Abstract
Patients with severe immune thrombocytopenic purpura (ITP) may require an acute increase in the platelet count for surgery or ongoing hemorrhage as well as long-term maintenance treatment. Certain of these patients may be refractory to steroids, intravenous anti-D, intravenous immunoglobulin (IVIG), and splenectomy. Therefore, acute platelet increases were studied in 35 patients completely unresponsive to IVIG or high-dose steroid treatment. Because of their lack of response to either or both single agents, these patients were administered a 3- or 4-drug combination including IVIG 1 g/kg, intravenous methylprednisolone 30 mg/kg, Vinca alkaloids (VCR 0.03 mg/kg), and/or intravenous anti-D (50-75 μg/kg). Subsequent maintenance therapy with the oral combination of danazol (10-15 mg/kg) and azathioprine (2 mg/kg) was given to 18 of the 35 patients. Seventy-one percent of the patients responded to the intravenous combination treatment with acute platelet increases of at least 20×109/L to a level greater than 30×109/L. Two thirds of the patients given maintenance therapy achieved stable platelet counts greater than 50×109/L without other treatments. One patient developed an ileus, but otherwise there was little toxicity of combination treatment. Combination chemotherapy is a useful approach for patients with ITP refractory to conventional treatments both for acute induction and for long-term maintenance therapy.
Introduction
Immune thrombocytopenic purpura (ITP) is an autoimmune disorder of children and adults in whom antiplatelet antibodies lead to increased destruction of platelets.1 These same antibodies may also impede platelet production as a result of effects on megakaryocytes.2-4 If the platelet count is sufficiently low, there may be clinically significant bleeding, including life-threatening hemorrhages (eg, intracranial [ICH] and gastrointestinal). Therefore, treatment may be needed to acutely increase platelet counts to prevent or stop ongoing hemorrhage or prior to surgery. Most treatments that acutely increase the platelet count do so for relatively short periods of time. In many patients, there is also a need for effective, tolerable, and safe maintenance treatment.
Standard therapies used to acutely increase the platelet count in both children and adults with ITP include steroids, intravenous gamma globulin (IVIG), and intravenous anti-D. A small percentage of patients is severely affected and does not respond to treatment. Currently, there are no well-defined, efficacious treatment options for these patients unresponsive to conventional first-line therapies.
If marked thrombocytopenia persists, it is associated with an increased risk of bleeding complications.5,6 Many clinicians would use a series of other treatments including danazol, azathioprine, plasmapheresis, vincristine, cyclophosphamide, platelet transfusion, and especially splenectomy.7-10 The latter is often but not always effective.11 However, the long-term complications of splenectomy, in addition to sepsis, are not well-defined.12 The chronic use of high-dose immunosuppressants, particularly steroids, can lead to infectious complications.6 The recently developed thrombopoietic agents appear promising in clinical trials, but they require further study before their use can be recommended in these refractory patients.13 Therefore in this study, the most active single agents were combined to explore their effects in patients refractory to single agents.
The first part of this study investigated combinations of agents in patients unresponsive to single-agent therapy requiring an acute platelet increase. The goal was to use a 3- or 4-drug intravenous combination (Table 1) to rapidly increase the platelet count. This tested the hypothesis that resistance to one or more treatments would be overcome by the administration of multiple simultaneous treatments and that these combinations would be well tolerated.
The second focus of the study was the development of oral maintenance treatment. Both danazol and azathioprine were given to patients needing ongoing maintenance therapy either before or generally after splenectomy (Table 1). Danazol and azathioprine were chosen because the efficacy of each had been well described in relatively large numbers of patients and also because of their tolerability and low, generally nonoverlapping toxicity profiles.8-10 Danazol also suppresses menses, providing additional benefit to women who may have had clinically significant menometrorrhagia. Hirsutism with this agent is less of a problem than development of acne. Acceleration of bone age makes the use of danazol problematic in prepubertal children.10 None of the patients in this study received rituximab.
Other potential candidates were not selected (eg, colchicines, mycophenolate mofetil) because there were few data describing them when the study was initiated.14-16 Many of the patients receiving maintenance treatment had received the combination treatment described in the first part of the study to acutely increase their platelet counts but also required ongoing treatment. The hypothesis tested in this section was that by combining 2 active agents with different mechanisms of effect and largely nonoverlapping toxicities, a higher response rate would be seen without an increase in toxicity.17
Patients and methods
Acute phase
Patients could be enrolled if they had been diagnosed with ITP according to the guidelines of the American Society of Hematology. They were eligible for the induction phase of the study if they had completely failed to respond to high-dose oral prednisone (greater than 1 mg/kg per day) and/or IVIG (1 g/kg) in that their platelet counts either did not change or increased less than 10×109/L to a level less than 30×109/L following treatment. Most commonly, patients were not having major bleeding but they were severely thrombocytopenic and the platelet increase was sought to minimize the chance of a serious hemorrhage. Patients were eligible for the maintenance phase of the study if they had persistent thrombocytopenia with bleeding signs and symptoms and therefore required long-term support of their platelet count. The majority of these patients had failed to respond to prior splenectomy. There were no age restrictions on study entry. Neither part of the study was randomized as to which treatments were administered.
Thirty-two of the 35 patients were evaluated at New York Presbyterian Hospital (NYPH)–Cornell Medical Center. Most received treatment there but several were treated by their referring hematologist. Three patients were treated at Children's National Medical Center by one of the authors (M.C.D.). Patients treated at Cornell often received follow-up monitoring, and in some cases, further treatment, with their referring hematologist. Data were collected from medical charts including age, date of diagnosis of ITP, history of treatments and responses to ITP therapy, as well as other medically relevant information. All of the patients received 3- or 4-drug acute therapy (Table 2) including IVIG and methylprednisolone in all cases with the addition of intravenous anti-D and/or vincristine at the doses specified in Table 1. Eleven of the 35 patients received the 3-drug combination of IVIG/steroid/anti-D to avoid the potential toxicity of vincristine. All patients who were initially treated at NYPH from November 1996 to November 2000, on the protocol listed in Table 1 were included in this study. The agents selected and their doses were at the discretion of the treating physician. In general, if the direct antiglobulin test (DAT) was positive or the patient was Rh−, anti-D was not used. If the patient had Evans syndrome, anti-D was not used. Anti-D was used in combination in splenectomized patients. Patients, such as the elderly and those who had previously received weekly vincristine were deemed to be at risk of neuropathy and were not given vincristine. The perception of greater urgency or greater refractoriness resulted in all 4 agents being combined. Institutional Review Board approval was obtained from New York Hospital-Cornell for a retrospective chart review, and written consent was obtained in accordance with the Declaration of Helsinki from the patients.
Maintenance phase
Therapy with the oral combination of danazol and azathioprine (Table 1) was started in 18 of the 35 patients in this study. Patients who continued to have severe thrombocytopenia were offered long-term maintenance therapy. Some patients had transient responses to acute combination therapy, warranting different approaches to obtain a prolonged elevation of platelet counts. Patients with underlying human immunodeficiency virus (HIV) infection, liver disease, or diabetes were not started on these oral immunosuppressants. Patients were monitored monthly for transaminitis, a potential toxicity of both danazol and azathioprine.
Response to intravenous induction combination therapy was defined as an increase in platelet count greater than 20×109/L to a count greater than 30×109/L. Response to maintenance therapy was defined as an increase to a stable platelet count greater than 50×109/L without the use of other agents. If after 4 months of treatment with azathioprine and danazol no response was seen, these medications were discontinued.
The analysis of the data was descriptive. For the acute platelet increase, platelet counts were recorded on study day 1 (prior to treatment), and then on days 2 to 5, 6 to 10, and 11 to 17. For each time point, the mean, median, standard deviation, and quartiles in the adult patients, pediatric patients, and the group as a whole were calculated. Other results according to subgroups (ie, HIV-infected patients or Evans syndrome) were also calculated (Table 3). For patients on maintenance treatment, the focus was on the number responding, the time to response, and the duration of response.
Results
This report describes 35 patients with refractory ITP (Table 3) who had initially been diagnosed with ITP a median of 5.3 years (range, 1 month to 30 years) before study treatment. Prior to receiving combination therapy, all 35 of the patients had failed to increase their platelet count by as much as 10×109/L with IVIG (1-2 g/kg) and/or steroids (usually prednisone 2 mg/kg per day for ≥ 2 weeks; following intravenous methylprednisolone). Twenty-five patients were adults, including 19 who had failed to respond to splenectomy. Thirty-three of the 35 patients had chronic ITP (greater than 6 months' duration).
Overall 25 (71%) of 35 patients responded to the intravenous combination chemotherapy with a platelet increase of greater than 20×109/L to a count greater than 30×109/L (Figures 1,2). We used the response criteria that were used to license intravenous anti-D, which required an increase in the platelet count of greater than 20×109/L to a level greater than 30×109/L. If instead the criteria for response required an increase in the platelet count of greater than 30×109/L to a level greater than 50×109/L, 2 responders would be converted to nonresponders. This still leads to an overall response rate of 66%, which is impressive in this population that was selected for the severity of disease. Using doubling of the platelet count as a response criterion, 83% of patients responded to acute intravenous therapy.
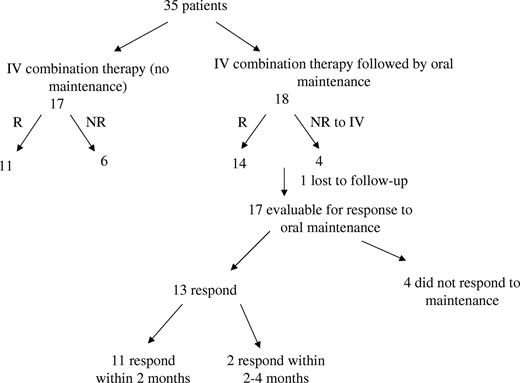
Overview of study results: acute intravenous and oral maintenance combination therapies for patients with refractory ITP. Thirty-five patients were treated with acute intravenous combination therapy, of whom 25 responded with at least a platelet increase of greater than 20×109/L to a platelet count of greater than 30×109/L. Eighteen patients received oral maintenance combination therapy following acute intravenous combination therapy of whom 17 were evaluable; 13 responded to oral maintenance therapy within 4 months. Response to oral maintenance therapy was defined as a sustained platelet count greater than 75×109/L without the need for interim acute treatments of any kind.
Overview of study results: acute intravenous and oral maintenance combination therapies for patients with refractory ITP. Thirty-five patients were treated with acute intravenous combination therapy, of whom 25 responded with at least a platelet increase of greater than 20×109/L to a platelet count of greater than 30×109/L. Eighteen patients received oral maintenance combination therapy following acute intravenous combination therapy of whom 17 were evaluable; 13 responded to oral maintenance therapy within 4 months. Response to oral maintenance therapy was defined as a sustained platelet count greater than 75×109/L without the need for interim acute treatments of any kind.
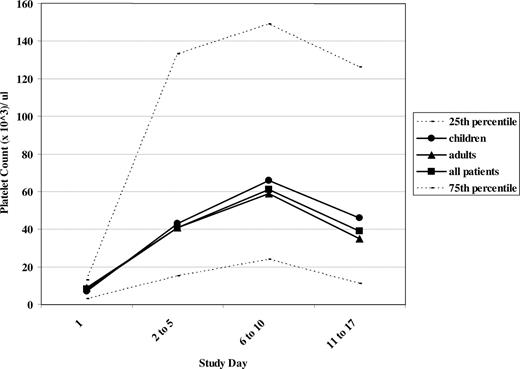
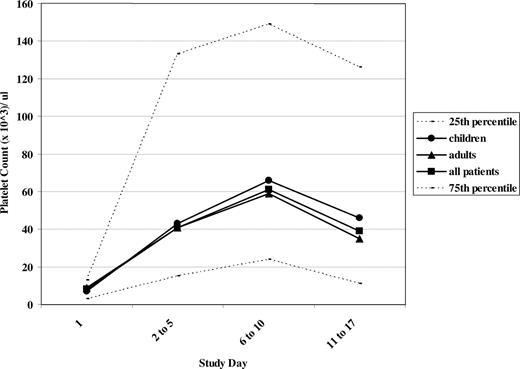
Platelet counts following acute intravenous combination therapy. Median platelet counts are illustrated at study days 1 (prior to treatment), 2 to 5, 6 to 10, and 11 to 17. ● represent children in the study; ▴, adults; and □, all patients. The 25th and 75th percentiles at each time point are demonstrated by - - -.
Platelet counts following acute intravenous combination therapy. Median platelet counts are illustrated at study days 1 (prior to treatment), 2 to 5, 6 to 10, and 11 to 17. ● represent children in the study; ▴, adults; and □, all patients. The 25th and 75th percentiles at each time point are demonstrated by - - -.
Treatment subgroups, the 2 different 3-drug groups and the 4-drug combination, were not selected randomly. Possibly as a result, no differences were seen in responses according to treatment subgroups. Patients receiving combinations including vincristine responded in 18 of 24 cases, which was not different from the 6 of 11 responses in those receiving IVIG–intravenous anti-D–intravenous methylprednisolone (Table 2).
Similarly, given the limited sample size, no significant differences in response rate were seen based on clinical characteristics of the treated patients. These characteristics included age, sex, the presence of HIV, prior splenectomy, or presence or absence of Evans syndrome (all P values > .1). Duration of prior ITP did not predict response.
Fifty percent of these refractory patients, despite a median initial platelet count of 8×109/L, had peak platelet counts at days 6 to 10 of at least 61×109/L (Figure 2). The initial platelet count did not predict response. Only 4 of 35 patients had pretreatment platelet counts greater than 20×109/L prior to treatment, whereas 27 of 35 did so at days 6 to 10 of the study, the time of the peak platelet count. The mean platelet count prior to initiating the intravenous combination treatment was 12×109/L, reaching 68×109/L at days 2 to 5 and 111/L at days 6 to 10 following treatment. While the platelet counts at days 11 to 17 in both children and adults had decreased compared with the peak counts on days 6 to 10, the median platelet count was sustained at a level greater than 35×109/L in both children and in adults at days 11 to 17 (Figure 2), and the mean platelet counts were both greater than 50×109/L.
Prior to treatment, half of the patients had signs and symptoms of severe thrombocytopenia, including purpura, petechiae, and mucosal bleeding, manifested by wet purpura and epistaxis. However, none of the patients presented with severe, life-threatening bleeding (eg, an ongoing ICH). In all patients, bleeding signs and symptoms improved when the platelet count increased. Three patients were able to undergo procedures.
Ninety percent of the patients had failed IVIG prior to treatment with the combination therapy, most of whom had also gotten high-dose steroids. Thirty percent of patients had previously failed anti-D. Eighty-two percent of the IVIG failures were converted to IVIG responders when given in combination with the other drugs and 86% of the anti-D failures who got anti-D as part of their combination treatment did respond. Of the 6 patients in the study who had failed to respond to IVIG and anti-D given as single agents, 5 of them responded when given in combination.
In this series of 35 patients with ITP, 18 were subsequently started on oral maintenance therapy with azathioprine and danazol (Figure 1) because their persistent thrombocytopenia warranted continued treatment for the combination of severe thrombocytopenia and bleeding sings and symptoms. The 6 HIV-positive patients were not started on oral immunosuppressant agents. One patient was not started on danazol due to severe liver disease, and 1 patient had uncontrolled diabetes warranting the avoidance of further immunosuppression. Of the 18 patients started on oral maintenance therapy, 14 had had a prior splenectomy and 4 (3 children) had not. One patient was noncompliant with the oral medications. After 2 months of treatment with azathioprine and danazol, 11 of the 17 patients on maintenance therapy had achieved platelet counts stably greater than 75×109/L. Of the 6 patients with platelet counts less than 35×109/L at 2 months, 2 responded to the oral medications after 2 more months of treatment, with the overall result that 13 of the 17 evaluable patients on the maintenance regimen had platelet responses with counts greater than 50×109/L.
One of the early responders to the oral combination therapy was subsequently lost to follow-up. The other 12 patients maintained platelet counts of greater than 75×109/L 4 months after the start of therapy with oral medications. Response to intravenous treatment predicted response to the oral combination therapy. Two patients who did not respond to the intravenous combination treatment responded to the oral combination (and vice versa). Thirteen of the 17 patients were concordant in their responses to the intravenous and the oral combination regimens.
Seventeen of this series of 35 patients, 12 adults and 5 children, were treated with acute intravenous combination therapy more than once to keep the platelet count from persisting at a very low level, in most cases while awaiting an anticipated response to oral maintenance therapy. Some patients were unable to start maintenance therapy due to underlying HIV infection or the concern of additional toxicities to patients on multiple medications for other conditions. There were 58 responses (78%) to 74 combination treatments in these 17 patients, demonstrating that combination chemotherapy could be repeated if needed without the patients becoming refractory to it (Table 4). For example, patients A and B received a total of 15 combination treatments with response to all 15. Nonresponders to subsequent treatments were generally those who had minimal initial responses such that a small decrement in their response on a subsequent treatment resulted in their being a nonresponder (Table 4, patients M and N).
Regarding toxicity, no cases of thrombosis or aggravated hemolysis were seen; in particular, giving IVIG and intravenous anti-D together did not result in additive acute inflammatory reactions or hemoglobin decreases. Oral combination therapy did not result in transaminitis in the 18 treated patients, nor were there other substantial complications of treatment appreciated (ie, worsening anemia, leukopenia, diarrhea, or depression). There was evidence of severe toxicity in only one patient.
An 82-year-old man with a 1-year history of ITP, not at all responsive to anti-D or IVIG was treated with IVIG, methylprednisolone, vincristine, and anti-D. His platelet count rose from 2×109/L to greater than 50×109/L. He received multiple infusions of combination therapy and achieved platelet counts greater than 100×109/L with subsequent infusions.
A 6-year-old girl with a 2-month history of ITP was unable to achieve a platelet count greater than 20×109/L and experienced long-lasting episodes of epistaxis in spite of single-agent treatments with anti-D, IVIG, and steroids. She received intravenous combination therapy with IVIG, methylprednisolone, and vincristine on 2 occasions due to bleeding in association with severe thrombocytopenia. The first time, her platelet count rose from 4×109/L to greater than 200×109/L and the second time from 23×109/L to greater than 100×109/L. With concomitant danazol and azathioprine, her platelet count then stabilized at greater than 75×109/L.
A 25-year-old woman with a 6-year history of ITP had undergone splenectomy with little effect, became unresponsive to steroids, and then did not respond to IVIG. She responded to combination treatment with methylprednisolone, IVIG, and vincristine with an overnight platelet increase from 4×109/L to 160×109/L. She was briefly hospitalized with severe constipation and a presumed vincristine ileus (she had previously received several doses of vincristine). She was the only patient with significant toxicity as a result of combination therapy.
A 65-year-old woman with chronic ITP suffered a myocardial infarction and needed to undergo cardiac catheterization and possible coronary artery bypass grafting. IVIG and intravenous methylprednisolone (IVIG at 1 g/kg per day × 2 days and intravenous methylprednisolone at 1 g/day × 2 days) failed to increase the platelet count as much as 5×109/L (the counts went from 32×109/L to 33×109/L). The procedure was rescheduled and a combination of all 4 agents was administered intravenously during one day with a peak count of 160×109/L, allowing the cardiac catheterization to be performed 2 days later without hemorrhagic complications.
A 51-year-old woman with chronic ITP failed to respond to IVIG and intravenous methylprednisolone. She received 4-drug combination therapy prior to splenectomy with an increase of counts from 30×109/L to 154×109/L, allowing the surgery to proceed safely.
Discussion
Combination intravenous therapy with IVIG, methylprednisolone, the vinca alkaloids, and/or anti-D appears to be the optimal treatment for patients with immune thrombocytopenic purpura refractory to standard approaches who require an acute platelet increase. IVIG and intravenous anti-D were combined to take advantage of their different mechanisms of effect based on interactions with different Fcγ receptors.18-20 In this study, combination intravenous therapy was effective in acutely raising the platelet count in 71% of these refractory patients. From the subset analysis, it did not appear that there was any one group particularly responsive or unresponsive to this therapy. For example, it was effective in patients with Evans syndrome (without intravenous anti-D) and also in those with HIV-related ITP. In the 35 treated patients, there were no identifiable predictors of response in these patients. Although none of these patients presented with life-threatening bleeding, this combination therapy provides a reasonable approach for patients in critical clinical situations to stop the bleeding. The therapy was effective in treating mucosal bleeding and in allowing invasive procedures.
Many different approaches to achieving a stable, adequate platelet count have been tried in patients with chronic ITP. Reviews of the use of single agents in this patient population show less than 30% success rates for the maintenance of adequate counts while on treatment, illustrating the need for improved management in this group of patients.21,22 Long-term steroids, liposomal doxorubicin, cyclophosphamide, vincristine, procarbazine, dexamethasone, and etoposide have had limited benefit for patients with refractory ITP in this setting.23,24 Cyclosporine has benefit that is offset to a variable degree by its toxicity.25 Mycophenolate mofetil also has limited efficacy.14 Furthermore, long-term immunosuppressive therapy has an infectious risk.6 Patients may have concurrent illnesses that preclude the use of certain agents in the management of their chronic ITP. In particular, ongoing hepatitis would be a contraindication for the use of danazol and azathioprine. Current treatment options are not far different from those that have existed for a number of years with the exception of the recent availability of rituximab.26 Responses to this agent lasting greater than 1 year are seen in one third of patients; approximately one half of these will subsequently relapse.27 The study described here was largely completed prior to the now-widespread use of this agent.
The therapy offers several advantages over conventional treatment especially in these cases reported here in which single treatments were completely ineffective. The effect of acute treatment generally lasted at least 2 weeks (Figure 2). These responses are consistent with achieving the goals of preventing future hemorrhage, controlling ongoing hemorrhage, and/or taking a patient to surgery. The combination treatment was generally well tolerated and was typically infused on a single day in the outpatient department over 5 to 8 hours. Response should be seen within a week of treatment. Seventeen patients received multiple courses of combination treatment without becoming refractory to it. Many patients experienced dramatic platelet increases despite their lengthy histories of poor and/or nonexistent responses to prior treatment with other medications.
While not all patients with ITP respond to any treatment as seen in 10 of the 35 patients in this study, complete failure to respond to the 3- or 4-drug intravenous combination treatment may suggest a diagnosis other than ITP and at least warrants further investigation and a complete review of the history. One child (not in this report) with newly presenting, apparent ITP (including a normal initial bone marrow examination) completely failed to respond to 4-drug combination intravenous therapy. Following this lack of response, a repeat bone marrow evaluation, obtained 4 weeks after the initial marrow, revealed aplastic anemia. Another recent patient not included in this report who failed to respond to combination treatment was found to have thrombocytopenia secondary to systemic cytomegalovirus infection, which had been clinically unsuspected in part because he was afebrile without hepatosplenomegaly or a mono-like prodrome. His platelet count improved following effective antiviral therapy. The lack of sensitive and specific testing to include or exclude the diagnosis of immune thrombocytopenic purpura (ie, platelet antibody) leaves discrimination of these refractory cases to clinical judgment. Therefore when there is not a response to even 4-drug therapy, other etiologies of acute severe thrombocytopenia need to be carefully reconsidered.
Only 1 patient of the 35 in this series had a severe adverse event (ileus) requiring hospitalization. No other overt neurotoxicity as a complication of the vinca alkaloids was seen in this series of patients because of the limitation of dosing (1.0 to 1.5 mg vincristine), less frequent dosing, and smaller number of total doses of this agent (ie, maximum of 2-4 times at 2- to 4-week intervals). Therapy was switched from vincristine to vinblastine if repeated dosing was used to decrease the likelihood of neurotoxicity, particularly in the elderly or those who had previously received multiple doses of vincristine. Intravenous immunoglobulin often results in headaches, which may be severe as with cases of aseptic meningitis, but the concurrent use of intravenous methylprednisolone seemed to ameliorate these side effects. No augmented infusional or postinfusion toxicity of intravenous anti-D was noted when it was administered with IVIG. Similarly, the oral agents were well tolerated. Currently, it is our general practice to start all patients requiring maintenance treatment on 2 agents (ie, danazol and azathioprine).
ITP can have a self-limited course in a percentage of cases especially in children but also in adults, so treatment may be needed in some patients only until the disease improves spontaneously.28,29 If patients, even adults with chronic disease, are going to improve on their own, then repeated use of the single agents may be sufficient to “buy time” until “spontaneous” improvement occurs.28 However, the group of patients included in this report was particularly difficult to treat as exemplified by their absence of any acute response to single agents. Thirty-three had chronic disease, 19 of whom were refractory to splenectomy. Generally, therefore ongoing oral combination treatment was required to obtain stable adequate platelet counts in these patients. Since a response to these oral agents may require 2 to 4 months, the patients received repeated intravenous combination treatments to avoid potentially serious bleeding while awaiting a response to the oral agents. Oral combination treatment with danazol and azathioprine was effective in 13 (76%) of 17 of these refractory patients and was well tolerated. Eighty-five percent of the patients who had responded to the intravenous combination therapy and were started on oral medications also responded to the chronic medications, and 2 of 4 of the nonresponders to intravenous combination treatment responded to the oral combination maintenance therapy as well.
There are many other agents used in the management of ITP, both those intended to acutely increase the platelet count and those whose use is generally to maintain the count on a long-term basis. Individual patients may be treated with one or more of these agents depending upon circumstances. Patients with preexisting transaminitis were not encountered in this series, but this would have precluded the combination oral treatment used in this study. Danazol is a particularly key component of it because it is not immunosuppressive. It does not appear that either azathioprine, mycophenolate mofetil, or cyclosporine on its own is likely to result in opportunistic infections, but the use of 2 of these agents together would presumably increase the risk of such an eventuality. One advantage of the combination treatment also is that it may avoid long-term use of high doses of the 2 agents since they can be tapered in responders—thus minimizing, for patients taking danazol (especially in girls or young women), the development of acne, hirsutism, and deepening of the voice.
In conclusion, combination therapies given both intravenously to acutely increase the platelet count and orally as maintenance treatment in those with chronic refractory disease provide a benefit to a majority of children and adults with primary and secondary refractory ITP with minimal risk of adverse side effects. No subset of patients was identified that was particularly responsive or unresponsive to this approach.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by the Children's Cancer and Blood Foundation.
We thank all of the nurses for their care of these patients, especially Erin Shine Fodero. We thank Allison Schindler for her help in chart review.
Authorship
Contribution: D.M.B. analyzed data and wrote the paper; J.B.B. designed and performed the research and wrote the paper; S.G. analyzed data; M.C.D. performed research and contributed patients to the research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: James B. Bussel, Departments of Pediatrics, Medicine and Obstetrics and Gynecology, New York Presbyterian Hospital, Weill Cornell Medical Center, 525 E 68th St, Payson 695, New York, NY 10021; e-mail:jbussel@med.cornell.edu.