Abstract
Cyclosporin A (CSA) is commonly used to prevent graft-versus-host disease. The influence of CSA on T-cell function has been extensively investigated; however, the effect of CSA on natural killer (NK) cells is less understood. NK cells were cultured with IL-2 and IL-15 with and without CSA for 1 week. Compared with controls, CSA-treated cultures showed fewer CD56+CD16+KIR+ NK cells and a reciprocal increase in CD56+CD16−KIR− cells. These changes were due mainly to a reduced proliferation of the CD56dim NK-cell subpopulation and a relative resistance of CD56bright NK cells to CSA. Following coculture with K562 targets, CSA-exposed NK cells differed from controls and lacked Ca2+ oscillations, nuclear factor of activated T cells (NFAT) dephosphorylation, and NFAT nuclear translocation. NK cells cultured in CSA retained cytotoxicity against K562, Raji, and KIR ligand-expressing lymphoblastoid cells. NK cells cultured in CSA showed increases in NKp30 and reductions in NKp44 and NKG2D. Following IL-12 and IL-18 stimulation, CSA-treated NK cells showed more IFN-γ–producing cells. Using in vitro NK-cell differentiation, progenitor cells gave rise to more CD56+KIR− NK cells in the presence of CSA than controls. Collectively, these studies show that CSA influences NK-cell function and phenotype, which may have important implications for graft-versus-leukemia effects.
Introduction
Following allogeneic hematopoietic cell transplantation (allo-HCT), graft-versus-host disease (GVHD) is a major cause of morbidity and mortality. In the late 1980s, drugs such as cyclosporin A (CSA) were introduced as posttransplantation immune suppression. CSA is a cyclic oligopeptide derived from the fungus Tolypocladium inflatum and was heralded as a revolutionary agent with T-cell–selective immune-suppressive properties.1 However, it is now clear that CSA modifies the function of many cell types, including non-T-cell lymphocyte populations. To date, the effects of CSA on natural killer (NK)–cell function have not been fully investigated.
Both murine and human studies show that NK cells mediate a number of potentially beneficial functions following allo-HCT, including eliminating residual malignant cells, removing host antigen-presenting cells (thereby reducing GVHD), and mediating immunity to viral pathogens directly through the cytolysis of virally infected tissues or indirectly by elaborating inflammatory cytokines, such as interferons (IFNs).2,3 NK-cell function is governed by a series of both inhibitory and activating surface receptors (reviewed in Lanier4 and Moretta et al5 ). Such receptors include killer immune globulin-like receptors (KIRs), NKG2D, and the natural cytotoxicity receptors (NCRs) NKp30, NKp44, and NKp46. The ligands for these receptors have partially been identified and include major histocompatibility complex (MHC) class I (for KIR), MHC class I–like proteins (for NKG2D), and viral-derived proteins (for NCRs).6-9
Recently the Perugia group (Ruggeri et al3,10 ) published results suggesting that NK cells play a critical role in graft-versus-leukemia (GVL) reactions. Following conditioning chemotherapy, patients received T-cell–depleted and CD34+-selected haploidentical grafts. Posttransplantation immune suppression was not used due to stringent T-cell depletion. In some recipient-donor pairs, the donor possessed MHC alleles (ie, KIR ligands) that were not expressed by the recipient. In this “KIR ligand-mismatched” situation, NK-cell clones that are not restrained by host MHC class I can exist and have GVL potential.10 Recipients with acute myelogenous leukemia (AML) who received a haploidentical transplant from a KIR ligand-mismatched donor had a marked reduction in relapse compared with an otherwise similar group of AML patients without this mismatch (0% vs 75%).3 In contrast, a large retrospective analysis of unrelated donor, T-cell–replete transplantations from the National Marrow Donor Program showed no decrease in AML relapse following KIR-mismatched transplantations.11 Similar results have been found by other groups.12-16 There were a number of differences between these 2 transplantation settings, including donor source (sibling/parent vs unrelated), HLA matching (haploidentical vs matched unrelated), the presence of T cells (severe depletion vs T-cell replete), and the use of posttransplantation immune suppression (absent vs present). This latter issue led us to investigate the influence of CSA on NK-cell function. Here we show that CSA has a number of profound and unexpected effects on NK-cell phenotype and function.
Materials and methods
CD3−CD56+ NK-cell enrichment
Peripheral blood mononuclear cells (PBMCs) were isolated from deidentified healthy donor buffy coats using Lymphocyte Separation Media (Mediatech, Herndon, VA). PBMCs were washed and NK cells were isolated by negative selection (Rosett-Sep NK Cell Isolation; StemCell Technologies, Vancouver, BC) according to the manufacturer's recommendations, with modifications.17 Enriched NK cells were more than 85% to 90% CD56+CD3− (not shown). Samples from deidentified donors were obtained from Memorial Blood Center (St Paul, MN) in accordance with its protocols.
Cell culture
Enriched NK-cell populations were cultured in Delbecco modified Eagle medium (DMEM)/Hams F12 (2:1) with 10% human AB− sera (SeraCare, Oceanside, CA), 2-β mercaptoethanol (24 μM), l-ascorbic acid (24 mg/L), sodium selenite (50 μg/L), ethanolamine (50 μM), and penicillin-streptomycin (100 U/mL of each). At the start of cultures, IL-2 (100 U/mL; Chiron, Emeryville, CA) and IL-15 (10 ng/mL; Peprotech, Rocky Hill, CT) were added. Cyclosporin A (Bedford Laboratories, Bedford, OH) was diluted in ethanol (1 mg/mL) and added at final concentrations from 0.1 to 5 μg/mL. Vehicle control cultures were set up in parallel using ethanol (EtOH).
Flow cytometry and FACS sorting
Antibodies against the following antigens were used: CD3, CD16, CD56, CD158a, CD158b, CD158e1, NKG2D, IFN-γ, perforin, granzyme B, and FasL (BD Biosciences, San Diego, CA); CD158j, NKp30, NKp44, and NKp46 (Beckman Coulter, Fullerton, CA). In some experiments (IFN-γ and perforin/granzyme), intracellular staining was performed with Fix/Perm Buffer (BD Biosciences). Apoptotic cells were detected using annexin-V (BD Biosciences) and propidium iodine (Sigma, St Louis, MO). Cells were analyzed on a fluorescence-activated cell sorter, FACS Calibur (BD Biosciences). Data were analyzed using Flowjo software (TreeStar, Ashland, OR).
In some experiments, NK-cell subpopulations were obtained by FACS sorting. Briefly, enriched NK cells were stained with antibodies against CD3, CD56, CD16 and sorted on a FACS Diva (BD Biosciences) gating on CD3−CD56brightCD16− and CD3−CD56dimCD16+ subpopulations.
CFSE staining
NK cells were stained using the Vybrant CFDA SE Cell Tracer Kit (Invitrogen, Frederick, MD; Molecular Probes, Eugene, OR). Following this, NK cells were cultured and CFSE (carboxy fluoroscein succinimidyl ester) content was determined by FACS at various time points.
Cytotoxicity assay
Chromium release assays were performed as previously described.18 Briefly, target cells were labeled with 11.1 MBg (300 μCi) chromium-51 (51Cr, DuPont/NEN, Boston, MA) for 1 to 2 hours at 37°C, 5% CO2. Labeled cells were washed 3 times with PBS and plated in triplicate in 96-well round-bottom plates (104 cells/well). Specified ratios of NK cells were added and incubated for 4 hours at 37°C, 5% CO2. Assay supernatant was collected and counted using a gamma counter. The percentage lysis was calculated using the following equation: % specific lysis = 100 × (test release − spontaneous release)/(maximal release − spontaneous release).
Where stated, target cells were treated with the pan-MHC class I blocking mAb HP-1F7 (kindly provided by Dr M. López-Botet, Hospital de la Princessa, Madrid, Spain). Briefly, 51Cr-labeled target cells were washed and resuspended (106 cells/mL). Monoclonal antibody (mAb) (25 μg) was added to 200 μL target cells for 30 minutes. Target cells (with or without Ab) were diluted 10-fold with RPMI 1640 containing 10% fetal bovine serum (FBS), and 100 μL was plated in 96-well round-bottom plate. NK cells were add at specified ratios (in 100 μL) and incubated for 4 hours at 37°C, 5% CO2. The final concentration of mAb was 6.25 μg/mL.
Ca2+ imaging
To assess dynamic [Ca2+]i changes in individual NK cells, the Ca2+-sensitive fluorophore fura-2 AM (Molecular Probes) was used. NK cells were allowed to adhere to 25-mm2 glass slides and were placed on the stage of an inverted microscope (Nikon Diaphot; Melville, NY). Cells were loaded with 10 nM fura-2 AM and 2.5 μg/mL pluronic acid (Molecular Probes) for 20 minutes, followed by 20 minutes in a Ca2+-containing solution to allow for de-esterification before the experiment. Following this, K562 cells were added and ratiometric imaging was performed with the excitation wavelengths of 340 nm and 380 nm and an emission wavelength of 510 nm. Images were acquired with an intensified charge-coupled device camera (Photonic Science, Robertsbridge, United Kingdom) with Nikon CGI Super Fluor Series optics (40×/1.3 NA objective lens at 400× magnification) and using Metafluor image capture and analysis software (Fryer, Bloomington, MN). Ca2+ calibration was achieved by measuring a maximum (with 1 mM ionomycin) and a minimum (with 10 mM EGTA). Intracellular free Ca2+ was calculated assuming a dissociation constant of 220.19 For each experiment 10 to 20 cells were visualized and ratiometric data were acquired from individual cells.
Western blotting
Cell lysates (50 μg) were prepared from NK cells after culture for 7 days using freshly made lysis buffer (10 mM Tris-HCl [pH = 8], 150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.5% deoxycholate, 0.1% SDS, protease inhibitor cocktail [complete protease inhibitor, Roche, Indianapolis, IN]) and PMSF. Samples were separated on a 4% to 15% gradient polyacrylamide gel and transferred to a PVDF membrane. Membranes were blocked with 5% nonfat milk. One portion of the membrane was probed with an NFAT-1 antibody (BD Biosciences) while the other portion was probed with an antibody against actin (Santa Cruz Biotechnology, Santa Cruz, CA). Both were probed with a species-specific secondary HRP-conjugated antibody and developed using chemiluminescence (ECL; Amersham, Piscataway, NJ).
ImageStream analysis
At day + 7, NK cells were washed and cocultured with K562 cells (1:1 ratio) for 10 minutes, then fixed with 4% paraformaldehyde and permeabilized using an equal volume of 0.1% Triton X-100. Cells were then stained with 1 μg NFAT-1-specific antibody, followed by secondary staining with goat antimouse IgG. Thereafter, cells were blocked with mouse Ig for 30 minutes and stained with CD56 and glycophorin A for 30 minutes. Cells were washed and resuspended in PBS and 5 μL 7-AAD (0.5 mg/mL) was added. Analysis was performed using an ImageStream 100 (Amnis, Seattle, WA) and approximately 5000 events/sample were acquired. Nuclear focus versus intensity was used to examine only cells in focus. DNA content was used to identify single cells, eliminating conjugates and debris. Next, cells were gated on CD56 and then NFAT translocation was evaluated using a similarity score for 7-AAD versus NFAT (a monotonic function of Pearson correlation coefficient between the pixel values of 2 image pairs, described in Arechiga et al20 ). In total, data were analyzed on approximately 2500 events per sample (∼ 50% of the collected sample).
NK-cell differentiation cultures
NK-cell differentiation from progenitor cells was performed as described.21 Briefly, CD34+CD38−Lin− cells were isolated by CD34 positive selection (Miltenyi Biotech, Auburn, CA) followed by FASC sorting, gating on the CD34+CD38−lin− population. mAbs against CD2, CD3, CD4, CD5, CD7, CD8, CD10, and CD19 (all from BD Biosciences) served as lineage antigens. Cells were cultured in either 24-well or 96-well plates on irradiated (3000 cGy) AFT024 cells (murine stromal cell line) in medium (DMEM: Hams F12 [2:1]) containing IL-3 (5 ng/mL, at the culture initiation only; R&D systems, Minneapolis, MN), IL-7 (20 ng/mL; R&D Systems), IL-15 (10 ng/mL; R&D systems), FLT-3L (10 ng/mL; gift from Amgen, Thousand Oaks, CA), and SCF (20 ng/mL; gift from Amgen).
Statistical analysis
All statistical analyses were performed with Statistical Analysis System statistical software version 9.1 (SAS Institute, Cary, NC). For nonnormally distributed data, the nonparametric Wilcoxon signed rank test and Mann-Whitney rank sum test were used in the evaluation of the statistical differences between vehicle controls (EtOH) and CSA-treated cells. One-way and 2-way ANOVA were calculated when the data were approximately normally distributed using the general linear models procedure (PROC GLM). Adjustments for multiple comparisons were done with the Tukey method. Groups with P values less than or equal to .05 were considered to be statistically different.
Results
Effect of CSA on NK-cell expansion
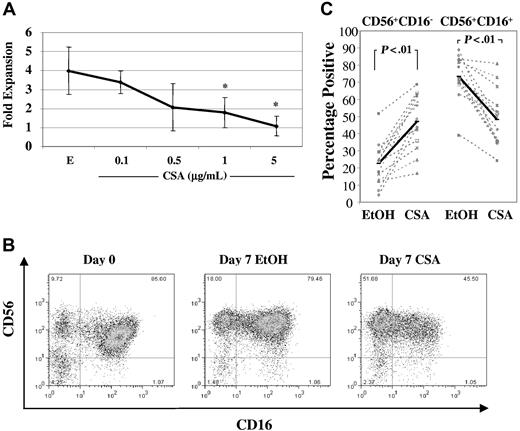
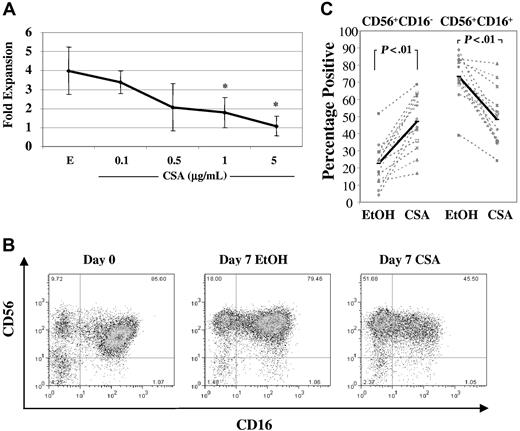
NK cells were isolated from healthy donor PBMCs using negative selection and were cultured in IL-2 (100 U/mL) and IL-15 (10 ng/mL) with increasing concentrations of CSA (0.1-5 μg/mL) or vehicle (EtOH). After 1 week, there was a dose-dependent reduction in NK-cell expansion (Figure 1A). Following allogeneic hematopoietic cell transplantation, peak CSA levels reach 0.5 μg/mL to 1 μg/mL,22,23 thus all further experiments were performed in the presence of CSA at 1 μg/mL. At this dose, NK-cell fold expansion was consistently lower than vehicle controls (0.8 ± 0.06 vs 2.4 ± 1.5, n = 10, P < .01). To investigate whether this reduction in fold expansion in CSA-treated NK cells was due to an increase in apoptosis, cultures were analyzed for both fold expansion and the percentages of apoptotic cells at days 3, 5, and 7. As shown in Table 1, at day 7, CSA-treated NK cells showed a significant reduction in fold expansion and an increase in apoptotic cells relative to controls (n = 8).
Culture with CSA reduces overall NK-cell fold expansion and results in higher percentages of CD56+CD16− NK cells. (A) Fold expansion of NK cells cultured in IL-2 (100 U/mL) and IL-15 (10 ng/mL) in the presence of either vehicle control (EtOH, E) or increasing concentrations of CSA (0.1, 0.5, 1, and 5 μg/mL) after 7 days. Results are the average plus and minus the SD from 4 donors. Asterisk (*) indicates values that showed significantly less proliferation (P < .05) compared with vehicle controls using ANOVA with Tukey adjustment. (B) FACS plots from a representative donor showing staining for CD56 and CD16 before (left) and after 1 week of culture with IL-2 (100 U/mL) and IL-15 (10 ng/mL) and either vehicle control (EtOH) (middle) or CSA (1 μg/mL) (right). (C) The percentages of CD56+CD16− and CD56+CD16+ NK-cell subpopulations after 7 days of culture in IL-2 (100 U/mL) and IL-15 (10 ng/mL) with or without CSA or vehicle (EtOH). Results of 14 individual donors are shown (gray dashed line) as well as averages (heavy solid line). Statistical analysis was performed using Wilcoxon singed rank test.
Culture with CSA reduces overall NK-cell fold expansion and results in higher percentages of CD56+CD16− NK cells. (A) Fold expansion of NK cells cultured in IL-2 (100 U/mL) and IL-15 (10 ng/mL) in the presence of either vehicle control (EtOH, E) or increasing concentrations of CSA (0.1, 0.5, 1, and 5 μg/mL) after 7 days. Results are the average plus and minus the SD from 4 donors. Asterisk (*) indicates values that showed significantly less proliferation (P < .05) compared with vehicle controls using ANOVA with Tukey adjustment. (B) FACS plots from a representative donor showing staining for CD56 and CD16 before (left) and after 1 week of culture with IL-2 (100 U/mL) and IL-15 (10 ng/mL) and either vehicle control (EtOH) (middle) or CSA (1 μg/mL) (right). (C) The percentages of CD56+CD16− and CD56+CD16+ NK-cell subpopulations after 7 days of culture in IL-2 (100 U/mL) and IL-15 (10 ng/mL) with or without CSA or vehicle (EtOH). Results of 14 individual donors are shown (gray dashed line) as well as averages (heavy solid line). Statistical analysis was performed using Wilcoxon singed rank test.
CSA differentially affects NK-cell subpopulations
NK cells can be separated into subsets on the basis of CD56 receptor density (reviewed in Cooper et al24 ). The majority of peripheral blood NK cells are CD56dim. Freshly isolated, they coexpress CD16 and KIR receptors and mediate potent cytotoxicity against K562 cells. In contrast, most CD56bright cells do not express CD16 and have minimal KIR receptor expression. CD56bright and CD56dim NK-cell subpopulations are readily discernable by flow cytometry when freshly isolated. However, after in vitro culture, distinguishing these subsets may be difficult due to less obvious differences in CD56 intensity. Therefore, CD16 expression was used as a marker of CD56dim cells after 1 week of culture. Comparing CSA-containing cultures to vehicle controls, there was an increase in the percentage of CD56+CD16− NK cells and a reciprocal reduction of CD56+CD16+ NK cells (Figure 1B shows a representative donor). Similar findings were observed in 14 donors, with an increase in CD56+CD16− NK cells (22.6% ± 12.3% vs 47% ± 15.9%, P < .01) and a decrease in CD56+CD16+ subpopulations (73.2% ± 12.6% vs 48.2% ± 16.7%, P < .01) (Figure 1C).
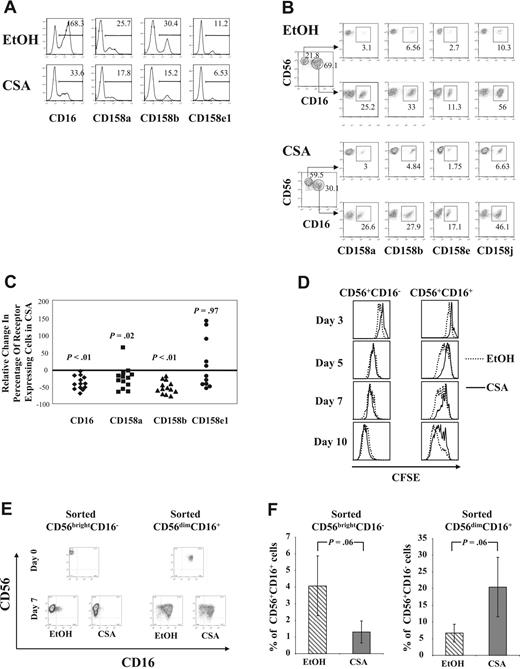
In addition to changes in CD56+CD16− and CD56+CD16+ NK cells, there were also alterations in the percentage of KIR-expressing cells following culture with CSA. A representative donor is shown in Figure 2A, demonstrating that a significant proportion of vehicle-treated NK cells expressed KIR receptors. In contrast, NK cells from the same donor cultured with CSA showed reductions in CD158a, CD158b, and CD158e1. Gating on CD56+CD16− and CD56+CD16+ cell subpopulations after 1 week of culture demonstrated that CD16+ cells showed higher percentages of KIR-expressing cells, regardless of whether NK cells cultured with or without CSA (Figure 2B). These results validate our approach of using CD16 as a marker of CD56bright and CD56dim NK-cell subpopulations after culture. Changes in CD16 and KIR were investigated across a series of donors by determining the percentage of NK cells staining positive for CD16 or a particular KIR and then comparing this to vehicle controls. As shown in Figure 2C, significant reductions were consistently noted for CD16, CD158a, and CD158b (n = 13), while CD158e1 (n = 11) showed heterogeneous changes following culture with CSA.
Following culture with CSA, there are fewer KIR-expressing cells due to a reduction in CD56+CD16+ cell proliferation. (A) Results from cells from a representative donor after 7 days of culture with IL-2 (100 U/mL) and IL-15 (10 ng/mL), with and without CSA, showing a reduction in CD16 or KIR (CD158a, CD158b, CD158e1) in the CSA-treated cultures (bottom), relative to controls (EtOH) (top). Results are representative of more than 3 individual donors. Numbers in upper right are the percentage of positive cells. (B) Cultured CD56+CD16− cells differ in KIR expression compared with CD56+CD16+ cells regardless of whether they are cultured in CSA or vehicle (EtOH). Purified NK cells were cultured with IL-2 (100 U/mL) and IL-15 (10 ng/mL) with and without CSA for 7 days and then analyzed by FACS. Results are from cells from a single donor and are representative of more than 3 donors. Numbers in upper right are the percentage of positive cells. (C) Reduction in CD16 and KIR on NK-cell exposure to CSA. NK cells were cultured in IL-2 (100 U/mL) and IL-15 (10 ng/mL) with and without CSA for 7 days and analyzed by FACS. Shown is the percentage relative change in CD16 or KIR between CSA- and vehicle control–treated NK cultures (percent relative change = 100 × (% receptor-expressing cells in CSA − % receptor-expressing cells in control)/% receptor-expressing cells in control). Results are the average of cells from 13 healthy donors for CD16, CD158a, and CD158b and from 11 donors for CD158e1. Cells that expressed less than 5% CD158e1 were considered to not express this gene and samples from those donors were excluded (n = 2). Wilcoxon signed rank test was used to calculate statistics. (D) CSA differentially affects the proliferation of CD56+CD16− and CD56+CD16+ NK-cell subpopulations. NK cells were freshly isolated and stained with the membrane dye CFSE. Cells were analyzed at days 3, 5, 7, and 10 of culture after staining with CD56-APC and CD16-PE. CFSE content was determined after gating on the CD56+CD16− and the CD56+CD16+ subpopulations and the differences between CSA- and vehicle control–treated cultures are shown as overlaid histograms. Results are representative of samples from 3 individual donors. (E,F) CD56brightCD16− and CD56dimCD16+ NK-cell subpopulations were FACS purified from healthy donor buffy coats. Purity after sorting was more than 97%. After culture in IL-2 (100 U/mL) and IL-15 (10 ng/mL) with and without CSA for 7 days, cell phenotype was determined using FACS. As shown, some CD56brightCD16− cells acquired CD16 and some CD56dimCD16+ NK cells lost CD16. (E) Cells from a representative donor are shown. (F) The average percentage of CD56+CD16+ cells found after CD56brightCD16− cells were cultured with (▧) or without (▒) CSA (left). The average percentage of CD56+CD16− cells generated after culture of the CD56dimCD16+ purified fraction either with (▧) or without (▒) CSA (right). Results are the average plus and minus the SD for cells from 5 separate donors.
Following culture with CSA, there are fewer KIR-expressing cells due to a reduction in CD56+CD16+ cell proliferation. (A) Results from cells from a representative donor after 7 days of culture with IL-2 (100 U/mL) and IL-15 (10 ng/mL), with and without CSA, showing a reduction in CD16 or KIR (CD158a, CD158b, CD158e1) in the CSA-treated cultures (bottom), relative to controls (EtOH) (top). Results are representative of more than 3 individual donors. Numbers in upper right are the percentage of positive cells. (B) Cultured CD56+CD16− cells differ in KIR expression compared with CD56+CD16+ cells regardless of whether they are cultured in CSA or vehicle (EtOH). Purified NK cells were cultured with IL-2 (100 U/mL) and IL-15 (10 ng/mL) with and without CSA for 7 days and then analyzed by FACS. Results are from cells from a single donor and are representative of more than 3 donors. Numbers in upper right are the percentage of positive cells. (C) Reduction in CD16 and KIR on NK-cell exposure to CSA. NK cells were cultured in IL-2 (100 U/mL) and IL-15 (10 ng/mL) with and without CSA for 7 days and analyzed by FACS. Shown is the percentage relative change in CD16 or KIR between CSA- and vehicle control–treated NK cultures (percent relative change = 100 × (% receptor-expressing cells in CSA − % receptor-expressing cells in control)/% receptor-expressing cells in control). Results are the average of cells from 13 healthy donors for CD16, CD158a, and CD158b and from 11 donors for CD158e1. Cells that expressed less than 5% CD158e1 were considered to not express this gene and samples from those donors were excluded (n = 2). Wilcoxon signed rank test was used to calculate statistics. (D) CSA differentially affects the proliferation of CD56+CD16− and CD56+CD16+ NK-cell subpopulations. NK cells were freshly isolated and stained with the membrane dye CFSE. Cells were analyzed at days 3, 5, 7, and 10 of culture after staining with CD56-APC and CD16-PE. CFSE content was determined after gating on the CD56+CD16− and the CD56+CD16+ subpopulations and the differences between CSA- and vehicle control–treated cultures are shown as overlaid histograms. Results are representative of samples from 3 individual donors. (E,F) CD56brightCD16− and CD56dimCD16+ NK-cell subpopulations were FACS purified from healthy donor buffy coats. Purity after sorting was more than 97%. After culture in IL-2 (100 U/mL) and IL-15 (10 ng/mL) with and without CSA for 7 days, cell phenotype was determined using FACS. As shown, some CD56brightCD16− cells acquired CD16 and some CD56dimCD16+ NK cells lost CD16. (E) Cells from a representative donor are shown. (F) The average percentage of CD56+CD16+ cells found after CD56brightCD16− cells were cultured with (▧) or without (▒) CSA (left). The average percentage of CD56+CD16− cells generated after culture of the CD56dimCD16+ purified fraction either with (▧) or without (▒) CSA (right). Results are the average plus and minus the SD for cells from 5 separate donors.
To investigate whether the changes observed after culture with CSA were due to differential proliferation of CD56bright and CD56dim NK cells, freshly isolated NK cells were labeled with the membrane dye CFSE at the start of cultures. CFSE content was determined by gating on CD56+CD16− and CD56+CD16+ subpopulations on days 3, 5, 7, and 10 of culture. At nearly all time points, CD56+CD16− cells showed a similar number of divisions determined by loss of CFSE, regardless of the culture conditions. In contrast, CD56+CD16+ cells cultured in CSA underwent fewer cell divisions than vehicle controls (Figure 2D).
To further prove that CSA differentially affects NK-cell subsets, freshly isolated NK cells were FACS sorted into CD56brightCD16− and CD56dimCD16+ fractions and cultured with IL-2/IL-15 with and without CSA. As shown in Table 2, CSA inhibited the proliferation of both subpopulations, but not equally. The expansion of sorted CD56brightCD16− cells in CSA was 1.3-fold less than vehicle (P < .05), whereas CD56dimCD16+ cells expanded, on average, 3.5-fold less than controls (P < .05). Thus CSA induced significantly more inhibition in purified CD56dimCD16+ cells compared with the CD56brightCD16− population (P = .02). Analyzing the sorted NK subsets for KIR expression showed similar results to those obtained after gating on the CD56+CD16− and CD56+CD16+ fractions of bulk NK cells (as in Figure 2B, not shown). Interestingly, while the majority of both sorted NK subsets retained their starting phenotype, some cells either acquired or lost CD16 after a week in culture. This occurred despite a high degree of purity (> 97%; a representative donor is shown in Figure 2E). Regardless of the starting population, CSA favored the CD56+CD16− phenotype. More specifically, when CD56brightCD16− cells were cultured with CSA, lower percentages of CD16-expressing NK cells were observed compared with controls. Likewise, when CD56dimCD16+ cells were cultured in CSA, higher percentages of cells without CD16 were recovered (P = .06 for both, Figure 2F). Taken together, these results show that CSA inhibits proliferation and induces apoptosis of NK cells. This is more pronounced in CD56dimCD16+ cells relative to the CD56brightCD16− fraction and is the primary explanation for the increase in CD56+CD16− NK cells after culture in CSA. However, CSA also influences the interconversion of CD56+CD16− to CD56+CD16+ populations (and vice versa), favoring the CD56+CD16− phenotype.
Ca2+ flux and NFAT translocation in CSA-exposed NK cells
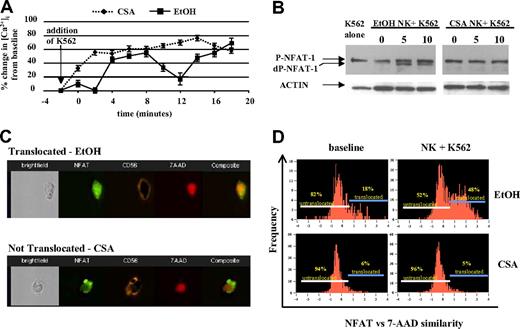
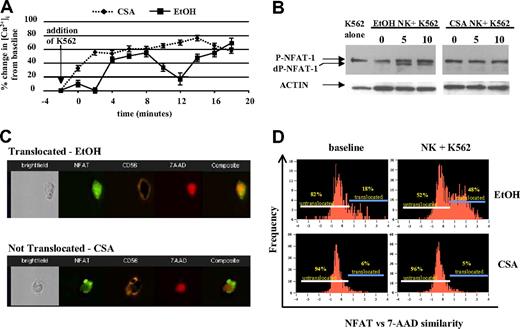
NK cells use a series of surface receptors to recognize malignant cells. Following receptor ligation, free intracellular Ca2+ increases, triggering a series of downstream events including the activation of calcineurin, a calcium-regulated phosphatase that dephosphorylates cytosolic NFAT. Dephopsphorylated NFAT then enters the nucleus and regulates gene transcription.25 By inhibiting calcineurin, CSA blocks nuclear translocation of NFAT.26-28 We investigated whether there were differences in Ca2+ flux in CSA-treated NK cells upon target cell contact, using fura-2-loaded NK cells. Vehicle-treated NK cells showed the expected biphasic increase in intracellular Ca2+ following the addition of K562 cells.29 In contrast, CSA-treated NK cells showed a rise in intracellular Ca2+ upon contact with K562 cells, but no oscillations were noted (Figure 3A).
CSA-exposed NK cells do not show Ca2+ oscillations, NFAT dephosphorylation, and/or nuclear translocation following engagement with K562 cells. (A) NK cells were cultured with IL-2 (100 U/mL) and IL-15 (10 ng/mL) with and without CSA for 7 days and used to assess dynamic intracellular calcium changes in individual cells (described in “Ca2+ imaging, Materials and methods”). Following the addition of K562 target cells, vehicle-treated (EtOH) NK cultures showed the expected calcium oscillations. In contrast, CSA-treated NK cells showed no oscillations. Results are representative of cells from 5 individual donors. Error bars are plus or minus standard error. (B) The addition of K562 cells leads to rapid NFAT dephosphorylation in control (EtOH)-treated cells, but not in CSA-exposed NK cells. Freshly isolated NK cells were cultured for 7 days with IL-2 (100 U/mL) and IL-15 (10 ng/mL), with and without CSA. Following this, NK cells were cocultured with K562 target cells (E/T = 1:1) for 5 and 10 minutes. Controls included K562 cells alone (left) and NK cells without the addition of K562 (time 0). Immediately at the time points stated (5 and 10 minutes), cells were lysed and subjected to Western blotting. Actin served as the loading control. Results are representative of cells from 3 individual donors. (C,D) Multispectral imaging demonstrating that NFAT nuclear translocation is inhibited following K562 engagement in CSA-treated NK cells, but not vehicle-treated control cultures. (C) Individual images of vehicle (EtOH) control-treated (top) and CSA-treated (bottom) cells showing nuclear NFAT translocation and nontranslocation after coculture with K562 cells (10 minutes), respectively. Shown are bright field, FL-1, FL-4, FL-2, and composite images from left to right. (D) NFAT versus 7-AAD similarity score for vehicle control–treated (top row) and CSA-treated (bottom row) NK cultures both at baseline (left columns) and following coculture with K562 cells (right columns) after 10 minutes. Gates identify cells that have not (left) and have (right) undergone NFAT translocation. Results are representative of 2 experiments on approximately 2500 events for each condition.
CSA-exposed NK cells do not show Ca2+ oscillations, NFAT dephosphorylation, and/or nuclear translocation following engagement with K562 cells. (A) NK cells were cultured with IL-2 (100 U/mL) and IL-15 (10 ng/mL) with and without CSA for 7 days and used to assess dynamic intracellular calcium changes in individual cells (described in “Ca2+ imaging, Materials and methods”). Following the addition of K562 target cells, vehicle-treated (EtOH) NK cultures showed the expected calcium oscillations. In contrast, CSA-treated NK cells showed no oscillations. Results are representative of cells from 5 individual donors. Error bars are plus or minus standard error. (B) The addition of K562 cells leads to rapid NFAT dephosphorylation in control (EtOH)-treated cells, but not in CSA-exposed NK cells. Freshly isolated NK cells were cultured for 7 days with IL-2 (100 U/mL) and IL-15 (10 ng/mL), with and without CSA. Following this, NK cells were cocultured with K562 target cells (E/T = 1:1) for 5 and 10 minutes. Controls included K562 cells alone (left) and NK cells without the addition of K562 (time 0). Immediately at the time points stated (5 and 10 minutes), cells were lysed and subjected to Western blotting. Actin served as the loading control. Results are representative of cells from 3 individual donors. (C,D) Multispectral imaging demonstrating that NFAT nuclear translocation is inhibited following K562 engagement in CSA-treated NK cells, but not vehicle-treated control cultures. (C) Individual images of vehicle (EtOH) control-treated (top) and CSA-treated (bottom) cells showing nuclear NFAT translocation and nontranslocation after coculture with K562 cells (10 minutes), respectively. Shown are bright field, FL-1, FL-4, FL-2, and composite images from left to right. (D) NFAT versus 7-AAD similarity score for vehicle control–treated (top row) and CSA-treated (bottom row) NK cultures both at baseline (left columns) and following coculture with K562 cells (right columns) after 10 minutes. Gates identify cells that have not (left) and have (right) undergone NFAT translocation. Results are representative of 2 experiments on approximately 2500 events for each condition.
We next investigated NFAT-1 expression in cultured NK cells and found no significant differences in CSA-treated NK cells relative to controls (Figure 3B, time 0). Coculture of NK cells with K562 targets led to rapid NFAT-1 dephosphorlyation in controls, but not in CSA-treated NK cells, even after prolonged (30 minutes) coincubation with K562 targets (Figure 3B and not shown). To further assay nuclear translocation of NFAT-1, multispectral cell imaging was used. This technique allows for the differentiation of effector and target cells by membrane staining as well as the cellular localization of antigens in individual cells (ie, cytoplasmic vs nuclear) (Figure 3C). Approximately 45% of control-treated NK cells translocated NFAT into the nucleus after a 10-minute incubation with K562 cells (n = 1763 events). In contrast, only approximately 5% of CSA-treated cells showed nuclear translocation (n = 1674 events) (Figure 3D). Collectively, these results show that vehicle control–treated NK cells dephosphorylate and translocate NFAT-1 upon interaction with K562 cells, while CSA-treated NK cells do not.
Functionality of CSA-exposed NK cells
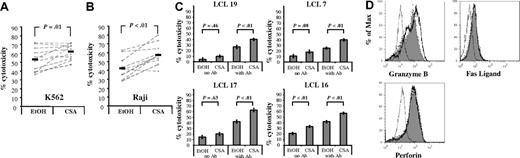
To investigate the effect of CSA on NK-cell cytotoxicity, CSA- or vehicle control–treated NK cells were used for 51Cr release assays. Targets included both K562 (MHC class I−) and Raji (MHC class I+) cell lines. Comparing CSA-treated NK cells to vehicle controls, there was a small but significant increase in average cytotoxicity against K562 cells (62% ± 8.7% vs 53.3% ± 13.2%, P = .01, n = 11, Figure 4A). Likewise, CSA-treated cultures showed higher cytotoxicity against Raji cells (57.4% ± 11.8% vs 42.3% ± 12.1%, P < .01, n = 11, Figure 4B). Thus, surprisingly, NK cells cultured in CSA had higher cytotoxicity than controls, and this was more pronounced for the MHC class I–expressing Raji targets.
CSA-treated NK cells have higher cytotoxicity, in part due to the reduction in KIR-expressing cells. Cytotoxicity against (A) K562 cells or (B) Raji cells after 7 days of culture. The results for individual donors (n = 11, - - - -) and average cytotoxicity (▬) (E/T = 5:1) are shown. Statistics were calculated using the Wilcoxon signed rank test. (C) Cytotoxicity of NK cells (cultured with vehicle or CSA) against KIR-L–expressing LCLs (HLA-B Bw4 and HLA-C C1/C2). The average cytotoxicity of 4 donors plus and minus the SE is shown (E/T = 5:1). Statistics calculated using the Wilcoxon signed rank test. (D) FACS histograms for granzyme B (left), perforin (middle), and FasL (right) for cells cultured with CSA (heavy line, open histogram) or vehicle (EtOH, dotted line, closed histogram). Isotype controls are shown in dashed line and open histogram. Granzyme B and perforin were detected using intracellular staining, while surface staining was used for FasL. Results are representative of samples from 4 donors.
CSA-treated NK cells have higher cytotoxicity, in part due to the reduction in KIR-expressing cells. Cytotoxicity against (A) K562 cells or (B) Raji cells after 7 days of culture. The results for individual donors (n = 11, - - - -) and average cytotoxicity (▬) (E/T = 5:1) are shown. Statistics were calculated using the Wilcoxon signed rank test. (C) Cytotoxicity of NK cells (cultured with vehicle or CSA) against KIR-L–expressing LCLs (HLA-B Bw4 and HLA-C C1/C2). The average cytotoxicity of 4 donors plus and minus the SE is shown (E/T = 5:1). Statistics calculated using the Wilcoxon signed rank test. (D) FACS histograms for granzyme B (left), perforin (middle), and FasL (right) for cells cultured with CSA (heavy line, open histogram) or vehicle (EtOH, dotted line, closed histogram). Isotype controls are shown in dashed line and open histogram. Granzyme B and perforin were detected using intracellular staining, while surface staining was used for FasL. Results are representative of samples from 4 donors.
Given that CSA-exposed cultures had higher percentages of CD56+CD16−KIR− cells, we investigated whether increased killing was secondary to a reduction in KIR. To do this, cytotoxicity was tested against a panel of lymphoblastoid cell lines (LCLs) that express all known KIR ligands (KIR-Ls; ie, HLA-Bw4 and HLA C1/C2). The average cytotoxicity of 4 donors is shown for each target (Figure 4C). For 2 of the 4 LCLs tested, there was significant (LCL 16, P < .01) or near significantly (LCL 7, P = .08) higher cytotoxicity in CSA-treated cultures. No differences between EtOH- and CSA-treated cells were detected for LCL 19 (P = .46) and LCL 17 (P = .63). If cytotoxicity was entirely due to differences in KIR, blocking such interactions should result in equivalent killing. To test this, a pan-MHC class I blocking mAb (HP-1F7) was used.30,31 The addition of the blocking mAb resulted in increased cytotoxicity for all 4 LCLs tested; however, CSA-treated cells still showed higher cytotoxicity relative to EtOH controls (P < .01 for all). Given that CSA-treated cells had less CD16 expression than controls (Figure 1C), such findings are probably not due to antibody-dependent cellular cytotoxicity (ADCC). Collectively these results suggest that the reduction of KIR-expressing cells (in CSA-treated cultures) is not the sole reason for the increased cytotoxicity.
Given the higher cytotoxicity in CSA-treated NK cells (despite MHC class I masking), differences in the expression of effector molecules were tested. As shown in Figure 4D, in 3 of 4 donors tested was a slight but nonsignificant increase in the geometric mean fluorescence intensity (gMFI) for intracellular granzyme B after culture with CSA compared with vehicle controls (113.5 ± 74.9 vs 73.5 ± 45.4, P = .25). Similarly, there was no difference for intracellular perforin (67.0 ± 31.7 vs 60.8 ± 16.9, NS) or surface FasL (6.7 ± 2.1 vs 6.2 ± 2.6, NS) between the various culture conditions.
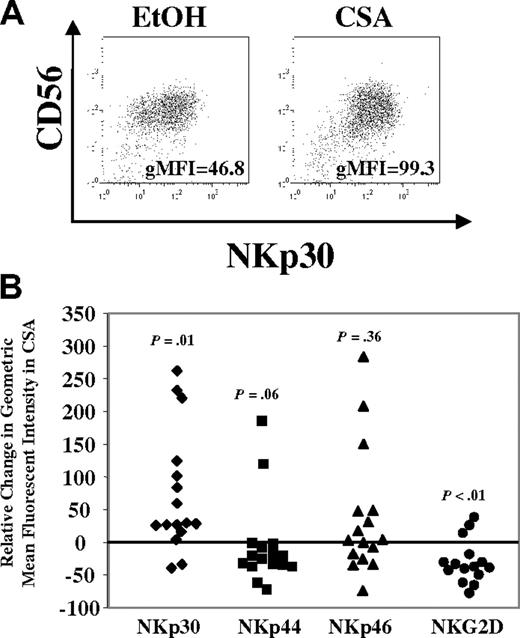
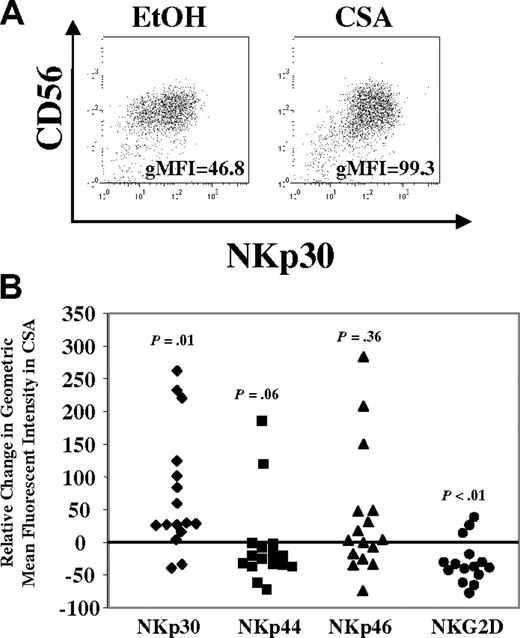
Next, differences in activating receptor expression were investigated. After one week, the majority of cells in either condition (CSA or vehicle) expressed NKp30, NKp44, NKp46, and NKG2D. However, differences were noted and a representative donor is shown in Figure 5A. To quantify these, the relative change in the gMFI was calculated across a series of donors (n = 16). In CSA-treated cultures the gMFI for NKp30 increased relative to controls (P = .01) (Figure 5B). In some donors (7/16), increases were also noted for NKp46, but this was not significant (P = .36). Interestingly, decreases in the gMFI were noted for NKp44 (P = .06) and NKG2D (P < .01) in CSA-treated cultures. Similar changes for activating receptors were noted in the FACS-purified CD56brightCD16− and CD56dimCD16+ NK-cell subsets (as in Figure 2E, n = 2, not shown).
Changes in NCRs (NKp30, NKp44, and NKp46) and NKG2D after culture with CSA. (A) NK cells were cultured in IL-2 (100 U/mL) and IL-15 (10 ng/mL) with and without CSA for 7 days and analyzed by FACS. NKp30 staining for cells from a representative donor is shown. gMFI is listed in right lower portion of figure. (B) Relative change in gMFI for NKp30, NKp44, NKp46, and NKG2D after culture with CSA. Relative change in gMFI = 100 × (gMFI of receptor in CSA − gMFI of receptor in control)/gMFI of receptor in control. Results are the average of samples from 16 healthy donors. Statistics were calculated using the Wilcoxon signed rank test.
Changes in NCRs (NKp30, NKp44, and NKp46) and NKG2D after culture with CSA. (A) NK cells were cultured in IL-2 (100 U/mL) and IL-15 (10 ng/mL) with and without CSA for 7 days and analyzed by FACS. NKp30 staining for cells from a representative donor is shown. gMFI is listed in right lower portion of figure. (B) Relative change in gMFI for NKp30, NKp44, NKp46, and NKG2D after culture with CSA. Relative change in gMFI = 100 × (gMFI of receptor in CSA − gMFI of receptor in control)/gMFI of receptor in control. Results are the average of samples from 16 healthy donors. Statistics were calculated using the Wilcoxon signed rank test.
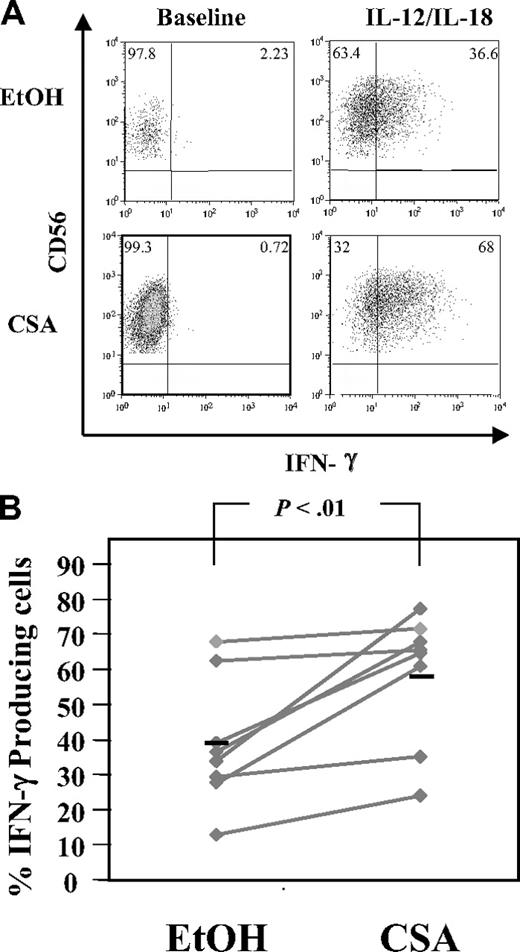
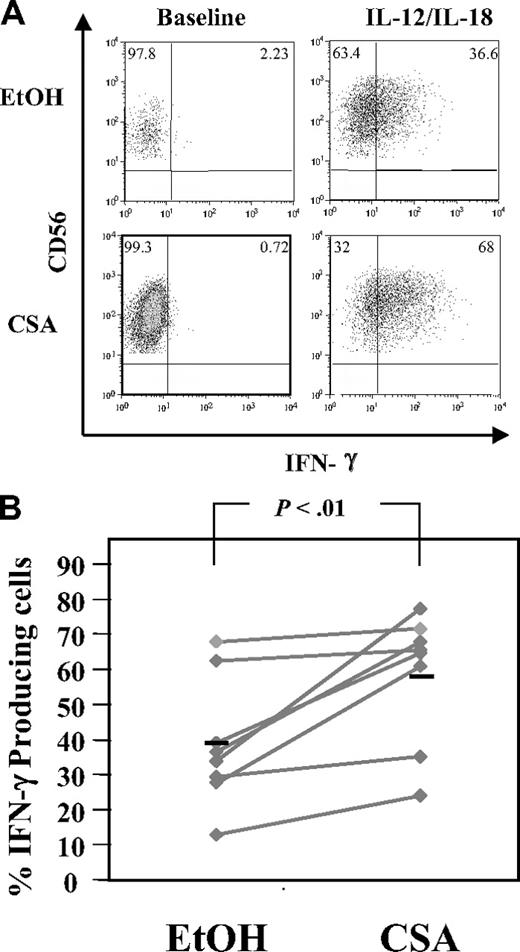
NK cells link the innate and adaptive immune responses, in part through the rapid elaboration of cytokines, including IFN-γ.32 To test the ability of CSA-exposed NK cells to secrete IFN-γ in response to monocyte-derived cytokines, NK cells were stimulated with IL-12 and IL-18.32 A higher percentage of CSA-treated NK cells secreted IFN-γ compared with controls (58.7% ± 18.6% vs 39.1% ± 18.0%, n = 8, P < .01) (Figure 6A,B). In addition, CSA-exposed NK cells had brighter channelfluorescence for IFN-γ (Figure 6A bottom right panel) relative to controls, suggesting that on a per-cell basis, each CSA-treated NK cell may make more IFN-γ. PMA and ionomycin stimulation served as a positive control and IFN-γ elaboration was seen in the majority of cells regardless of treatment (vehicle or CSA, not shown).
Intracellular IFN-γ detection in CSA-exposed NK cells. NK cells were cultured with IL-2 (100 U/mL) and IL-15 (10 ng/mL) with and without CSA for 7 days and then IFN-γ production in NK cells was induced by culturing cells in IL-12 (10 ng/mL) and IL-18 (100 ng/mL) for 18 hours. Brefeldin A (10 μg/mL final concentration) was added for the last 4 hours. Samples were stained with CD56 first, permeabilized, and stained with IFN-γ. (A) Cells from a representative donor. (B) The percentage of IFN-γ–expressing cells from 8 consecutive donors. Black bars represent the mean. Statistics were calculated using the Wilcoxon signed rank test.
Intracellular IFN-γ detection in CSA-exposed NK cells. NK cells were cultured with IL-2 (100 U/mL) and IL-15 (10 ng/mL) with and without CSA for 7 days and then IFN-γ production in NK cells was induced by culturing cells in IL-12 (10 ng/mL) and IL-18 (100 ng/mL) for 18 hours. Brefeldin A (10 μg/mL final concentration) was added for the last 4 hours. Samples were stained with CD56 first, permeabilized, and stained with IFN-γ. (A) Cells from a representative donor. (B) The percentage of IFN-γ–expressing cells from 8 consecutive donors. Black bars represent the mean. Statistics were calculated using the Wilcoxon signed rank test.
The impact of CSA on NK-cell development
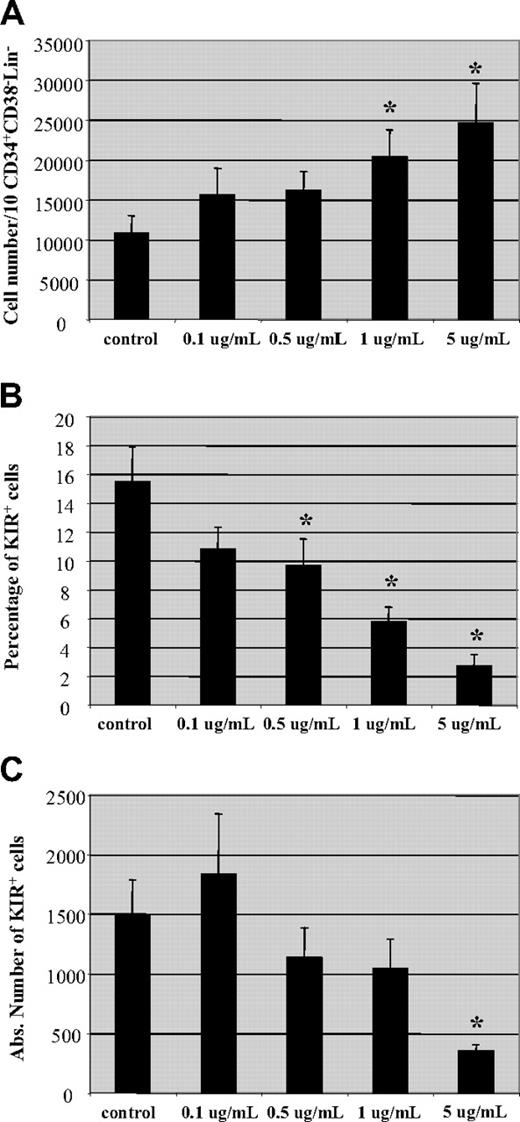
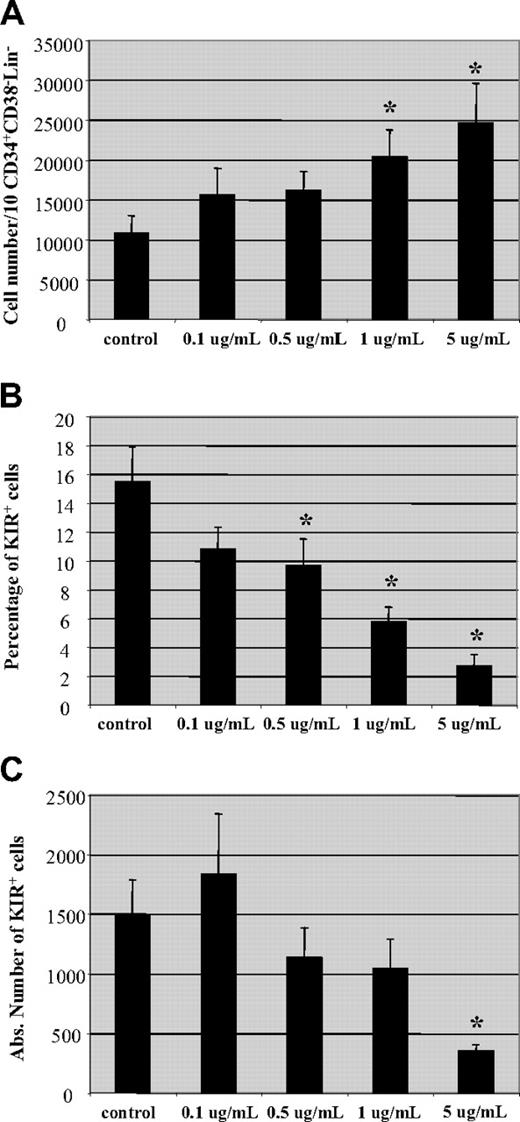
Following allo-HCT, CSA may not only affect the transferred mature NK cells but it may also impact NK cells developing from progenitor cells. To investigate the influence of CSA on NK-cell development, we used an in vitro NK-cell developmental system.21 Briefly, CD34+CD38−lin− cells were isolated from umbilical cord blood and cultured on a murine stromal cell line (AFT-024) in the presence of IL-3 (for the first week), IL-7, IL-15, stem cell factor, and FLT-3L.21 After 5 to 7 weeks, CD56+ cells were generated. Increasing concentrations of CSA had no impact on the percentage of CD56+ cells in the culture (not shown). Interestingly, an increase in the number of cells derived from a fixed number of progenitor cells (10 CD34+CD38−Lin− cells/well) was seen with increasing concentrations of CSA (Figure 7A). On the contrary, reductions in both the percentage and absolute number of KIR-expressing CD56+ cells were observed with increasing CSA concentrations (Figure 7B,C). These results demonstrate that CSA affects both mature PB NK cells as well as the development of NK cells from progenitor cells. In both cases, NK cells expressing KIR receptors are affected most by CSA.
Hematopoietic progenitor cell-derived NK cells express less KIR in the presence of CSA. Umbilical cord blood (UCB)–derived CD34+CD38−Lin− cells cultured on a stromal cell line (AFT024) in the presence of cytokines (IL-3, IL-7, IL-15, SCF, and FLT-3L) and increasing amounts of CSA (0.1-5 μg/mL) as described in “NK-cell differentiation cultures, Materials and methods.” On day + 50, cultures were examined for (A) total cell number, (B) percentage, and (C) absolute number of KIR-expressing NK cells. Results are the average of cells from 4 donors plus and minus the SEM. Asterisk (*) indicates values that were significantly less than controls (P < .05) by Mann-Whitney rank sum test
Hematopoietic progenitor cell-derived NK cells express less KIR in the presence of CSA. Umbilical cord blood (UCB)–derived CD34+CD38−Lin− cells cultured on a stromal cell line (AFT024) in the presence of cytokines (IL-3, IL-7, IL-15, SCF, and FLT-3L) and increasing amounts of CSA (0.1-5 μg/mL) as described in “NK-cell differentiation cultures, Materials and methods.” On day + 50, cultures were examined for (A) total cell number, (B) percentage, and (C) absolute number of KIR-expressing NK cells. Results are the average of cells from 4 donors plus and minus the SEM. Asterisk (*) indicates values that were significantly less than controls (P < .05) by Mann-Whitney rank sum test
Discussion
During allo-HCT, a complex mixture of lymphocytes and progenitor cells is transferred to the recipient. Mature donor T cells can respond to recipient alloantigens, resulting in GVHD. To prevent GVHD, immune-suppressive drugs, such as CSA, are administered. The influence of CSA on T-cell function has been extensively investigated (reviewed in Hollander et al33 ). In contrast, the impact of CSA on NK-cell function is not fully established. Here we demonstrate that CSA induces a number of unexpected and significant changes in the phenotype and function of cultured NK cells. Our studies show that CSA results in a dose-dependent reduction in NK-cell expansion, consistent with other investigators.34 However, we significantly extend these earlier observations by demonstrating a differential effect of CSA on CD56brightCD16− and CD56dimCD16+ NK subsets. After one week of culture in CSA, there were significantly higher percentages of CD56+CD16− cells and a reciprocal decrease in CD56+CD16+ cells. In bulk cultures, there were increased numbers of apoptotic cells in the presence of CSA. Using CSFE staining, we demonstrated that these effects are predominately on the CD56dimCD16+ NK-cell population. These results were confirmed using FACS-purified CD56brightCD16− and CD56dimCD16+ populations. CSA significantly inhibited the CD56dimCD16+ cell expansion relative to CD56brightCD16− cell expansion. We also noted that these 2 populations can interconvert after a week of culture. Such interconversion between CD56brightCD16− and CD56dimCD16+ populations has recently been described by other groups.35,36 While this occurred in only a minority of purified cells, CSA favored CD56+CD16− cells, regardless of the starting population (either CD56brightCD16− or CD56dimCD16+). Keskin et al have recently reported that the conversion of CD56dimCD16− cells to a CD56brightCD16− phenotype was, in part, mediated by transforming growth factor β (TGF β).35 Interestingly, CSA has been shown to induce TGF β secretion/release from T cells37-39 ; however, this has not been investigated in NK cells.
Conflicting results have been reported regarding the influence of CSA on NK-cell cytotoxicity. Introna et al showed that short-term exposure to CSA (20 hours) significantly inhibited K562 cytolysis,40 while Shao-Hsien et al found no impact of CSA on K562 killing using the identical experimental design.41 After culture of peripheral blood leukocytes (PBLs) with CSA for 3 days, there was no adverse effect on NK-cell cytotoxicity.34 In a rodent model, CSA inhibited T-cell responses to alloantigen but did not affect NK-cell responses, supporting the concept that T cells may be affected at a lower CSA dose compared with NK cells.42 More recently Poggi et al have shown that CSA inhibits NK-cell apoptosis upon target encounter.43 Across a series of donors, we observed slight increases in the cytotoxicity of CSA-treated cultures. Differences in cytotoxicity between CD56dim and CD56bright cells are well documented. Freshly isolated CD56brightCD16− cells are weakly cytotoxic against malignant targets, while the opposite is true for CD56dimCD16+ cells.24,44 However, CD56bright NK cells acquire cytotoxicity comparable with CD56dim cells after in vitro culture44 or in vivo stimulation with IL-2.45 While it was tempting to speculate that the increase in cytotoxicity was due entirely to a reduction in KIR-expressing cells, higher killing was also observed for MHC class I− targets (K562). CSA-treated cells did not differ from controls in the intracellular content of granzyme B, perforin, or surface expression of FasL. However, we noted significant increases in surface expression of NKp30 and in some donors NKp46 was also increased. This, along with a reduction in KIR- expressing cells, could account for increased cytotoxicity observed in our experiments. Moreover, in individual donors, we could demonstrate that an increase in the density of a particular activating receptor translated into higher cytolysis using redirected killing assay (not shown).
Following receptor ligation, one of the earliest phases in NK- cell activation is the increase in intracellular Ca2+ concentration that links membrane proximal events to downstream signaling pathways.46-48 Included in this process is activation of calcineurin, a Ca-dependent serine/threonine phosphatase that dephosphorylates NFAT, facilitating its nuclear translocation.26,27 Once in the nucleus, NFAT binds regulatory upstream elements and initiates gene transcription (reviewed in Rao et al28 ). CSA exerts its immunosuppressive properties by binding to cyclophilin A and antagonizing the phosphatase activity of calcineurin, preventing the nuclear translocation of NFAT.27,49,50 Using single-cell imaging, CSA-treated NK cells differed from controls, with regard to Ca2+ flux. Consistent with others,29 control-treated NK cells showed an oscillatory pattern of intracellular Ca2+ following K562 engagement. In contrast, CSA-exposed NK cells showed a progressive increase in Ca2+ and no such oscillations, suggesting that either the activation of calcineurin and/or the nuclear translocation of NFAT may be responsible for the oscillatory pattern of Ca2+ translocation following target encounter. Further experiments demonstrated that engagement of K562 cells leads to the rapid dephosphorylation and nuclear translocation of NFAT in control-treated NK cells, while neither occurred in cells cultured in CSA. Despite this, CSA-treated cells showed target cell killing, suggesting that the effector stage of cytotoxicity (ie, granule release) is not dependent upon nuclear translocation of NFAT. Other investigators have shown that CSA can inhibit mitochondrial permeability (ie, cytochrome c release), protecting cells from apoptosis induced by oxidative stress.51 Protection from apoptosis may also account for the increased cytotoxicity seen in CSA-treated NK cells, as has previously been reported.43 However, the lack of nuclear translocation of NFAT in CSA-exposed NK cells suggests that following target engagement, the downstream genetic programs driven by NFAT are probably not triggered. What effects this has on later stages of NK-cell activation are not well understood.
One of the main immune-suppressive actions of CSA is thought to be through inhibition of cytokine gene transcription.25 Surprisingly, higher percentages of IFN-γ–producing NK cells were detected in CSA-treated cultures. Considering that CSA-exposed NK-cell cultures had higher percentages of CD56+CD16− NK cells and that CD56bright NK cells secrete more IFN-γ in response to monokine stimulation,32 such findings may not be entirely unexpected. However, in some experiments there were more IFN-γ–producing cells than there were CD56+CD16− cells. Interestingly, CSA-treated T cells show similar increases in IFN-γ after T-cell receptor/CD28 stimulation52 or CD3 triggering with exogenous IL-12.53 These findings may be due to elevated c-fos expression and increased AP-1 binding to the IFN-γ promoter.53 Increased IFN-γ synthesis and secretion following CSA exposure may have important consequences for both preserving GVL activities and exacerbating GVHD.54-57
Here we have shown that CSA induces a dose-dependent and selective inhibition in the IL-2– and IL-15–induced proliferation of CD56dim NK cells and that CD56bright NK cells are relatively resistant to this effect. This calcineurin inhibitor does not reduce cytotoxicity and leads to higher quantities of IFN-γ–secreting cells. As a result, the repertoire of NK cells in CSA-treated patients following allo-HCT may be expected to reflect these features. In fact, some investigators have reported such observations. For instance, Chklovskaia et al58 and Jacobs et al59 both showed that, following transplantation, the ratio of CD56bright and CD56dim cells is reversed for 4 to 6 months. We have recently observed that at day + 100, patients who underwent allo-HCT have fewer KIR-expressing cells that secrete more IFN-γ compared with the donor.60 Likewise, Shilling et al demonstrate that KIR-expressing NK cells increase slowly (during the first 9 months after transplantation).61 Such reductions in KIR-expressing cells (due to CSA) may, in part, explain the inconsistent findings regarding any effect of KIR-L mismatch on leukemia relapse following T-cell–replete transplantations.11-16 Previously, reductions in KIR and increases in CD56bright NK cells seen after transplantation were ascribed to a response to cytokines present during the period of lymphopenia during transplantation or perhaps the NK-cell developmental process itself (ie, CD56bright giving rise to/or preceding CD56dim cell reconstitution). Our studies suggest that calcineurin inhibitors may also account for these findings and that these drugs impact NK cells in unexpected ways.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by NIH K08 HL04505-03 (M.R.V.), Viking's Children's Research Fund (M.R.V.), P01 CA65493 (J.S.M.), R01 HL55417 (J.S.M.), R01 AI34495 (B.R.B.), and R01 CA72669 (B.R.B.).
We would like to thank Paul Leibson and Dan Billadeau for their thoughtful comments in the early stages of this work.
National Institutes of Health
Authorship
Contribution: H.W. designed, performed, and analyzed experiments, and wrote the paper; B.G. designed and analyzed experiments, and wrote the paper; D.S. designed, performed, and analyzed experiments; V.M. designed and analyzed experiments and contributed to writing the paper; Q.C. performed statistical analyses; A.B.L. performed and analyzed experiments; B.R.B. designed experiments and contributed to writing the paper; D.N.C. designed and analyzed experiments; J.S.M. designed experiments and contributed to writing the paper; M.R.V. designed and analyzed experiments and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael R. Verneris, Suite 660, 425 E River Rd, Minneapolis, MN 55455; e-mail: verneris@umn.edu.