Abstract
Latency enables human cytomegalovirus (HCMV) to persist in the hematopoietic cells of infected individuals indefinitely and prevents clearance of the pathogen. Despite its critical importance to the viral infectious cycle, viral mechanisms that contribute to latency have not been identified. We compared the ability of low-passage clinical and laboratory-adapted strains of HCMV to establish a latent infection in primary human CD34+ cells. The low-passage strains, Toledo and FIX, established an infection with the hallmarks of latency, whereas the laboratory strains, AD169 and Towne, replicated producing progeny virus. We hypothesized that ULb′ region of the genome, which is unique to low-passage strains, may encode a latency-promoting activity. We created and analyzed recombinant viruses lacking segments or individual open reading frames (ORFs) in the ULb′ region. One 5-kb segment, and more specifically the UL138 ORF, was required for HCMV to establish and/or maintain a latent infection in hematopoietic progenitor cells infected in vitro. This is the first functional demonstration of a virus-coded sequence required for HCMV latency. Importantly, UL138 RNA was expressed in CD34+ cells and monocytes from HCMV-seropositive, healthy individuals. UL138 might be a target for antivirals against latent virus.
Introduction
Human cytomegalovirus (HCMV) is a ubiquitous β-herpesvirus that infects a broad range of cell types in its human host, contributing to its complex and varied pathogenesis. HCMV infection in healthy individuals is typically asymptomatic. Following this primary infection, HCMV will establish a life-long relationship with its host by way of a latent infection.1 Reactivation from latency to a productive infection can result in life-threatening disease in individuals who are immunosuppressed, including stem cell and solid organ transplant recipients, and HIV–infected individuals. The precise mechanisms governing HCMV latency are unknown, but it is likely that both cellular and viral factors contribute to the establishment and maintenance of latency. Elucidating the factors controlling latency is critical to ultimately controlling HCMV disease
Latent HCMV resides in cells of the myeloid lineage. Monocytes are the predominant mononuclear cell latently infected with HCMV,2 and virus has been recovered from peripheral blood monocytes after stimulation in culture.3 It has been reported that direct infection of monocytes4 or polymorphonuclear leukocytes5-8 results in an abortive infection suggesting that latently infected monocytes may acquire HCMV at an earlier stage of differentiation. More recent work suggests that monocytes infected in vitro harbor viral genomes in a quiescent state and acquire permissivity following differentiation.9 Monocytes are an important reservoir for latent HCMV, however, the primary reservoir for latent HCMV is a more primitive cell.10-15 Kondo et al detected latent genomes in granulocyte-macrophage progenitor cells (GM-Ps) after infection in vitro and in mononuclear cells from HCMV-seropositive individuals.10,16 Latent genomes have also been detected in myeloid dendritic cell precursors derived from HCMV-seropositive, healthy individuals and could be stimulated to reactivate ex vivo.17 Further, an infection consistent with latency is established in CD34+/CD38− cells in vitro but not in more mature subpopulations of CD34+ cells.18 In CD34+/CD38− cells, HCMV transiently expresses a unique subset of the viral genes in the absence of substantial virus replication and virus can be reactivated from these cells. Taken together, these findings suggest that the establishment of a latent infection is dependent on the cellular environment unique to specific subsets of hematopoietic cells.
Viral transcripts or proteins have long been suspected to play a role in HCMV latency. The identity of HCMV latency determinants, however, has remained elusive. Two latency-associated transcripts that originate from the major immediate-early promoter region of the genome have been identified in GM-Ps.16,19 While these transcripts and the proteins they encode have been detected in healthy, seropositive individuals,16,20 they were dispensable for establishing viral latency in vitro.21 In addition, a latency-associated transcript that is antisense to UL81–82 of HCMV was detected in monocytes from healthy seropositive individuals.22 This finding is intriguing since the transcript is antisense to the mRNA encoding the essential UL82 protein and could inhibit its expression and, consequently, immediate-early gene expression.23-27 Similarly, a variant of the viral IL-10 homologue encoded by the UL111.5A region of the HCMV genome was detected in latently infected GM-Ps and in monocytes from healthy seropositive individuals.28 As yet, there is no direct role for the UL81–82 and UL111.5A gene products in latency.
Historically, HCMV research has relied heavily on attenuated, laboratory-adapted strains (herein referred to as laboratory strains) that have been extensively passaged in fibroblasts. HCMV strains adapted for growth in cultured fibroblasts have acquired genome rearrangements that distinguish them from clinical and low-passage strains. Namely, low-passage strains of CMV contain a 13- to 15-kb DNA segment that has been lost during passage in laboratory strains.29-31 This region, representing the majority of ULb′ region, encodes as many as 20 open reading frames including UL133 through UL151, as well as a newly predicted, conserved open reading frame, ORF11.31 Laboratory strains lacking ULb′ sequences replicate more efficiently and to higher titers than low-passage strains. As we develop more sophisticated means to study HCMV infection, these sequences and their possible contribution to latency and pathogenesis are of growing interest.
Using the hematopoietic progenitor cell model for latency, we compared the ability of low-passage strains (FIX and Toledo) and laboratory strains (Towne and AD169) to establish a latent infection in CD34+ cells. Low-passage but not laboratory strains established an infection consistent with latency. Based on this observation, we hypothesized that the ULb′ region of the HCMV genome unique to clinical isolates may encode factors that promote the latent infection. We identified a 5-kb region, and specifically the UL138 ORF, required for HCMV to establish and maintain a latent infection. Viruses lacking these sequences failed to establish/maintain a latent infection and replicated like laboratory strains. Importantly, UL138-specific RNA was detected in monocytes and CD34+ cells from healthy, seropositive individuals. This is the first functional demonstration of a virally encoded factor required for HCMV latency.
Materials and methods
Cells and viruses
Primary human lung fibroblasts (MRC5) were maintained in Dulbecco-modified Eagle medium (DMEM) supplemented with 8% FBS, 1 mM sodium pyruvate, 10 mM HEPES, 2 mM l-glutamine, 0.1 mM nonessential amino acids, 100 U/mL penicillin, and 100 mg/mL streptomycin. The M2–10B4 murine stromal cell line expressing human interleukin-3 (IL-3) and granulocyte-colony stimulating factor (G-CSF) and the S1/S1 murine stromal cell line expressing human IL-3 and stem cell factor (SCF) were generously provided by Stem Cell Technologies Ltd. on behalf of D. Hogge (Terry Fox Laboratory, University of British Columbia, Vancouver, BC) and cultured as recommended.32
Informed consent for human subjects work was provided in accordance with the Declaration of Helsinki. For most experiments, bone marrow cells were obtained from waste produced during bone marrow harvest from healthy donors at Wake Forest University Baptist Medical Center via a protocol approved by the institutional review board. Alternatively, bone marrow from cadavers was purchased from the National Disease Research Interchange (Philadelphia, PA). For reverse-transcriptase polymerase chain reaction (PCR) assays, peripheral blood mononuclear cells were obtained from healthy donors who had been mobilized with G-CSF prior to leukopheresis under a protocol approved by the Cambridge Local Research Ethics Committee. CD34+ cells were isolated as described previously.11 For some reverse-transcriptase PCR experiments, CD34+ cells purified from the peripheral blood of G-CSF–mobilized donors were purchased (Cambrex Bioscience, Workingham, United Kingdom). Monocytes were purified from peripheral blood mononuclear cell preparations by incubating cells for 1 hour in PBS at 37°C, 5% CO2. Nonadherent cells were aspirated and cells were incubated for 3 hours in RPMI to remove residual T lymphocytes. Donor HCMV serostatus was tested using a diagnostic IgG ELISA kit (Capita; Trinity Biotech, Bray, Ireland).
For infection, newly isolated or thawed CD34-enriched bone marrow cells were exposed to virus at a multiplicity of 2 plaque-forming units (PFU) per cell for 20 hours in Iscoves modified DMEM (IMDM) supplemented with 10% BIT9500 serum substitute (Stem Cell Technologies, Vancouver, CA), 2 mM l-glutamine, 20 ng/mL low-density lipoprotein (Sigma, St Louis, MO), and 50 μM 2-mercaptoethanol. Following infection, cells were washed in citrate buffer (40 mM Na citrate, 10 mM KCl, 135 mM NaCl, pH 3.0) for 1 minute to inactivate unabsorbed virus.
For long-term bone marrow cultures, a 1:1 suspension of M210B4 (IL-3, G-CSF) and S1/S1 (IL-3, SCF) cells were irradiated (20Gy, Cesium 137 source, Gammacell 40; Nordion International, Kamata, ON) and plated onto collagen-coated tissue culture dishes at a density of 3 × 105 (7.5 × 104 cells of each cell type per mL) cells per 35-mm well.32 CD34+ cells were established in transwells (Costar, Cambridge, MA) above irradiated stromal cells. CD34+ cells were differentiated into dendritic cells as described previously.17
The FIX, Toledo, Towne, and AD169 virus strains have been cloned as bacterial artificial chromosomes (BACs) and engineered to express the green fluorescent protein (GFP).31 Virus stocks were propagated by electroporation of infectious BAC DNA into MRC5 cells and purified by density gradient centrifugation through a sorbitol cushion. Virions were resuspended in IMDM containing 1% bovine serum albumin and stored at −80°C. Before infection, virus was thawed and sonicated. Infectious virus yields were determined by median tissue culture infectious dose (TCID50) on MRC5 cells.
Mutant viruses were constructed by linear recombination33 using PCR products with homologous flanking sequences (Table 1) to the targeted region of the HCMV genome in the FIX strain where GFP had been inserted into the BAC backbone (unpublished results, D. Yu and T.S., February 2004). Primer sequences used for each substitution mutant are shown in Table 1. PCR reactions to synthesize fragments for linear recombination included 50 ng pKan/LacZ (for 5- to 15-kb substitutions) or pKanfrtFLAG (for individual ORF substitutions) as a template. Linear fragments were synthesized, digested with DpnI, gel purified, and transfected into E coli strain DY380 containing the FIXGFP-BAC. The structures of the substitutions in kanamycin-resistant, ampicillin-sensitive clones were confirmed by sequencing. FIX recombinant BACs were reconstituted by electroporation of purified, infectious BAC genomic DNA into MRC5 cells.
For multistep growth curves, MRC5 cell monolayers were infected at a multiplicity of 0.05 PFU/cell. At various times after infection, cells were collected in their medium and sonicated. Virus yields were determined by TCID50.
Flow cytometry
Infected CD34+/CD38− cell populations were labeled with fluorescently conjugated monoclonal antibodies in PBS supplemented with 0.5% FBS. Subpopulations of cells were isolated using a FACSVantage flow cytometer (BD Biosciences Immunocytometry Systems, San Jose, CA) using monoclonal antibodies conjugated to phycoerythrin (PE) for CD34 (BD Pharmingen, San Diego, CA) and allophycocyanin (APC) for CD38 (BD Biosciences Immunocytometry Systems). For all experiments, the purity of cells recovered was 97% or higher.
Limiting dilution reactivation assay
The frequency of reactivation in infected CD34+ cell populations was determined by adapting a limiting dilution assay as described previously.18 Briefly, 40 000 infected hematopoietic cells/mL were serially diluted 2-fold in reactivation medium (RPMI supplemented with 20% FBS, 50 μM 2-mercaptoethanol, 100 U/mL penicillin, and 100 mg/mL streptomycin) and 20 ng/mL each of stem cell factor (FLT-3 ligand, IL-3, IL-6, G-CSF, GM-CSF; cytokines from R&D Systems, Minneapolis, MN) after 11 to 14 days in long-term bone marrow culture. An aliquot (0.1 mL) of each dilution was added to 12 wells of 96-well tissue culture plates containing human fibroblasts. Fibroblasts were monitored for GFP expression for a period of 24 days. To differentiate virus made as a result of reactivation from virus pre-existing in the cell cultures, an equal number of cells was serially diluted and plated on fibroblasts after being mechanically disrupted.18 The frequencies of infectious center formation were based on the faction of wells of each dilution that scored positively for GFP expression and statistically determined using a modified TCID50 assay based on a logistic model as described previously.18
HCMV arrays
RNA was isolated using Absolutely RNA (Stratagene, La Jolla, CA) and subjected to 2 rounds of linear amplification using MessageAmp (Ambion, Austin, TX). The resulting cDNA was labeled with Cy3-dUTP using the CyScribe First-Strand cDNA Labeling kit (Amersham Biosciences, Piscataway, NJ), hybridized to HCMV arrays, and analyzed as described previously.11 HCMV cDNAs, cellular cDNAs for normalization, and positive and negative controls were prepared and printed onto glass slides as described previously11 using an Omnigrid Arrayer (GeneMachine, San Carlos, CA). Hybridization to arrays was analyzed using a GenePix 4000B scanner and GENEPIX PRO VERSION 3.0 software (Molecular Devices, Axon Instruments, Sunnyvale, CA).
Detection of CMV transcripts in endogenous infection
Total RNA from 106 cells was extracted using RNA-Bee (Biogenesis, Poole, United Kingdom) as described by the manufacturer and the final pellet was resuspended in 50 μL nuclease-free water. Reverse transcription (RT) of mRNA was achieved using an AMV reverse transcriptase kit (Promega, Madison, WI).
Following RT, cDNAs were amplified using UL123 (IE1), UL138, or GAPDH-specific primers (Table 2). All PCR reactions were performed using a PCR-ready mix (Bioline, London, United Kingdom) supplemented with primers (final concentration, 200 ng/mL), MgCl2 (1.5-3.0 mM), sterile water, and cDNA template (to 50 μL) and amplified for 25 to 50 cycles. RNA was amplified by PCR to confirm that there was no DNA contamination. PCR products were separated on 2% agarose gel by electrophoresis. The UL138 and IE1 PCR products were transferred to nitrocellulose and hybridized with a radiolabeled product-specific probe generated using internal primers (Table 2). GAPDH PCR products were analyzed following ethidium bromide staining.
Results
Low-passage strains, but not laboratory strains, establish and maintain a latent infection in hematopoietic cells
Sequence analysis of HCMV strains has revealed well-defined differences between the low-passage and laboratory strains.29-31 Since laboratory strains have been selected for efficient lytic replication, it is possible that some of these differences may play an important role in establishing a balance between lytic replication and latency. In our studies, we have used 2 low-passage strains, FIX and Toledo, and 2 laboratory strains, Towne and AD169. FIX and Toledo both retain the ULb′ region, although the region is inverted in Toledo relative to other low-passage strains.29 The laboratory strains used in our studies, Towneshort and AD169, are missing 13.1 and 15.2 kb of ULb′ region, respectively.29 All virus strains were engineered to express GFP.11
We compared the ability of HCMV laboratory and low-passage strains to establish a latent infection in CD34+ cells infected in vitro.11,18 A latent infection is defined by the persistence of viral genomes in the absence of virus replication until an appropriate stimulus is provided to reactivate viral replication. In our experiments, CD34+ cells were infected with low-passage or laboratory strains of HCMV without prior ex vivo culture. Twenty hours later, infected cells were purified by fluorescent activated cell sorting (FACS) for GFP expression. At a multiplicity of 2 PFU/cell, 16.5% ± 6.5%, 22.8% ± 3.6%, 10.7% ± 5.2%, and 32.3% ± 11.8% of CD34+ cells exposed to FIX, Toledo, Towne, and AD169, respectively, were GFP+ and, therefore, successfully infected. Following purification by FACS, infected CD34+ cells were cultured over a murine stromal cell support that has been shown to maintain progenitor cell phenotype and function.32,34 At 11 to 14 days after infection (dpi), when the HCMV genome has become silent,18 infected hematopoietic cells were transferred by limiting dilution to coculture with permissive human lung fibroblasts (MRC5) to quantify infectious center formation as a direct measure of reactivation. As a control, an equivalent number of infected CD34+ cells were mechanically lysed prior to seeding onto fibroblasts. The lysate control is critical for distinguishing infectious centers formed as a result of reactivation from those produced during the latency period prior to reactivation. Using the limiting dilution assay, we can quantitatively compare infectious center formation by each of the HCMV strains.
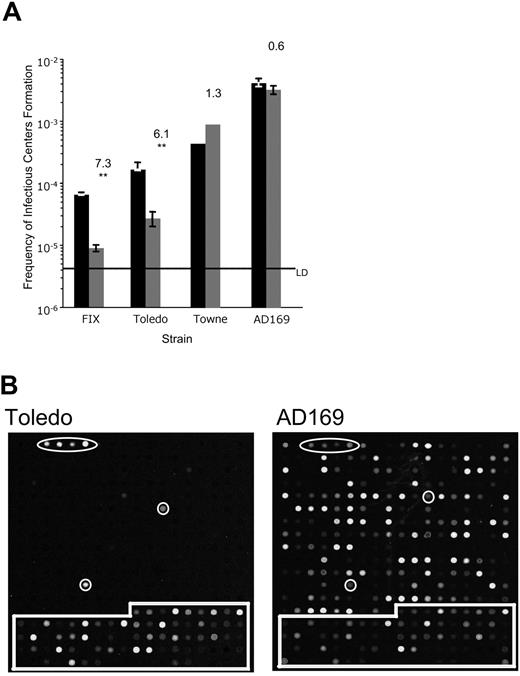
The low-passage strains, FIX and Toledo, produced fewer infectious centers compared with the laboratory strains, Towne and AD169 (Figure 1A). Formation of an infectious center following reactivation required 15 291 cells infected with FIX, 6012 cells infected with Toledo, 1818 cells infected with Towne, or 241 cells infected with AD169. These results suggest that increased adaptation to growth in cultured fibroblasts correlates with more efficient replication in hematopoietic progenitor cells. Importantly, the low-passage strains generated 6- to 7-fold more infectious centers in the reactivation samples than in the lysate control, whereas the laboratory strains generated similar amounts of infectious centers with or without a reactivation stimulus. Prior to reactivation (lysate control), 111 421 or 36 765 cells infected with FIX or Toledo, respectively, resulted in an infectious center. By contrast, infectious center formation required 1111 or 308 cells infected with Towne or AD169, respectively. For laboratory strains, the reactivation-to-lysate ratio is close to one indicating that the laboratory strains replicated persistently through the culture period. We conclude that low-passage but not laboratory strains can establish and maintain an infection in cultured CD34+ cells consistent with latency.
Low-passage clinical strains of HCMV, but not laboratory strains, establish a latent infection in cultured CD34+ hematopoietic progenitor cells. (A) Limiting dilution analysis was used to determine frequency of infectious center formation in CD34+ cells infected with low-passage (FIX and Toledo) or laboratory (Towne and AD169) strains at 10 to 14 dpi. The frequency is calculated as 1 over the number of cells required to yield one infectious center. The bars represent the average frequency of infectious center formation in the reactivation experiment (■) or the lysate control (▒) in at least 3 independent experiments for FIXwt, Toledo, and AD169 and two independent experiments for Towne. The standard error of the means is shown. The top number above each pair of bars reports the fold increase in infectious center formation in the reactivation compared with the lysate control. Using the paired Student t test, the probability (P) that the difference between the 2 averages is significant was calculated. Statistical significance is indicated by ** for P ≤ .01. All other pairs do not represent a significant difference (P > .05). The line marks the limit of detection (LD) for the assay. (B) CD34+ cells infected with Toledo or AD169 at a multiplicity of 5 PFU/cell were purified 20 hours later by FACS and seeded into long-term bone marrow cultures. Linearly amplified RNA was analyzed using the HCMV array at 20 dpi infection. All RNAs were hybridized in triplicate. Arabidopsis cDNA spots used as positive controls are circled. Cellular cDNA spots used for normalizing arrays are boxed. All other spots represent HCMV ORFs.
Low-passage clinical strains of HCMV, but not laboratory strains, establish a latent infection in cultured CD34+ hematopoietic progenitor cells. (A) Limiting dilution analysis was used to determine frequency of infectious center formation in CD34+ cells infected with low-passage (FIX and Toledo) or laboratory (Towne and AD169) strains at 10 to 14 dpi. The frequency is calculated as 1 over the number of cells required to yield one infectious center. The bars represent the average frequency of infectious center formation in the reactivation experiment (■) or the lysate control (▒) in at least 3 independent experiments for FIXwt, Toledo, and AD169 and two independent experiments for Towne. The standard error of the means is shown. The top number above each pair of bars reports the fold increase in infectious center formation in the reactivation compared with the lysate control. Using the paired Student t test, the probability (P) that the difference between the 2 averages is significant was calculated. Statistical significance is indicated by ** for P ≤ .01. All other pairs do not represent a significant difference (P > .05). The line marks the limit of detection (LD) for the assay. (B) CD34+ cells infected with Toledo or AD169 at a multiplicity of 5 PFU/cell were purified 20 hours later by FACS and seeded into long-term bone marrow cultures. Linearly amplified RNA was analyzed using the HCMV array at 20 dpi infection. All RNAs were hybridized in triplicate. Arabidopsis cDNA spots used as positive controls are circled. Cellular cDNA spots used for normalizing arrays are boxed. All other spots represent HCMV ORFs.
Viral gene expression is silenced in Toledo-infected CD34+ cells but not in cells infected with AD169
The striking difference in the ability of low-passage and laboratory strains to form infectious centers during long-term bone marrow culture prompted us to analyze the differences in viral gene expression. We previously found that CD34+/CD38− cells infected with Toledo transiently expressed a unique subset of viral RNAs following infection.18 The RNAs detected were encoded by both early and late kinetic classes of genes and many have no known function or may not encode a protein.35 Viral gene expression was silenced by 8 to 10 days after infection, a time when viral genomes were most abundant.18 We compared viral gene expression in CD34+ cells infected with the low-passage strain, Toledo, or the laboratory strain, AD169, using an HCMV gene array (Figure 1B). Consistent with previous results, no viral gene expression was detected in CD34+ cells infected with Toledo at 20 dpi. By contrast, AD169 expressed its genes as would be expected for a lytic infection. These results are consistent with the substantial amount of virus detected in lysate controls for laboratory strains in the latency/reactivation assays (Figure 1A).
HCMV genome sequences promote viral latency
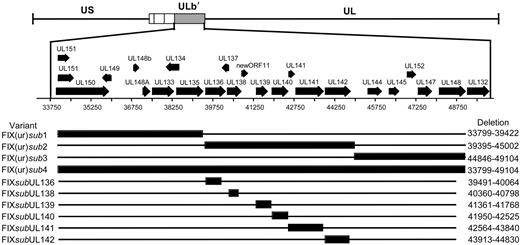
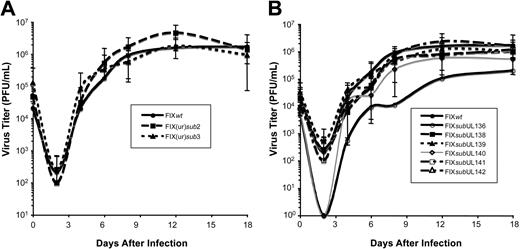
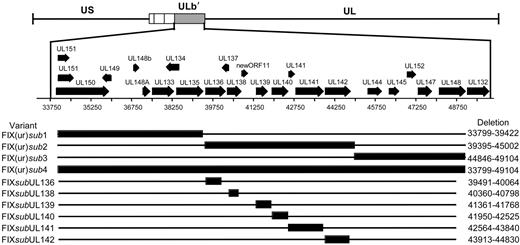
Given the differences in the ability of low-passage and laboratory strains to establish a latent infection in our experimental system, we asked if sequences within the ULb′ region unique to the low-passage strains contributed to viral latency. Using linear recombination into the FIXBAC, we substituted a marker cassette encoding kanamycin resistance for 3 contiguous 5-kb regions (FIX(ur)sub1-3) within the ULb′ region or for the entire region encoding UL133 through UL151 (FIX(ur)sub4) (Figure 2A). A prerequisite for studying sequences for a role in latency is that they are dispensable for viral replication in culture. Otherwise, we could not distinguish mutant viruses with a defect in reactivation from those with a defect in replication. We analyzed the growth kinetics of each mutant compared with the parental FIX strain (FIXwt) by performing multistep growth curves. FIX(ur)sub2, lacking UL136 through UL142, and FIX(ur)sub3, lacking UL144 through UL148, replicated with kinetics equivalent to FIXwt (Figure 3A). These results are consistent with previously published results for a mutant virus lacking UL138 through UL148 constructed in Toledo.36 Similar to results published by Hahn et al,37 where the entire ULb′ region was deleted in FIX, FIX(ur)sub4 exhibited a major growth defect and was not further studied (data not shown). Interestingly, FIX(ur)sub1 exhibited a growth defect similar to FIX(ur)sub4 and also was not further studied (data not shown).
Schematic of ULb′ region recombinant viruses. Large regions or individual ORFs within the ULb′ region were substituted by linear recombination into the FIX strain cloned as a BAC. Black bars represent the region missing in the recombinant virus, and the nucleotides missing are shown to the right of each variant.
Schematic of ULb′ region recombinant viruses. Large regions or individual ORFs within the ULb′ region were substituted by linear recombination into the FIX strain cloned as a BAC. Black bars represent the region missing in the recombinant virus, and the nucleotides missing are shown to the right of each variant.
Growth kinetics of recombinant viruses. MRC5 cells were infected at a multiplicity of 0.05 PFU/cell with the FIXwt or each recombinant containing (A) a large substitution in the ULb′ region or (B) a substitution in an individual ORF within the FIX(ur)sub2 region. Lysates were collected over a time course and virus yields determined by TCID50 assays on MRC5 cells. The standard error is shown for 3 to 4 independent experiments.
Growth kinetics of recombinant viruses. MRC5 cells were infected at a multiplicity of 0.05 PFU/cell with the FIXwt or each recombinant containing (A) a large substitution in the ULb′ region or (B) a substitution in an individual ORF within the FIX(ur)sub2 region. Lysates were collected over a time course and virus yields determined by TCID50 assays on MRC5 cells. The standard error is shown for 3 to 4 independent experiments.
FIX(ur)sub2 and FIX(ur)sub3 were next analyzed for their ability to establish a latent infection in CD34+/CD38− cells relative to FIXwt and AD169 strains. Pure populations of infected cells were isolated by FACS and seeded into long-term bone marrow cultures. Infectious centers produced prior to reactivation or as a result of reactivation were analyzed for each infection by limiting dilution assay.18 As expected, FIXwt established an infection consistent with latency (7-fold more infectious centers produced in the reactivation as in the lysate), whereas the AD169 strain did not establish a latent infection and instead replicated productively (Figure 4A). Strikingly, FIX(ur)sub2 produced high levels of infectious centers in both the reactivation assay and the lysate control similar to AD169. When cells were infected with FIX(ur)sub2, 7498 and 5402 cells were required to produce an infectious center in the reactivation and lysate experiments, respectively. In the AD169 infection, 1940 cells in the reactivation assay and 1803 cells in the lysate control were required to form an infectious center. By contrast, FIX(ur)sub3 produced significantly more infectious centers in the reactivation compared with the lysate, a phenotype more closely resembling the FIXwt strain. In the reactivation, 15 654 cells infected with FIX(ur)sub3 were required to form an infectious center compared with 52 809 cells in the lysate experiment. From these data, we conclude that sequences within the 5-kb region encoding UL136 to UL142 are required for the establishment or maintenance of a latent infection in vitro.
The UL138 ORF is required for a latent infection of cultured CD34+ cells
We then asked if specific ORFs were required to establish or maintain a latent infection in vitro by substituting the gene encoding kanamycin resistance for individual open reading frames within the UL136 to UL142 region missing in the FIX(ur)sub2 mutant virus (Figure 2). With the exception of FIXsubUL136, which exhibited a modest growth defect, the substitution viruses grew with wild-type kinetics in human fibroblasts (Figure 3B). Each mutant virus infected fibroblast or hematopoietic cells with equivalent efficiencies as detected by GFP (data not shown). In order to screen a large number of recombinant viruses for a latency phenotype, the reactivation was modified to use a single dilution for analysis of each recombinant virus. We seeded 10 000 infected CD34+/CD38− cells or an equivalent cell lysate into each of 24 wells of a 96-well dish containing permissive fibroblasts (Figure 4B). At 24 dpi, the fraction of with GFP+ fibroblasts was scored. FIXwt and FIX(ur)sub3 again exhibited a wild-type latency phenotype where a greater fraction of wells had infectious centers in the reactivation compared with the lysate control. All but one of the single ORF substitutions also exhibited a wild-type latency phenotype. Only a substitution in the UL138 ORF (FIXsubUL138) resulted in a virus that replicated equivalently in the reactivation and the lysate control. The FIXsubUL138 phenotype strikingly resembles that of the AD169 and Towne laboratory strains and the FIX(ur)sub2-mutant virus. When further analyzed in a limiting dilution format, the UL138-deficient virus again behaved similarly to the FIX(ur)sub2 mutant virus. Formation of an infectious center required 30 063 cells infected with FIXsubUL138 in the reactivation experiment and 43 304 cells in the lysate control. These data suggest that sequences encoding UL138 promote a latent infection in cultured hematopoietic progenitor cells. This is the first functional demonstration of HCMV sequences required to promote a latent infection.
UL138 promotes a latent infection in cultured hematopoietic progenitor cells. (A) The latency phenotype of each virus in CD34+/CD38− cells was analyzed by limiting dilution assay. The bars represent the average frequency of infectious center formation in the reactivation experiment (black) or the lysate control (gray) in at least 3 independent experiments for FIXpar, FIX(ur)sub2, FIX(ur)sub3, and AD169. The top numbers above each pair of bars represent the fold increase in infectious center formation in the reactivation compared with the lysate control. The line indicates the limit of detection (LD) for the assay. (B) The latency phenotype was analyzed for single ORF substitution viruses by seeding 10 000 cells or an equivalent cell lysate per well into each of 24 wells. The bars represent the fraction of GFP+ wells in the reactivation experiment (■) or the lysate control (▒) in at least 3 independent experiments or 2 independent experiments for FIXsubUL141 and Towne. For panels A and B, statistical significance is indicated by * for P < .05 and ** for P ≤ .01 where there were 3 or more experiments. All other pairs do not represent a significant difference (P ≥ .05). The standard error of the means is shown.
UL138 promotes a latent infection in cultured hematopoietic progenitor cells. (A) The latency phenotype of each virus in CD34+/CD38− cells was analyzed by limiting dilution assay. The bars represent the average frequency of infectious center formation in the reactivation experiment (black) or the lysate control (gray) in at least 3 independent experiments for FIXpar, FIX(ur)sub2, FIX(ur)sub3, and AD169. The top numbers above each pair of bars represent the fold increase in infectious center formation in the reactivation compared with the lysate control. The line indicates the limit of detection (LD) for the assay. (B) The latency phenotype was analyzed for single ORF substitution viruses by seeding 10 000 cells or an equivalent cell lysate per well into each of 24 wells. The bars represent the fraction of GFP+ wells in the reactivation experiment (■) or the lysate control (▒) in at least 3 independent experiments or 2 independent experiments for FIXsubUL141 and Towne. For panels A and B, statistical significance is indicated by * for P < .05 and ** for P ≤ .01 where there were 3 or more experiments. All other pairs do not represent a significant difference (P ≥ .05). The standard error of the means is shown.
UL138 RNAs are detected in latently infected, healthy individuals
If UL138 is required for HCMV latency, then it is possible that UL138 is expressed endogenously in healthy, seropositive individuals infected with HCMV. We analyzed RNA from peripheral blood CD14+ monocytes of 5 seropositive donors or 8 seronegative donors for the presence of the UL138 transcript by PCR in the presence or absence of a reverse-transcriptase (RT) step followed by hybridization with an internal radioactive probe (Figure 5A). As a control, we also screened for the presence of IE1 RNA, which would be indicative of a lytic infection. UL138 transcript was detected strongly in 2 positive donors (Figure 5 lanes 4 and 6) and weakly in 3 positive donors (lanes 7, 9, 11), but not in the seronegative donors. UL138 was not detected in the absence of reverse transcriptase. IE1 RNA was not detected in any sample, indicating the UL138 was not expressed as part of a productive infection.
Detection of UL138 RNA in hematopoietic cells isolated from seropositive donors. (A) RNA isolated from CD34+ cells of seropositive (lanes 1-5) or seronegative (lanes 6-8) donors was analyzed by RT-PCR for (i) UL138, (iii) IE1, and (iv) GAPDH gene expression. RNA was amplified in panel ii with no prior RT. (B) RNA isolated from peripheral blood monocytes of seropositive (lanes 4, 6, 7, 9, and 11) or seronegative (lanes 1-3, 5, 8, 10, 12, 13) donors was analyzed by RT-PCR for (i) UL138, (iii) IE1, and (iv) GAPDH gene expression. RNA was also amplified in panel ii with no prior RT. (C) RNA isolated from CD34+ cells (lanes 1, 2, 5, 6) and CD34-derived dendritic cells (DDCs) (lanes 3, 4, 7, 8) of 2 further seropositive donors was analyzed by RT-PCR (lanes 1-4) for (i) UL138, (ii) IE1, and (iii) GAPDH gene expression. RNA was also amplified in an (i) UL138-PCR and (ii) with no prior RT (lanes 5-8). In all experiments, PCR products were transferred to a nitrocellulose membrane and probed with internal sequences to UL138 and IE1 genes.
Detection of UL138 RNA in hematopoietic cells isolated from seropositive donors. (A) RNA isolated from CD34+ cells of seropositive (lanes 1-5) or seronegative (lanes 6-8) donors was analyzed by RT-PCR for (i) UL138, (iii) IE1, and (iv) GAPDH gene expression. RNA was amplified in panel ii with no prior RT. (B) RNA isolated from peripheral blood monocytes of seropositive (lanes 4, 6, 7, 9, and 11) or seronegative (lanes 1-3, 5, 8, 10, 12, 13) donors was analyzed by RT-PCR for (i) UL138, (iii) IE1, and (iv) GAPDH gene expression. RNA was also amplified in panel ii with no prior RT. (C) RNA isolated from CD34+ cells (lanes 1, 2, 5, 6) and CD34-derived dendritic cells (DDCs) (lanes 3, 4, 7, 8) of 2 further seropositive donors was analyzed by RT-PCR (lanes 1-4) for (i) UL138, (ii) IE1, and (iii) GAPDH gene expression. RNA was also amplified in an (i) UL138-PCR and (ii) with no prior RT (lanes 5-8). In all experiments, PCR products were transferred to a nitrocellulose membrane and probed with internal sequences to UL138 and IE1 genes.
We then analyzed RNA isolated from CD34+ cells of 5 healthy seropositive and 3 seronegative donors for the presence of the UL138 transcript by RT-PCR (Figure 5B). Interestingly, UL138 RNA was detected in one of the seropositive donors but not in any of the seronegative donors. No IE1 expression was detected in donor samples. UL138 expression was further analyzed in CD34+ cells and dendritic cells derived from CD34+ cells from 2 additional seropositive donors (Figure 5C). UL138 RNA was evident in CD34+ cells from both seropositive donors. However, when CD34+ cells from these donors were differentiated into dendritic cells, a cell type permissive for HCMV replication,17 UL138 expression dropped to a level that was no longer detected, and IE1 RNA could be detected. No transcripts were detected in the absence of the reverse-transcriptase step. These results indicate that UL138 RNA is frequently expressed in healthy seropositive individuals latently infected with HCMV, and that its expression may be down-regulated following differentiation into a cell type permissive for HCMV replication.
Discussion
Latency is a key feature of HCMV pathogenesis. Defining the viral and cellular determinants of viral latency will be critical to controlling HCMV disease. Here, we have identified the first viral sequence with a latency-promoting function. Three lines of evidence support this conclusion. First, low-passage strains establish and maintain an infection with the hallmarks of latency in CD34+ cells, whereas laboratory strains cannot (Figure 1). Second, the deletion of the UL138 ORF from low-passage strains blocked its ability of the virus to establish or maintain a latent infection in CD34+/CD38− cells (Figure 4). Third, UL138 RNA is present in latently infected CD14+ monocytes and CD34+ progenitor cells isolated from seropositive donors (Figure 5).
From viral array and PCR experiments, UL138 is expressed in productively infected fibroblasts (data not shown) and CD34+ or CD34+/CD38− cells infected in vitro,11,18 and in healthy seropositive individuals (Figure 5). The mechanisms by which UL138 promotes the establishment and/or maintenance of a latent infection are unknown. The UL138 transcript could be a functional RNA similar to the polyadenylated latency-associated transcript of herpes simplex virus or may encode a protein.38 UL138 is likely to encode a protein since it has a 5′ ATG with a Kozak motif, it is predicted to encode a protein by the biodictionary-based gene-finder algorithm, and it is conserved in chimpanzee CMV.35 The U94/REP protein of human herpesvirus-6 (HHV-6), another member of the β-herpesvirus family distantly related to HCMV, restricts HHV-6 replication39 and that of HCMV.40 U94/REP is expressed in healthy individuals infected with HHV-6.39 It is intriguing to speculate that UL138 may encode a similar function that down-regulates HCMV gene expression and replication, perhaps by antagonizing one or more IE products to promote viral latency. Viruses lacking UL138 did not show a dramatic growth advantage in cultured fibroblasts relative to FIXwt (Figure 3B) and the same was true for viruses lacking 5-kb segments of the ULb′ region (Figure 3A). However, such an activity may be dependent on the cell type and fail to score in fibroblasts.
While our studies have identified UL138 as a gene having a prominent role in establishing and/or maintaining latency, other genes within the ULb′ region might be involved as well. Evidence for this includes the failure of mutant viruses lacking only UL138 to produce infectious centers as efficiently as the FIX(ur)sub2 virus, which lacks a block of genes including UL138 (Figure 4B). These differences could be interpreted to indicate that sequences deleted in the FIX(ur)sub2 virus, other than UL138, facilitate latency in hematopoietic cells. While FIX(ur)sub3 phenotype resembles that of the FIXwt strain, the difference between infectious centers resulting from reactivation and those in the lysate is reduced to 3-fold (P value derived from Student t test is .04). One possible explanation for the increased leakiness in infectious center formation prior to reactivation (lysate) in cells infected with FIX(ur)sub3 is that sequences within UL144-UL148 contribute to the efficient maintenance of the latent infection. While all single ORF substitution viruses other than UL138 exhibited a wild-type latency phenotype, several substitutions resulted in viruses that reactivated to produce infectious centers in a greater fraction of wells compared with FIXwt (Figure 4B). This included substitutions in UL140, UL141, and UL142. Further analyses in a limiting dilution format might identify sequences in this region that may subtly modulate latency.
Interestingly, none of the recombinant viruses analyzed exhibited a defect in reactivation. This suggests that lytic replication may be the default cycle of HCMV when it encounters a cell with a permissive environment. Latency, by contrast, is a survival mechanism that becomes a possibility only in the appropriate cellular background. In our work, we have observed a distinctive gradation regarding HCMV strains and their ability to establish latency (Figure 1A). FIXwt is the strain most similar to the original clinical isolate and establishes the most quiescent infection (ie, we detect the least virus in lysates of infected CD34+ cells). Toledo, like FIX, retains most of the ULb′ region but has been more extensively passaged in fibroblasts. Toledo produced a greater number of infectious centers after reactivation and exhibits greater leakiness during the latency period prior to reactivation compared with FIXwt (Figure 1A). Towneshort has lost most of the ULb′ region like AD169, but retains UL146, UL147, and UL148. Towneshort fails to establish latency in CD34+/CD38− cells similarly to AD169, and produces infectious centers more efficiently than low-passage strains. AD169, the most attenuated and highly passaged strain in our studies, replicates the most efficiently in hematopoietic progenitor cells.
Our finding that the laboratory strains replicate productively in hematopoietic progenitor cells is surprising given reports that the laboratory strains AD16928 and Towne10,28,41 establish a latent infection in GM-Ps. While the discrepancy between our results and those of others is difficult to reconcile, one possible explanation is that the variable outcomes after infection with laboratory strains are due to differences in the model systems. In comparison with CD34+ or CD34+/CD38− cell populations used in our studies, GM-Ps (CD33+, CD14+ or CD15+, CD1a+, and CD10+) used in other studies are derived from fetal liver and expanded prior to infection.10 GM-Ps are a more highly differentiated population of cells.10 It is possible that establishing latency in the GM-P system does not require ULb′ sequences. Consistent with our results, previous studies have also reported viral replication in hematopoietic progenitor cells infected with Towne.42 It should be noted that it the original Towne strain was a mixture of 2 genomic variants, TowneLong and TowneShort (or TownevarRIT3).43 TowneLong resembles low-passage strains in that it contains the ULb′ region including UL138, whereas TowneShort resembles AD169. The TowneShort variant was used in our studies. Towne strains containing ULb′ sequences would be expected to establish a latent infection. Therefore, differences in the strains used by different laboratories may also contribute to differences in latency phenotypes.
It has been reported that HCMV strains with a mutation in the UL131-128 locus fail to infect endothelial and epithelial cells, and also fail to efficiently transfer virus to monocytes and polymorphonuclear (PMN) leukocytes.13,37,44 However, we observed little difference in the ability of strains with an altered UL131–128 locus (AD169, Towne, and Toledo) to infect CD34+ cells compared with the FIX strain. Our results are consistent with other studies that have demonstrated CD34+ infection with Towne.15,42,45,46 One possible explanation is that entry and infection of CD34+ progenitor cells does not require the UL131–128 locus as does infection of more differentiated hematopoietic cells such as monocytes and PMNs. While unlikely, it is conceivable that our strains have reacquired PMN-tropism by a reversion event.37,47,48
The expression of UL138 transcripts in cells derived from healthy seropositive donors taken together with the functional data in cultured hematopoietic cells strongly implicate UL138 as a viral product that promotes HCMV latency. Loss of UL138 RNA concomitant with immediate-early RNA expression upon differentiation of CD34+ cells into dendritic cells (Figure 5) suggests that UL138 may be down-regulated during viral reactivation. A currentfocus of our work is to elucidate the mechanism by which UL138 functions to promote latency.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from NIH: K01CA111343 to F.G. and R01CA087586 to T.S. F.G. was supported by a Special Fellowship from the Leukemia and Lymphoma Society.
We thank Christina DeCoste for flow cytometry expertise and Melissa Trader for coordinating bone marrow delivery.
National Institutes of Health
Authorship
Contribution: F.G. and T.S. designed experiments, analyzed data, and wrote the paper; K.H. provided essential reagents; M.R. and J.S. designed and executed the experiment in Figure 5.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Felicia Goodrum, Department of Immunobiology, BIO5 Institute, University of Arizona, Tucson AZ 85711; e-mail: fgoodrum@email.arizona.edu.