protein kinases (MAPKs).29,30 We then examined whether Spi-1 down-regulation was associated with a default in EpoR activation. Tyrosine phosphorylation of EpoR was analyzed by Western blotting in conditions in which EpoR was reactivated by 10 U/mL Epo with or without dox. A tyrosine-phosphorylated form of EpoR was detected at a comparable level in immunoprecipitates from cells grown in the absence of dox (Figure 6A) and in cells with dox. This suggested that a decrease in Spi-1 level does not impede the ability of the cells to phosphorylate EpoR. Next, we examined the EpoR downstream signaling pathways. Activation of MAPK, PI3K, and Jak2/STAT5 pathways was followed by analyses of ERK1/2, AKT/PKB, and STAT5 phosphorylation, respectively, on Western blots using appropriate antibodies (Figure 6B). Protein extracts were prepared from cells cultured with serum and Epo in the absence or presence of dox for 2, 3, and 4 days. Results are only shown for A2B cells because data obtained with the different clones were similar (Figure 6B). As expected from the Epo dependence of proerythroblasts, when EpoR was not activated (column S), each of the downstream effectors was at rest. In dox-treated A2B cells, ERK1/2 phosphorylation started to decrease on day 3 and was detectable at a low level on day 4, whereas no change in total ERK1/2 expression level was observed (Figure 6B). Conversely, a significant increase of AKT phosphorylation was found after 2 days of dox treatment as compared with cells grown without dox. This increase remained constant for up to 4 days (Figure 6B). Finally, modulation of STAT5 phosphorylation associated with a Spi-1 decrease (dox+) was qualitatively similar to that observed for AKT, that is, an increase of phosphorylation as early as 2 days after treatment (Figure 6B). Such modification in the level of ERK1/2, AKT, and STAT5 phosphorylation by dox was not seen in control cells.
Inhibition of EpoR activation is not responsible for changes in proliferation and survival associated with the release of erythroid differentiation blockage. (A) Cells were grown either in the presence or absence of dox for 3 days (100 ng/mL) and then deprived in both serum and Epo for 4 hours (S) before being stimulated 5 minutes with 10 U/mL Epo and 5% serum (R). Cell lysates were subjected to immunoprecipitation with anti-EpoR antibodies and analyzed by Western blotting using antiphosphotyrosine antibodies (PY) followed by anti-EpoR antibodies (EPOR). A representative image for A2B and control cells is shown. (B) Western blot analysis of STAT5, ERK, and AKT phosphorylation as readout of signaling pathways activated downstream of the EpoR in cells treated for 2, 3, or 4 days with or without dox (100 ng/mL) in the presence of Epo (1 U/mL). S stands for starved cells that have been deprived from serum and Epo during 4 hours. Total protein extracts were subjected to immunoblotting with anti–phospho-AKT (specific for phosphorylated serine 473) or anti-AKT antibodies, with anti–phospho-ERK1/2 or anti-ERK1/2 and with anti–phospho-STAT5 (recognizing tyrosine 694–phosphorylated form) or anti-STAT5. For each cell type, analysis was performed at least 3 times with similar results. A representative image for A2B and control cells is shown.
Inhibition of EpoR activation is not responsible for changes in proliferation and survival associated with the release of erythroid differentiation blockage. (A) Cells were grown either in the presence or absence of dox for 3 days (100 ng/mL) and then deprived in both serum and Epo for 4 hours (S) before being stimulated 5 minutes with 10 U/mL Epo and 5% serum (R). Cell lysates were subjected to immunoprecipitation with anti-EpoR antibodies and analyzed by Western blotting using antiphosphotyrosine antibodies (PY) followed by anti-EpoR antibodies (EPOR). A representative image for A2B and control cells is shown. (B) Western blot analysis of STAT5, ERK, and AKT phosphorylation as readout of signaling pathways activated downstream of the EpoR in cells treated for 2, 3, or 4 days with or without dox (100 ng/mL) in the presence of Epo (1 U/mL). S stands for starved cells that have been deprived from serum and Epo during 4 hours. Total protein extracts were subjected to immunoblotting with anti–phospho-AKT (specific for phosphorylated serine 473) or anti-AKT antibodies, with anti–phospho-ERK1/2 or anti-ERK1/2 and with anti–phospho-STAT5 (recognizing tyrosine 694–phosphorylated form) or anti-STAT5. For each cell type, analysis was performed at least 3 times with similar results. A representative image for A2B and control cells is shown.
Together, the data show that the number of functional EpoR at the cell surface and EpoR activation are not reduced by a decrease in Spi-1 expression. Moreover, although ERK1/2 phosphorylation is reduced, STAT5 and AKT phosphorylation are augmented when Spi-1 was knocked down. All these results suggest that apoptotic death and inhibition of proliferation are not due to an impediment of the EpoR activation.
Discussion
In this study, we describe a new experimental system that allowed a graded expression of Spi-1 protein in preleukemic cells using a conditional expression of 1 or 2 hits of stably integrated-interfering RNAs. We determined that a decrease in Spi-1 expression was sufficient to resume the erythroid differentiation process. One major finding was the demonstration that, in addition to blocking erythroid differentiation, Spi-1 participates in the development of erythroleukemia also by protecting erythroid cells against apoptotic death. We found that apoptosis and/or proliferative defects induced by a decrease in Spi-1 level did not involve the Fas/Fas-L signaling or a failure in EpoR activation. Finally, we investigated the effect of decreasing Spi-1 expression level on downstream effectors of the EpoR activation, and our data show that STAT5 and AKT activations were up-regulated, whereas ERK1/2 phosphorylation was reduced when Spi-1 knock-down allowed erythroid differentiation.
The gene silencing method used with 2 conditional vectors producing distinct siRNAs into preleukemic cells, allowed a fine modulation of Spi-1 expression levels. With this approach, we demonstrate that the effects of Spi-1 on preleukemic proerythroblasts were directly dependent on its concentration. A high Spi-1 expression level, as observed in transgenic cells (defined as 100%), completely blocks erythroid differentiation. An intermediate Spi-1 expression (30%) is linked to an accumulation of cells in S phase of the cell cycle, a G0/G1 arrest, and differentiation of a part of the cell population. A very low Spi-1 expression (10%) provokes a transient increase in the number of cells in S phase, a strong G0/G1 arrest, and differentiation of a large proportion of cells. We believe the experimental procedure described here may be applicable for knocking-down genes highly expressed, as is spi-1 oncogene in the preleukemic proerythroblasts.
Previous studies have indicated that overexpressing Spi-1 in murine and chicken erythroid cells blocked differentiation,15,31-34 and attempts to inhibit its expression resulted in a partial reduction of proliferation and/or differentiation block.35,36 However, experiments described in these reports used Spi-1 overexpression in erythroleukemic cells harboring a deregulation in Epo/EpoR signaling,34,37,38 thus impeding a clear definition of the contribution of Spi-1 in the differentiation blockage. In contrast, preleukemic proerythroblasts derived from spi-1 transgenic mice as used in the present study are dependent on Epo for their survival and growth, and Epo signaling is normal in that cells.16,39 We show that reduction in Spi-1 expression was sufficient to release the arrest in differentiation, establishing that Spi-1 overexpression is the sole causal event responsible for erythroid differentiation blockage. In addition, reduction of Spi-1 levels triggered apoptotic death of a part of the preleukemic population/proerythroblasts, indicating that Spi-1 is involved in the survival of preleukemic proerythroblasts despite an active Epo signaling. Alternatively, apoptosis may be a consequence of the differentiation process, either as a final issue of in vitro–differentiated cells or by affecting immature cells as a control of the number of mature cells,27,40 or, yet, because some of the cells have lost the potential to differentiate and died as a default issue. Experiments done to analyze in further detail apoptosis suggested that Spi-1 displays an antiapoptotic function per se, which is distinct from the differentiation process. First, the percentage of apoptotic cells was not correlated with the percentage of differentiated cells. Second, Spi-1 protected proerythroblasts from apoptosis in the absence of Epo signaling when cultured in the presence of SCF alone, a condition that was not associated with differentiation.22-25 Finally, as it is reported that the Fas/FasL level is a critical parameter to induce apoptosis during physiologic erythropoiesis,26,27 we looked at whether the surface expression of Fas was modulated by Spi-1 in our model. Our data show that the cell-surface expression of Fas was not up-regulated in the situation when Spi-1 was decreased, thus excluding an involvement of Fas in the control of apoptosis of preleukemic cells. This observation was consistent with the published Epo-mediated suppression of Fas/FasL expression and sensitivity of the erythroid cells in humans26 and adult mice,27 as our experiments were performed in the presence of a high Epo concentration (1 U/mL).26,27 Previous reports have shown that Spi-1 sustains a pool of immature erythroid progenitors during fetal development in mice by exerting proliferative and antiapoptotic actions.7 These activities are transient because Spi-1 is rapidly silenced during normal erythroid differentiation.7,8 Our results indicate that Spi-1 renewal and antiapoptotic activities can be functional in engaged erythroid cells from adult mice when its expression is enforced and that these activities may be implicated in the conversion of a normal into a preleukemic proerythroblast. The mechanisms controlled by Spi-1 and responsible for protection against apoptosis in the preleukemic proerythroblasts remain to be determined. We have excluded a possible involvement of Fas/FasL signaling. We also investigated whether Spi-1 knock-down would impede EpoR activation and downstream signaling. Absence of modification in the number of functional EpoR at the cell surface and in Epo-stimulated phosphorylation of EpoR, combined with the increased phosphorylation of AKT and STAT5, 2 effectors of Epo signaling seen when Spi-1 expression was reduced, prompted us to propose that apoptosis is not due to a failure of EpoR activation. Furthermore, Spi-1 overexpression and the presence of Epo in the culture medium only partially compensate for the loss of each other in preventing apoptosis. These results show that Spi-1 overexpression and associated transformation of proerythroblasts are not due to an hyperactivation of the EpoR. Moreover, it suggests that Spi-1 and Epo signaling provide mechanistically independent and additive control of the preleukemic development of the proerythroblasts.
Interestingly, the phosphorylations of AKT and STAT5 were increased in parallel to Spi-1 diminution and erythroid differentiation. Presently, it is not known whether STAT5/AKT phosphorylation and/or potential activation are required for the process of erythroid differentiation following Spi-1 decline or whether it is a compensatory response of proerythroblasts in which Spi-1 was down-regulated. A crucial role for STAT5 in EpoR antiapoptotic signaling mediated by the immediate-early induction of Bcl-XL in erythroid cells has been described.41,42 The increase in STAT5 phosphorylation might be responsible for the survival of cells during differentiation associated with Spi-1 decrease, as Bcl-XL was up-regulated at the mRNA level during proerythroblasts differentiation (data not shown). A recent article describes a SHP-1 overexpression in SFFV-infected erythroleukemic cells that might be responsible for inhibition of STAT1 phosphorylation in the presence of PU.1 overexpression.43 It will be interesting to examine whether SHP-1 phosphatase activity is responsible for the lower STAT5 phosphorylation described in our study when Spi-1/PU.1 was overexpressed. The Epo-activated PI3K/AKT signaling pathway has been recently involved in erythroid differentiation.44,45 Ghaffari et al46 brought also arguments showing that AKT function is required for regulation of erythroid differentiation. In view of these reports, our data showing an activation of AKT following Spi-1 down-regulation and erythroid differentiation are particularly interesting, and our model will be fruitful to investigate the relationship between Spi-1 differentiation blockage and AKT function. Activation of the Ras/Raf/ERK1/2 pathway has been extensively involved in neoplasia through its role in permitting proliferation and survival (for review, see Steelman et al47 ). Recently, Raf-1/ERK1/2 inhibition associated with Fas-mediated caspase activation has been involved in the control of differentiation.48 Here, in differentiating preleukemic proerythroblasts, ERK1/2 decrease was not associated with modulation of Fas expression or due to a decrease in EpoR activation. Further work is necessary to determine the implication of the ERK1/2 activation in the preleukemic state of Spi-1 transgenic proerythroblasts.
Several reports describe that PU.1 impedes GATA-1 transcriptional activity in erythroid tissues49-51 by organizing a complex of proteins that creates a repressive chromatin structure.52 The derepression of GATA-1 target genes associated with Spi-1 knock-down may explain the release of differentiation. However, it does not explain the higher apoptosis as GATA-1 protects against cell death.53 So, the mechanism of the antiapoptotic function of Spi-1 involved in the expansion of preleukemic proerythroblasts must be investigated through an alternative pathway.
Future studies using global transcriptome analysis of the preleukemic spi-1 transgenic proerythroblasts according to Spi-1 expression level may offer a valuable approach to unravel the multifaceted functions of Spi-1/PU.1 in erythroleukemogenesis.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christel Guillouf, Institut Curie-Recherche, INSERM U528, 26 rue d'Ulm, 75248 Paris Cedex 05, France; e-mail: guillouf@curie.fr.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank N. Brandon, N. Denis, and Z. Maciorowski for technical assistance and J. Sanceau for RT-PCR analysis of the OAS-1 gene; I. Gallais, R. Monni, and F. Rosselli for helpful discussions; and F. Wendling for comments on the manuscript. We are grateful to Drs Morlé, De Murcia, and Clevers for providing the plasmids pGJ10, 10G6, and pTER, respectively.
This work was supported by grants from the Association pour la Recherche sur le Cancer and the association Christelle Bouillot as well as funds from the Institut Curie (Paris, France) and INSERM. P.R. was supported by a fellowship from the Ministère de la Recherche et de la Technologie.