Leukemia stem cells (LSCs) are thought to play a central role in the pathogenesis of acute leukemia and likely contribute to both disease initiation and relapse. Therefore, identification of agents that target LSCs is an important consideration for the development of new therapies. To this end, we have previously demonstrated that the naturally occurring compound parthenolide (PTL) can induce death of human LSCs in vitro while sparing normal hematopoietic cells. However, PTL has relatively poor pharmacologic properties that limit its potential clinical use. Consequently, we generated a family of PTL analogs designed to improve solubility and bioavailability. These studies identified an analog, dimethylamino-parthenolide (DMAPT), which induces rapid death of primary human LSCs from both myeloid and lymphoid leukemias, and is also highly cytotoxic to bulk leukemic cell populations. Molecular studies indicate the prevalent activities of DMAPT include induction of oxidative stress responses, inhibition of NF-κB, and activation of p53. The compound has approximately 70% oral bioavailability, and pharmacologic studies using both mouse xenograft models and spontaneous acute canine leukemias demonstrate in vivo bioactivity as determined by functional assays and multiple biomarkers. Therefore, based on the collective preclinical data, we propose that the novel compound DMAPT has the potential to target human LSCs in vivo.
Introduction
Recent studies have demonstrated that myeloid leukemia and certain forms of lymphoid leukemia arise from malignant stem cells (called leukemia stem cells [LSCs]).1,–3 LSCs are typically found in a quiescent state and are thus unlikely to respond to standard chemotherapeutic agents that preferentially eradicate actively cycling cells.4,,–7 Indeed, the persistence of LSCs following chemotherapy may be a major factor contributing to clinical relapse.8,9 In addition, conventional leukemia therapy is also substantially toxic to normal hematopoietic cells and frequently results in severe myelosuppression. Therefore, given the drug-refractory nature of LSCs, and the importance of normal hematopoiesis, identification of less toxic and more specific forms of therapy are important priorities for the development of better therapeutic regimens
As a foundation for developing more selective leukemia treatments, our previous experiments have investigated basic properties of primitive acute myelogenous leukemia (AML) cells. These studies showed that LSCs from different AML subtypes share characteristics10 that are unique to AML and thus represent potential therapeutic targets for the selective ablation of LSCs relative to their normal counterparts.11,12 Specifically, we reported that NF-κB, a known regulator of growth and survival, is constitutively active in LSCs but not in normal hematopoietic stem cells (HSCs).13 Notably, many traditional cancer therapies induce activation of NF-κB, a potentially undesirable characteristic likely to facilitate survival of malignant cells.14,15 Given the ability of many cancer cells to evade apoptosis, we hypothesized that NF-κB inhibition could be used to facilitate LSC-selective cell death, a concept supported by studies using the proteasome inhibitor MG-132 (known to inhibit NF-κB) with the anthracycline idarubicin (IDR).16 However, molecular genetic approaches demonstrated that NF-κB inhibition alone is not sufficient to strongly induce AML-specific apoptosis. Further investigation of pathways induced by MG-132 plus IDR treatment revealed activation of p53 and increased oxidative load as prevalent components of the AML cell death process.7 Collectively, these data suggest that the mechanism of LSC death involves combined inhibition of survival pathways and activation of tumor suppressor and/or stress pathways.17 More recently, we have shown that robust apoptosis of primary AML cells can be achieved with a single agent, the plant-derived compound parthenolide (PTL), which is known to induce oxidative stress and inhibit NF-κB.7 Importantly, PTL also effectively eradicates AML stem and progenitor cells in vitro while sparing normal hematopoietic cells. Hence, PTL has the ability to eradicate AML stem cells as well as to ablate bulk leukemia blast cells, properties that should make this compound an attractive agent for clinical evaluation. However, despite the utility of PTL determined by in vitro studies, its solubility is relatively poor, making pharmacologic use of the compound difficult. In animal studies, maximum attainable serum levels were 200 nM,18 a concentration approximately 30-fold less than required to mediate LSC cell death in vitro. Therefore, we have synthesized and screened PTL analogs to identify a compound with improved solubility and bioavailability. These studies have generated a dimethylamino analog of parthenolide (DMAPT). When formulated as a fumarate salt, DMAPT demonstrates more than 1000-fold greater solubility in water relative to PTL (S.N. et al, manuscript in preparation). Moreover, as shown in the present studies, DMAPT effectively eliminates human AML stem and progenitor cells without apparent harm to normal hematopoietic stem and progenitor cells. The compound also eradicates phenotypically primitive blast-crisis chronic myeloid leukemia (bcCML) and acute lymphoblastic leukemia (ALL) cells. The molecular responses to DMAPT, both in vitro and in vivo, include activation of cellular stress responses and inhibition of NF-κB. Together, the data suggest that DMAPT represents a clinical candidate for leukemia therapy with the potential to target leukemia stem and progenitor cells.
Materials and methods
Cell isolation and culture
Primary human AML, bcCML, and ALL cells, and normal bone marrow (BM) cells were obtained from volunteer donors. Informed consent was obtained in accordance with the Declaration of Helsinki. All manipulation and analysis of human specimens was approved by the University of Rochester Institutional Review Board. Umbilical cord blood (CB) was obtained from the National Disease Research Interchange (NDRI). Dog samples were obtained from Bellingham Veterinary Clinic, Colorado State University (Department of Pathology), or Redbank Veterinary Hospital (Case Study II). Mononuclear cells were isolated from the samples using Ficoll-Plaque (Pharmacia Biotech, Piscataway, NY) density gradient separation. For canine case studies, white blood cell (WBC) numbers were determined using the HESKA (Loveland, CO) CBC-Diff system. In some cases, cells were cryopreserved in freezing medium of Iscove modified Dulbecco medium (IMDM), 40% fetal bovine serum (FBS), and 10% dimethylsulfoxide (DMSO) or in CryoStor CS-10 (VWR, West Chester, PA). Cells were cultured in serum-free medium (SFM)19 for 1 hour before the addition of DMAPT. PTL was obtained from Biomol (Plymouth Meeting, PA).
DMAPT synthesis and pharmacology
DMAPT was prepared from the reaction of PTL with dimethylamine, and the resulting dimethylamino analog was then converted to its water-soluble fumarate salt. The detailed synthesis, structural identity, and stereochemistry of DMAPT are reported elsewhere (S.N. et al, manuscript in preparation). Bioavailability and pharmacokinetic assays for rodents were performed by the Developmental Therapeutics Program from the National Cancer Institute and for dogs by Integrated Analytical Solutions (Berkeley, CA).
Methylcellulose colony-forming assay
AML, BM, or CB cells were cultured in SFM as stated for 18 hours in the presence or absence of DMAPT. Cells were plated at 50 000 cells/mL in Methocult GFH4534 (Stem Cell Technologies, Vancouver, BC) supplemented with 3 U/mL erythropoietin and 50 ng/mL granulocyte colony-stimulating factor (G-CSF). Colonies were scored after 10 to 14 days of culture.
EMSA and immunoblot analysis
Electrophoretic mobility shift assay (EMSA) was performed as described.13 Briefly, nuclear extracts equivalent to 200 000 cells were incubated with 2 μg of poly-d(I-C) (Roche Molecular Biochemicals, Indianapolis, IN) and 10−14 mol 32P-labeled NF-κB probe in 10 mM HEPES, 5 mM Tris, 50 mM KCl, 1.2 mM EDTA, and 10% glycerol for 15 minutes at room temperature. Protein/DNA complexes were resolved on a native polyacrylamide gel in 0.25 × TBE. For immunoblots, cells were prepared and analyzed as previously described.11 Blots were probed with anti-phospho–p53 (ser15) from Cell Signaling (Beverly, MA), and antiactin (AC-15) from Sigma (St Louis, MO).
Confocal microscopy
Cells were fixed in methanol at −20°C. The cells were permeabilized with blocking buffer (10% FBS and 0.1% Tween 20 in 1× phosphate-buffered saline [PBS; pH 7.4]) as described.20 Cells were stained using either rabbit polyclonal anti-p65 (C-20), anti–Nrf-2 (C-20) (Santa Cruz Biotechnology, Santa Cruz, CA), anti-HO1 (GeneTex Inc, San Antonio, TX), or mouse monoclonal anti-γH2AX (Upstate Biotech, Charlottesville, VA) in blocking buffer for 2 hours at room temperature. Cells were washed and stained with either goat anti-rabbit Alexa 488 or goat anti-mouse Alexa 488 (Invitrogen, Carlsbad, CA) secondary antibodies and ToPro3 for nuclear stain (Invitrogen). Slides were mounted using Fluoromount (no. 63722; Southern Biotech, Birmingham, AL). Slides were left to dry overnight. Fluorescence was observed using a 100× objective (1.4 numeric aperture), further magnified by a 2× zoom, on a Leica SPl inverted scanning confocal microscope (Heidelberg, Germany). Images were acquired using Leica confocal software v. 2.6.1 Build 1537.
Flow cytometry
Apoptosis assays were performed as described.13 Briefly, after 18 to 24 hours of treatment, normal and AML specimens were stained for the surface antibodies CD38-allophycocyanin (APC), CD34-PECy7, and CD123-phycoerythin (Becton Dickinson, San Jose, CA) for 15 minutes. Cells were washed in cold PBS and resuspended in 200 μL of annexin-V buffer (0.01 M HEPES/NaOH, 0.14 M NaCl, and 2.5 mM CaCl2). Annexin-V–fluorescein isothiocyanate (FITC) and 7-aminoactinomycin (7-AAD; Molecular Probes, Eugene, OR) were added, and the tubes were incubated at room temperature for 15 minutes then analyzed on a BD LSRII flow cytometer (BD Biosciences, San Jose, CA). Analyses for phenotypically described stem cell subpopulations were performed by gating CD34+/CD38− populations. To analyze human cell engraftment in the nonobese diabetic/severe combined immunodeficient (NOD/SCID) xenotransplantation model, BM cells were blocked with the anti-Fc receptor antibody 2.4G2 and 25% human serum and later labeled with anti–human CD45-PE antibody (BD Biosciences). For canine studies, cells were stained with CD45-FITC (YKIX716.13; Serotec, Raleigh, NC), CD14-PeCy5 (TUK4; Serotec), CD34-PE (1H6; BD Biosciences) for 30 minutes. Cells were washed and resuspended in fluorescence-activated cell sorter (FACS) buffer (0.5% FBS in PBS) with 5 μg/mL of DAPI. To analyze canine cell engraftment in the NOD/SCID xenotransplantation model, cells were labeled with anti-canine CD45-APC antibody (Serotec) and anti-mouse CD45-FITC (BD Biosciences) and anticanine CD34. Cells were washed and resuspended in FACS buffer (0.5% FBS in PBS) with 5 μg/mL of DAPI.
NOD/SCID mouse assays
NOD/SCID mice were sublethally irradiated with 2.7 Gy (270 rad) using a RadSource-2000 x-ray (Rad Source Technologies, Alpharetta, GA) irradiator before transplantation. Cells to be assayed were injected via tail vein in a final volume of 0.2 mL PBS with 0.5% FBS. For analysis of human cells, 5 to 10 million primary CB specimens were used for each recipient animal. For analysis of canine cells, 5 million peripheral blood leukocytes were used for each recipient animal. For secondary transplantation studies of canine cells, total marrow from primary NOD/SCID mice was harvested, analyzed for canine cell content (by FACS), and then immediately retransplanted into irradiated secondary recipients at a cell dose adjusted to contain 3 million canine cells. After 6 to 8 weeks, animals were killed, and BM was analyzed for the presence of human or canine cells by flow cytometry.
Canine studies
In vitro and in vivo studies were performed with owner's consent on 3 animals with a diagnosis of CD34+ acute leukemia. Case study 1 was an 8-year-old male Labrador retriever. At the initiation of treatment, the animal was in advanced stages of disease and received anti-inflammatory agents, sedatives, diuretics, antibiotics, and prokinetic agents as needed during the course of DMAPT treatments. In addition, the dog received 40 mg of prednisone starting at day 5 and reduced to 20 mg on day 11, and 500 mg mesna 3 times a day starting on day 8. At day 14, the animal succumbed to multiple symptoms associated with advanced disease. Case study 2 was an 8-year-old male mixed breed dog. At the beginning of treatment, the WBC was highly elevated (81 ×109/L) and the animal was receiving 100 mg deracoxib once a day. At day 15, the animal also began receiving amoxicillin and prednisone. At day 24, the animal was withdrawn from study and euthanized at the owner's request due to concern there was not sufficient likelihood of cure to warrant continued treatment. Case study 3 was a 12-year-old male Pug. Prior to the beginning of DMAPT treatment, the WBC was 96.5 × 109/L, and the animal was receiving predisone (5 mg), famotidine, amoxicillin, and hydrocodone. At day 14, the animal was withdrawn from study and euthanized at the owner's request due to concern there was not sufficient likelihood of cure to warrant continued treatment.
U937 differentiation assay
U937 cells were plated at 400 000 cells/mL and treated with DMAPT (2.5 μM or 5 μM) or 5 μM all-trans retinoic acid (ATRA). Cells were counted and analyzed 72 hours after treatment for expression of CD11b and viability (DAPI).
Statistical analysis
Statistical analyses and graphs were performed using GraphPad Prism software (GraphPad Software, San Diego, CA). For statistical analysis, the data were log-transformed and analyzed by 1-way analysis of variance (ANOVA) followed by the Tukey post-hoc test. For 2 group comparisons, significance was determined by 2-tailed t tests.
Results
DMAPT selectively eradicates primitive leukemia cells
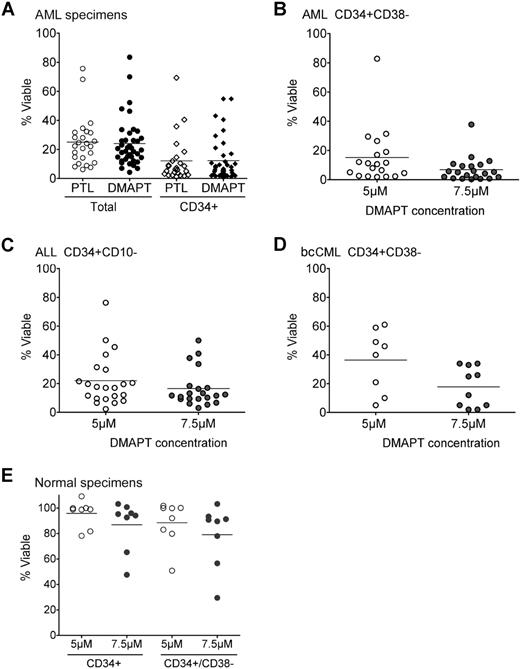
DMAPT was prepared from the reaction of PTL with dimethylamine, and the resulting dimethylamino analog was then converted to its water-soluble fumarate salt (S.N. et al, manuscript in preparation). Initially, we performed detailed biological studies to determine the efficiency and specificity of antileukemia properties for DMAPT. Figure 1A shows that 24-hour exposure of primary human AML cells to either 7.5 μM PTL or DMAPT results in similar mean viability in total and CD34+ cell populations (25% vs 24%, and 12% vs 12%, respectively; n = 25 for PTL and n = 39 for DMAPT). Table S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article) shows the percentage of viability for each of the primary AML specimens after treatment with either PTL or DMAPT. We further investigated the levels of cell death in phenotypically primitive AML stem cells (CD34+CD38−CD123+). Figure 1B shows the results of cells treated with DMAPT in which 5.0 μM and 7.5 μM concentrations resulted in 15.10% (n = 19) and 6.84% (n = 21) mean viability, respectively. Dose response studies show that the LD50 for DMAPT in primary AML cells is 1.7 μM. Taken together, the data in Figure 1A and 1B indicate that DMAPT is highly cytotoxic to both overall AML blast cells as well as the AML stem cell population. To extend our analyses of DMAPT, we also examined other hematologic disorders known to derive from malignant stem cells. Table S2 provides the percentage of viability for primary blast crisis (bc) CML and B-ALL specimens after treatment with either PTL or DMAPT. Figure 1C and 1D show the effect of DMAPT on phenotypically described B-ALL stem/progenitor cells (CD34+CD10−)3 and CML stem/progenitor cells (CD34+CD38−).4 These experiments indicate DMAPT also has utility for lymphoid and chronic myeloid forms of leukemia. Finally, to verify the specificity of DMAPT for malignant cells, viability of normal hematopoietic cells was determined. Table S3 shows the percentage of viability for normal mononuclear cells obtained from healthy donors after treatment with either PTL or DMAPT. The data in Figure 1E demonstrate that DMAPT does not significantly affect viability of normal CD34+ or CD34+CD38− hematopoietic cells obtained from healthy donors (n = 8; P > .05). Indeed, the mean viability at 5 μM DMAPT was 96% for CD34+ cells and 88% for CD34+CD38− cells. At 7.5 μM, the mean viability for both CD34+ and CD34+CD38− populations was more than 79%. Together, the data demonstrate that DMAPT not only induces rapid cell death in phenotypically described AML, bcCML, and ALL stem/progenitor cells, but is also well tolerated by normal stem and progenitor cells.
DMAPT induces death of primary human AML, ALL, and CML cells, but not normal hematopoietic cells. (A) Percentage of viable cells of primary human AML cells exposed to either 7.5 μM PTL or DMAPT. Viability was measured by labeling with annexin-V and 7-AAD. Analysis of total AML versus selected CD34+ cells are indicated. (B) Percentage of viable cells of CD34+CD38− AML cells at the indicated concentrations of DMAPT. (C) Percentage of viable cells of CD34+CD10− ALL cells at the indicated concentrations of DMAPT. (D) Percentage of viable cells of CD34+CD38− CML cells at the indicated concentrations of DMAPT. (E) Percentage of viable cells of normal CD34+ or CD34+CD38− hematopoietic cells obtained from healthy donors (CB or BM) and treated at the indicated concentrations of DMAPT. In all panels, the horizontal bars represent the mean, and each circle or diamond represents 1 specimen. Analysis of each specimen was performed in triplicate, and the average was used to represent the results for a single specimen. All viability values are relative to untreated controls.
DMAPT induces death of primary human AML, ALL, and CML cells, but not normal hematopoietic cells. (A) Percentage of viable cells of primary human AML cells exposed to either 7.5 μM PTL or DMAPT. Viability was measured by labeling with annexin-V and 7-AAD. Analysis of total AML versus selected CD34+ cells are indicated. (B) Percentage of viable cells of CD34+CD38− AML cells at the indicated concentrations of DMAPT. (C) Percentage of viable cells of CD34+CD10− ALL cells at the indicated concentrations of DMAPT. (D) Percentage of viable cells of CD34+CD38− CML cells at the indicated concentrations of DMAPT. (E) Percentage of viable cells of normal CD34+ or CD34+CD38− hematopoietic cells obtained from healthy donors (CB or BM) and treated at the indicated concentrations of DMAPT. In all panels, the horizontal bars represent the mean, and each circle or diamond represents 1 specimen. Analysis of each specimen was performed in triplicate, and the average was used to represent the results for a single specimen. All viability values are relative to untreated controls.
Functional assays demonstrate that DMAPT ablates primary human AML stem and progenitor cells
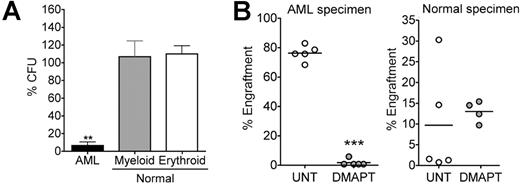
In vitro colony assays and NOD/SCID xenotransplantation experiments were used to determine whether DMAPT targets functionally defined leukemia progenitor and stem cells. Treatment of normal hematopoietic cells with 5 μM DMAPT did not affect myeloid or erythroid colony formation relative to untreated controls (Figure 2A; P > .05, n = 5). In contrast, DMAPT treatment strongly inhibited the ability of AML cells to form colonies (mean viability = 6.58%; P < .001, n = 10), indicating selective targeting of leukemic progenitor cell populations. Similarly, 18-hour treatment of primary AML cells with DMAPT dramatically inhibited engraftment of sublethally irradiated NOD/SCID mice (representative example shown in Figure 2B). Analysis of 4 independent AML specimens decreased engraftment by 98.2% (n = 5 mice; P < .001), 91% (n = 5; P = .001), 90% (n = 9; P < .002), and 85% (n = 4; P < .001) compared with untreated controls. In contrast, of 3 independent normal specimens tested, engraftment levels for DMAPT-treated cells were 144% (n = 5; P = .178), 166% (n = 5; P = .77), and 65% (n = 5; P = .06) relative to untreated controls, with no changes reaching statistical significance. Together, these data indicate that DMAPT specifically ablates AML stem and progenitor cells without affecting the growth or engraftment potential of normal primitive cells.
Progenitor/stem cell functional assays for DMAPT treated cells. (A) AML versus normal cells were treated with 5 μM DMAPT for 18 hours in suspension culture, followed by plating in methylcellulose culture. Horizontal bars represent the mean. **P < .001 AML versus erythroid and AML versus myeloid. The percentage of colony-forming units (CFU) was normalized to untreated control. All assays were performed in triplicate. Error bars represent SEM. (B) Representative examples of the percentage of engraftment achieved in NOD/SCID mice receiving AML (left panel) or normal CB (right panel) cells after 18 hours of culture with or without 7.5 μM DMAPT. Each symbol represents a single animal analyzed at 6 to 8 weeks after transplantation. Mean engraftment is indicated by the horizontal bars. ***P < .001 DMAPT versus untreated (UNT).
Progenitor/stem cell functional assays for DMAPT treated cells. (A) AML versus normal cells were treated with 5 μM DMAPT for 18 hours in suspension culture, followed by plating in methylcellulose culture. Horizontal bars represent the mean. **P < .001 AML versus erythroid and AML versus myeloid. The percentage of colony-forming units (CFU) was normalized to untreated control. All assays were performed in triplicate. Error bars represent SEM. (B) Representative examples of the percentage of engraftment achieved in NOD/SCID mice receiving AML (left panel) or normal CB (right panel) cells after 18 hours of culture with or without 7.5 μM DMAPT. Each symbol represents a single animal analyzed at 6 to 8 weeks after transplantation. Mean engraftment is indicated by the horizontal bars. ***P < .001 DMAPT versus untreated (UNT).
DMAPT treatment induces stress responses, inhibits NF-κB, and activates p53
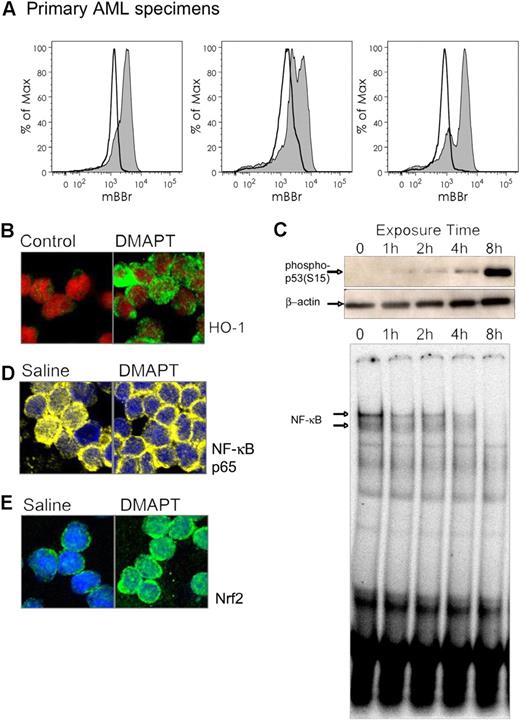
We have previously shown that induction of oxidative stress, inhibition of NF-κB, and activation of p53 are functions associated with anti-LSC activity in primary AML cells.7 In addition, we have identified prevalent pathways and genes affected by PTL using global gene expression analysis of CD34+ primary AML specimens (D.C.H. et al, manuscript in preparation). These experiments have identified strong up-regulation of NF-E2–related factor 2 (Nrf2) and its transcriptional target heme oxygenase 1 (HO-1) in response to PTL treatment. Both genes are part of a cytoprotective response against oxidative stress,21 and provide potential biomarkers for PTL-based drug responses. To evaluate the molecular consequences of DMAPT treatment, we first examined changes in oxidative state using the free thiol reactive dye mBBr. As shown in Figure 3A, labeling with mBBr was consistently reduced in specimens treated with DMAPT, indicating a strong decrease in intracellular free thiol groups. Subsequent analysis of stress response mechanisms showed that HO-1 levels strongly increase after 2 hours of treatment (Figure 3B), indicating that DMAPT induces a protective response against oxidative stress in the cell (n = 3). An induction of Nrf2 nuclear localization was also observed (data not shown). Interestingly, inhibition of NF-κB occurs more slowly, with some decrease evident within an hour, but maximal reduction is not apparent until 4 to 8 hours (Figure 3C bottom panel). Similarly, activation of p53 as detected by phosphorylation at ser15 is not evident until after approximately 8 hours of treatment (Figure 3C top panel). Notably, the antileukemia effect of DMAPT on AML cells becomes nonreversible only after 8 or more hours of exposure (data not shown), suggesting that changes in NF-κB and p53 may represent final (or late) steps in the commitment to cell death process. Together, these data indicate that DMAPT induces a rapid induction of oxidative stress followed by a series of cellular responses that include downstream stress control proteins and modulation of both survival and tumor suppressor mechanisms. These findings are in good agreement with previous hypotheses on the mechanisms that regulate selective targeting of LSCs.10
DMAPT induces stress responses and inhibits NF-κB. (A) Primary AML cells labeled with thiol-reactive dye mBBr before (shaded histograms) and after (open histograms) exposure to DMAPT. (B) Confocal micrograph of primary human AML cells with treated with 7.5 μM DMAPT for 2 hours. HO-1 (green) and nucleus (ToPro3, represented in red). (C) Immunoblots (top 2 panels) for phospho p53ser15 (top) or actin (middle) of CD34+ primary human AML cells treated with 7.5 μM DMAPT for the indicated times. Bottom panel shows an EMSA for NF-κB binding for the same treatment. (D-E) NOD/SCID mice engrafted with human AML cells 6 weeks prior to the experiment were treated with a single intraperitoneal dose of 100 mg/kg DMAPT or saline control. At 1 hour later, animals were killed, and BM was harvested and analyzed by confocal microscopy. (D) NF-κB (p65 subunit in yellow). (E) Nrf2 (green). The nucleus is shown in blue for both panels.
DMAPT induces stress responses and inhibits NF-κB. (A) Primary AML cells labeled with thiol-reactive dye mBBr before (shaded histograms) and after (open histograms) exposure to DMAPT. (B) Confocal micrograph of primary human AML cells with treated with 7.5 μM DMAPT for 2 hours. HO-1 (green) and nucleus (ToPro3, represented in red). (C) Immunoblots (top 2 panels) for phospho p53ser15 (top) or actin (middle) of CD34+ primary human AML cells treated with 7.5 μM DMAPT for the indicated times. Bottom panel shows an EMSA for NF-κB binding for the same treatment. (D-E) NOD/SCID mice engrafted with human AML cells 6 weeks prior to the experiment were treated with a single intraperitoneal dose of 100 mg/kg DMAPT or saline control. At 1 hour later, animals were killed, and BM was harvested and analyzed by confocal microscopy. (D) NF-κB (p65 subunit in yellow). (E) Nrf2 (green). The nucleus is shown in blue for both panels.
DMAPT pharmacology
To further characterize the potential utility of DMAPT, preliminary pharmacologic studies were performed. Not surprisingly, substantial pharmacokinetic (PK) differences were observed in rodent and canine studies. In mice, an oral DMAPT dose of 100 mg/kg achieved a concentration maximum (Cmax) of 25 μM and a half-life (T1/2) of 0.63 hours in serum. In contrast, canine studies showed a Cmax of 61 μM, with a T1/2 of 1.9 hours when DMAPT was dosed at 100 mg/kg orally. For both the mouse and canine models, oral bioavailability was approximately 70% compared with intravenous administration. These characteristics represent a significant improvement over PTL, which demonstrated a maximum serum concentration of 200 nM in mice when dosed at 40 mg/kg, which is the highest dose attainable given the relative insolubility of PTL.18 Furthermore, in preliminary toxicology studies, daily administration of 100 mg/kg DMAPT to mice for 10 consecutive days was well tolerated with no evidence of acute toxicity or changes in hematologic parameters. Similarly, daily oral dosing of dogs at 50 to 100 mg/kg for 14 consecutive days was well tolerated. Collectively, these studies indicate that the pharmacologic properties of DMAPT are superior to PTL.
In vivo biological activity of DMAPT in a murine model
Increased oxidative stress and inhibition of NF-κB are consistent in vitro features of DMAPT treatment (Figure 3A-C). Thus, we tested whether such responses were also evident in vivo where they could serve as potential biomarkers for DMAPT activity. To this end, we injected primary human AML cells into sublethally irradiated NOD/SCID mice to establish xenografts. At 6 weeks after injection (a time at which the AML cells have strongly engrafted in bone marrow), mice were treated with a single oral dose of 100 mg/kg DMAPT. At 1 hour after treatment, animals were killed to evaluate the bioactivity of DMAPT in human AML cells isolated from the bone marrow. Figure 3D shows that the NF-κB p65 subunit (yellow) is localized to the cytoplasm upon drug treatment (nucleus shown in blue), an activity indicating substantial inhibition of NF-κB (n = 3). In addition, Figure 3E shows that Nrf2 (green) localized to the nucleus, indicating its typical activation in response to oxidative stress (n = 3). Together, these data indicate that DMAPT induces both the activation of stress responses and inhibition of NF-κB in vivo, and that both of these measurements can be used as biomarkers to monitor drug activity.
In vivo biological activity of DMAPT in spontaneous canine leukemias
Spontaneous leukemias are well documented in dogs, and thus provide a large animal system in which to investigate drug activities. In addition, as mentioned, the pharmacology observed for DMAPT was notably better in dogs than in mice. Therefore, we used studies of primary canine leukemia as a means to further characterize the activity of DMAPT. First, in vitro studies were conducted to examine whether NF-κB is constitutively active in canine leukemias and whether such cells are sensitive to DMAPT. Constitutive NF-κB activity (as measured by EMSA) was evident in 7 of 8 specimens tested (Figure S2A). Further, in vitro exposure to DMAPT for 24 hours resulted in decreased NF-κB activity (Figure S2C) as well as decreased cell survival (36% mean viability at 10 μM, n = 6; Figure S2B).
After establishing that DMAPT had similar effects in spontaneous canine leukemia cells as in human cells in vitro, we proceeded to test the in vivo biological activity of DMAPT by treating dogs with spontaneous leukemias. Notably, canine leukemias are usually only detected at advanced stages, when dogs diagnosed with acute disease have a median time to death of approximately 16 days. Thus, the clinical window of opportunity to evaluate treatment regimens is generally very brief. Nonetheless, canine studies provide a useful large animal model in which to assess basic parameters of drug activity.
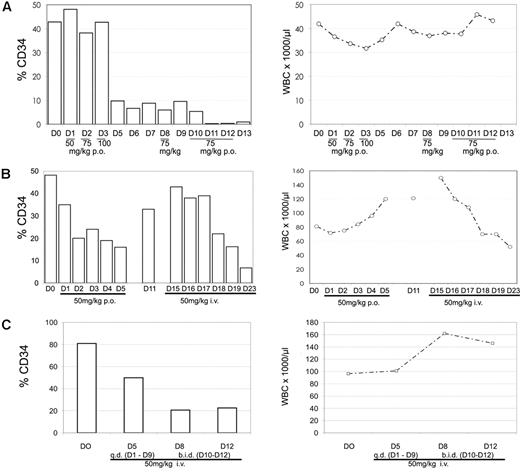
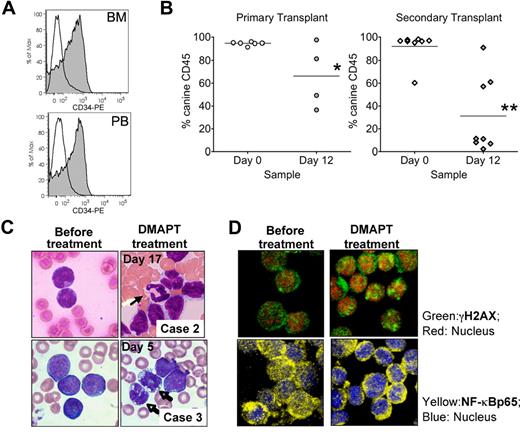
DMAPT was tested in 3 dogs diagnosed with CD34+ spontaneous leukemia. Both oral and intravenous routes of administration were used over daily dosing regimens ranging from 3 to 12 days. A summary of the peripheral blood analysis from each study is shown in Figure 4. Although the animals were from different breeds and under different stages of supportive care (“Canine studies”), we observed a rapid and consistent reduction in the level of CD34+ cells. Further, marrow analysis of 1 animal before and after treatment also showed a strong reduction in CD34+ cells (Figure 5A), indicat-ing that the effects of DMAPT treatment were similar for marrow-resident versus peripheral leukemia cells. In contrast, overall WBC counts were variable during the treatment regimen for each dog, with no clear reduction except in animal no. 2 (Figure 4B). The marked loss of CD34+ cells for each animal led us to speculate that either differentiation and/or selective ablation of more primitive cells may have occurred. Analysis of blood cell morphology showed increased levels of peripheral neutrophils, as well as a change in the morphology of blast cells (Figure 5C), supporting the concept that differentiation of the tumor was increased. Further, to assess LSC-specific effects, we performed functional assays using the NOD/SCID xenotransplantation system. Analysis of pretreatment peripheral blood cells from each dog showed that leukemia specimens from animals no. 2 and no. 3 readily engrafted NOD/SCID mice (Figure S3). Canine cells in NOD/SCID marrow were almost entirely CD34+ and had a blastlike morphology as determined by hematoxylin and eosin (H&E) stain, thereby indicating the leukemic origin of engrafting cells. For animal no. 3, we had sufficient material to compare pre- and posttreatment specimens to determine whether NOD/SCID engraftment (ie, LSC activity) varied. As shown in Figure 5B left panel), a significant reduction in the level of canine cells in NOD/SCID marrow was observed after DMAPT treatment (P = .043). To further assess the self-renewal potential of canine LSCs, marrow from primary NOD/SCID animals was retransplanted into secondary recipient mice (Figure 5B right panel). These studies showed an even more profound reduction in engraftment potential for canine cells treated with DMAPT (P = .002), suggesting that the canine LSCs were significantly impaired by the drug in vivo.
DMAPT decreases CD34 surface expression in canine studies. The percentage of CD34+ cells was determined for PB samples obtained at the indicated day of treatment (left panels, □). WBC counts for the same samples are shown in the right panels. The dose of DMAPT and day and route of administration are shown on the horizontal axis for each study. (A) Canine study case no. 1. (B) Case no. 2. (C) Case no. 3.
DMAPT decreases CD34 surface expression in canine studies. The percentage of CD34+ cells was determined for PB samples obtained at the indicated day of treatment (left panels, □). WBC counts for the same samples are shown in the right panels. The dose of DMAPT and day and route of administration are shown on the horizontal axis for each study. (A) Canine study case no. 1. (B) Case no. 2. (C) Case no. 3.
DMAPT demonstrated biological activity in vivo. (A) Overlays for CD34 expression in BM (top) and PB (bottom) from pretreatment (filled histograms) and posttreatment (open histograms) specimens from canine case no. 1. (B) Percentage of canine cell engraftment achieved in NOD/SCID mice receiving pretreatment (Day 0) versus post-treatment (Day 12) cells from canine case no. 3. Each symbol represents a single animal analyzed at 6 to 8 weeks after transplantation. Primary and secondary transplantations are shown in the left and right graphs, respectively. Mean engraftment is indicated by the horizontal bars. *P = .043; **P = .002. (C) Blood smears for PB samples obtained at the indicated days and cases. (D) Confocal micrograph for canine cells obtained before treatment (day 0) or from day 5 after initial treatment with 50 mg/kg oral dose DMAPT. Top panels show cells stained for NF-κB p65 (yellow) and ToPro3 (blue). Bottom panels show γH2AX (green) and ToPro3 (red).
DMAPT demonstrated biological activity in vivo. (A) Overlays for CD34 expression in BM (top) and PB (bottom) from pretreatment (filled histograms) and posttreatment (open histograms) specimens from canine case no. 1. (B) Percentage of canine cell engraftment achieved in NOD/SCID mice receiving pretreatment (Day 0) versus post-treatment (Day 12) cells from canine case no. 3. Each symbol represents a single animal analyzed at 6 to 8 weeks after transplantation. Primary and secondary transplantations are shown in the left and right graphs, respectively. Mean engraftment is indicated by the horizontal bars. *P = .043; **P = .002. (C) Blood smears for PB samples obtained at the indicated days and cases. (D) Confocal micrograph for canine cells obtained before treatment (day 0) or from day 5 after initial treatment with 50 mg/kg oral dose DMAPT. Top panels show cells stained for NF-κB p65 (yellow) and ToPro3 (blue). Bottom panels show γH2AX (green) and ToPro3 (red).
Finally, to examine in vivo biomarkers of DMAPT activity, the levels of γH2AX22 and the NF-κB p65 subunit were analyzed in cells from specimens before and after treatment. As shown in Figure 5D, increased γH2AX and decreased nuclear NF-κB p65 were detected after treatment, indicating that DMAPT induced oxidative stress and inhibited NF-κB in vivo.
Discussion
In vivo targeting of LSCs represents a formidable challenge to the leukemia research field. Not only must future therapies more effectively eradicate LSCs, they must do so with less collateral damage to normal tissues. Several biological features of normal stem cells are retained in malignant populations and likely contribute to the difficulty in targeting the LSC population. For example, a mostly quiescent cell-cycle status, expression of xenobiotic efflux pumps, and a protective microenvironmental niche are all factors that may shield LSC from therapeutic insult.23 Thus, these parameters and possibly other aspects of in vivo biology must be considered for the development of improved regimens.
From basic studies of primary human tissue, we have previously proposed that 2 types of events are necessary to induce preferential induction of cell death in LSCs: (1) inhibition of survival signals (such as NF-κB) and (2) activation of stress responses.17 Importantly, neither of these events alone appears to mediate substantial killing of LSCs.16 We hypothesize that stress responses such as increased activity of heat-shock proteins, DNA damage pathways, and oxidative stress response factors (ie, HO-1 and Nrf2) are a direct result of drug-induced cellular damage, and that increased NF-κB is a protective reaction to the insult. Thus, by inhibiting elements of the NF-κB pathway (or similar survival factors), the detrimental effects of stress, such as increased oxidative load, are uncovered by regimens that mediate both effects. We further hypothesize the PTL-based drugs fall into this class of drug, in which both induction of stress and inhibition of survival signals are central to the therapeutic mechanism.
In the present report, we have enhanced the antileukemia features of PTL by creating a more pharmacologically useful form of the drug, DMAPT, and by demonstrating that the analog retains key properties of the parent molecule. In vitro treatment of primary human AML, ALL, and bcCML cells with DMAPT demonstrated potent eradication of leukemic stem and progenitor cells, as well as the overall blast population (Figure 1). Importantly, functional assays demonstrated that DMAPT specifically ablated primitive human leukemia cells without impairing their normal counterparts. Together, these data indicate that DMAPT is a novel therapeutic candidate for targeting human LSCs, and may have utility against a broad range of hematologic cancers.
These findings provide a strong rationale for taking DMAPT forward to human clinical trials for leukemia. However, we were cognizant that further characterization of the drug in some type of preclinical in vivo model could also be of value. Although numerous studies have used human-mouse xenografts to study biological properties of stem cells in vivo, there has been almost no use of such systems for therapeutic modeling. Indeed, the metabolic differences between mouse and human physiology make pharmacologic comparisons difficult. Thus, our human-mouse xenograft experiments were limited to relatively simple single-dose pharmacodynamic studies. In order to derive data more pertinent to human disease, we extended our in vivo studies to include analysis of spontaneous acute leukemia in dogs. While the analysis of canine disease is attractive because it provides a unique means to investigate authentic leukemia in a large animal system, it is also challenging due to the advanced stage in which disease is typically detected. Nonetheless, we were able to conduct 3 case studies, roughly equivalent to human phase 1 clinical trials, in which pilot feasibility and pharmacodynamic analyses were performed (Figures 4,5). The findings from those studies indicate clear in vivo activity of DMAPT, as assessed by 2 independent biomarkers, in good agreement with previous data from in vitro studies and the mouse xenograft model. In addition, we also observed biological changes in tumor cells suggestive of drug efficacy. In all treated animals, a rapid decrease in the percentage of CD34+ cells was detected, accompanied by an increase in the frequency of differentiated cells. More importantly, functional analysis of specimens taken from animal no. 3 before and after DMAPT treatment showed a clear reduction in the engraftment potential of canine LSCs in the NOD/SCID xenograft system. To our knowledge, these data are the first to indicate in vivo targeting of LSCs in any kind of large animal. While the underlying mechanism requires further analysis, the overall findings indicate that drug-induced differentiation may be responsible. Notably, in vitro studies using U937 myelomonocytic cells indicate subtoxic concentrations of DMAPT may induce differentiation (Figure S4). Further, a recent report by Gopal et al has demonstrated that parthenolide is a potent inhibitor of HDAC1,24 which validates the concept that parthenolide-based drugs may be epigenetic modifiers and thereby function via mechanisms that include differentiation induction.
Taken together, the data indicate that DMAPT mediates in vivo biological changes in leukemia cells that will lead to their impairment and/or death. Moreover, given the strong efficacy of the drug for AML stem and progenitor cells in vitro, we propose that a similar effect is possible in vivo, and provide preliminary evidence that LSC-specific targeting can occur in spontaneous canine leukemia. Based on these preclinical findings, its oral bioavailability, and a favorable toxicology profile, DMAPT is proceeding to human phase 1 clinical trials in the near future.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We dedicate this manuscript in loving memory of Isaac Greenlaw, whose spirit lives on through his contributions to our work.
We sincerely thank Dr Timothy Bushnell for expert advice in flow cytometry and Dr Anne Avery for assistance with canine pathology studies.
This work was supported by the Douglas Kroll Research Foundation, the Leukemia and Lymphoma Society (6099-06), the National Cancer Institute (NCI: R01CA90446), and the US Department of Defense (DAMD17-03-1-0263). C.T.J. is a Scholar of the Leukemia and Lymphoma Society. Pharmacology studies were supported in part through the National Cancer Institute Rapid Access to International Development Program of the Developmental Therapeutics Program, Division of Cancer Treatment and Diagnosis, NCI, National Institutes of Health.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services nor does mention of trade names, commercial products, or any organization imply endorsement by the US Government.
National Institutes of Health
Authorship
Contribution: M.L.G. designed and performed research, analyzed data, and helped write the paper. R.M.R., X.L., C.C., J.L.L., and A.V. performed research. S.N. designed and performed research, and analyzed data. D.C.H., M.W.B., and E.S. performed research and analyzed data. J.M.B. analyzed data. C.J.S. contributed vital reagents and analyzed data. W.M. analyzed data and helped write the paper. M.C. contributed vital reagents and analyzed data. J.L.L. contributed vital reagents. P.A.C. designed research and analyzed data. C.T.J. designed research, analyzed data, and helped write the paper.
Conflict-of-interest disclosure: W.M. is president of Leuchemix, which has a financial interest in DMAPT. C.J.S., P.A.C., and C.T.J. are stockholders in Leuchemix. All other authors declare no competing financial interests.
Correspondence: Craig T. Jordan, University of Rochester Medical Center, 601 Elmwood Ave, Box 703, Rochester, NY 14642; e-mail:craig_jordan@urmc.rochester.edu.