Abstract
Adaptive mechanisms to hematocrit levels of 0.9 in our erythropoietin-overexpressing mice (tg6) include increased plasma nitric oxide levels and erythrocyte flexibility. Doubled reticulocyte counts in tg6 suggest an increased erythrocyte turnover. Here we show that compared with wild-type (wt) animals, erythrocyte lifespan in tg6 is 70% lower in tg6 mice. Transgenic mice have a younger erythrocyte population as indicated by higher intercellular water and potassium content, higher flexibility, decreased density, increased surface to volume ratio, and decreased osmotic fragility. Interestingly, despite being younger, the tg6 erythrocyte population also harbors characteristics of accelerated aging such as an increased band 4.1a to 4.1b ratio, signs of oxidative stress, or decreased surface CD47 and sialic acids. In tg6, in vivo tracking of PKH26-labeled erythrocytes revealed dramatically increased erythrocyte incorporation by their liver macrophages. In vitro experiments showed that tg6 macrophages are more active than wt macrophages and that tg6 erythrocytes are more attractive for macrophages than wt ones. In conclusion, in tg6 mice erythrocyte aging is accelerated, which results, together with an increased number and activity of their macrophages, in enhanced erythrocyte clearance. Our data points toward a new mechanism down-regulating red cell mass in excessive erythrocytosis in mice.
Introduction
During aging, the erythrocytes considerably change the internal ion and protein composition, and the biomechanical and biochemical properties of their cell membrane, as well as size, shape, and surface to volume ratio. In humans, these changes finally result in selective elimination of senescent erythrocytes from the circulation.1 The clearance of erythrocytes in mice and rabbits is in part age dependent and in part random.2 Nevertheless, the age-dependent erythrocyte clearance in these mammals is well controlled, involving oxidative damage, phosphatidylserine exposure,3,4 desialylation,5 and immunoglobulins.6 The involvement of immunoglobulins in clearance of senescent erythrocytes in mice must, however, differ from that in humans, because naturally occurring antibodies in mice are exclusively of the IgM class. Under pathological conditions such as sickle cell anemia the phosphatidylserine exposure to the outer leaflet of the red cell membrane presumably plays a predominant role since in this disease the percentage of phosphatidylserine-expressing red cells is increased 2- to 10-fold.4
Our transgenic (tg6) mice that constitutively overexpress human Epo in an oxygen-independent manner have a 12-fold elevated Epo plasma level7 leading to hematocrit values of up to 0.9. As to the question how tg6 mice cope with excessive erythrocytosis, we reported earlier that they show chronic vasodilatation due to excessive NO production7 and regulate blood viscosity by elevating erythrocyte flexibility.8 Here we were especially interested to define the process of erythrocyte turnover in tg6 animals by focusing on mechanisms of erythrocyte sequestration that prevent the polycythemic mice to further elevate their hematocrit until death. Indeed, we discovered that these animals down-regulate erythrocyte numbers by accelerated red cell aging and by highly efficient erythrocyte clearance by macrophages.
Materials and methods
Animals
The transgenic mouse line (termed tg6) overexpresses human Epo cDNA driven by the human platelet-derived growth factor B-chain promotor7 and shows increased Epo levels in plasma and brain.9 Breeding was performed by mating hemizygous males to wild-type C57Bl/6 females. Half of the offspring was hemizygous for the transgene while the other half was wild type (wt) and served as controls. Animals used for assessment of erythrocyte properties were killed with CO2. Then the blood was collected through cardiac puncture into heparinized syringes. All experiments were performed in accordance with the Swiss animal protection laws (Kantonales Veterinäramt Zürich) and institutional guidelines.
Measurements of the unidirectional K+ influx and cellular ion and water content
The unidirectional K+ influx measurements in mouse erythrocytes are described in detail elsewhere.10 Briefly, blood from 2 to 3 animals was pooled, and erythrocytes were isolated and suspended in the incubation medium (125 NaCl, 25 NaHCO3, 5 KCl, 1 CaCl2, 0.15 MgCl2, 10 Tris-MOPS, 10 glucose, 10 sucrose, all in mM, pH 7.4). Incubation was performed in Eschweiler tonometers with a gas phase consisting of 5% CO2, 20% O2, and 75% N2. The cells were preincubated for 20 minutes with or without inhibitors of the Na/K ATPase (1 mM ouabain) or Na-K-2Cl cotransporter (NKCC, 100 μmol bumetanide). Thereafter, 86Rb was added as a radioactive tracer for K+ and aliquots of the suspension were taken 20, 40, and 60 minutes after addition of the tracer. Flux was terminated by immediate dilution of the 86Rb with excessive amounts of ice-cold washing solution containing 100 mM Mg(NO3) and 10 mM imidazol titrated with HNO3 to a pH of 7.4 when on ice. Cells were washed 3 times to remove not internalized radioactivity and then deproteinized with 0.2 mL of 5% trichloracetic acid. The amount of 86Rb in the medium and deproteinized cell pellet extracts were measured in water phase (Cherenkov effect) using a Tri-Carb 1600TR liquid scintillation counter (Packard, Palo Alto, CA).
For the measurements of ion/water content of erythrocytes, blood was immediately centrifuged for 5 minutes at 6000g and 4°C in predried preweighed Eppendorf tubes. Plasma and buffy coat were discarded and erythrocytes were washed 3 times with an ice-cooled buffer. Packed cell pellets were weighed and dried at 80°C for 72 hours. After reweighing, the dried pellets were burned in ultra-pure concentrated HNO3 and cellular Na+ and K+ content was determined using a flame photometer.
Osmotic gradient ektacytometry
Erythrocyte deformability was measured using laser diffraction technique with an ektacytometer (Technikon Products, Bayer, Germany). Erythrocytes were suspended in a viscous solution containing Dextran T70 (GE Healthcare, Uppsala, Sweden) and osmoscans were recorded as described elsewhere.11
Osmotic fragility
Blood (10 μL) was dissolved in 3 mL distilled water containing either 0.9%, 0.75%, 0.65%, 0.55%, 0.5%, 0.45%, 0.4%, 0.35%, 0.3%, 0.2%, or 0% NaCl each adjusted to a pH of 7.4 using concentrated HCl. Ten minutes later, the tubes were centrifuged for 5 minutes at 2000g. Then 2 mL of the supernatant was added to 1 mL hemoglobin transformation solution (Dr Lange AG, Hegnau, Switzerland), mixed, and measured photometrically at 546 nm. The extinction was then plotted on a percentage basis of the hemolysis in distilled water (set to 100%) against the NaCl concentration in the test solution. The NaCl concentration at 50% hemolysis was determined by regression analysis.
Erythrocyte life span
Erythrocytes were labeled ex vivo with Biotin-x-N-hydroxysuccimide ester (BxNHS; Calbiochem, Dietikon, Switzerland) according to Hoffmann-Fezer et al.12 In brief, anesthesia was induced with a gas mixture containing 4% halothane, 70% N2O, remainder O2 and maintained by reducing the inspired halothane concentration to 1% to 1.5%. Body temperature was maintained at 37°C using a temperature-controlled heating pad. A catheter was inserted into the left femoral vein and 300 (wt) to 600 (tg6) μL blood was withdrawn into a syringe containing about 25 units of heparin. The blood loss was substituted with 200 (wt) to 400 (tg6) μL saline. The blood was then incubated with 1 mg BxNHS dissolved in 20 μL dimethylformamide and 120 μL phosphate-buffered saline (PBS) containing 0.1% glucose (PBS-G) at 37°C for 30 minutes with gentle shaking. Then the erythrocytes were washed twice with PBS-G, resuspended in PBS-G, and reinfused via the catheter in the femoral vein. After removal of the catheter and wound closure the mice were housed under standard conditions. Then every 3 to 4 days, approximately 2 μL blood was collected from each animal and incubated for 10 minutes with FITC-labeled avidin (Calbiochem) before determining the percentage of labeled erythrocytes with a fluorescence-activated cell sorting (FACS) analyzer (FACSCalibur; Becton Dickinson, Oxford, United Kingdom). Sampling was stopped when less than 0.5% labeled cells was found. The percentage of labeled erythrocytes was plotted against the day of blood collection and erythrocyte half life was calculated for each individual animal using exponential regression analysis.
In vivo erythrocyte tracking
Blood was collected as described in “Erythrocyte life span.” After washing the erythrocytes twice with PBS-G containing 0.2 mM phenylmethylsulfonyl fluoride (PMSF), the packed cells were diluted in “diluent C” of the PKH26-labeling Kit (Sigma, Buchs, Switzerland) and mixed with the same volume of “diluent C” containing 4 μM of the dye according to the supplier's instructions. The erythrocytes were then incubated at 37°C for 20 minutes under constant agitation. Four days after reinfusion of the labeled erythrocytes, the presence of labeled erythrocytes was confirmed by analyzing a blood smear and the animals were transcardially perfused with PBS containing 25 IU heparin/mL at 115 mmHg for at least 10 minutes to wash away all erythrocytes from the vasculature. Finally, fixation was achieved by perfusing with 3% paraformaldehyde (PFA) in PBS. Thereafter, liver and spleen were excised, stored for 3 days in 3% PFA followed by 4 days storage in 20% sucrose in PBS, and frozen for further analysis. In one mouse, in addition to these 2 organs also brain, kidney, heart, adrenal gland, muscle, and gut with Peyer patches were harvested and analyzed. Except for liver and spleen, no PKH26 fluorescence was detected in any other organ, confirming the complete removal of free erythrocytes from the animals' organs (not shown).
Subsequently, liver and spleen were sectioned into 10-μm slices using a cryomicrotome and stained with a FITC-labeled rat antibody directed against the F4/80 antigen (dilution 1:100; THP, Vienna, Austria) that is known to be present on the major subpopulation of resident tissue macrophages.13,14 Sections were analyzed using an incident fluorescence microscope (Axiovert 200M, Plan-Neofluar objective lens 20×/0.50; Zeiss, Deisenhofen, Germany) and arbitrary areas of liver and spleen were documented using an Axiocam HRm CCD camera and Zeiss Axiovision software version 4.5 (Zeiss). The resulting images were used to assess the total area of PKH26 fluorescence per tissue area. The area occupied by empty vessels was excluded using an image analyzing system (MCID Analysis 7.0, St. Catharines, ON) that allows to discriminate the vessels from the slightly autofluorescent tissue by their nearly black appearance. Tissue area could then be calculated from the difference of the total image area and the vessel area. Incorporated erythrocytes appeared as white dots within the tissue (Figure 3B).
Macrophage assay
These experiments were performed according to Bratosin et al15,16 with slight modifications. In brief, peritoneal macrophages were isolated by washing the peritoneal cavity with 10 mL Hanks balanced salt solution (HBSS) followed by centrifugation of the washing solution. The cells were then resuspended in 1 mL DMEM containing 20% FCS and incubated for 4 hours at 37°C in humidified air containing 5% CO2. Nonadherent macrophages were removed by 2 washings with DMEM. Then PKH26-labeled erythrocytes were added to the macrophages (macrophage-erythrocyte ratio = 1:10, approximately 2 × 106 cells/mL). After incubation for an additional 2 hours, unbound erythrocytes were removed by washing with DMEM. The remaining unincorporated erythrocytes were lysed using a hypotone buffer (140 mM NH4Cl, 17 mM Tris, pH 7.2) and the macrophages were fixed by flushing the Petri dishes with 3% paraformaldehyde in PBS and treated with 70% ethanol in order to elute the PKH26 from ghosts still adherent to the Petri dishes or macrophages. Arbitrary areas of the macrophage culture were then photographed at dark field and at appropriate fluorescence excitation light for PKH26. Using the image analyzing system mentioned in “Materials and methods, In vivo erythrocyte tracking,” the percentage of labeled macrophages and the total fluorescent area within each single macrophage containing labeled erythrocytes were determined.
Assessment of properties typical for senescent erythrocytes
Direct Coombs test.
Erythrocytes of tg6 and wt mice were washed 3 times in 20 volumes of PBS-G. Thereafter, packed cells (centrifuged at 350g) were mixed with 5 volumes of pure, 1:1, 1:3, 1:7, 1:15 diluted antimouse polyvalent immunoglobulin (anti-IgG, anti-IgM whole molecule; Sigma) and PBS-G as negative control. Ten minutes later, the agglutination was recorded semiquantitatively.
Band 4.1a to 4.1b ratio.
After washing erythrocytes 3 times in PBS-G containing 30 μg/mL PMSF, they were hemolysed in 30 volumes of lysis buffer (5 Na2HPO4, 1 EDTA, 0.4 di-isopropyl fluorophosphate, all in mM, pH 7.4). Then the erythrocyte ghosts were washed several times in lysis buffer until the pellet appeared completely pale after centrifugation (Sorvall RC-5B rotor (Thermo Electron Corp., Franklin, MA): SS34 at 47800g, 20°C, 10 minutes). Packed membranes were then mixed with one volume of a solution containing 1% sodium dodecyl sulfate (SDS) and 5 mM N-ethylmalaeimide (NEM) and frozen at −70°C. After thawing and determination of the protein content, the samples were mixed with electrophoresis buffer containing 40 mM DTT and heated to 100°C for 3 minutes. Then an excess of NEM was added and the proteins were separated on 8% SDS–polyacrylamide gels and visualized using silver staining as described elsewhere.17 The ratio of band 4.1a and 4.1b was quantified using the image analyzing system mentioned above.
Nonprotein thiols.
The amount of cellular glutathione (GSH) and oxidized glutathione (GSSG) was assayed in erythrocytes as described elsewhere.10 Briefly, blood and plasma samples were collected from wt and tg6 mice and mixed 1:1 with deproteinizing solution containing 1.67 g glacial metaphosphoric acid, 0.2 g Na2EDTA, and 30 g NaCl in 100 mL ddH2O. After centrifugation, GSH and GSSG (upon reduction to GSH in the presence of glutathione reductase and NADH) concentration was determined in supernatants using 5,5′-dithiobis(2-nitrobenzoic acid) (Ellman reagent). Optical density of the colored complex was measured photometrically at 412 nm.
CD47.
The amount of CD47 on the surface of erythrocytes was determined using a FITC-labeled monoclonal antibody directed against mouse CD47 (BD Biochemistry, Allschwil, Switzerland). After 3 washes with PBS-G and 1-hour incubation at room temperature with the antibody diluted 1:50, 1:100 (recommendation of the supplier), or 1:200, fluorescence intensity was quantified by FACS.
Surface sialic acids.
The amount of sialic acid residues on the erythrocyte surface was determined as described by Aminoff.18 Briefly, mouse erythrocytes were washed twice in 0.9% NaCl and finally in a solution containing 10 mM CaCl2 and 0.9% NaCl titrated with NaHCO3 to a pH of 7.0 at 37°C. Final hematocrit of the erythrocyte suspension was adjusted to 17% to 25% using the same solution. Aliquots of the suspension (200 μL) were incubated with neuraminidase (final activity of 0.2 U/mL) for 1 hour at 37°C. After centrifugation (5 minutes at 2000g), the supernatants were collected and deproteinized by boiling for 2 to 3 minutes. After acidification to a pH of 4.0 with 1 drop of glacial acetic acid, 100 μL of the deproteinized supernatant was mixed with 50 μL periodic acid (25 mM of periodic acid in 0.125 N H2SO4) and incubated for additional 30 minutes at 37°C. Thereafter the excess of periodate was neutralized by adding 40 μL sodium arsenide (2% in 0.5 N HCl) and the mixture incubated with 400 μL 2-thiobarbituric acid for 7.5 minutes at 100°C in a water bath. The colored complex was then extracted with 1 mL acidic butan-1-ol and absorbance was measured photometrically at 549 nm. A calibration curve was made using 1-40 μg N-acetylneuraminic acid dissolved in distilled water.
In addition, a qualitative test for assessment of surface sialic acids was performed19 by mixing one drop of PBS-G washed erythrocyte suspension adjusted to a hematocrit of 0.2 with 2 drops of PBS containing 1% hexadimetrin bromide (Polybrene; Sigma) at room temperature. Ten minutes later, the suspension was transferred to Neubauer chambers and observed for red cell aggregation in an Axioskop 2 microscope with a Plan-Neofluar 20×/0.50 objective lens. Images were captured using an AxioCam CCD color camera and processed using AxioVision 4.2 and converted to grayscale.
Statistical analysis
Data were compared among the experimental groups using ANOVA and a 2-tailed Student t test for unpaired samples with Welch correction or a Fischer test (GraphPad Instat. V3.05; GraphPad Software, San Diego, CA). The level of statistical significance was set at P < .05.
Results
Ion and water content
The plasma concentrations of the major cations did not differ between tg6 and wt mice (not shown). In contrast, both K+ and water content of tg6 erythrocytes exceeded that of the wt, whereas Na+ concentration was similar (Figure 1A). Figure 1B shows that the enhancement of the active (ouabain-sensitive, ATPase-mediated) K+ influx in transgenic erythrocytes is coupled to a partial suppression of the passive (bumeteanude-sensitive, NKCC-mediated) K+ flux. The transmembrane electrochemical gradient for potassium directed NKCC-mediated net K+ flux outwardly. Thus, its suppression along with activation of ouabain-sensitive K+ uptake explains the increased intracellular K+ content of transgenic erythrocytes that is typical for young erythrocytes.
Concentration of the major intracellular cations, water content, and potassium fluxes of wt (open bars) and tg6 (black bars) erythrocytes. (A) The intracellular sodium concentration was unaffected, whereas the potassium concentration was significantly elevated in tg6 erythrocytes. Accordingly, the water content of tg6 erythrocytes was higher compared with wt ones. (B) The increased intracellular potassium concentration was due to an increased ouabain-sensitive (active, Na/K-ATPase) transmembral potassium flux coupled with a suppression of the bumetanide-sensitive (passive, NKCC) flux. Means ± SD; *P < .05; **P < .01.
Concentration of the major intracellular cations, water content, and potassium fluxes of wt (open bars) and tg6 (black bars) erythrocytes. (A) The intracellular sodium concentration was unaffected, whereas the potassium concentration was significantly elevated in tg6 erythrocytes. Accordingly, the water content of tg6 erythrocytes was higher compared with wt ones. (B) The increased intracellular potassium concentration was due to an increased ouabain-sensitive (active, Na/K-ATPase) transmembral potassium flux coupled with a suppression of the bumetanide-sensitive (passive, NKCC) flux. Means ± SD; *P < .05; **P < .01.
Osmotic gradient ektacytometry
Apart from the osmolarity of the suspension medium, the deformability of the erythrocytes is determined by their membrane elasticity, their surface to volume ratio, and their internal viscosity, the latter being a function of the mean corpuscular hemoglobin concentration that is inversely proportional to the density of the erythrocytes. Osmoscans were performed with erythrocytes from 3 to 4 individual wt or tg6 mice. The osmoscans (not shown) were highly reproducible and revealed that, compared with the wt control, tg6 erythrocytes have an increased surface to volume ratio, a reduced cell density, and an increased flexibility. Enhanced flexibility confirms our previous results8 and fits, together with the decreased cell density, to the alterations in ion and water content shown in Figure 1. Once again, these observations point toward the presence of a younger erythrocyte population.
Osmotic fragility
Considering that the erythrocyte population in tg6 mice is more juvenile, we tested their resistance to osmotic stress. While 50% of the wt erythrocytes lysed at a NaCl concentration of 0.54% ± 0.04%, tg6 erythrocytes were more stable, lysing at 0.43% ± 0.04% NaCl (n = 7, P < .05). Most likely the lower osmotic fragility of the tg6 erythrocytes is due to their higher surface to volume ratio.
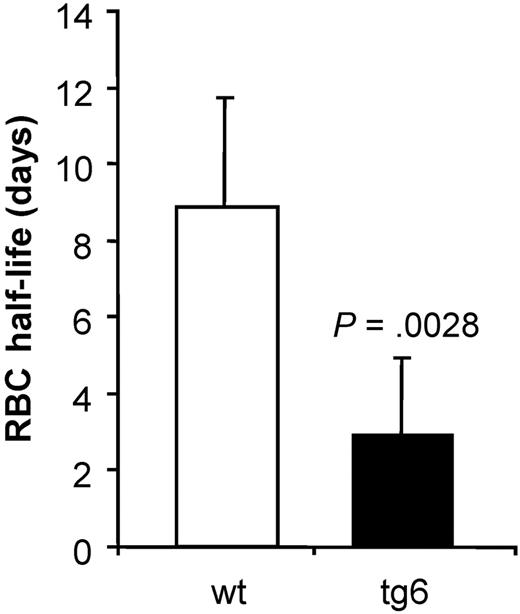
Red cell life span
The life span of wt erythrocytes was in accordance with the values reported in the literature20 namely about 60 days. Interestingly, in tg6 mice the life span of the erythrocytes was reduced to almost a third compared with wt (Figure 2). Obviously, there are mechanism(s) that allow efficient removal of transgenic erythrocytes, thereby counteracting the fatal effects of excessive erythrocytosis in our tg6 mice. Tg6 red cell survival in vivo was also measured in wt mice and wt red cell survival in tg6 mice. In both experiments, the percentage of labeled cells dropped below 0.5% within 8 to 12 days (data not shown), confirming the abnormal red cell life span in tg6 mice.
Decay of biotin-x-N-hydroxysuccimide ester–labeled erythrocytes. The percentage of labeled cells (day 0, set to 100%) was measured every 3 to 4 days until less than 0.5% labeled cells was found and the data were plotted against the day of sampling. For each individual mouse, an exponential regression equation was determined that was used to calculate the erythrocytes' half live as shown in the figure. Life span of tg6 erythrocytes is reduced to almost a third compared with wt erythrocytes. Means ± SD; n = 5-6.
Decay of biotin-x-N-hydroxysuccimide ester–labeled erythrocytes. The percentage of labeled cells (day 0, set to 100%) was measured every 3 to 4 days until less than 0.5% labeled cells was found and the data were plotted against the day of sampling. For each individual mouse, an exponential regression equation was determined that was used to calculate the erythrocytes' half live as shown in the figure. Life span of tg6 erythrocytes is reduced to almost a third compared with wt erythrocytes. Means ± SD; n = 5-6.
In vivo red cell tracking
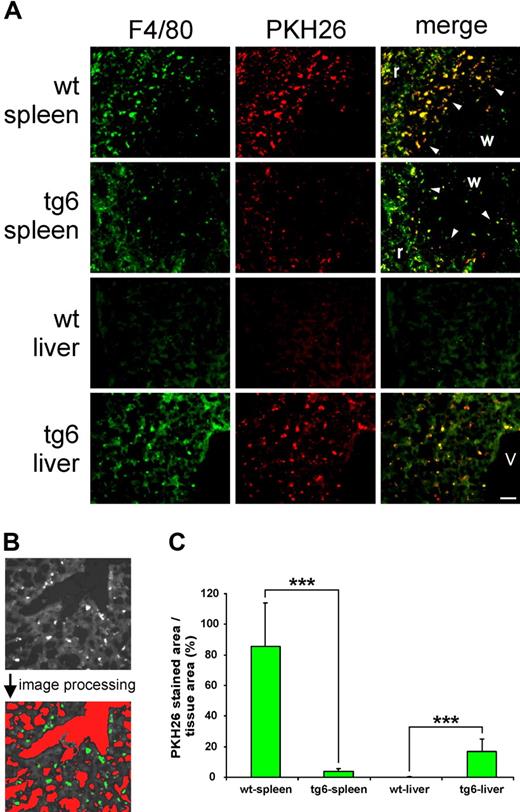
Four days after reinfusion, PKH26-labeled autologous erythrocytes were still present in the circulation as confirmed by blood smears. PKH26 fluorescence was detected in spleen and liver only, but in none of the other organs tested (not shown). In spleen and liver, PKH26 colocalized with F4/80 staining, indicating that the labeled erythrocytes were indeed incorporated by tissue macrophages.14 Moreover, Figure 3A shows major differences in the distribution of the incorporated erythrocytes between spleen and liver in wt and tg6 mice. The wt spleen contained many F4/80-positive cells, mainly in the red pulp. Especially at the marginal zone between white and red pulp, macrophages were highly positive for PKH26. Interestingly, F4/80 as well as colocalized PKH26 staining was reduced in the tg6 spleen. Very low F4/80 or PKH26 staining was found in the wt liver, whereas the tg6 liver contained an increased number of F4/80-positive cells that had incorporated PKH26-labeled erythrocytes. The PKH26-positive area per tissue area in tg6 spleen, about 22 times smaller in and tg6 liver, was 150 times larger compared with wt organs (Figure 3C). Most likely, tg6 splenic macrophages were superseded by the dramatically increased number of erythropoietic precursor cells populating the transgenic spleen.8 Considering the weight of the liver (tg6: 1.503 ± 0.18 g; wt: 0.997 ± 0.05 g; P < .05) and spleen (tg6: 407 ± 43 mg; wt: 87 ± 8.5 mg8 ) together with the PKH26-positive area per tissue area, tg6 mice have a nearly 8 times increased macrophage mass than do incorporate erythrocytes.
In vivo tracking of erythrocytes. (A) Macrophages were visualized by F4/80 staining and incorporated erythrocytes, by prior PKH26 labeling. Compared with the wt organ, F4/80 and PKH26 staining is decreased in the tg6 spleen. The most intense phagocytosis of erythrocytes was found in the marginal zone (arrowheads) between white (w) and red (r) pulp. In contrast, F4/80 as well as PKH26 staining was dramatically increased in the tg6 liver (same magnification of all images; bar represents 50 μm; v indicates central vein). (B) Illustration of the quantification procedure of the PKH26 fluorescent area using image analysis. Tissue area was defined as the total image area minus the cross-sectional area of all vessels (higher optical density compared with the tissue, marked red after image processing). The PKH26-stained area is evident from a much lower optical density compared with the tissue (marked green after image processing). (C) Compared with wt, the PKH26-positive area per tissue area was about 22 times smaller in the transgenic spleen but approximately 150 times larger in the liver of tg6 mice. Means (± SD) of 10 analyzed images of liver and spleen each of 4 animals of each line; ***P < .001.
In vivo tracking of erythrocytes. (A) Macrophages were visualized by F4/80 staining and incorporated erythrocytes, by prior PKH26 labeling. Compared with the wt organ, F4/80 and PKH26 staining is decreased in the tg6 spleen. The most intense phagocytosis of erythrocytes was found in the marginal zone (arrowheads) between white (w) and red (r) pulp. In contrast, F4/80 as well as PKH26 staining was dramatically increased in the tg6 liver (same magnification of all images; bar represents 50 μm; v indicates central vein). (B) Illustration of the quantification procedure of the PKH26 fluorescent area using image analysis. Tissue area was defined as the total image area minus the cross-sectional area of all vessels (higher optical density compared with the tissue, marked red after image processing). The PKH26-stained area is evident from a much lower optical density compared with the tissue (marked green after image processing). (C) Compared with wt, the PKH26-positive area per tissue area was about 22 times smaller in the transgenic spleen but approximately 150 times larger in the liver of tg6 mice. Means (± SD) of 10 analyzed images of liver and spleen each of 4 animals of each line; ***P < .001.
Macrophage assay
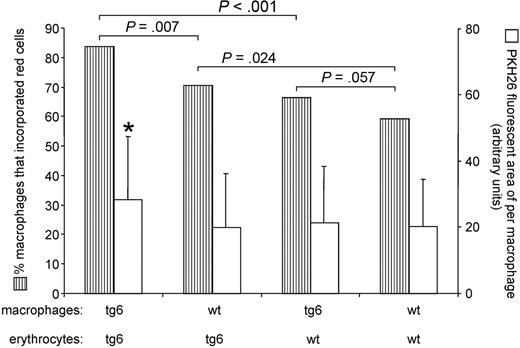
Next we exposed macrophages isolated from wt and tg6 mice to either wt or transgenic erythrocytes. Figure 4 shows that tg6 macrophages coincubated with tg6 erythrocytes had the highest phagocytic activity followed by wt macrophages coincubated with tg6 erythrocytes. In contrast, the overall phagocytic activity of wt macrophages was lower than that of tg6 macrophages. Of note, tg6 erythrocytes showed a tendency to be more attractive also for the wt macrophages. Moreover, tg6 macrophages coincubated with tg6 erythrocytes incorporated significantly more erythrocytes per single macrophage than all other combinations of macrophages and erythrocytes (Figure 4). Taken together, tg6 macrophages have an increased erythro-phagocytic activity compared with wt macrophages and tg6 erythrocytes are more attractive for both tg6 and wt macrophages.
Assessment of macrophage activity. Hatched bars represent the percentage of macrophages that incorporated at least one red cell. Tg6 macrophages incubated together with tg6 erythrocytes showed the highest phagocytotic activity followed by wt macrophages incubated with tg6 erythrocytes. Wt macrophages were less active compared with tg6 macrophages when coincubated with tg6 or wt erythrocytes, respectively. Tg6 erythrocytes were significantly more attractive than wt erythrocytes for both tg6 and wt macrophages. Moreover, the PKH26 fluorescent area of per single macrophage (open bars) was significantly larger in tg6 macrophages coincubated with tg6 erythrocytes compared with all other combinations of macrophages and erythrocytes. P values for hatched bars were calculated using a Fischer test, and for open bars using a 2-tailed Student t test for unpaired samples. Means ± SD of 4 independent experiments each; *P < .05.
Assessment of macrophage activity. Hatched bars represent the percentage of macrophages that incorporated at least one red cell. Tg6 macrophages incubated together with tg6 erythrocytes showed the highest phagocytotic activity followed by wt macrophages incubated with tg6 erythrocytes. Wt macrophages were less active compared with tg6 macrophages when coincubated with tg6 or wt erythrocytes, respectively. Tg6 erythrocytes were significantly more attractive than wt erythrocytes for both tg6 and wt macrophages. Moreover, the PKH26 fluorescent area of per single macrophage (open bars) was significantly larger in tg6 macrophages coincubated with tg6 erythrocytes compared with all other combinations of macrophages and erythrocytes. P values for hatched bars were calculated using a Fischer test, and for open bars using a 2-tailed Student t test for unpaired samples. Means ± SD of 4 independent experiments each; *P < .05.
Assessment of properties typical for senescent erythrocytes
In the last set of experiments, we aimed to determine the mechanism(s) leading to enhanced phagocytosis of transgenic erythrocytes.
Testing for autoantibodies against erythrocytes.
To exclude that tg6 develop autoantibodies against their erythrocytes explaining their reduced life span, we performed a series of direct Coombs assays. The semiquantitative assessment of agglutination of erythrocytes suspended in different dilutions of antimouse polyvalent immunoglobulin revealed slightly higher antibody binding in wt compared with tg6 erythrocytes (not shown). Thus, we have no evidence that tg6 animals develop autoantibodies against their own erythrocytes.
Band 4.1a to 4.1b ratio.
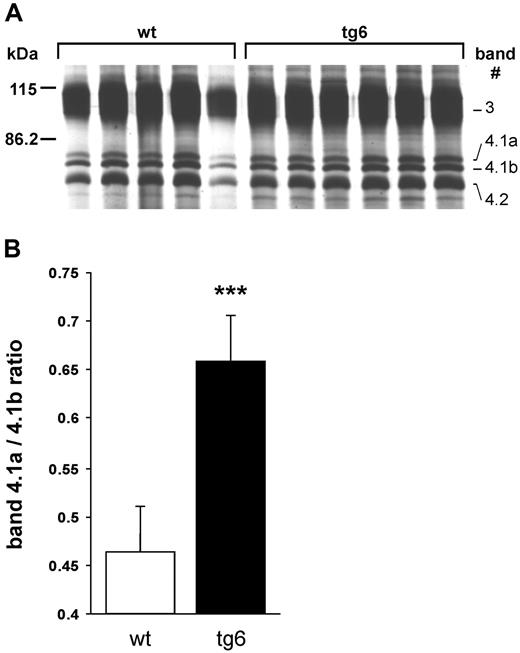
Erythrocyte ghost proteins showed an increased band 4.1a to 4.1b ratio in the whole tg6 erythrocyte population (Figure 5). While the value for control mice was in accordance to values determined earlier by others,22 the ratio in tg6 mice was unexpectedly higher, suggesting that the tg6 erythrocyte population contains more aged than young cells. Indeed, density separation of tg6 erythrocytes revealed a different density distribution compared with wt controls with, most importantly, much lighter cells in tg6 mice (not shown).
Band 4.1a to 4.1b ratio. (A) Silver-stained SDS–polyacrylamide gel electrophoresis (PAGE) of erythrocyte membrane proteins. Samples were isolated form 5 wt and 6 tg6 individuals and the part of the gel containing bands 3 through 4.2 labeled according to Steck21 is shown. (B) Quantification of the band 4.1a to 4.1b ratio by densitometry revealed an increased ratio in tg6 red cell membranes, which is typical for senescent erythrocytes. Means (± SD); n = 5-6; ***P < .001.
Band 4.1a to 4.1b ratio. (A) Silver-stained SDS–polyacrylamide gel electrophoresis (PAGE) of erythrocyte membrane proteins. Samples were isolated form 5 wt and 6 tg6 individuals and the part of the gel containing bands 3 through 4.2 labeled according to Steck21 is shown. (B) Quantification of the band 4.1a to 4.1b ratio by densitometry revealed an increased ratio in tg6 red cell membranes, which is typical for senescent erythrocytes. Means (± SD); n = 5-6; ***P < .001.
Nonprotein thiols.
As tg6 mice developed excessive erythrocytosis that nearly doubled their arterial oxygen content,23 we speculated that tg6 erythrocytes suffer from oxidative damage. Thus, we measured the nonprotein thiols in tg6 and wt erythrocytes. Transgenic erythrocytes contained significantly less GSH compared with wt erythrocytes (2.04 ± 0.2 vs 2.55 ± 0.14 μmol/mL erythrocytes, n = 9, P < .05). Accordingly, GSSG levels of tg6 erythrocytes were slightly higher (wt: 0.031 ± 0.013 vs tg6: 0.073 ± 0.02 μmol/mL erythrocytes, n = 8), although the latter observation did not reach statistical significance. These findings indicate an increased in vivo oxidative stress of tg6 erythrocytes.
CD47.
Elevated phagocytosis of transgenic erythrocytes might be due to reduced expression of CD47 that is known to inhibit erythro-phagocytosis by binding the inhibitory receptor signal regulatory protein alpha (SIRPα) present on macrophages.14,24 Table 1 shows the histogram statistics of the FACS analysis of labeled (monoclonal antimouse CD47 antibody, dilution: 1:100) erythrocytes obtained from wt and tg6 mice (5-6 individual animals each). At this dilution and both other dilutions tested (1:50 and 1:200, not shown), mean, geometrical mean, median, and fluorescence intensity channel with the highest counts (peak channel) were highly significantly shifted toward lower fluorescence intensities in histograms obtained with tg6 erythrocytes. This clearly indicates a reduced expression of CD47 on transgenic erythrocytes.
In addition, the histograms of tg6 erythrocytes appeared to be wider compared with those of wt erythrocytes, suggesting a higher variance of the fluorescence intensities. This can be quantified by the standard deviation, the coefficient of variation, and the height of the peak. For transgenic erythrocytes, the peak height was significantly lower at all dilutions of the antibody, the coefficient of variation was significantly higher only at the highest dilution, and the standard deviation, at none of the dilutions.
Surface sialic acids.
While aging, erythrocytes lose sialic acids25-28 among other surface carbohydrates. As a reduction of sialic acids on the erythrocyte's surface is known to be associated with elevated erythro-phagocytosis,16 we quantified sialic acid on wt and tg6 erythrocytes. Sialic acids at the cell's surface were 22% lower in tg6 mice compared with wt (Figure 6A). Furthermore, this observation was qualitatively confirmed by the lower tendency of tg6 erythrocytes to agglutinate in PBS containing 1% polybrene (Figure 6B).
Erythrocyte surface sialic acids. Compared with wt, surface sialic acids were about 22% reduced in tg6 erythrocytes (left) despite the fact that the population of the transgenic erythrocytes is younger. The polybrene agglutination test (right) confirmed these quantitative measurements. Many red cell clusters (arrowheads) could be observed with wt erythrocytes, whereas hardly any could be detected with tg6 erythrocytes. Original magnification 200 ×; means (± SD) of 4 independent experiments each.
Erythrocyte surface sialic acids. Compared with wt, surface sialic acids were about 22% reduced in tg6 erythrocytes (left) despite the fact that the population of the transgenic erythrocytes is younger. The polybrene agglutination test (right) confirmed these quantitative measurements. Many red cell clusters (arrowheads) could be observed with wt erythrocytes, whereas hardly any could be detected with tg6 erythrocytes. Original magnification 200 ×; means (± SD) of 4 independent experiments each.
Discussion
Paradoxically, erythrocytes from tg6 mice share features of young (increased intracellular potassium concentration, water content, flexibility, surface to volume ratio, and decreased cell density) as well as those of senescent (increased band 4.1a to 4.1b ratio, decreased GSH levels, reduced surface CD47 and sialic acids) erythrocytes. Presumably, tg6 erythrocytes age faster than normal ones resulting in enhanced phagocytosis of tg6 erythrocytes by wt and tg6 macrophages in vitro. This is reflected in vivo by the dramatically reduced erythrocyte life span in tg6 mice. In addition, tg6 macrophages showed an increased phagocytic activity when coincubated with both transgenic and wt erythrocytes. Moreover, in tg6 mice more macrophages per tissue area were found in the liver but less in the spleen. Considering that the transgenic liver and spleen are enlarged by 50% and 470%, respectively, tg6 mice have a drastically increased cell mass capable to incorporate erythrocytes. Together, these findings provide strong evidence for the existence of a so far unknown mechanism down-regulating the erythrocyte mass in excessive erythrocytosis most probably by enhancement of the normal physiological pathways to remove senescent erythrocytes. This includes first an adaptive increase in number and activity of tissue macrophages that remove erythrocytes and second accelerated erythrocyte aging that might be induced by oxidative damage as indicated by the lowered GSH levels. As a consequence, for example, CD47 loss of the whole cell population would facilitate erythro-phagocytosis in tg6. However, a reduction of CD47 concentration per surface area on the red cells could be in principle also be due to a gain of surface area after the erythrocytes have left the bone marrow. This has been observed in patients suffering from cirrhosis of the alcoholic29 or in dogs after feeding with a cholesterol-rich diet30 and is mediated by cholesterol incorporation into the erythrocyte membrane. This phenomenon could be confirmed in vitro by incubating red cells with cholesterol-rich lipid dispersions.31 Indeed, our tg6 mice have a liver pathology since their liver is enlarged and shows hemosiderin dispositions and inflammatory foci—but tg6 mice do not show any signs of liver cirrhosis.32 Of note, cholesterol incorporation into the red cell membrane leads to a decrease of osmotic resistance as well as erythrocyte flexibility.30,31 However, as both parameters are increased in tg6 erythrocytes these observations argue against cholesterol overloading of their membrane. In addition, red cells of patients suffering from cirrhosis of the alcoholic show morphologic abnormalities such as target or spur cells29,31 that we have never detected in blood smears of our tg6 mice (not shown). Finally, we have measured the CD47 content using FACS analysis. Compared with wt controls, these measurements revealed a reduced CD47 content per erythrocyte in tg6 mice. Since FACS analysis measures the antigen content per complete cell, it is independent on changes in membrane surface area due to lipid incorporation and a consecutive dilution of the membrane proteins. Therefore it is not very likely that a gain of membrane surface area due to lipid incorporation is responsible for the reduction of CD47 on the surface of tg6 erythrocytes. Whether recognition by macrophages is further enhanced by exposure of penultimate galactose residues or by opsonins remains open, since the negative Coombs test excludes the presence of induced autoantibodies, but cannot differentiate between unopsonized and opsonized cells by naturally occurring antibodies. On the other hand, phosphatidylserine exposure to the outer leaflet of the erythrocyte membrane, another age-related erythrocyte feature,33,34 appears to be an important mechanism for red cell sequestration especially under pathological conditions such as sickle cell anemia since phosphatidylserine exposure is increased 2- to 10-fold in these patients.4 However phosphatidylserine exposure did not differ significantly between wt and tg6 (not shown) indicating other mechanism(s) responsible for the reduced erythrocyte life span in tg6 mice.
High hematocrit levels are found in patients suffering from chronic pulmonary failure, polycythemia vera, or chronic mountain disease, and in lowlanders at high altitude and in Epo-abusing athletes. Although humans rarely reach hematocrit values observed in our mice, there are cases described with hematocrit values up to 0.91.35 Excessive erythrocytosis results in clinical complications such as hypertension, thromboembolism, and even death.36
As to the question how tg6 mice adapt to the extreme hematocrit values, we previously showed that compensatory mechanisms include vasodilatation and regulation of blood viscosity.7,8,37 However vasodilatation leads to a decrease in blood flow velocity and thus fluid shear stress. Of note, as a non-Newtonian fluid, blood increases its viscosity with decreased shear stress.38 Therefore, regulation of blood viscosity appears to be an at least as important mechanism as vasodilatation. The simplest way to maintain the blood viscosity as low as possible despite the elevated hematocrit is to keep the erythrocyte population as young as possible. Indeed, increased reticulocyte counts, a higher mean corpuscular volume, and somewhat lower (although not significant) mean corpuscular hemoglobin concentration in tg6 mice have been reported previously.8 All these features characterize young erythrocytes.39,40 The lower bumetanide-sensitive (NKCC) K+ flux and higher ouabain-sensitive (active) K+ flux also point to a younger erythrocyte population in tg6. This finding is in accordance with previous studies showing low basal NKCC activity and high Na/K-ATPase activity in reticulocytes that increases and decreases, respectively, with maturation.41 The changes in the potassium transport kinetics we report here lead, via accumulation of intracellular K+ and water, to an increased cell volume. The lower mean corpuscular hemoglobin concentration as a consequence of the accumulation of cations and water results in a lower internal viscosity and thus, together with the higher surface to volume ratio (especially in humans another feature of young erythrocytes42 ), to increased red cell flexibility as observed with osmotic gradient ectacytometry.
Under conditions of hypoxia-independent chronically elevated Epo plasma levels, as in our transgenic mice, so far unknown mechanism(s) might be activated to actively counteract the surplus of erythrocytes finally resulting in a markedly reduced erythrocyte life span. As shown here, one part of such a mechanism might be the increase in number and activity of tissue macrophages that are able to incorporate erythrocytes. If erythrocytes would be removed randomly whole blood viscosity would not change. In contrast, whole blood viscosity would decrease by an enhanced elimination of older erythrocytes that might be the second part of this mechanism. This possibility appears likely, although tg6 erythrocytes appear chronologically older than control cells based on the increased band 4.1a/4.1b ratio. Since even the lightest one third of the cells from tg6 mice have a higher band 4.1a/4.1b ratio (although not significant, not shown) than the lightest cells from controls, we speculate that tg6 erythrocytes remain longer in the bone marrow, but maintain their enzymatic set up until they enter the circulation.
In summary, our findings indicate the existence of a mechanism negatively regulating the erythrocyte mass that is capable to override the consequence of extreme plasma Epo levels.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by Stiftung für wissenschaftliche Forschung an der Universität Zürich (J.V.); and Swiss National Science Foundation (3100B0-112449 and 3100A0-112216 to A.B. and M.G., respectively).
The authors thank S. Keller and B. Grenacher for technical support.
Authorship
Contribution: A.B. measured ion, water content, and surface sialic acids; D.M. measured ion fluxes and nonprotein thiols; H.L. performed osmoscans of CD47 and band 4.1 measurements and suggested article text revisions; B.S. performed band 4.1 measurements; M.G. generated the tg6 mice and revised article text and data presentation; J.V. designed the study, wrote the article, performed erythrocyte in vivo tracking, macrophage assay, polybrene testing and Coombs testing, and measured erythrocyte life span, osmotic fragility, and CD47.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Johannes Vogel, Institute of Veterinary Physiology, Vetsuisse Faculty University of Zürich, Winterthurerstr. 260, CH-8057 Zürich, Switzerland; e-mail: jvogel@vetphys.uzh.ch.