Abstract
Nbs1, a member of the Mre11-RAD50-Nbs1 complex, is phosphorylated by ATM, the product of the ataxia-telangiectasia mutated gene and a member of the phosphatidylinositol 3-kinase–related family of serine-threonine kinases, in response to DNA double-strand breaks (DSBs) to regulate DNA damage checkpoints. Here we show that BCR/ABL stimulated Nbs1 expression by induction of c-Myc–dependent transactivation and protection from caspase-dependent degradation. BCR/ABL-related fusion tyrosine kinases (FTKs) such as TEL/JAK2, TEL/PDGFβR, TEL/ABL, TEL/TRKC, BCR/FGFR1, and NPM/ALK as well as interleukin 3 (IL-3), granulocyte-macrophage colony-stimulating factor (GM-CSF), and stem cell factor (SCF) also stimulated Nbs1 expression. Enhanced ATM kinase–dependent phosphorylation of Nbs1 on serine 343 (S343) in response to genotoxic treatment was detected in leukemia cells expressing BCR/ABL and other FTKs in comparison to normal counterparts stimulated with IL-3, GM-CSF, and SCF. Expression of Nbs1-S343A mutant disrupted the intra–S-phase checkpoint, decreased homologous recombinational repair (HRR) activity, down-regulated XIAP expression, and sensitized BCR/ABL-positive cells to cytotoxic drugs. Interestingly, inhibition of Nbs1 phosphorylation by S343A mutant enhanced the antileukemia effect of the combination of imatinib and genotoxic agent.
Introduction
BCR/ABL oncogenic tyrosine kinase, the product of the translocation between the c-ABL gene from chromosome 9 and the BCR gene on chromosome 22 [t(9,22)],1 is present in chronic myeloid leukemia (CML) patients2 as well as in a cohort of acute lymphocytic leukemia (ALL) patients.3
Imatinib mesylate (IM), a selective inhibitor of ABL kinase activity, revolutionized the treatment of BCR/ABL-positive leukemias.4 IM induces hematologic remissions in nearly all CML–chronic phase (CML-CP) patients; complete cytogenetic remission (CCR) is achieved in 76% of newly diagnosed patients.5 However, almost all patients remained reverse transcriptase–polymerase chain reaction (RT-PCR) positive for BCR/ABL for up to 4 years,6 arguing that some leukemia cells are not eliminated.
Strategies to enhance the effect of IM and eventually overcome the resistance are dose escalation, addition of a growth factor, and combination with novel tyrosine kinase inhibitors like AMN107 and dasatinib or with inhibitors targeting downstream BCR/ABL effectors (eg, PI-3k).7-9 Unfortunately, resistance to small-molecule inhibitors is likely to appear.10 Moreover, the inhibitors may not eliminate all leukemia stem cells because they remove the proliferative advantage but do not induce apoptosis.11 Altogether, it appears that IM therapy should be combined with other agents to achieve a curative effect.
BCR/ABL plays a number of roles in transformation including protection from apoptosis, rendering cells independent of growth factors, promoting invasion and metastasis, and modulating responses to DNA damage causing drug resistance and genomic instability.12-14 BCR/ABL is a founder of the family of fusion tyrosine kinases (FTKs) such as TEL/ABL, TEL/JAK2, TEL/PDGFβR, TEL/TRKC, BCR/FGFR, NPM/ALK, and others, resulting from chromosomal translocations and causing changes in response to genotoxic treatment.15-17
FTK-positive cells may accumulate even higher levels of DNA damage in comparison to their normal counterparts,18,19 but the former cells repair the lesions more proficiently.20-23 FTK-mediated drug resistance has not been completely characterized, although at least 3 mechanisms have been described. The first mechanism involves the prolongation of the G2/M and intra-S cell-cycle checkpoints, which may allow more time for repair of damaged DNA.19,24,25 In the second mechanism, BCR/ABL protects from apoptosis via overexpression of Bcl-xL, an antiapoptotic protein that inhibits caspase-3 activation.26 In the third mechanism, BCR/ABL-positive cells may display higher activity of homologous recombinational repair (HRR) and nonhomologous end-joining (NHEJ), 2 major mechanisms responsible for DNA double-strand breaks (DSBs).18,23,27 Finally, we have reported that G2/M arrest, Bcl-xL, and HRR work in concert to protect FTK-positive cells from genotoxicity.28
The Mre11-RAD50-Nbs1 (MRN) protein complex is a major player in cellular responses to DSBs, which include break sensing and activation of signaling pathways that control cell-cycle checkpoints in response to damage and also repair.29 Nbs1 plays an important role in activation of intra-S30 and G2/M31 cell-cycle checkpoints. ATM, the product of the ataxia-telangiectasia mutated gene and a member of the phosphatidylinositol 3-kinase–related family of serine-threonine kinases, senses DNA damage resulting in autophosphorylation and interacts with and phosphorylates Nbs1, which can then reciprocally modulate the phosphorylation of ATM.32-34 This leads to the activation of an intra–S-phase checkpoint by phosphorylation of SMC135 and/or FANCD236 or a G2/M checkpoint via the phosphorylations of Chk2 and the phosphatase Cdc25a.31 In addition to the regulation of checkpoints, Nbs1 may also play a direct role in the DSB repair by HRR37,38 and NHEJ.39 In agreement, expression of an Nbs1 mutant, which disrupts the MRN complex, was associated with an increased sensitivity to cisplatin.40
On the other hand, overexpression of the wild-type Nbs1 has been implicated as a marker for aggressive head and neck squamous cell carcinoma and melanoma.41,42 In addition, the overexpression of Nbs1 was demonstrated to contribute to transformation through activation of phosphatidylinositol 3-kinase/Akt in Rat1a and HeLa cells.43 These reports implicate Nbs1 in carcinogenesis.
Here, we report that expression and phosphorylation of Nbs1 is elevated in tumor cells expressing BCR/ABL and other FTKs. This enhanced phosphorylation of Nbs1 leads to increased resistance to genotoxic agents in BCR/ABL-positive cells most likely by prolonging the intra–S-phase checkpoint and allowing more time for DNA repair to occur. In addition, we show that targeting both Nbs1 phosphorylation and BCR/ABL kinase activity leads to abrogation of drug resistance in leukemia cells.
Patients, materials, and methods
Cells
Murine cells (32Dcl3, Baf3, and FL512) and human cells (M07e and UT7), and their FTK-transformed counterparts, have already been described.28,44 Cell lines were cultured in Iscove modified Dulbecco medium (IMDM) supplemented with 10% fetal bovine serum (FBS) and pretested concentrations of WEHI- and BHK-MKL–conditioned medium as a source of interleukin-3 (IL-3; for murine cells) or stem cell factor (SCF; for human cells), respectively, required to maintain the proliferation of parental cells. Human embryonic kidney 293T cells and amphotropic Phoenix packaging cells (American Type Culture Collection, Manassas, VA) were cultured in DMEM supplemented with 10% FBS. CD34+ normal bone marrow cells were from Cambrex Bio Science Walkersville (Walkersville, MD) and those from CML–chronic phase (CML-CP) and CML–blast crisis (CML-BC) patients were obtained from the Stem Cell and Leukemia Core Facility of the University of Pennsylvania (Philadelphia, PA) and the Institute of Hematology and Blood Transfusions (Warsaw, Poland) after receiving informed consent in accordance with the Declaration of Helsinki. Primary cells were seeded in IMDM supplemented with 10% FBS and conditioned medium from BHK-MKL cells (10% vol/vol), as a source of SCF, and 5 ng/mL of recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF; PeproTech, Rocky Hill, NJ).
Inhibitors
ABL kinase inhibitor imatinib mesylate (IM) was obtained from Novartis Pharma AG (Basel, Switzerland). The caspases inhibitor Z-VAD-FMK was purchased from Bachem AG (Bubendorf, Switzerland) and proteasome inhibitor epoxomycin was purchased from Biomol (Plymouth Meeting, PA).
Hematopoietic cell transfections
Human Nbs1 and Nbs1-S343A cDNAs were kindly obtained from Dr John Petrini (Memorial Sloan-Kettering Cancer Center, New York, NY). Wild-type Nbs1 and Nbs1-S343A mutant were cloned into pMig1-IRES-GFP retroviral construct (gift from Dr Warren Pear, University of Pennsylvania, Philadelphia, PA). Amphotropic Phoenix packaging cells were used to produce retroviral particles, and infections were performed as described.28 Green fluorescent protein (GFP)+ cells were isolated by sorting.
Cytotoxic treatment
The following cytostatic agents were used: mitomycin C (MMC), hydroxyurea (HU; Sigma Chemical, St Louis, MO), and cisplatin (Cis; Platinol-AQ; Bristol-Myers Squibb, Princeton, NJ). Indicated concentrations of the drugs were added to cells growing in suspension (105/mL) or in semisolid medium (MethoCult H4230; Stem Cell Technologies, Vancouver, BC, Canada) supplemented with the pretested concentrations of SCF and/or GM-CSF necessary to maintain proliferation of normal cells. Viable cells in suspension cultures were detected 24 and 48 hours later by Trypan blue exclusion. Colonies growing in semisolid medium were counted after 7 days. Cell-cycle analysis was performed as described.28
Western blot analysis
Cells were lysed in 1 × SDS sample buffer by boiling for 3 minutes, followed by sonication for 10 to 15 seconds. Cell lysates were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and examined by Western blot analysis using antibodies recognizing the following proteins: Mre11 (BD Biosciences, Franklin Lakes, NJ); RAD50 (Novus Biologicals, Littleton, CO); Nbs1, pNbs1-S343, Bcl-xL, active caspase-3, p53, actin (all from Santa Cruz Biotechnology, Santa Cruz, CA); phosphorylated tyrosine (Upstate Biotechnology, Lake Placid, NY); tubulin, c-ABL (Calbiochem, San Diego, CA); XIAP (Abcam, Cambridge, MA); and PARP (Cell Signaling Technology, Beverly, MA).
Luciferase assay
The assay was performed as previously described.28 Briefly, 293T cells were transiently transfected by calcium phosphate with 10 μg pMig plasmids containing IRES-GFP only, BCR/ABL wild-type-IRES-GFP, or BCR/ABL kinase–deficient mutant (K1172R)-IRES-GFP (kindly obtained from Dr Warren Pear), as well as 10 μg of Nbs1 promoter-reporter plasmids NBSLuc1500 and NBSLuc1500Mut (generously provided by Dr K.J. Wu, National Yang-Ming University, Taipei, Taiwan).45 In addition, 293T cells were transfected with pMig plasmids containing IRES-GFP or BCR/ABL wild-type IRES-GFP and pSRαMSVtkneo empty plasmid or that containing c-Myc–In373 dominant-negative mutant (DNM).46 In addition, M07e parental and B/A-M07e cells were electroporated with 10 μg of NBSLuc1500 and NBSLuc1500Mut plasmids. A β-galactosidase plasmid (5 μg) was cotransfected as a transfection efficiency control. Luciferase was quantified by the Luciferase Assay System (Promega, Madison, WI). Transfection efficiency was normalized by measuring β-galactosidase activity.
Immunofluorescence
Cells growing in suspension were cytospun and cell staining and images were processed as previously described.47 pNbs1-S343 (Santa Cruz Biotechnology) and γ-H2AX (Upstate Biotechnology) primary antibodies were used. Secondary antibodies conjugated to Alexa Fluor 488 or Alexa Fluor 568 were applied (Molecular Probes, Eugene, OR). Negative controls were performed with nonimmune isotype counterparts of the primary antibodies. DNA was counterstained with fluorochrome 4′,6′ diamedino-2-phenylindole (DAPI, Molecular Probes, Eugene, OR). Specific staining was visualized with an inverted Olympus IX70 fluorescence microscope equipped with a U Plan Apochromat 100×/1.35 oil objective and a Cook Sensicom ER camera (Olympus America, Melville, NY). A series of three-dimensional images of each individual picture (cell) were stored in the SlideBook 3.0.1 (Intelligent Imaging Innovations, Denver, CO). De-convolution was applied to increase resolution and contrast of the images. A collection of three-dimensional images describing individual cells was converted to one two-dimensional picture. Pictures were prepared with Adobe Photoshop 6.0 (Adobe Systems, San Jose, CA). Two hundred cells from each group were scored as negative (< 4 foci), low-positive (4-20 foci), or high-positive (> 20 foci) for pNbs1 foci.
Radio-resistant DNA synthesis assay
Radio-resistant DNA synthesis (RDS) assay was performed as described48 with modifications. Briefly, cells were grown in appropriate media and labeled with 20 nCi/mL (740 Bq/mL) of 14C-thymidine for 48 hours to control DNA content prior to DNA damage. Cells were washed free of label, rested in nonradioactive medium for 12 hours, treated or not with 0.25 μg/mL MMC for 4 hours, incubated with 2.5 μCi/mL (92.5 kBq/mL) of 3H-thymidine for 1 hour, and then washed and placed onto Whatman glass microfiber filters. TCA precipitation was performed followed by cell fixing with 100% ethanol.
HRR assay
HRR efficiency was measured as described before49,50 with modification. Cells were electroporated with 100 μg of pCβA-Sce expression plasmid encoding I-SceI endonuclease and 20 μg of pDsRed1-Mito (Clontech, Palo Alto, CA). Expression of I-SceI causes a DSB in the I-SceI restriction site included in the DR-GFP cassette, and an HRR event restores the functional GFP gene containing the BcgI restriction site. because the cells used here already have GFP cDNA after the transfection with pMig1-IRES-GFP and pMig1-IRES-Nbs1-S343A mutant, semiquantitative PCR was applied to examine HRR. A similar approach was used before to evaluate NHEJ in this experimental model.51 HRR-specific and control products were simultaneously amplified from genomic DNA isolated from Red1-positive cells using the pairs of primers specific for DR-GFP cassette: 15 pmol each of 2133-2150 (BcgI sequence) and 3092-3112 primer, generating a 980-bp HRR-specific product; and 1.5 pmol each of 1340-1359 and 1695-1716 primer, producing a reaction control fragment. Ten-fold less control primers were used to increase the sensitivity of detection of the HRR product. PCR did not amplify any fragments from pMig1-IRES-GFP cDNA, and HRR products were detected only after induction of a DSB by I-SceI.
NHEJ assay
NHEJ was measured in nuclear protein extracts as described before,21 using pBluescript KS + linear plasmid digested XhoI + XbaI to generate noncompatible 5′ overhangs. Products of NHEJ reaction were resolved in 0.5% agarose gel containing 0.5 μg/mL ethidium bromide, scanned with Adobe Photoshop 6.0 (Adobe Systems), and analyzed by ImageQuant TL (Amersham Bioscience, Piscataway, NJ).
Results
BCR/ABL kinase stimulates the expression of Nbs1 by c-Myc–mediated transcriptional activation and protection from caspase-dependent degradation
Western blot analysis of various murine and human cell lines showed that Mre11 and Nbs1, but not RAD50, protein expression was up-regulated approximately 6- and 4-fold, respectively, in various BCR/ABL-positive cells in comparison to parental counterparts in the absence of growth factors (IL-3, SCF), whereas expression of all 3 proteins was equal in the presence of growth factors (Figure 1A).
BCR/ABL kinase elevates the expression of Nbs1 by stimulation of c-Myc–mediated transactivation and inhibition of caspase-dependent degradation. (A) Mre11, Nbs1, and RAD50 expression was examined by Western-blot analysis in total cell lysates from BCR/ABL (B/A)–positive or parental (P) mouse (32D, Baf3, and FL512) and human (M07e and UT7) cell lines. Cells were incubated for 12 hours in the presence or absence of growth factors (IL-3 or SCF) as indicated. Actin was detected as a loading control. (B-D) M07e parental (P) and BCR/ABL-positive (B/A) cells were starved from growth factor for 12 hours in the presence or absence of 1 μM IM (B), 1 μM epoxomycin (Epox) (C), and 20 μM Z-VAD-FMK (Z-VAD) (D). Expression of Mre11, Nbs1, RAD50, phosphotyrosine proteins (p-Tyr), p53, active caspase-3, and PARP (intact top and cleaved bottom band) was detected by Western-blot analysis. (E) The 293T cells were transiently transfected with the plasmid containing IRES-GFP (1), BCR/ABL-IRES-GFP (2), or BCR/ABL kinase–deficient mutant-IRES-GFP (3) along with the plasmids encoding the luciferase reporter gene driven by either the NBS promoter (NBSLuc1500, ■) or the NBS promoter containing the mutation in the E-box site preventing c-Myc binding (NBSLuc1500Mut, ▩). (F) M07e parental (1) and B/A-positive (2) cells were electroporated with NBSLuc1500 (■) or NBSLuc1500Mut (▩). (G) The 293T cells were transiently transfected with the plasmids containing NBSLuc1500 and IRES-GFP (1), BCR/ABL-IRES-GFP (2), or BCR/ABL-IRES-GFP and c-Myc–In373 (3). Luciferase activity is expressed in arbitrary units and results represent mean (± the standard deviation [SD]) of 3 independent experiments. P < .03 *in comparison to ■ in groups 1 and 3 in panel E and group 1 in panel F and to group 1 in panel G; **in comparison to ■ in group 2 in panels E and F and to bar 2 in panel G. Insets show the expression of BCR/ABL, c-Myc, and actin in particular groups.
BCR/ABL kinase elevates the expression of Nbs1 by stimulation of c-Myc–mediated transactivation and inhibition of caspase-dependent degradation. (A) Mre11, Nbs1, and RAD50 expression was examined by Western-blot analysis in total cell lysates from BCR/ABL (B/A)–positive or parental (P) mouse (32D, Baf3, and FL512) and human (M07e and UT7) cell lines. Cells were incubated for 12 hours in the presence or absence of growth factors (IL-3 or SCF) as indicated. Actin was detected as a loading control. (B-D) M07e parental (P) and BCR/ABL-positive (B/A) cells were starved from growth factor for 12 hours in the presence or absence of 1 μM IM (B), 1 μM epoxomycin (Epox) (C), and 20 μM Z-VAD-FMK (Z-VAD) (D). Expression of Mre11, Nbs1, RAD50, phosphotyrosine proteins (p-Tyr), p53, active caspase-3, and PARP (intact top and cleaved bottom band) was detected by Western-blot analysis. (E) The 293T cells were transiently transfected with the plasmid containing IRES-GFP (1), BCR/ABL-IRES-GFP (2), or BCR/ABL kinase–deficient mutant-IRES-GFP (3) along with the plasmids encoding the luciferase reporter gene driven by either the NBS promoter (NBSLuc1500, ■) or the NBS promoter containing the mutation in the E-box site preventing c-Myc binding (NBSLuc1500Mut, ▩). (F) M07e parental (1) and B/A-positive (2) cells were electroporated with NBSLuc1500 (■) or NBSLuc1500Mut (▩). (G) The 293T cells were transiently transfected with the plasmids containing NBSLuc1500 and IRES-GFP (1), BCR/ABL-IRES-GFP (2), or BCR/ABL-IRES-GFP and c-Myc–In373 (3). Luciferase activity is expressed in arbitrary units and results represent mean (± the standard deviation [SD]) of 3 independent experiments. P < .03 *in comparison to ■ in groups 1 and 3 in panel E and group 1 in panel F and to group 1 in panel G; **in comparison to ■ in group 2 in panels E and F and to bar 2 in panel G. Insets show the expression of BCR/ABL, c-Myc, and actin in particular groups.
Abrogation of BCR/ABL kinase activity by IM caused down-regulation of Mre11 and Nbs1, but not RAD50, in BCR/ABL-positive cells (Figure 1B), similar to levels seen in parental cells. To further characterize the mechanism(s) leading to the overexpression of Mre11 and Nbs1, the proteasome inhibitor epoxomycin as well as the broad caspase inhibitor Z-VAD-FMK were used in the absence of growth factor. Figure 1C shows that epoxomycin had no visible effect on Mre11 and Nbs1 protein expression, while as expected it protected p53 from degradation.52 However, the caspase inhibitor Z-VAD-FMK was able to increase Mre11 and Nbs1 protein expression in parental cells to levels seen in BCR/ABL-positive cells as well as inhibit the expression of an active caspase-3 fragment that was associated with reduced degradation of PARP (Figure 1D).
Furthermore, the luciferase assay detected transactivation of the Nbs1 promoter (NBSLuc1500) in 293T cells transfected with BCR/ABL (B/A), in comparison to those transfected with empty vector or kinase-deficient (KD) BCR/ABL mutant (Figure 1E), and in BCR/ABL-M07e leukemia cells, in comparison to parental counterparts (Figure 1F). Thus, not only Nbs1 protein but also the expression of Nbs1 mRNA is stimulated by the kinase activity of BCR/ABL. c-Myc was recently shown to directly regulate Nbs1 transcription in multiple cell lines, including lymphoblastoid CB33 cells, Rat1 fibroblasts, 293T embryonic kidney cells, and HeLa carcinoma, and is also known to be a critical component of transformation by BCR/ABL.45,46 Therefore NBSLuc1500Mut promoter, which is mutated in the E-box site located in the intron 1 region so that it can no longer bind c-Myc,45 was used. When binding of c-Myc to the Nbs1 promoter is prevented in the NBSLuc1500Mut, BCR/ABL-induced transactivation is abrogated in 293T and M07e cells (Figure 1E and F, respectively). In addition, c-Myc–In373 DNM abolished BCR/ABL-mediated NBSLuc1500 transactivation (Figure 1G). In similar conditions, c-Myc–In373 DNM reduced BCR/ABL-dependent transactivation of Werner syndrome gene by 2- to 3-fold (data not shown).
Enhanced phosphorylation of Nbs1 on serine 343 in BCR/ABL-positive cells treated with genotoxic agents
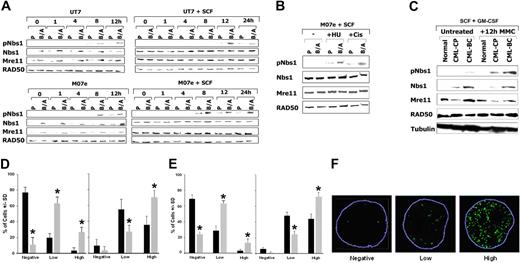
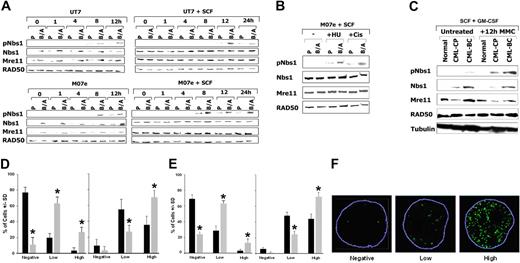
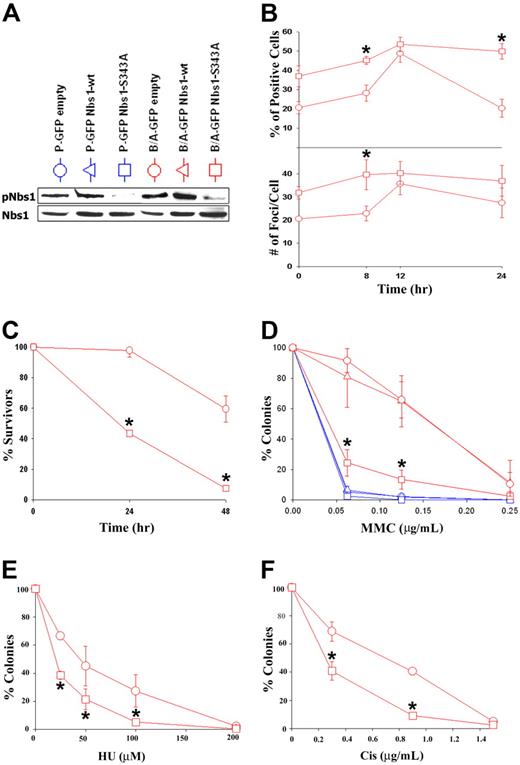
Nbs1-deficient cells are hypersensitive to mitomycin C (MMC).36 Therefore, the phosphorylation status of Nbs1 in response to MMC was examined. UT7 and M07e parental human hematopoietic cell lines and their BCR/ABL-positive counterparts were treated or not with 0.5 μg/mL MMC, and Nbs1 containing the phosphorylated S343 residue (pNbs1) was detected by Western blot analysis. Figure 2A shows that in the presence and absence of growth factors, Nbs1 is usually phosphorylated on S343 in parental cells after 8 to 12 hours and 8 to 24 hours of MMC treatment, respectively (the phosphorylation could not be assessed after 24 hours of MMC treatment because of the substantial apoptosis in parental cells due to lack of growth factor). pNbs1 is enhanced in MMC-treated BCR/ABL-positive cells in comparison to parental cells. This effect is associated with increased ATM kinase activity in B/A-M07e cells treated with MMC (Figure S1, available on the Blood website; see the Supplemental Figures link at the top of the online article). However, MMC treatment did not change the expression of Mre11, RAD50, and Nbs1 proteins (Figure 2A). In addition, other genotoxic agents such as hydroxyurea (HU) and cisplatin (Cis) led to enhanced pNbs1 in BCR/ABL-positive cells (Figure 2B).
Enhanced phosphorylation of Nbs1 on serine 343 in BCR/ABL-positive cells on DNA damage. (A) M07e and UT7 parental (P) and their BCR/ABL (B/A) counterparts were treated with 0.5 μg/mL MMC in the presence or absence of SCF. pNbs1, total Nbs1, Mre11, and RAD50 were examined by Western-blot analysis at the indicated time points during MMC treatment. (B) M07e (P) and their BCR/ABL (B/A) counterparts were treated with 0.3 μg/mL cisplatin (Cis) or with 10 mM hydroxyurea (HU) for 12 hours in the presence of SCF. pNbs1, Nbs1, Mre11, and RAD50 were examined by Western-blot analysis. (C) CD34+ cells were isolated from healthy donors (Normal) and CML-CP and CML-BC patients. Cells were treated or not with 0.5 μg/mL MMC for 12 hours in the presence of GM-CSF and SCF. pNbs1, Nbs1, Mre11, RAD50, and tubulin were detected by Western blot analysis. Results are representative for 3 donors in each group. (D) M07e (■) and B/A-M07e (▩) cells and (E) CD34+ cells from healthy donors (■) and CML-BC patients (▩) were untreated (left panels) or treated (right panels) with 0.5 μg/mL MMC for 12 hours in the presence of GM-CSF and SCF. pNbs1 foci were detected in the nuclei by immunofluorescence; results represent mean (± SD) of the number of cells classified as negative (< 4 foci), low-positive (4-20 foci), or high-positive (> 20 foci). *P < .05, ▩ versus ■ in particular groups. (F) Representative nuclei scored as negative, low-positive, and high-positive for pNbs1 foci; nuclear borders are outlined in blue.
Enhanced phosphorylation of Nbs1 on serine 343 in BCR/ABL-positive cells on DNA damage. (A) M07e and UT7 parental (P) and their BCR/ABL (B/A) counterparts were treated with 0.5 μg/mL MMC in the presence or absence of SCF. pNbs1, total Nbs1, Mre11, and RAD50 were examined by Western-blot analysis at the indicated time points during MMC treatment. (B) M07e (P) and their BCR/ABL (B/A) counterparts were treated with 0.3 μg/mL cisplatin (Cis) or with 10 mM hydroxyurea (HU) for 12 hours in the presence of SCF. pNbs1, Nbs1, Mre11, and RAD50 were examined by Western-blot analysis. (C) CD34+ cells were isolated from healthy donors (Normal) and CML-CP and CML-BC patients. Cells were treated or not with 0.5 μg/mL MMC for 12 hours in the presence of GM-CSF and SCF. pNbs1, Nbs1, Mre11, RAD50, and tubulin were detected by Western blot analysis. Results are representative for 3 donors in each group. (D) M07e (■) and B/A-M07e (▩) cells and (E) CD34+ cells from healthy donors (■) and CML-BC patients (▩) were untreated (left panels) or treated (right panels) with 0.5 μg/mL MMC for 12 hours in the presence of GM-CSF and SCF. pNbs1 foci were detected in the nuclei by immunofluorescence; results represent mean (± SD) of the number of cells classified as negative (< 4 foci), low-positive (4-20 foci), or high-positive (> 20 foci). *P < .05, ▩ versus ■ in particular groups. (F) Representative nuclei scored as negative, low-positive, and high-positive for pNbs1 foci; nuclear borders are outlined in blue.
When CD34+ cells from healthy donors and CML-CP and CML-BC patients were treated with MMC for 12 hours in the presence of GM-CSF and SCF, enhanced phosphorylation of Nbs1 was associated with the malignant stage of the disease (Figure 2C). As expected, total RAD50 and Mre11 protein levels were not elevated in CML cells versus normal cells in the presence of growth factors; however, Nbs1 protein was up-regulated in leukemia cells (Figure 2C). The latter phenomenon may be attributed to the deregulated growth of CML CD34+ cells.53 In the absence of growth factors, CML-BC cells in comparison to normal counterparts displayed elevated levels of Mre11 and Nbs1, but not RAD50, and time-dependent enhancement of pNbs1 on MMC treatment (Figure S2), in concordance with the results obtained in cell lines (Figure 2A).
Immunofluorescence studies showed that a greater percentage of B/A-M07e and CD34+ CML-BC cells (▩) were highly positive (> 20 foci per nuclei) for pNbs1 nuclear foci than their parental/normal counterparts (■) after 12 hours of MMC treatment (Figure 2D and E, respectively, right panels). Interestingly, before MMC treatment B/A-M07e and CD34+ CML-BC cells had a greater percentage of pNbs1-positive cells (mostly low-positive [4-20 foci] but also some high-positive) than parental/normal cells (Figure 2D and E, respectively, left panels). Representative nuclei for negative, low-positive, and high-positive pNbs1 cells are shown in Figure 2F.
FTK-transformed cells display enhanced expression and phosphorylation of Nbs1 on serine 343 in response to genotoxic agent
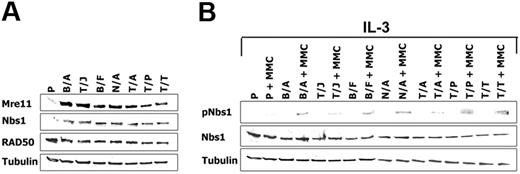
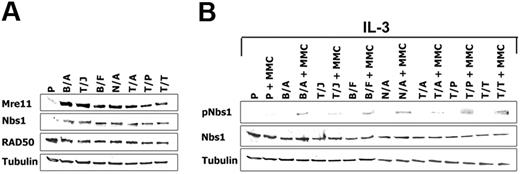
A number of cell lines transformed by BCR/ABL-related FTKs were also examined using Western-blot analysis to determine whether regulation of the M/R/N complex may be applicable to other leukemias besides those carrying the Philadelphia chromosome. Nbs1 protein expression was up-regulated by approximately 5-fold in the FTK-transformed cell lines starved from IL-3 for 12 hours in comparison to parental cells (Figure 3A), whereas equal expression was detected in the presence of growth factor (not shown). Moreover, Mre11 but not RAD50 was also elevated by FTKs (Figure 3A).
Cells transformed by FTKs display enhanced expression and phosphorylation of Nbs1. (A) Mre11, Nbs1, and RAD50 expression was examined by Western blot analysis in total cell lysates from Baf3 (P) or Baf3 cells expressing the following FTKs: BCR/ABL (B/A), TEL/JAK2 (T/J), BCR/FGFR (B/F), NPM/ALK (N/A), TEL/ABL (T/A), TEL/PDGFβR (T/P), and TEL/TRKC(L) (T/T). Cells were starved from IL-3 for 12 hours. (B) pNbs1 and Nbs1 expression was examined in the untreated and MMC-treated (0.5 μg/mL for 12 h) cells in the presence of IL-3. Tubulin was detected as a loading control.
Cells transformed by FTKs display enhanced expression and phosphorylation of Nbs1. (A) Mre11, Nbs1, and RAD50 expression was examined by Western blot analysis in total cell lysates from Baf3 (P) or Baf3 cells expressing the following FTKs: BCR/ABL (B/A), TEL/JAK2 (T/J), BCR/FGFR (B/F), NPM/ALK (N/A), TEL/ABL (T/A), TEL/PDGFβR (T/P), and TEL/TRKC(L) (T/T). Cells were starved from IL-3 for 12 hours. (B) pNbs1 and Nbs1 expression was examined in the untreated and MMC-treated (0.5 μg/mL for 12 h) cells in the presence of IL-3. Tubulin was detected as a loading control.
Stimulation of pNbs1 was examined after 12-hour treatment with MMC in the presence of IL-3 to maintain similar levels of Nbs1 expression in parental and FTK-positive cells. pNbs1 was elevated by 3- to 10-fold in leukemia cells treated with MMC in comparison to parental counterparts (Figure 3B).
Enhanced Nbs1 phosphorylation on DNA damage in BCR/ABL-positive cells contributes to resistance to genotoxic drugs
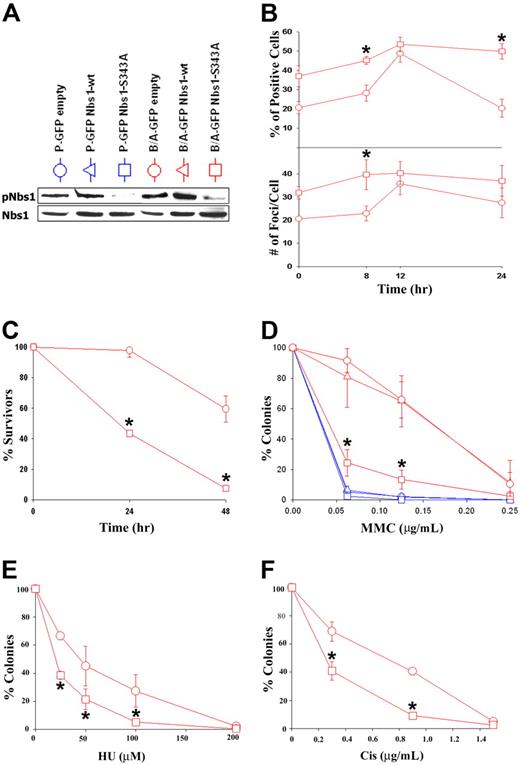
To determine whether the enhanced S343 phosphorylation of Nbs1 in BCR/ABL-positive cells contributes to their resistance to genotoxic agents, M07e and B/A-M07e cells were infected with MigR1 (GFP empty), MigR1-Nbs1–wild-type (wt), or MigR1-Nbs1-S343A retroviral particles, and GFP+ cells were isolated by fluorescence-activated cell sorter (FACS). Inhibition of Nbs1 phosphorylation after MMC treatment was confirmed by Western-blot analysis in cells transfected with the S343A mutant but not Nbs1-wt or empty plasmid (Figure 4A). This effect probably depended on reduction of expression of endogenous Nbs1 by the S343A mutant. GFP+ cells were treated with 0.125 μg/mL MMC for 8, 12, and 24 hours; cytospun; and probed for γ-H2AX nuclear foci to determine the influence of pNbs1 on DSB formation.54 B/A-M07e cells expressing the S343A mutant have a higher percentage of γ-H2AX–positive cells (> 20 foci) prior to MMC treatment and also after 8 hours of treatment in comparison to cells transfected with empty virus (Figure 4B top panel). At 12 hours after treatment, levels of γ-H2AX foci-positive cells are similar in both groups; however, at 24 hours the percentage of positive B/A-GFP empty cells came back to pretreatment levels, whereas B/A-GFP Nbs1-S343A cells continued to have high levels of γ-H2AX nuclear foci. B/A-GFP Nbs1-S343A cells also appeared to have a higher number of foci per positive cell than the B/A-GFP empty cells at 0 and 8 hours after MMC treatment (Figure 4B, bottom panel).
Nbs1-S343A mutant sensitizes BCR/ABL-M07e cells to MMC treatment. (A) M07e (P) and B/A-M07e (B/A) cells were infected with empty pMig retroviral construct or those containing Nbs1-wt or Nbs1-S343A mutant. GFP+ cells were sorted and cultured in the presence of GM-CSF, and phosphorylation of Nbs1 on S343 was verified by Western blot analysis (pNbs1). (B) B/A-GFP empty and B/A-GFP Nbs1-S343A cells were then treated or not with 0.125 μg/mL of MMC for the indicated time periods in the presence of GM-CSF. γ-H2AX nuclear foci were detected by immunofluorescence. Percentage of highly positive living cells (> 20 foci/cell) was calculated (top panel) and the number of foci per cell was counted (bottom panel). (C) Cells were treated with 0.125 μg/mL MMC and their survival was determined by trypan blue exclusion assay. (D-F) Cells were plated in methylcellulose in the presence of GM-CSF and the indicated concentrations of MMC, HU, or Cis. Colonies were counted after 7 days. Results in panels B-F represent mean (± SD) from 3 different experiments. *P < .05 (B), *P < .004 (C), *P < .007 (D), *P < .05 (E), and *P < .03 (F) in comparison to B/A-GFP empty and B/A-GFP Nbs1-wt (if present).
Nbs1-S343A mutant sensitizes BCR/ABL-M07e cells to MMC treatment. (A) M07e (P) and B/A-M07e (B/A) cells were infected with empty pMig retroviral construct or those containing Nbs1-wt or Nbs1-S343A mutant. GFP+ cells were sorted and cultured in the presence of GM-CSF, and phosphorylation of Nbs1 on S343 was verified by Western blot analysis (pNbs1). (B) B/A-GFP empty and B/A-GFP Nbs1-S343A cells were then treated or not with 0.125 μg/mL of MMC for the indicated time periods in the presence of GM-CSF. γ-H2AX nuclear foci were detected by immunofluorescence. Percentage of highly positive living cells (> 20 foci/cell) was calculated (top panel) and the number of foci per cell was counted (bottom panel). (C) Cells were treated with 0.125 μg/mL MMC and their survival was determined by trypan blue exclusion assay. (D-F) Cells were plated in methylcellulose in the presence of GM-CSF and the indicated concentrations of MMC, HU, or Cis. Colonies were counted after 7 days. Results in panels B-F represent mean (± SD) from 3 different experiments. *P < .05 (B), *P < .004 (C), *P < .007 (D), *P < .05 (E), and *P < .03 (F) in comparison to B/A-GFP empty and B/A-GFP Nbs1-wt (if present).
To study survival, living cells were examined using trypan blue exclusion 24 and 48 hours after MMC treatment. B/A-GFP Nbs1-S343A cells had approximately 2.5 and 12 times fewer living cells than the empty counterparts at 24 and 48 hours, respectively (Figure 4C). Next, to verify that Nbs1-S343A mutant reduces the resistance of B/A-M07e cells to MMC, we plated M07e and B/A-M07e cells containing either GFP empty, GFP Nbs1-wt, or GFP Nbs1-S343A in methylcellulose along with or without various doses of MMC. The Nbs1-S343A mutant sensitized B/A-M07e cells to MMC treatment to levels similar to those displayed by parental cells (Figure 4D). In addition, Nbs1-S343A mutant also reduced resistance to HU and Cis (Figure 4E and F, respectively) in B/A-M07e cells.
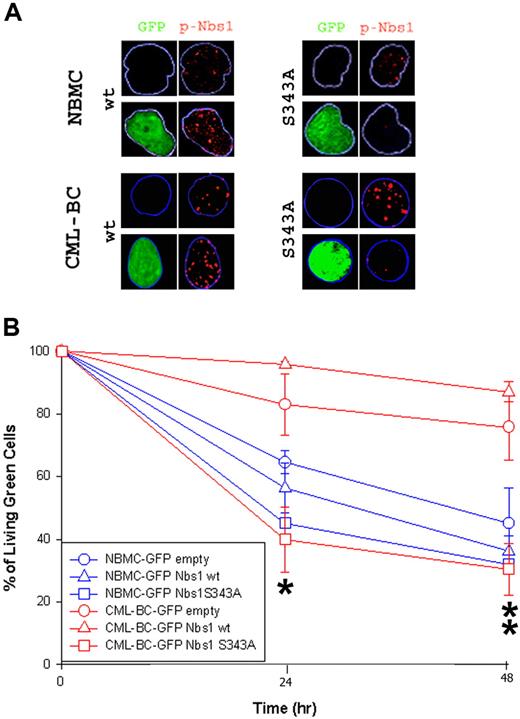
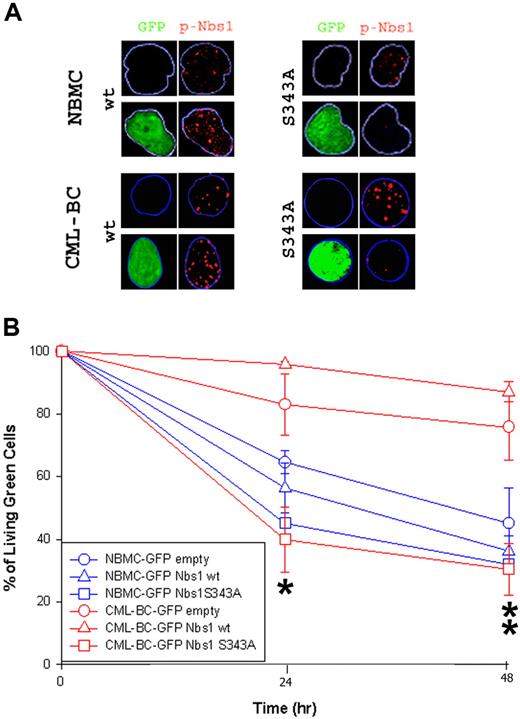
A similar experiment was performed using CD34+ normal bone marrow (NBMC) and CML patient cells in the blast-crisis phase (CML-BC) of the disease. Down-regulation of the Nbs1-S343 phosphorylation in cells infected with the S343A mutant was verified via immunofluorescence studies (Figure 5A). Please note that pNbs1 foci were reduced only in GFP+ cells expressing Nbs1-S343A mutant. GFP+ cells surviving 0.1 μg/mL MMC treatment were counted using the trypan blue exclusion test (Figure 5B). The Nbs1-S343A mutant significantly reduced resistance to MMC in CML-BC cells at both 24 and 48 hours, whereas the mutant only had a slight effect on NBMC at 24 hours (Figure 5B). In addition, down-regulation of total Nbs1 expression by siRNA reduced drug resistance on B/A-M07e cells (Figure S3).
Nbs1-S343A mutant decreases MMC resistance in CML-BC cells. CD34+ NBMC and CML-BC cells were infected with pMig retroviral particles containing Nbs1-wt, Nbs1-S343A, or empty plasmid. (A) pNbs1 was examined by immunofluorescence after 0.1 μg/mL MMC treatment for 24 hours. Expression of GFP and detection of pNbs1 foci is shown in representative nuclei. (B) Survival of GFP+ cells 24 and 48 hours after MMC treatment was determined using trypan blue exclusion. Results represent mean (± SD) for 2 donors each for NBMC and CML-BC. *P < .04, CML-BC-GFP Nbs1-S343A in comparison to CML-BC-GFP empty and CML-BC-GFP Nbs1-wt, and NBMC-GFP-Nbs1-S343A in comparison to NBMC-GFP empty and NBMC-GFP Nbs1-wt; **P < .02, CML-BC-GFP Nbs1-S343A in comparison to CML-BC-GFP empty and CML-BC-GFP Nbs1-wt.
Nbs1-S343A mutant decreases MMC resistance in CML-BC cells. CD34+ NBMC and CML-BC cells were infected with pMig retroviral particles containing Nbs1-wt, Nbs1-S343A, or empty plasmid. (A) pNbs1 was examined by immunofluorescence after 0.1 μg/mL MMC treatment for 24 hours. Expression of GFP and detection of pNbs1 foci is shown in representative nuclei. (B) Survival of GFP+ cells 24 and 48 hours after MMC treatment was determined using trypan blue exclusion. Results represent mean (± SD) for 2 donors each for NBMC and CML-BC. *P < .04, CML-BC-GFP Nbs1-S343A in comparison to CML-BC-GFP empty and CML-BC-GFP Nbs1-wt, and NBMC-GFP-Nbs1-S343A in comparison to NBMC-GFP empty and NBMC-GFP Nbs1-wt; **P < .02, CML-BC-GFP Nbs1-S343A in comparison to CML-BC-GFP empty and CML-BC-GFP Nbs1-wt.
Enhanced phosphorylation of Nbs1 on serine 343 in BCR/ABL-positive cells after genotoxic treatment leads to prolongation of the intra–S-phase cell-cycle checkpoint, stimulation of homologous recombination repair, and up-regulation of XIAP
Nbs1 has been implicated in intra-S48 and G2/M31 checkpoints, which play a role in resistance of BCR/ABL leukemias to DNA damage.19,25,28 Therefore, we chose to examine whether the enhanced phosphorylation of Nbs1 in leukemia cells is affecting cell cycle after MMC treatment.
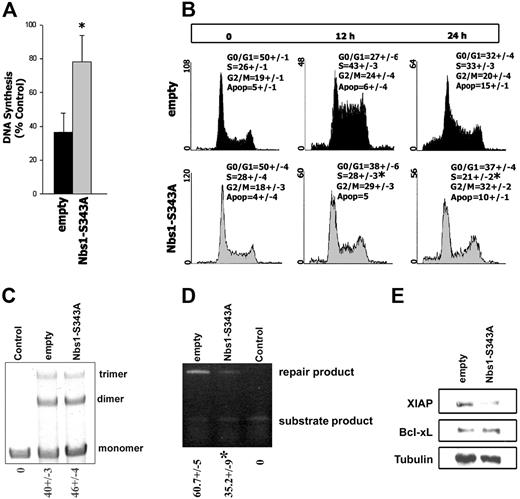
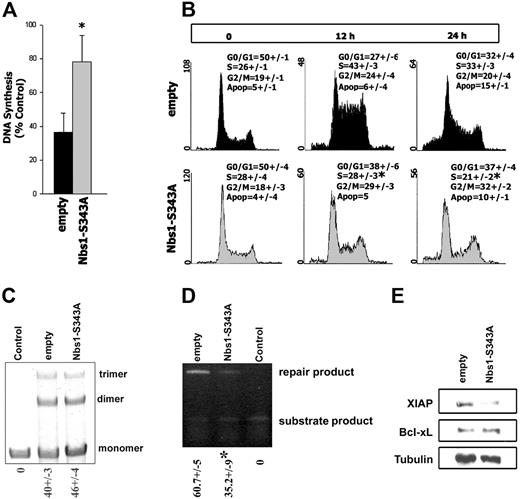
To study the intra–S-phase checkpoint, the RDS assay was performed in B/A-M07e cells expressing GFP (empty) or GFP and Nbs1-S343A mutant (Nbs1-S343A). MMC-treated B/A-M07e empty cells incorporated 40% (± 13%) of 3H-thymidine, which was increased to 78% (± 16%) by the expression of Nbs1-S343A mutant (Figure 6A).
Nbs1-S343A mutant disrupts the intra–S-phase checkpoint, inhibits HRR, and down-regulates XIAP. B/A-M07e cells expressing GFP (empty) or GFP and Nbs1-S343A mutant (Nbs1-S343A) were characterized in Figure 4A (B/A-GFP empty and B/A-GFP Nbs1-S343A cells, respectively). (A) RDS assay. Results show the ratio of 3H-thymidine to 14C-thymidine normalized to the corresponding untreated control to determine the relative amount of DNA synthesis. Results are representative of 5 experiments. *P < .05. (B) Cells were untreated or treated with 0.25 μg/mL MMC and cell-cycle distribution was analyzed 0, 12, and 24 hours later by flow cytometry after staining with propidium iodide. Results represent 3 independent experiments. *P < .05 in comparison to corresponding S-phase in empty group. (C) NHEJ-mediated end-ligation of the XhoI + XbaI–digested plasmid substrate (monomers) by the lysis buffer (Control) or cell lysates from B/A-GFP empty and B/A-GFP Nbs1-S343A cells generated multiplasmid products (dimers, trimers). The mean percentages of repair products (dimmer, trimer) (± SD) are shown below the lanes. (D) HRR-mediated repair of I-SceI–induced DSBs in DR-GFP cassette in B/A-GFP empty and B/A-GFP Nbs1-S343A cells could be evaluated by semiquantitative PCR generating products from repaired and unrepaired (substrate) DR-GFP cassette. Control consisted of cells carrying only unrepaired DR-GFP cassette. The mean percentages of repair product (± SD) are shown below the lanes. *P < .05. (E) XIAP and Bcl-xL expression was examined by Western blot analysis in total cell lysates; tubulin served as loading control.
Nbs1-S343A mutant disrupts the intra–S-phase checkpoint, inhibits HRR, and down-regulates XIAP. B/A-M07e cells expressing GFP (empty) or GFP and Nbs1-S343A mutant (Nbs1-S343A) were characterized in Figure 4A (B/A-GFP empty and B/A-GFP Nbs1-S343A cells, respectively). (A) RDS assay. Results show the ratio of 3H-thymidine to 14C-thymidine normalized to the corresponding untreated control to determine the relative amount of DNA synthesis. Results are representative of 5 experiments. *P < .05. (B) Cells were untreated or treated with 0.25 μg/mL MMC and cell-cycle distribution was analyzed 0, 12, and 24 hours later by flow cytometry after staining with propidium iodide. Results represent 3 independent experiments. *P < .05 in comparison to corresponding S-phase in empty group. (C) NHEJ-mediated end-ligation of the XhoI + XbaI–digested plasmid substrate (monomers) by the lysis buffer (Control) or cell lysates from B/A-GFP empty and B/A-GFP Nbs1-S343A cells generated multiplasmid products (dimers, trimers). The mean percentages of repair products (dimmer, trimer) (± SD) are shown below the lanes. (D) HRR-mediated repair of I-SceI–induced DSBs in DR-GFP cassette in B/A-GFP empty and B/A-GFP Nbs1-S343A cells could be evaluated by semiquantitative PCR generating products from repaired and unrepaired (substrate) DR-GFP cassette. Control consisted of cells carrying only unrepaired DR-GFP cassette. The mean percentages of repair product (± SD) are shown below the lanes. *P < .05. (E) XIAP and Bcl-xL expression was examined by Western blot analysis in total cell lysates; tubulin served as loading control.
Cell-cycle analysis was performed on B/A-M07e cells, expressing either GFP empty or the GFP and Nbs1-S343A mutant, treated with MMC for 0, 12, and 24 hours (Figure 6B). B/A-M07e-GFP cells displayed accumulation, whereas B/A-M07e-GFP+ Nbs1-S343A cells showed a significant decrease in the accumulation of cells in S-phase 12 hours after MMC treatment (Figure 6B, compare upper and lower middle panels). However, Nbs1-S343A mutant did not affect the G2/M phase in MMC-treated B/A cells. Expression of GFP-Nbs1 wild-type did not affect the cell-cycle response after MMC treatment (data not shown). Despite activation of the intra–S-phase checkpoint, M07e parental cells did not accumulate in S-phase, instead 26% and 40% contained a subdiploid amount of DNA, at 12 and 24 hours after MMC treatment, respectively, indicating apoptosis (data not shown).
In addition to intra–S-phase checkpoint activation, Nbs1 can affect DSB repair mechanisms NHEJ and HRR.37-39 Inhibition of pNbs1 in B/A-M07e-GFP+ Nbs1-S343A cells did not affect the efficiency of NHEJ (Figure 6C) but reduced HRR by approximately 2-fold in comparison to that in B/A-M07e-GFP cells (Figure 6D).
BCR/ABL kinase provides additional protection against genotoxic treatment by elevation of the expression of Bcl-xL.28 Down-regulation of pNbs1 did not affect the expression levels of Bcl-xL in B/A-M07e-GFP+ Nbs1-S343A cells in comparison to B/A-M07e-GFP cells (Figure 6E). However, both BCR/ABL and Nbs1 were reported to be involved in regulation of expression of X-chromosome–linked inhibitor of apoptosis protein (XIAP).55 Expression of XIAP protein is elevated by approximately 5-fold in BCR/ABL-M07e-GFP cells in comparison to M07e-GFP counterparts (data not shown). Interestingly, inhibition of pNbs1 reduced expression of XIAP in B/A-M07e-GFP+ Nbs1-S343A cells by approximately 3-fold compared with B/A-M07e-GFP cells (Figure 6E).
Nbs1-S343A mutant in combination with IM decreases MMC resistance in BCR/ABL-positive cells
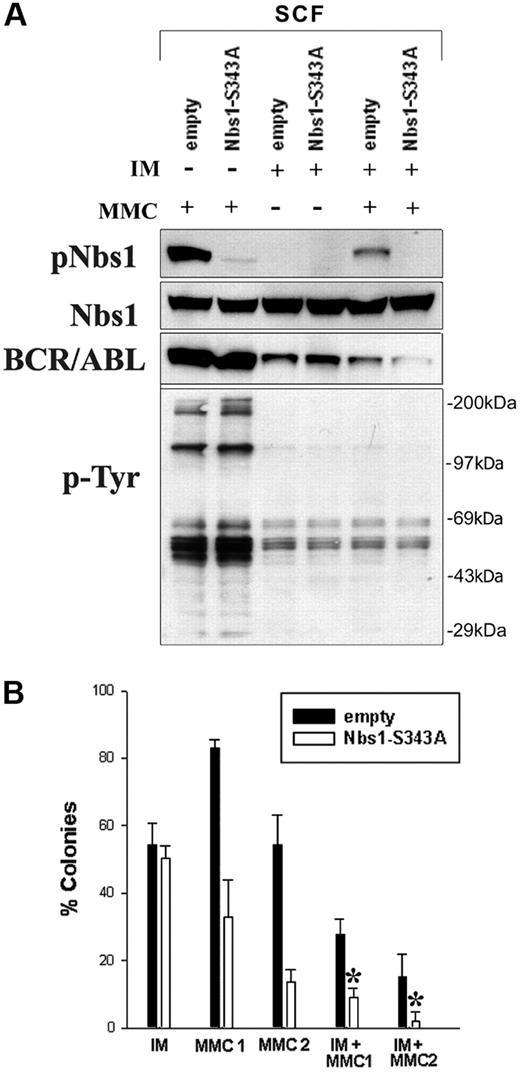
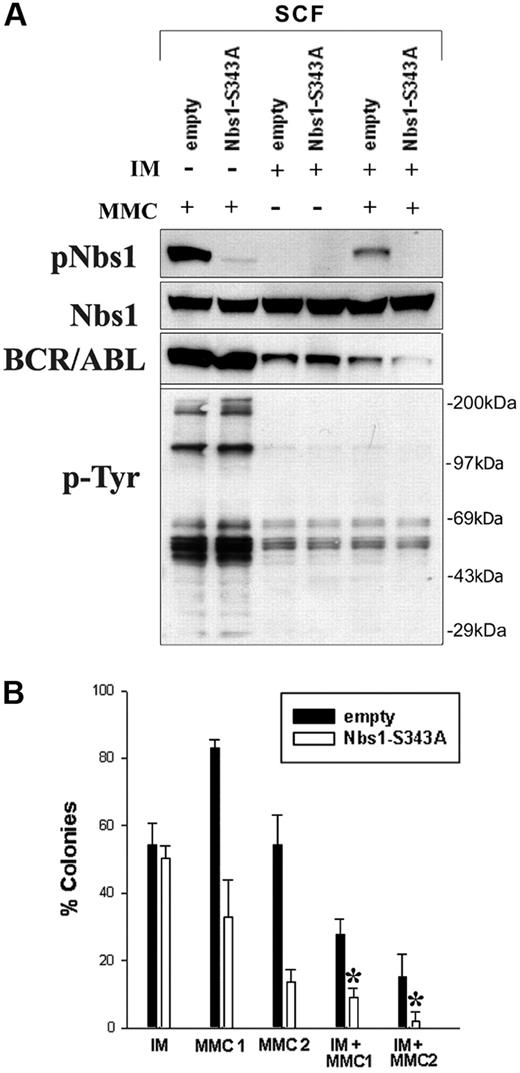
Finally, to investigate whether MMC resistance could be further reduced in BCR/ABL-positive cells, a combinatorial strategy was used targeting enhanced Nbs1 phosphorylation and the deregulated BCR/ABL tyrosine kinase activity, using the Nbs1-S343A phosphorylation-less mutant and a suboptimal concentration of IM eliminating approximately 50% of leukemia cells, respectively. B/A-M07e cells containing either GFP empty (■) or Nbs1-S343A mutant (□) were treated with IM, MMC, or IM + MMC. Down-regulation of pNbs1 and phosphotyrosine (p-Tyr)–containing proteins was verified by Western blot analysis (Figure 7A). Reduction of BCR/ABL protein expression after prolonged treatment with IM was observed before and associated with down-regulation of Hsp90, which protects BCR/ABL kinase from degradation.56
Down-regulation of Nbs1-S343 phosphorylation followed by inhibition of BCR/ABL kinase sensitizes BCR/ABL-positive cells to MMC. (A) B/A-M07e (A/B) cells were infected with empty pMig retroviral construct (empty) or containing Nbs1-S343A mutant (Nbs1-S343A). GFP+ cells were sorted and cultured in the presence of SCF. Cells were treated (+) or not (−) with 1 μM IM for 24 hours and/or 0.5 μg/mL MMC for 12 hours in the presence of GM-CSF. Expression of pNbs1, Nbs1, BCR/ABL, and phosphotyrosine proteins (p-Tyr) was examined by Western-blot analysis. (B) Clonogenic assay was performed on cells preincubated with 1 μM IM that were then treated with 0.025 μg/mL MMC (MMC1), 0.05 μg/mL MMC (MMC2), or both (IM + MMC1, IM + MMC2) in the presence of GM-CSF. Colonies were counted 7 days later. Results represent mean percentage of colonies (± SD) in comparison to the colonies formed by untreated cells. *P < .005 in comparison to the corresponding empty cells treated with MMC + IM; *P < .05 in comparison to corresponding Nbs1-S343A cells treated with MMC or IM.
Down-regulation of Nbs1-S343 phosphorylation followed by inhibition of BCR/ABL kinase sensitizes BCR/ABL-positive cells to MMC. (A) B/A-M07e (A/B) cells were infected with empty pMig retroviral construct (empty) or containing Nbs1-S343A mutant (Nbs1-S343A). GFP+ cells were sorted and cultured in the presence of SCF. Cells were treated (+) or not (−) with 1 μM IM for 24 hours and/or 0.5 μg/mL MMC for 12 hours in the presence of GM-CSF. Expression of pNbs1, Nbs1, BCR/ABL, and phosphotyrosine proteins (p-Tyr) was examined by Western-blot analysis. (B) Clonogenic assay was performed on cells preincubated with 1 μM IM that were then treated with 0.025 μg/mL MMC (MMC1), 0.05 μg/mL MMC (MMC2), or both (IM + MMC1, IM + MMC2) in the presence of GM-CSF. Colonies were counted 7 days later. Results represent mean percentage of colonies (± SD) in comparison to the colonies formed by untreated cells. *P < .005 in comparison to the corresponding empty cells treated with MMC + IM; *P < .05 in comparison to corresponding Nbs1-S343A cells treated with MMC or IM.
Clonogenic assay was performed on cells pretreated with IM that were then treated with 0.025 μg/mL MMC (MMC1), 0.05 μg/mL MMC (MMC2), or both (IM + MMC1, IM + MMC2). Figure 7B shows that B/A-GFP Nbs1-S343A cells treated with IM had 3-fold and 7-fold fewer colonies after 0.025 μg/mL MMC (IM + MMC1) and 0.05 μg/mL MMC (IM + MMC2) treatment, respectively, than those cells without IM treatment (MMC1, MMC2). In addition, B/A-GFP Nbs1-S343A cells in IM + MMC1 and IM + MMC2 groups formed approximately 3 × and approximately 8 × fewer colonies, respectively, than B/A-GFP cells. Therefore, targeting both BCR/ABL kinase activity and Nbs1 phosphorylation in combination significantly sensitizes B/A-positive cells to MMC treatment, nearly eliminating all leukemia cells at higher MMC doses.
Discussion
Mechanisms protecting FTK-positive leukemia cells from apoptosis induced by DNA damage are important, especially because malignant cells may accumulate more “spontaneous” and genotoxic treatment-induced DNA lesions.18,19,21-23,27 Reports from several laboratories, including ours, have shown that in addition to enhanced DSB repair and antiapoptotic activity, BCR/ABL-positive cells display pronounced S and G2/M delays in response to a number of chemotherapeutics such as cisplatin, MMC, etoposide, and daunorubicin.19,24,25,28,57,58 Thus, drug resistance mechanisms represent legitimate targets for novel antileukemia therapies because their disruption may sensitize FTK-positive cells to genotoxic treatment. This speculation is supported by reports that inhibition of RAD51-mediated DSB repair, down-regulation of the antiapoptotic protein Bcl-xL, and disruption of intra-S and G2/M cell-cycle checkpoints sensitized leukemia cells to genotoxic treatments.19,28,49,52
ATM and ATR, and their major downstream effectors Chk1 and Chk2, play a major role in regulation of intra-S and G2/M checkpoints, most likely with both signaling pathways working together.59,60 The ATR→p53 and ATR→Chk1 axes were recently reported to be strongly activated in BCR/ABL-positive cells, playing a critical role in the accumulation of leukemia cells in G2/M and S cell-cycle phases, respectively, and contributing to the resistance to DNA cross-linking agents.19,52,61 These results, however, are in contrast with the report by Dierov et al62 that after treatment with etoposide, BCR/ABL causes a defect in the intra–S-phase checkpoint, leading to a radio-resistant DNA synthesis (RDS) phenotype. Although the reason for these discrepancies is unknown, the use of an inducible model by Dierov et al62 and stably expressing BCR/ABL cell lines (our studies) may be responsible. We believe that constitutive but not inducible expression of BCR/ABL better mimics the conditions in established Philadelphia chromosome–positive leukemia cells.
Nbs1, a member of the MRN complex, plays an essential role in activation of intra-S30 and G2/M31 cell-cycle checkpoints, both of which have been implicated in BCR/ABL drug resistance. Nbs1 is phosphorylated on S343 by ATM in the S-phase checkpoint pathway30 and also has been demonstrated to modulate the activity of ATM.34 In addition, Nbs1 is required for ATR-dependent phosphorylation events.63
The results presented here show that BCR/ABL kinase activity enhances the expression of Nbs1 protein due to stimulation of c-Myc transactivation and inhibition of caspase-dependent degradation, but additional mechanisms should not be ruled out. In addition, Western-blot analysis and immunofluorescence studies revealed that BCR/ABL-transformed cells in comparison to normal counterparts display enhanced phosphorylation of Nbs1 on S343 (pNbs1) after treatment with MMC, HU, and Cis. DNA damage-dependent enhancement of pNbs1 appears to be a broad phenomenon because it was also detected in MMC-treated tumor cells expressing other FTKs. The effect does not simply reflect the increased expression of Nbs1 protein in leukemia cells because elevation of pNbs1 was also detected in MMC-treated FTK-positive cells cultured in the presence of IL-3 when total levels of Nbs1 proteins were similar in normal and transformed cells.
Because BCR/ABL-positive leukemia cells in comparison to normal counterparts may accumulate more DNA lesions (including DSBs) after treatment with MMC and Cis,19 we hypothesize that the increase of pNbs1 in the former cells is associated with the elevated levels of DSBs at replication forks.64,65 This speculation is supported by the observation that pNbs1 was induced to a similar magnitude in leukemia and normal cells immediately after γ-irradiation (data not shown) when more cell-cycle–independent DSBs were counted in former cells.23
Replication stress induction by MMC treatment leads to the formation of nuclear foci containing phosphorylated Nbs1.66 Although pNbs1, before drug treatment, is not detectable by Western blot analysis, the immunofluorescence studies showed that BCR/ABL-transformed cells contain more pNbs1 foci than normal counterparts. This is in agreement with previous reports that BCR/ABL-positive leukemias contain elevated levels of “spontaneous” DNA damage most likely caused by elevated levels of reactive oxygen species (ROS).21 In summary, we postulate that enhanced pNbs1 observed in FTK-positive hematologic malignancies is dependent on elevated levels of DSBs accumulated due to ROS and drug treatment.
We found that enhanced phosphorylation of Nbs1 on S343 (pNbs1) leads to increased resistance to MMC, HU, and Cis in BCR/ABL cells. This is supported by a report showing that Nijmegen Breakage Syndrome (NBS) cells, which have defects in the NBS1 gene, are reported to be hypersensitive to MMC.36
Elevation of pNbs1 in BCR/ABL-positive cells was associated with activation of the intra–S-phase checkpoint and prolongation of the S but not G2/M cell-cycle phase, probably allowing longer time for the repair of numerous DSBs. In addition, although pNbs1 also contributed to the induction of an intra–S-phase checkpoint in normal cells, it did not result in accumulation in S-phase but in apoptosis. The latter observation supports the speculation that pNbs1 not only activates an intra–S-phase checkpoint but also may regulate or collaborate with other mechanisms such as stimulation of DSB repair and prevention of apoptosis to induce drug resistance in BCR/ABL cells.12
Inhibition of pNbs1 by expression of the S343A mutant led to prolonged accumulation of γ-H2AX foci in BCR/ABL-positive cells treated with MMC. Therefore, in accordance with a previous report,67 this suggests that persistent γ-H2AX foci found in B/A-GFP Nbs1-S343A cells might indicate retardation of DSB repair. We have shown that elevation of pNbs1 stimulates HRR but not NHEJ. This observation is in accordance with other reports implicating Nbs1 in facilitation of HRR,37,38,68 whereas its role in NHEJ remains controversial.39,68
In addition to prolonged intra-S and G2/M checkpoint activation and enhancement of HRR efficiency, BCR/ABL kinase also protects leukemia cells from caspase-3–mediated apoptosis in part by regulation of Bcl-2 family members including Bcl-xL.28 We showed here that pNbs1 does not appear to affect BCR/ABL-dependent up-regulation of Bcl-xL, but it stimulates expression of another BCR/ABL-dependent antiapoptotic protein, XIAP.69 XIAP may be one of the key elements in antiapoptotic machinery because it selectively binds and inhibits caspases-3, -7, and -9.70
In conclusion, pNbs1 may provide a molecular link between mechanisms regulating cell-cycle checkpoint, HRR, and protection from apoptosis, which collaborate to induce drug resistance in BCR/ABL-positive leukemias.28 More efficient but unfaithful DNA repair combined with enhanced survival capability of leukemia cells may contribute to drug resistance during consolidation treatment followed by bone marrow transplantation (BMT) and can also lead to genomic instability and malignant progression of CML.12,71 Thus t(9;22)–positive patients undergoing BMT may benefit from targeting the mechanisms responsible for resistance of leukemia cells to genotoxic treatment.
Treatment with IM increased the sensitivity of leukemia cells to genotoxic agents in vitro.20,21,49 However, IM and other compounds may not be effective in leukemia stem cells, in leukemia cells harbored in some organs such as central nervous system, and in CML-BC cells.72 In the latter cells, BCR/ABL kinase is often overexpressed,73 which is associated with enhanced pNbs1 (this work) and resistance to genotoxic treatment.74 Thus a concomitant targeting of the kinase and pNbs1 may be beneficial for antileukemia treatment. This hypothesis is supported by experimental data showing that simultaneous application of suboptimal concentrations of IM (eliminating ∼50% of leukemia cells) and inhibition of pNbs1 by the S343A mutant led to almost complete eradication of BCR/ABL-positive cells treated with MMC.
In conclusion, we showed that enhanced phosphorylation of Nbs1 prolongs intra–S-phase cell-cycle checkpoint contributing to drug resistance in BCR/ABL-positive cells. In addition, simultaneous targeting of both Nbs1 phosphorylation (S343) and BCR/ABL tyrosine kinase activity eradicates resistance to MMC in BCR/ABL-positive cells and may represent a novel therapeutic strategy for CML patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by CA89052 and CA87015 from the National Institutes of Health/National Cancer Institute (NIH/NCI; T. Skorski), Maureen Reed Research Fund (L.R.), and 2 P05A 155 29 from Polish Ministry of Education and Science (T. Stoklosa).
We would like to thank Dr Steve Jackson (The Grudon Institute, University of Cambridge, Cambridge, United Kingdom) and Dr John Petrini (Memorial Sloan-Kettering Cancer Center, New York, NY) for critical comments and helpful discussion.
National Institutes of Health
Authorship
Contribution: L.R. performed research, analyzed data, and cowrote the paper. A.S., M.N-S., and K.U. performed research. T. Stoklosa performed research and analyzed data. K.R. analyzed the data. I.S. prepared samples for research. T. Skorski designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tomasz Skorski, Department of Microbiology and Immunology, School of Medicine, Temple University, MRB, Room 548A, 3400 N Broad Street, Philadelphia, PA 19140; e-mail: tskorski@temple.edu.

![Figure 1. BCR/ABL kinase elevates the expression of Nbs1 by stimulation of c-Myc–mediated transactivation and inhibition of caspase-dependent degradation. (A) Mre11, Nbs1, and RAD50 expression was examined by Western-blot analysis in total cell lysates from BCR/ABL (B/A)–positive or parental (P) mouse (32D, Baf3, and FL512) and human (M07e and UT7) cell lines. Cells were incubated for 12 hours in the presence or absence of growth factors (IL-3 or SCF) as indicated. Actin was detected as a loading control. (B-D) M07e parental (P) and BCR/ABL-positive (B/A) cells were starved from growth factor for 12 hours in the presence or absence of 1 μM IM (B), 1 μM epoxomycin (Epox) (C), and 20 μM Z-VAD-FMK (Z-VAD) (D). Expression of Mre11, Nbs1, RAD50, phosphotyrosine proteins (p-Tyr), p53, active caspase-3, and PARP (intact top and cleaved bottom band) was detected by Western-blot analysis. (E) The 293T cells were transiently transfected with the plasmid containing IRES-GFP (1), BCR/ABL-IRES-GFP (2), or BCR/ABL kinase–deficient mutant-IRES-GFP (3) along with the plasmids encoding the luciferase reporter gene driven by either the NBS promoter (NBSLuc1500, ■) or the NBS promoter containing the mutation in the E-box site preventing c-Myc binding (NBSLuc1500Mut, ▩). (F) M07e parental (1) and B/A-positive (2) cells were electroporated with NBSLuc1500 (■) or NBSLuc1500Mut (▩). (G) The 293T cells were transiently transfected with the plasmids containing NBSLuc1500 and IRES-GFP (1), BCR/ABL-IRES-GFP (2), or BCR/ABL-IRES-GFP and c-Myc–In373 (3). Luciferase activity is expressed in arbitrary units and results represent mean (± the standard deviation [SD]) of 3 independent experiments. P < .03 *in comparison to ■ in groups 1 and 3 in panel E and group 1 in panel F and to group 1 in panel G; **in comparison to ■ in group 2 in panels E and F and to bar 2 in panel G. Insets show the expression of BCR/ABL, c-Myc, and actin in particular groups.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/2/10.1182_blood-2006-08-042630/4/m_zh80140705040001.jpeg?Expires=1763522936&Signature=1qm2M3XUVq0soInjyb8VYHsJRfdrCaiJl827b03M2CHWMfHlWikJux3wMXP3W0K46vTt2~4qwRWISRfTV41K2OQSUErErerWCHRYns2DXKVywRzDxj-hfzHKz3sLKbzuhl65b-kf65tg1BhqVHzFke0lKmY0FDuy0vVZl5smfl9hmry5JBToDDHAz860E3JwnSPhIVf~m8vXPe3p1wPVEskxPLiLFdMrupLO77XrSkSiwL0PstEVnNqkAbMueKF84T5glTPLbkdlPq3VfPTBFygD17XRszBLxpqPqcW4wN-VTSt7BMslvnwKp9emSFjNDVHowh-ZKnY3EJf5~BghIQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 1. BCR/ABL kinase elevates the expression of Nbs1 by stimulation of c-Myc–mediated transactivation and inhibition of caspase-dependent degradation. (A) Mre11, Nbs1, and RAD50 expression was examined by Western-blot analysis in total cell lysates from BCR/ABL (B/A)–positive or parental (P) mouse (32D, Baf3, and FL512) and human (M07e and UT7) cell lines. Cells were incubated for 12 hours in the presence or absence of growth factors (IL-3 or SCF) as indicated. Actin was detected as a loading control. (B-D) M07e parental (P) and BCR/ABL-positive (B/A) cells were starved from growth factor for 12 hours in the presence or absence of 1 μM IM (B), 1 μM epoxomycin (Epox) (C), and 20 μM Z-VAD-FMK (Z-VAD) (D). Expression of Mre11, Nbs1, RAD50, phosphotyrosine proteins (p-Tyr), p53, active caspase-3, and PARP (intact top and cleaved bottom band) was detected by Western-blot analysis. (E) The 293T cells were transiently transfected with the plasmid containing IRES-GFP (1), BCR/ABL-IRES-GFP (2), or BCR/ABL kinase–deficient mutant-IRES-GFP (3) along with the plasmids encoding the luciferase reporter gene driven by either the NBS promoter (NBSLuc1500, ■) or the NBS promoter containing the mutation in the E-box site preventing c-Myc binding (NBSLuc1500Mut, ▩). (F) M07e parental (1) and B/A-positive (2) cells were electroporated with NBSLuc1500 (■) or NBSLuc1500Mut (▩). (G) The 293T cells were transiently transfected with the plasmids containing NBSLuc1500 and IRES-GFP (1), BCR/ABL-IRES-GFP (2), or BCR/ABL-IRES-GFP and c-Myc–In373 (3). Luciferase activity is expressed in arbitrary units and results represent mean (± the standard deviation [SD]) of 3 independent experiments. P < .03 *in comparison to ■ in groups 1 and 3 in panel E and group 1 in panel F and to group 1 in panel G; **in comparison to ■ in group 2 in panels E and F and to bar 2 in panel G. Insets show the expression of BCR/ABL, c-Myc, and actin in particular groups.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/2/10.1182_blood-2006-08-042630/4/m_zh80140705040001.jpeg?Expires=1763522937&Signature=yzYRlUx3S9ubu2A7jGBLJa1cYOLBFOVTeGyOENQK8n-kYd2EK5UN1BOM3SFWkxiNlZNB-wE7LFaSbbK8D96Y7tgrDNqy83zeHktt0w3m3L88kX4xhEjqJ-pyaxGbjC1AF4u3PZZXaplqn9B3HoRoXgepDUmW7DPOvSnQjUVxIKrJ4ia-MbXtLsO1xMlkqfYNfueC6hS1IMt-5dJCn99BVXGeUQ9BVxyxX8afTyrCwJtVvTrAa-r5TGSHS2~z3gXn2OGcd62fo3wilWMQh9BXGDhOZFzMhdUG7yNGJ~tP~OnmJwW6XMrDdJf-5754aLZ1U8R-UNv7~WsS90fvmuMOiw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)