Abstract
The nuclear factor-κB (NF-κB) path-way has been implicated in tumor B-cell survival, growth, and resistance to therapy. Because tumor cells overcome single-agent antitumor activity, we hypothesized that combination of agents that target differentially NF-κB pathway will induce significant cytotoxicity. Therapeutic agents that target proteasome and Akt pathways should induce significant activity in B-cell malignancies as both pathways impact NF-κB activity. We demonstrated that perifosine and bortezomib both targeted NF-κB through its recruitment to the promoter of its target gene IκB using chromatin immunoprecipitation assay. This combination led to synergistic cytotoxicity in Waldenstrom macroglobulinemia (WM) cells that was mediated through a combined reduction of the PI3K/Akt and ERK signaling pathways, found to be critical for survival of WM cells. Moreover, a combination of these drugs with the CD20 monoclonal antibody rituximab further increased their cytotoxic activity. Thus, effective WM therapy may require combination regimens targeting the NF-κB pathway.
Introduction
Waldenstrom macroglobulinemia (WM) is a low-grade lymphoma characterized by the presence of lymphoplasmacytic cells in the bone marrow (BM) and a serum monoclonal immunoglobulin M protein in the circulation.1,2 Although indolent, it remains incurable and most patients die of disease progression with a median overall survival of 5 to 6 years.3 Therefore, there is an urgent need for rationally designed combinations of therapy in WM. Recent genomic and proteomic studies have demonstrated that several signaling pathways play an important role in the pathogenesis of WM compared with normal controls or other B-cell malignancies, including the nuclear factor-κB (NF-κB) and PI3K/Akt pathways.4
The NF-κB signaling pathway regulates the survival of normal and malignant B cells by controlling the expression of cell death regulatory genes.5,6 Depending on the cellular context, tumor necrosis factor alpha (TNFα) signaling and other stimuli activate the NF-κB pathway and augment the transcription of NF-κB target genes.5 These NF-κB target genes enhance cell survival, inhibit apoptosis, and limit the activity of proapoptotic BCL2 family members, in addition to multiple other effects.5 The NF-κB pathway undergoes a very tight, although complex, regulatory mechanism in which NF-κB controls its inhibitor IκBα transcription and in stabilizing IκB proteins.7,8 The NF-κB pathway-central role in plasma cell dyscrasia tumorigenesis has been recognized for a long time, partly through induction of cytokines and growth factors that promote tumor cell growth and survival.9,10 Data for its role in WM are limited, but there is some evidence to suggest that NF-κB is activated in WM cells.11,12
The PI3K/Akt pathway acts as a critical regulator of cell survival by stimulating cell proliferation and inhibiting apoptosis,13-15 and has been implicated in the pathogenesis of various cancers, including lymphoproliferative disorders.16-18 Akt indirectly activates NF-κB through direct phosphorylation and activation of IκB kinase alpha (IKKα), thereby inducing degradation of NF-κB inhibitor alpha (IκBα) by the ubiquitine-proteasome pathway.9,10
The degradation of cellular proteins is critical for normal cell cycling and function, such as transduction, transcriptional regulation, response to stress, and control of receptor function. The multicatalytic ubiquitin-proteasome pathway is responsible for the degradation of eukaryotic cellular proteins19,20 ; therefore, its dysregulation may play a role in tumor progression, drug resistance, and altered immune surveillance. This pathway also controls the activation of NF-κB by regulating degradation of IκBα,21 thus making the proteasome an appropriate and novel therapeutic target in cancer.22-24
We have previously demonstrated that perifosine, a novel Akt inhibitor that belongs to a class of lipid-related compounds called alkyl phospholipids, inhibits proliferation and induces apoptosis in WM cells.25 In addition, a phase 2 trial of perifosine in WM is ongoing with promising antitumor activity.26 In parallel, the proteasome inhibitor bortezomib has demonstrated significant clinical activity in patients with WM27,28 and induces apoptosis through several distinct mechanisms, including inhibition of NF-κB activity.29 We therefore hypothesized that therapeutic agents targeting the NF-κB pathway in WM may lead to significant antitumor activity. In this study, we demonstrated that the combination of perifosine and bortezomib leads to synergistic cytotoxic activity and inhibition of proliferation of WM cells. These results provide the framework for clinical studies of perifosine in combination with bortezomib in WM.
Methods
Cells
The WM cell lines, BCWM130 and WSU-WM (kind gift from Dr Al Khatib, Wayne State University, Detroit, MI), and IgM-secreting low-grade lymphoma cell lines, MEC1 (DMSZ, Braunschweig, Germany), RL (ATCC, Manassas, VA) were used in this study. All cell lines were cultured in RPMI-1640 containing 10% fetal bovine serum (Sigma-Aldrich, St Louis, MO), 2 μM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen, Carlsbad, CA). Patient samples were obtained after approval from the DFCI Institutional Review Board. Informed consent was obtained from all patients in accordance with the Declaration of Helsinki protocol. Primary WM cells were obtained from BM samples using CD19+ microbead selection (Miltenyi Biotec, Auburn, CA) with more than 90% purity, as confirmed by flow cytometric analysis with monoclonal antibody reactive to human CD20-PE (BD Biosciences, San Jose, CA). Peripheral blood mononuclear cells (PBMCs) were obtained from healthy volunteers by Ficoll-Hipaque density sedimentation.
Reagents
Perifosine was provided by Keryx Biopharmaceuticals (New York, NY). Bortezomib was obtained from Millennium Pharmaceuticals (Cambridge, MA). Rituximab was provided by Genentech (South San Francisco, CA). The following drugs were purchased at Sigma-Aldrich: dexamethasone, doxorubicine, fludarabine, melphalan, and chlorambucil. Interleukin-6 (IL-6), TNFα and CD40L were purchased from R&D Systems (Minneapolis, MN). The mouse antihuman anti IgG1 Fc monoclonal antibody was purchased at US Biological (Swampscott, MA).
Chromatin immunoprecipitation-based assays
Chromatin immunoprecipitation (ChIP) was performed as described31,32 using anti–p65NF-κB (Santa Cruz Biotechnology, Santa Cruz, CA). Enrichment for IκB promoter within the p65NF-κB–immunoprecipitated DNA was assessed by quantitative real-time PCR (q-PCR) using SYBR Green-based detection (Bio-Rad, Hercules, CA). Sequences of primers used in these assays are available on request. Data were normalized to inputs and internal negative controls.33
Quantitative reverse-transcribed polymerase chain reaction analysis
Quantitative reverse-transcribed polymerase chain reaction (RT-PCR) were performed and analyzed using RSP28 as an internal control.33 IκBα expression levels were determined using the following primers: 5′-GCCAGAGAGTGAGGATGAGG-3′ and 5′-CTTTGCGCTCATAACGTCAG-3′. Dissociation curve analyses were performed to ensure specific amplification of a single amplicon.
NF-κB activity assay using Active Motif
Active Motif TransAM Kits is a DNA-binding ELISA-based assay (Active Motif North America, Carlsbad, CA). NF-κBp65 transcription factor-binding to its consensus sequence on the plate-bound oligonucleotide was studied from nuclear extracts, following the manufacturer's procedure.
Proteasome activity assay
The 20S proteasome activity was measured by monitoring the release of fluorophore 7-amino-4-methylcoumarin (AMC) after cleavage from the labeled substrate LLVY-AMC following the manufacturer's protocol (Chemicon International, Temecula, CA).
Immunofluorescence
The baseline nuclear/cytoplasmic expression of phospho-NF-κBp65 was also examined using an immunocytochemical method, as described.34 Immunocytochemical analysis was performed using an epifluorescence microscope (Nikon Eclipse E800; Nikon, Avon, MA) and a Photometrics Coolsnap CF color camera (Nikon, Lewisville, TX).
Growth inhibition assay and DNA synthesis
Drug-induced cytotoxicity on WM cell survival was assessed by measuring the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Chemicon International) dye absorbance, as described.35 Proliferation was assessed by DNA synthesis, as described.35 DNA synthesis was measured by the [3H]-thymidine uptake (PerkinElmer Life and Analytical Sciences, Waltham, MA).
Effect on paracrine Waldenstrom macroglobulinemia cell growth in the bone marrow
To evaluate growth stimulation and signaling in WM cells adherent to bone marrow stromal cells (BMSCs), BMSCs were cultured in presence of either perifosine, bortezomib and the combination. DNA synthesis was measured using the [3H]-thymidine uptake assay as described.35
Colony forming cell assay
Colony forming cell (CFC) assays were performed using BM mononuclear fraction cultured in commercially available methylcellulose for human colony-forming cell assays (MethoCult; StemCell Technologies, Vancouver, BC), and treated with either perifosine, bortezomib and the combination. Burst-forming units erythroid (BFU-E), colony-forming units granulocyte (CFU-GM), macrophage (CFU-M), and granulocyte/erythroid/macrophage/megakaryocyte CFU-GEMM) colonies were counted at days 14 to 16.
Flow cytometric analysis
Cell-cycle analysis was profiled by flow cytometry using propidium iodide (PI) staining (5 μg/mL; Sigma-Aldrich) after 12 and 24 hours' culture with either perifosine, bortezomib, or the combination. Apoptosis was quantitated using Apo2.7 flow cytometric analysis (Beckman Coulter, Fullerton, CA), as described.35
Cell fractionation and immunoblotting
WM cells were lysed using lysis buffer (Cell Signaling Technology, Beverly, MA). For cell fractionation, once cytoplasmic extraction performed, nuclear fraction was next extracted using hypotonic lysis buffer, following the manufacturer's protocol (Panomics, Fremont, CA). Whole-cell lysates were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membrane (Bio-Rad Laboratories). The antibodies used for immunoblotting included: anti–phospho (p)-Akt (Ser473), -Akt, –p-ERK (Thr202/Tyr204), -ERK1/2, –p-GSK3α/β (Ser21/9), –p-S6 Ribosomal (Ser240/244), -IκBα, –p50/p105NF-κB, –p-p65NF-κB (Ser536), –p-CDK2 (Thr160), -CDK2, -CDK4, -CDK6, -p21waf1, -p27kip1, -p53, –p-Rb (Ser807/811), –Bcl-Xl, –Mcl-1, –caspase-8, –caspase-9, –caspase-3, and -PARP (Cell Signaling Technology), –β-actin, -nucleolin and –α-tubulin (Santa Cruz Biotechnology).
In vitro Akt kinase assay
In vitro Akt kinase assay (Cell Signaling Technology) was performed following manufacturer procedure. Briefly, BCWM1 cells were cultured with either perifosine, bortezomib, or the combination. Lysates were immunoprecipitated with immobilized Akt primary antibody. Kinase activity was detected by immunoblotting with phospho-GSK-3α/β (Ser21/9) antibody (Cell Signaling Technology).
Antibody-dependent cellular cytotoxicity assays
Antibody-dependent cellular cytotoxicity (ADCC) was performed as described.36 Briefly, IL-2 (BD Biosciences) activated PBMCs were used as effector cells and calcein-AM-(Invitrogen) labeled BCWM.1 cell line as targets. ADCC was performed in presence of rituximab or human control IgG1 at various effector-to-target ratios. After 4-hour incubation at 37°C, culture supernatants were read on Wallac VICTOR2 using 492/520 nm filters set (PerkinElmer). Calculation of percentage specific lysis was done using the following equation: % specific lysis = 100 × [(mean experimental release − mean spontaneous release)/(mean maximum release − mean spontaneous release)]. Experiments were also done in which target WM cells were treated with either perifosine, bortezomib, or the combination.
Statistical analysis
The interaction between drugs was analyzed by isobologram analysis using the CalcuSyn software program (Biosoft, Ferguson, MO) to determine whether the combinations were synergistic (combination index [CI] < 0.8). The program calculates a CI for a corresponding affected fraction of cells.
Results
Higher constitutive levels of NF-κB in bone marrow isolated CD19+ cells from patients with Waldenstrom macroglobulinemia compared with healthy donor
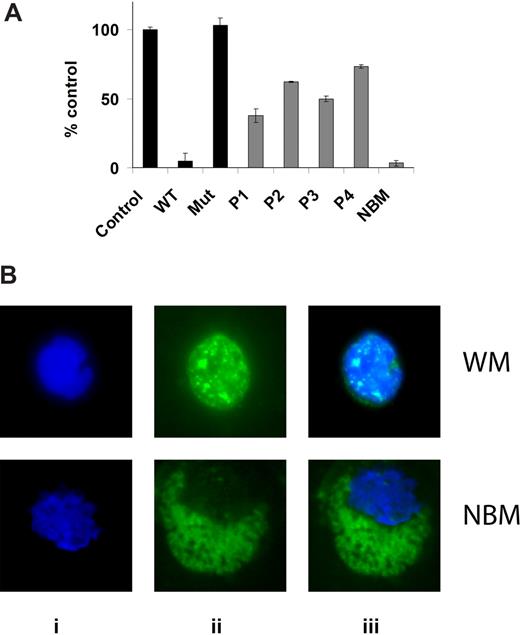
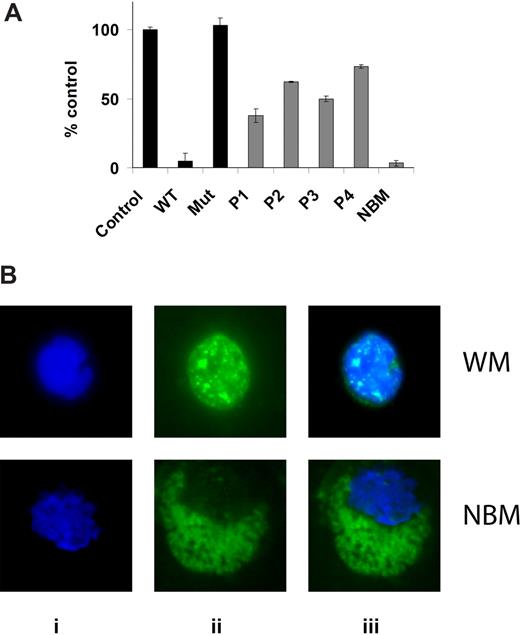
NF-κBp65 DNA binding activity was assessed in vitro using nuclear extracts using the Active Motif assay. We first determined the baseline expression of phospho-NF-κBp65 in BM isolated CD19+ cells from 4 patients with WM compared with healthy donor. As shown in Figure 1A, baseline expression was higher in all 4 patients samples compared with healthy donor, indicating constitutively high expression of NF-κBp65 activated form in WM cells. We confirmed these results using immunofluorescence for phospho-NF-κBp65 on BM isolated CD19+ cells from 12 patients with WM compared with healthy donor. As shown in Figure 1B, there was a significantly higher nuclear translocation of phospho-NF-κBp65 in WM cells compared with normal CD19+ cells.
Baseline NF-κB expression in WM cells. (A) NF-κBp65 DNA binding activity was assessed in vitro using nuclear extracts using the Active Motif assay. We compared BM isolated CD19+ cells from 4 patients with WM to one healthy donor. Jurkat nuclear extracts provided as a positive control in the kit were used as a control in the first 3 conditions. P indicates patient; NBM, healthy donor normal BM. WT and Mut are wild-type and mutated consensus competitor oligonucleotides, respectively. Data represent mean plus or minus SD of triplicate experiments. (B) Immunofluor-escence for phospho-NF-κBp65 on BM isolated CD19+ cells from one patient with WM compared with one healthy donor (NBM). Immunocytochemical analysis was assessed using anti–p-NF-κBp65 antibody (ii). DAPI was used to stain nuclei (i). (iii) The merge of i and ii panels.
Baseline NF-κB expression in WM cells. (A) NF-κBp65 DNA binding activity was assessed in vitro using nuclear extracts using the Active Motif assay. We compared BM isolated CD19+ cells from 4 patients with WM to one healthy donor. Jurkat nuclear extracts provided as a positive control in the kit were used as a control in the first 3 conditions. P indicates patient; NBM, healthy donor normal BM. WT and Mut are wild-type and mutated consensus competitor oligonucleotides, respectively. Data represent mean plus or minus SD of triplicate experiments. (B) Immunofluor-escence for phospho-NF-κBp65 on BM isolated CD19+ cells from one patient with WM compared with one healthy donor (NBM). Immunocytochemical analysis was assessed using anti–p-NF-κBp65 antibody (ii). DAPI was used to stain nuclei (i). (iii) The merge of i and ii panels.
Perifosine and bortezomib inhibit nuclear translocation of NF-κB in Waldenstrom macroglobulinemia
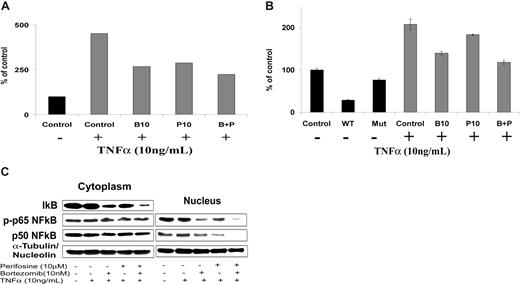
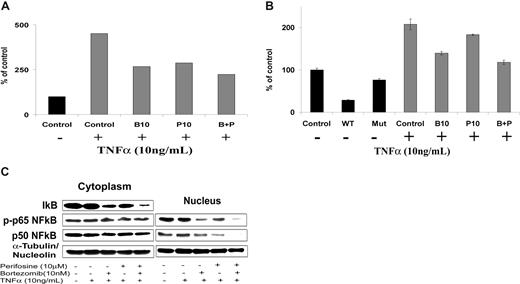
NF-κB is one of the major pathways implicated in the growth and survival of plasma cell dyscrasias.9,10 We first investigated the effect of perifosine and bortezomib on NF-κB recruitment to the promoter of its target gene IκB using ChIP assay in BCWM.1 WM cells. As expected, NF-κBp65 binding to IκB promoter was induced in BCWM.1 cells by TNFα treatment (Figure 2A). Both bortezomib (10 nM) and perifosine (10 μM) blunted this induction. Moreover, their combination showed a slightly stronger effect on NF-κBp65 recruitment (Figure 2A). Similar results were obtained when NF-κBp65 DNA binding activity was assessed in vitro using nuclear extracts from treated cells using the Active Motif assay in BCWM.1 (Figure 2B) and WSU-WM WM cell lines, and 2 other IgM-secreting cell lines, MEC1 and RL (data not shown). These results indicate that perifosine and bortezomib inhibit NF-κBp65 recruitment to the DNA in WM cells. Furthermore, immunoblotting of nuclear extracts demonstrated that p65 phosphorylation and p50NF-κB expression were inhibited by perifosine, bortezomib, and more significantly by the combination of both agents, as shown in Figure 2C. At the protein level, either agent alone, and more significantly their combination, inhibited IκB protein expression. This could possibly be related to their inhibition of NF-κB–induced IκB gene expression, along with a direct effect of the drugs on IκB itself. Together, these results indicate that perifosine and bortezomib significantly inhibit p65 and p50NF-κB subunit nuclear translocation and subsequent transcriptional activity. The combination of these drugs only slightly improved their inhibitory effect.
Perifosine and bortezomib inhibit NF-κB function in WM cells. (A) Chromatin immunoprecipitation (ChIP)-based assay. BCWM.1 cells were cultured with either perifosine (P, 10 μM), bortezomib (B, 10 nM), or the combination (B + P) overnight, then TNFα (10 ng/mL) was added for the last 30 minutes, and then anti–p65NF-κB immunoprecipitation was performed. Quantitative real-time PCR (Q-PCR) for IκB in the p65NF-κB-DNA immunoprecipitated fragments was assessed using SYBR. Experimental Q-PCR values were normalized against values obtained for 25 ng of input DNA with the same primer set. (B) NF-κB activity assay using Active Motif. BCWM.1 cells were cultured with either perifosine (10 μM), bortezomib (10 nM), or the combination for 6 hours, and then TNFα (10 ng/mL) was added for the last 30 minutes. NF-κBp65 transcription factor-binding to its consensus sequence on the plate-bound oligonucleotide was studied from nuclear extracts. WT and Mut are wild-type and mutated consensus competitor oligonucleotides, respectively. Data represent mean plus or minus SD of triplicate experiments. (C) BCWM.1 cells were cultured with either perifosine (10 μM), bortezomib (10 nM), or the combination for 6 hours, then TNFα (10 ng/mL) was added for the last 30 minutes, and then the effect on NF-κB pathway was studied using immunobloting on cell fractionation. Cytoplasmic and nuclear fractions were subjected to Western blotting using anti-IκBα, –NF-κBp50, –p-NF-κBp65, -nucleolin, and –α-tubulin antibodies.
Perifosine and bortezomib inhibit NF-κB function in WM cells. (A) Chromatin immunoprecipitation (ChIP)-based assay. BCWM.1 cells were cultured with either perifosine (P, 10 μM), bortezomib (B, 10 nM), or the combination (B + P) overnight, then TNFα (10 ng/mL) was added for the last 30 minutes, and then anti–p65NF-κB immunoprecipitation was performed. Quantitative real-time PCR (Q-PCR) for IκB in the p65NF-κB-DNA immunoprecipitated fragments was assessed using SYBR. Experimental Q-PCR values were normalized against values obtained for 25 ng of input DNA with the same primer set. (B) NF-κB activity assay using Active Motif. BCWM.1 cells were cultured with either perifosine (10 μM), bortezomib (10 nM), or the combination for 6 hours, and then TNFα (10 ng/mL) was added for the last 30 minutes. NF-κBp65 transcription factor-binding to its consensus sequence on the plate-bound oligonucleotide was studied from nuclear extracts. WT and Mut are wild-type and mutated consensus competitor oligonucleotides, respectively. Data represent mean plus or minus SD of triplicate experiments. (C) BCWM.1 cells were cultured with either perifosine (10 μM), bortezomib (10 nM), or the combination for 6 hours, then TNFα (10 ng/mL) was added for the last 30 minutes, and then the effect on NF-κB pathway was studied using immunobloting on cell fractionation. Cytoplasmic and nuclear fractions were subjected to Western blotting using anti-IκBα, –NF-κBp50, –p-NF-κBp65, -nucleolin, and –α-tubulin antibodies.
Perifosine and bortezomib synergistically inhibit growth of Waldenstrom macroglobulinemia cells
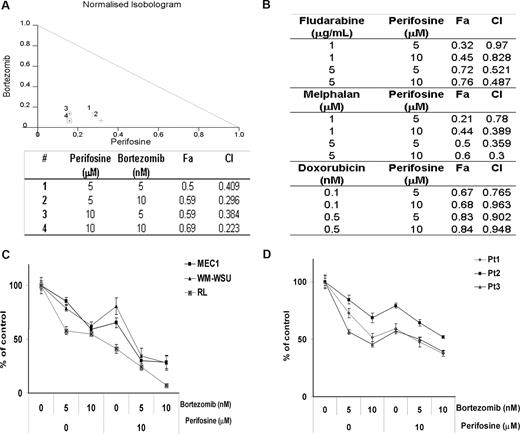
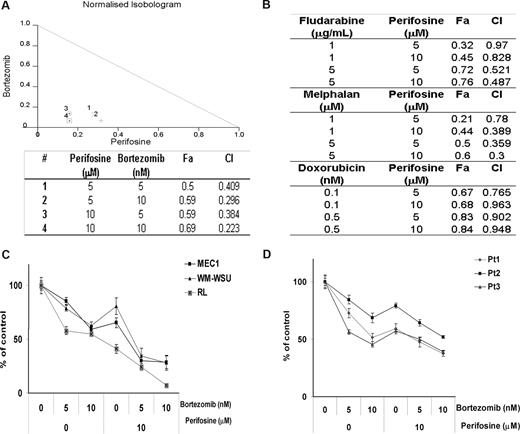
We have previously shown that perifosine inhibits the growth of WM tumor cell lines as well as other IgM-secreting cell lines with a 50% decrease in survival (IC50) at 5 to 20 μM.25 We sought to determine whether bortezomib could improve this cytotoxic effect. As shown in Figure 3A, perifosine (5 μM and 10 μM) showed a significant cytotoxic effect when combined with bortezomib (5 nM and 10 nM) in BCWM.1 cells using MTT assay at 48 hours. Indeed, perifosine (10 μM) induced 31% cytotoxicity that was synergistically enhanced to 59% (CI = 0.38) and 69% (CI = 0.22) when combined with bortezomib at 5 nM and 10 nM, respectively, and using the Calcusyn software to determine synergy. To further determine whether the combination of perifosine with other therapeutic agents used in WM therapy could lead to a comparable cytotoxic effect, we investigated the effect of perifosine (5 μM and 10 μM) in combination with chlorambucil (10 μM and 50 μM), dexamethasone (50 nM and 100 nM), fludarabine (1 μg/mL and 5 μg/mL), doxorubicin (0.1 nM and 0.5 nM), and melphalan (1 μM and 5 μM). As shown by the combination indices in Figure 3B, there was a synergistic activity of perifosine with fludarabine, melphalan, and doxorubicin, but not with chlorambucil and dexamethasone (not shown). Based on the combination indices and fractions affected, we demonstrated that the combination of perifosine and bortezomib led to the highest synergistic activity in WM. We next confirmed the synergistic cytotoxic activity of the combination of perifosine and bortezomib in BCWM.1 cells by studying the antiproliferative activity of the combination using the [3H]-thymidine uptake assay (not shown). Moreover, we investigated the cytotoxic effect of perifosine and bortezomib in other IgM-secreting cell lines and confirmed the effect of the combination of perifosine and bortezomib on transformed WM cell line WM-WSU and other IgM-secreting cell lines, at 48 hours (Figure 3C). Finally, we confirmed the cytotoxic activity of the combination of perifosine and bortezomib using primary CD19+ sorted cells from patients with WM as shown in Figure 3D. We then investigated the sequential addition of either bortezomib or perifosine 24 hours apart for a total of 48 hours before determining the effect of these agents in sequential delivery on the growth of WM cells. There was no difference in the cytotoxic effect of the sequential addition of either bortezomib or perifosine first compared with the treatment with perifosine and bortezomib together for 48 hours (not shown).
The combination of perifosine and bortezomib induces a decrease in proliferation and survival in WM tumor cells. (A) Normalized isobologram produced by Calcusyn software. BCWM.1 cells were cultured with either perifosine (5 μM and 10 μM), bortezomib (5 nM and 10 nM), or the combination. The table shows affected fractions and combination indices. Cytotoxicity was assessed using MTT assay. (B) Cytotoxicity induced with several agents known in WM disease therapy. Combination indices (CI) and fractions affected (FA) produced by Calcusyn software. BCWM.1 cells were cultured with the following agents: fludarabine (1 and 5 μg/mL), melphalan (1 μM and 5 μM), and doxorubicin (0.1 nM and 0.5 nM) in combination with perifosine (5 μM and 10 μM) for 48 hours. (C) Cytotoxicity was assessed on several IgM-secreting cell lines, MEC-1 (■), WM-WSU (Δ), and RL (x). Cells were cultured with either perifosine (10 μM), bortezomib (5 nM and 10 nM), or the combination for 48 hours. (D) Freshly isolated BM CD19+ samples from 3 patients with WM treated with the combination of perifosine (10 μM), bortezomib (5 nM and 10 nM), and the combination for 48 hours. Data represent mean plus or minus SD of triplicate experiments.
The combination of perifosine and bortezomib induces a decrease in proliferation and survival in WM tumor cells. (A) Normalized isobologram produced by Calcusyn software. BCWM.1 cells were cultured with either perifosine (5 μM and 10 μM), bortezomib (5 nM and 10 nM), or the combination. The table shows affected fractions and combination indices. Cytotoxicity was assessed using MTT assay. (B) Cytotoxicity induced with several agents known in WM disease therapy. Combination indices (CI) and fractions affected (FA) produced by Calcusyn software. BCWM.1 cells were cultured with the following agents: fludarabine (1 and 5 μg/mL), melphalan (1 μM and 5 μM), and doxorubicin (0.1 nM and 0.5 nM) in combination with perifosine (5 μM and 10 μM) for 48 hours. (C) Cytotoxicity was assessed on several IgM-secreting cell lines, MEC-1 (■), WM-WSU (Δ), and RL (x). Cells were cultured with either perifosine (10 μM), bortezomib (5 nM and 10 nM), or the combination for 48 hours. (D) Freshly isolated BM CD19+ samples from 3 patients with WM treated with the combination of perifosine (10 μM), bortezomib (5 nM and 10 nM), and the combination for 48 hours. Data represent mean plus or minus SD of triplicate experiments.
Combined inhibition of the PI3K/Akt and ERK MAPK survival pathways by perifosine and bortezomib in Waldenstrom macroglobulinemia
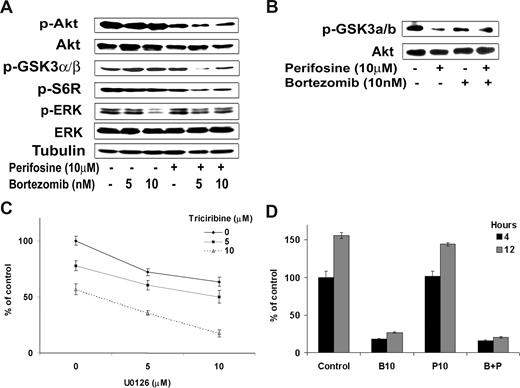
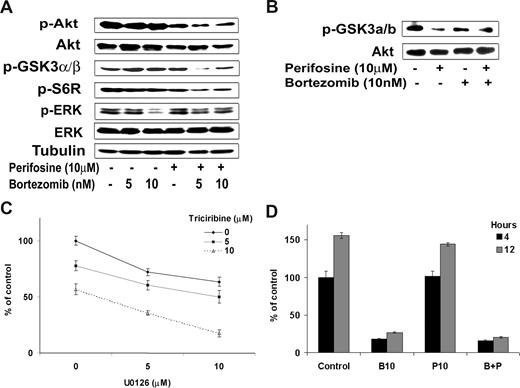
Because NF-κB inhibition by the combination of the 2 drugs was only slightly increased compared with either agent alone, we sought to further dissect the molecular mechanisms that lead to the synergistic cytotoxicity of both agents on WM cells. We thus examined their effect on PI3K/Akt activity as well as on ERK MAPK pathway, another important signaling cascade involved in survival of WM cells.37,38 As shown in Figure 4A, bortezomib (5 nM and 10 nM) inhibited phosphorylation of ERK MAPK pathway, whereas perifosine (10 μM) inhibited Akt phosphorylation (p-Akt) but induced activation of the ERK MAPK pathway. Interestingly, their combination was able to overcome the activation induced by the other agent, and the combination of perifosine and bortezomib led to a decrease in both p-Akt and p-ERK activity as shown in Figure 4A. In addition, the combination of perifosine and bortezomib significantly inhibited in a dose-dependent manner the phosphorylation of downstream target proteins of Akt, phospho-S6 ribosomal protein and phospho-GSK-3α/β (Figure 4A). We next confirmed the effect of the combination of perifosine and bortezomib on Akt activity using an in vitro Akt kinase assay. As shown in Figure 4B, the combination of perifosine (10 μM) with bortezomib (10 nM) decreased phosphorylation of fusion protein GSK3α/β.
Differential targeting of perifosine and bortezomib on Akt, Erk, and proteasome pathways. (A) BCWM.1 cells were cultured with the combination perifosine (10 μM) and bortezomib (10 nM) for 6 hours and rituximab (10 ng/mL) in the last hour, and then the effect on Akt and ERK MAPK pathways was assessed by immunobloting. Whole cell lysates were subjected to Western blotting using anti–p-Akt, -Akt, –p-GSK3α/β-p-S6R, -ERK1/2, –p-ERK, and –α-tubulin antibodies. (B) BCWM.1 cells were cultured with the combination perifosine (10 μM) and bortezomib (10 nM) for 6 hours, and then whole cell lysates were immunoprecipitated overnight with anti-Akt antibody. Then the immunoprecipitates were subjected to in vitro kinase assay according to manufacturer's protocol. Western blotting used -Akt and fusion protein -p-GSK3α/β antibodies. (C) BCWM.1 cells were cultured with either triciribine (5 μM and 10 μM), U0126 (5 nM and 10 μM), or the combination. Cytotoxicity was assessed using MTT assay. (D) 20S proteasome activity assay. BCWM.1 cells were cultured with either perifosine (10 μM), bortezomib (10 nM), or the combination for 4 and 12 hours, and then whole cell lysates were subjected to proteasome activity measurement. Data represent mean plus or minus SD of triplicate experiments.
Differential targeting of perifosine and bortezomib on Akt, Erk, and proteasome pathways. (A) BCWM.1 cells were cultured with the combination perifosine (10 μM) and bortezomib (10 nM) for 6 hours and rituximab (10 ng/mL) in the last hour, and then the effect on Akt and ERK MAPK pathways was assessed by immunobloting. Whole cell lysates were subjected to Western blotting using anti–p-Akt, -Akt, –p-GSK3α/β-p-S6R, -ERK1/2, –p-ERK, and –α-tubulin antibodies. (B) BCWM.1 cells were cultured with the combination perifosine (10 μM) and bortezomib (10 nM) for 6 hours, and then whole cell lysates were immunoprecipitated overnight with anti-Akt antibody. Then the immunoprecipitates were subjected to in vitro kinase assay according to manufacturer's protocol. Western blotting used -Akt and fusion protein -p-GSK3α/β antibodies. (C) BCWM.1 cells were cultured with either triciribine (5 μM and 10 μM), U0126 (5 nM and 10 μM), or the combination. Cytotoxicity was assessed using MTT assay. (D) 20S proteasome activity assay. BCWM.1 cells were cultured with either perifosine (10 μM), bortezomib (10 nM), or the combination for 4 and 12 hours, and then whole cell lysates were subjected to proteasome activity measurement. Data represent mean plus or minus SD of triplicate experiments.
Importantly, the synergistic effect of perifosine and bortezomib on WM cell viability may result from the combined inhibition of the PI3K/Akt and ERK MAPK pathways achieved when the drugs are associated. To confirm that targeting Akt and ERK MEK pathways was sufficient to induce significant cytotoxicity in WM cells, we used 2 specific inhibitors of these pathways: triciribine for the Akt pathway and U0126 for the ERK MEK pathway. We demonstrated that, after 48 hours of treatment, the combination of Akt and ERK MEK specific inhibitors significantly inhibited survival of BCWM.1 cells (Figure 4C). Indeed, U0126 (5 μM) induced 28% cytotoxicity that increased to 40% (CI = 0.65) and 65% (CI = 0.3) in the presence of 5 μM or 10 μM of triciribine, respectively. On the other hand, the combination of the drugs had no supplementary effect on proteasome inhibition compared with bortezomib alone (Figure 4D).
The combination of perifosine and bortezomib overcomes resistance induced by the BM microenvironment
Previous studies have shown that the BM microenvironment induces NF-κB activity in tumor cells through several mechanisms, including direct cell-cell contact with mesenchymal cells in the BM, as well as by cytokines present in the BM, including IL-6 and TNFα. Cytokines known to be highly expressed in the WM tumor microenvironment include CD40L (CD154)39 and IL-6.40 The BM microenvironment induces resistance and proliferation of tumor cells.41 Therefore, to mimic the effects of the BM milieu on the WM cells, we investigated the effect of coculture of mesenchymal cells with BCWM.1 cells as well as the addition of growth-inducing cytokines on proliferation, and the functional effect of bortezomib and perifosine on this system. As shown in Figure 5A, coculture of BCWM.1 cells and mesenchymal cells induced proliferation of WM cells. Adherence of BCWM.1 cells to mesenchymal cells triggered a 2-fold (P < .01) increase in the [3H]-thymidine uptake, indicating proliferation. Perifosine inhibited WM cell growth in the context of the BM microenvironment in a dose-dependent fashion (P < .01). This effect was significantly enhanced by the combination with bortezomib (P < .01; Figure 5A), confirming that bortezomib combination with perifosine enhances perifosine antitumor activity in the BM milieu. Similarly, IL-6 (25 ng/mL) and CD40L (3 μg/mL) induced proliferation in BCWM.1 cells as shown in Figure 5B, whereas the addition of either perifosine (5 μM and 10 μM), bortezomib (10 nM), and more significantly the combination inhibited the proliferation of WM cells, indicating that perifosine and bortezomib overcome activation of NF-κB by these cytokines. These results demonstrate that the combination of bortezomib and perifosine efficiently inhibits proliferation of WM cells in a context of cell-to-cell contact with mesenchymal cells or in the presence of BM milieu cytokines that induce NF-κB activation.
Growth factors and coculture with BM microenvironment cells do not protect WM cells against the combined perifosine and bortezomib-induced cytotoxicity. (A) BCWM.1 cells were cultured with the combination perifosine (5 μM and 10 μM) and bortezomib (10 nM) for 48 hours, in the presence or absence of BMSCs. Cell proliferation was assessed using the [3H]-thymidine uptake assay. (B) BCWM.1 cells were cultured with either perifosine (10 μM), bortezomib (5 nM and 10 nM), or the combination in presence of IL-6 (50 ng/mL) or CD40L (3 μg/mL) for 48 hours. Cytotoxicity was assessed by the MTT assay. (C) PBMCs from 3 healthy donors with either perifosine (10 μM), bortezomib (5 nM and 10 nM), and the combination for 48 hours. Cytotoxicity was assessed by MTT assay. (D) Colony-forming cell assay. Nonadherent mononuclear cells were cultured using methylcellulose semisolid technique cultured with either single agents or the combination. The plates were read at days 14 to 16. BFU-E indicates burst-forming units–erythroid; CFU-GM, colony-forming units–granulocyte/macrophage; CFU-M, colony-forming units–macrophage; CFU-GEMM, colony-forming units–granulocyte/erythroid/macrophage/megakaryocyte. Data represent mean plus or minus SD of triplicate experiments.
Growth factors and coculture with BM microenvironment cells do not protect WM cells against the combined perifosine and bortezomib-induced cytotoxicity. (A) BCWM.1 cells were cultured with the combination perifosine (5 μM and 10 μM) and bortezomib (10 nM) for 48 hours, in the presence or absence of BMSCs. Cell proliferation was assessed using the [3H]-thymidine uptake assay. (B) BCWM.1 cells were cultured with either perifosine (10 μM), bortezomib (5 nM and 10 nM), or the combination in presence of IL-6 (50 ng/mL) or CD40L (3 μg/mL) for 48 hours. Cytotoxicity was assessed by the MTT assay. (C) PBMCs from 3 healthy donors with either perifosine (10 μM), bortezomib (5 nM and 10 nM), and the combination for 48 hours. Cytotoxicity was assessed by MTT assay. (D) Colony-forming cell assay. Nonadherent mononuclear cells were cultured using methylcellulose semisolid technique cultured with either single agents or the combination. The plates were read at days 14 to 16. BFU-E indicates burst-forming units–erythroid; CFU-GM, colony-forming units–granulocyte/macrophage; CFU-M, colony-forming units–macrophage; CFU-GEMM, colony-forming units–granulocyte/erythroid/macrophage/megakaryocyte. Data represent mean plus or minus SD of triplicate experiments.
The combination of bortezomib and perifosine does not induce cytotoxicity in colony formation units or mononuclear cells
Given that perifosine and bortezomib induce significant cytotoxicity in WM cells even in the presence of the BM microenvironment, we sought to investigate the effect of these agents alone or in combination on nonmalignant hematopoietic cells. We first tested the effect of perifosine and bortezomib on normal mononuclear cells isolated from the peripheral blood of healthy volunteers. As shown in Figure 5C, perifosine (10 μM), bortezomib (5 nM and 10 nM), and the combination did not trigger cytotoxicity in PBMCs. We next investigated whether these 2 agents affect the proliferation of normal hematopoietic progenitor cells. As shown in Figure 5D, perifosine (10 μM), bortezomib (10 nM), and the combination did not inhibit the growth of colony forming units.
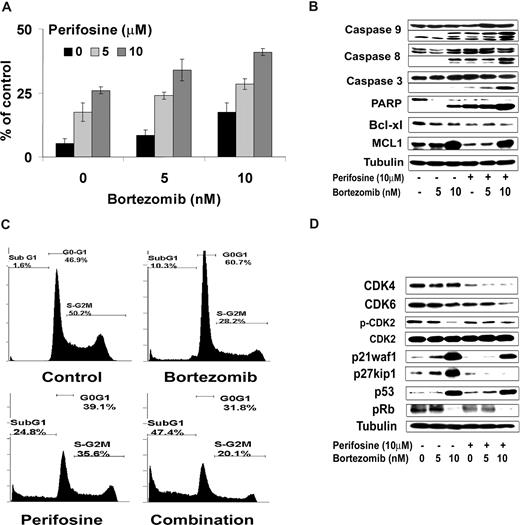
The combination of perifosine and bortezomib induces cell-cycle arrest and apoptosis in Waldenstrom macroglobulinemia cells
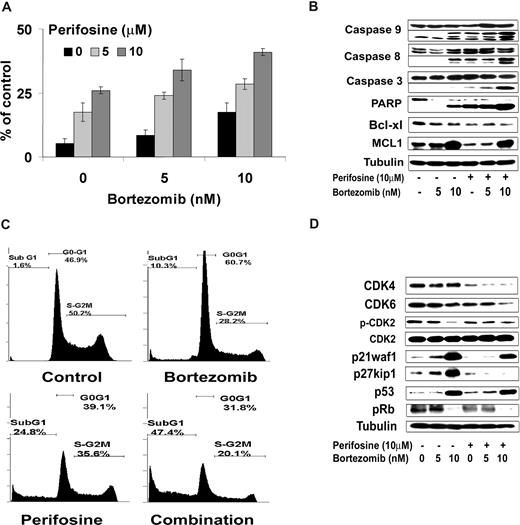
We next investigated the effect of perifosine in combination with bortezomib on apoptosis as evidenced by propidium iodide and Apo2.7 staining by flow cytometric analysis on BCWM.1 cells at 48 hours. As shown in Figure 6A, perifosine alone (5 μM and 10 μM) induced 17.5% and 26% apoptosis at 48 hours, which was enhanced to 28.5% and 41%, respectively, in combination with bortezomib 10 nM (P = .01). To determine the mechanism of perifosine and bortezomib-induced apoptosis, we investigated the effect of these agents on BCWM.1 cells using immunoblotting. As shown in Figure 6B, perifosine alone (10 μM) and bortezomib (5 nM and 10 nM) induced both intrinsic and extrinsic apoptotic pathways with caspase-9 and -8 cleavage after overnight incubation. This cleavage activity was enhanced when the 2 drugs were combined together. The combination of both agents also led to PARP and caspase-3 cleavage, as shown in Figure 6B. Both agents decrease the expression of the antiapoptotic protein Bcl-xL, whereas bortezomib (10 nM) but not perifosine (10 μM), increased expression of Mcl-1. Similarly, perifosine alone (10 μM, 24 hours) induced 25% subG1/G0 arrest, whereas bortezomib alone (10 nM, 24 hours) induced 10% subG1/G0 cells, which was increased to 47% when the 2 drugs were combined (Figure 6C). Interestingly, bortezomib (10 nM) induced a G1-S arrest on cell-cycle analysis using propidium iodide staining and flow cytometric analysis at 24 hours,23 whereas perifosine (10 μM) had minimal effect on cell cycle and induced mainly apoptosis with subG1/G0 arrest.42 We then studied by immunoblotting the molecular pathways of cell-cycle regulation induced by these agents in BCWM.1 cells. Bortezomib induced an increased expression of p53 tumor suppressor protein and of the cyclin-dependent kinase inhibitors p21waf1/cip1 and p27kip1, in a dose-dependent fashion at 24 hours (Figure 6D). Those proteins are known to regulate cyclin-dependent kinase 2 (CDK2), CDK4, and CDK6,43 which are important regulators of the G1 to S transition of cell cycle through inactivation of retinoblastoma tumor suppressor protein (Rb).44 Consequently, bortezomib induced a decrease in CDK2 phosphorylation and inactivation of Rb protein as shown by a decrease in Rb phosphorylation with 10 nM bortezomib (Figure 6D). Perifosine had no effect on proteins involved in G1-S transition of cell cycle confirming the cell-cycle analysis data but had some inhibitory effect on CDK4.
Perifosine and bortezomib-induced apoptosis and cell-cycle arrest were measured in WM cells. (A) BCWM.1 cells were cultured with either perifosine (5 μM and 10 μM), bortezomib (5 nM and 10 nM), and the combination for 48 hours. Then the percentage of cells undergoing apoptosis was studied using Apo2.7 staining and flow cytometry. (B) BCWM.1 cells were cultured with either perifosine (10 μM), bortezomib (5 nM and 10 nM), or the combination for 10 hours. Whole cell lysates were subjected to Western blotting using anticaspase 9, -caspase 8, -caspase 3, -PARP, -MCL1, Bcl-xl, and –α-tubulin antibodies. (C) Cell cycle was then studied using PI staining by flow cytometry. BCWM.1 cells were cultured with either perifosine (10 μM), bortezomib (10 nM), or the combination for 24 hours. Percentages indicate cells in sub-G1 phase, G0/G1 phase, and G2/M phase. (D) BCWM.1 cells were cultured with either perifosine (10 μM), bortezomib (5 nM and 10 nM), or the combination for 12 hours. Whole cell lysates were subjecting to Western blotting using anti-CDK4, -CDK6, -CDK2, -p-CDK2, -p27kip1, -p21Waf1/Cip1, -p53, -p-Rb, and –α-tubulin antibodies. Data represent mean plus or minus SD of triplicate experiment (A,C).
Perifosine and bortezomib-induced apoptosis and cell-cycle arrest were measured in WM cells. (A) BCWM.1 cells were cultured with either perifosine (5 μM and 10 μM), bortezomib (5 nM and 10 nM), and the combination for 48 hours. Then the percentage of cells undergoing apoptosis was studied using Apo2.7 staining and flow cytometry. (B) BCWM.1 cells were cultured with either perifosine (10 μM), bortezomib (5 nM and 10 nM), or the combination for 10 hours. Whole cell lysates were subjected to Western blotting using anticaspase 9, -caspase 8, -caspase 3, -PARP, -MCL1, Bcl-xl, and –α-tubulin antibodies. (C) Cell cycle was then studied using PI staining by flow cytometry. BCWM.1 cells were cultured with either perifosine (10 μM), bortezomib (10 nM), or the combination for 24 hours. Percentages indicate cells in sub-G1 phase, G0/G1 phase, and G2/M phase. (D) BCWM.1 cells were cultured with either perifosine (10 μM), bortezomib (5 nM and 10 nM), or the combination for 12 hours. Whole cell lysates were subjecting to Western blotting using anti-CDK4, -CDK6, -CDK2, -p-CDK2, -p27kip1, -p21Waf1/Cip1, -p53, -p-Rb, and –α-tubulin antibodies. Data represent mean plus or minus SD of triplicate experiment (A,C).
The combination of perifosine and bortezomib enhances cytotoxicity of the monoclonal antibody rituximab
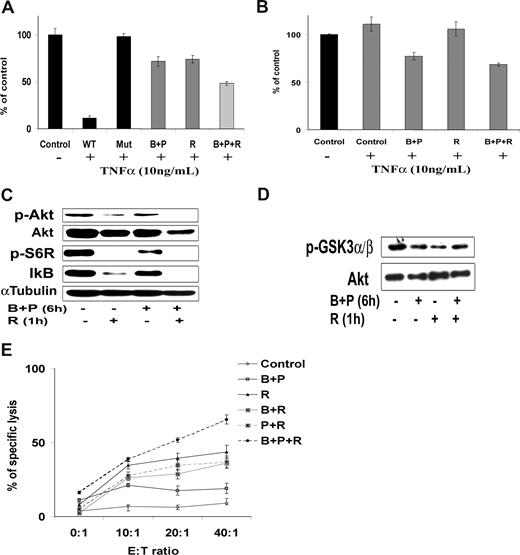
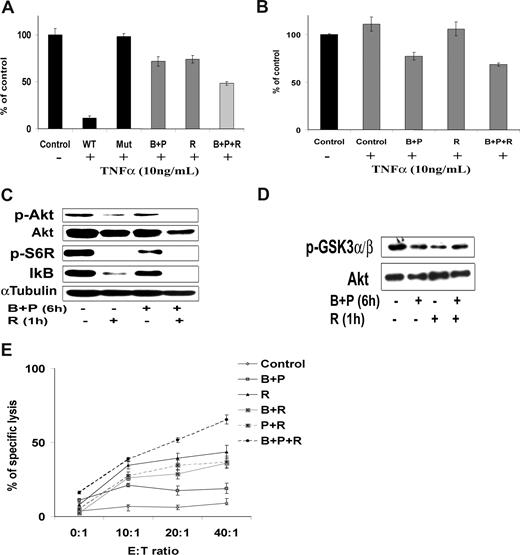
Although the combination of perifosine and bortezomib induced significant cytotoxicity, we sought to combine a third agent that could enhance tumor cell cytotoxicity. The monoclonal anti-CD20 antibody rituximab is one of the main therapeutic modalities used in WM. Rituximab is a known inhibitor of the PI3K/Akt and NF-κB pathways.45-47 We first showed that addition of rituximab (10 ng/mL) to bortezomib (10 nM) and perifosine (10 μM) increased their inhibitory effect on NF-κBp65 in WM cells using Active Motif assays (Figure 7A). This was correlated with a stronger inhibition of IκB gene expression compared with the combination of bortezomib and perifosine (Figure 7B). We next showed that the combination of bortezomib and perifosine, and rituximab induced inhibition of Akt phosphorylation and downstream activation of Akt pathway. Rituximab (10 μg/mL) and perifosine (10 μM) strongly decreased phosphorylation of Akt and downstream targets, such as S6 ribosomal and GSK3α/β using immunoblotting at 6 hours (Figure 7C). Similar results were observed after immunoprecipitation of Akt using an Akt kinase assay (Figure 7D). Bortezomib (10 nM) slightly increased Akt activity, but the combination of perifosine and bortezomib with rituximab decreased phosphorylation of Akt and downstream Akt activity. We next sought to determine the effect of the combination of perifosine and bortezomib with rituximab on WM cells. Because the main cytotoxic effect of rituximab in B cells is ADCC mediated (ADCC-mediated cytotoxicity),48 we examined the effect of either perifosine, bortezomib, or the combination with rituximab using ADCC. Rituximab (10 μg/mL) increased the percentage of specific lysis of BCWM.1 cells from 50% to 70% (Figure 7E). Pretreatment with perifosine and bortezomib overnight, before the addition of rituximab and effector cells, significantly enhanced specific lysis of BCWM.1 cells (P = .025; Figure 7E).
The combination of perifosine with bortezomib-induced cytotoxicity is enhanced in combination with the anti-CD20 monoclonal antibody, rituximab. (A,B) BCWM.1 cells were cultured with the combination of perifosine (10 μM) and bortezomib (10 nM) for 4 to 6 hours and with rituximab (10 μg/mL) during the last hour. (A) NF-κB activity assay using Active Motif. NF-κBp65 transcription factor-binding to its consensus sequence on the plate-bound oligonucleotide was studied from nuclear extracts. WT and Mut are wild-type and mutated consensus competitor oligonucleotides, respectively. (B) Relative quantitative PCR of IκB gene. (C) Whole cell lysates were subjected to Western blotting using anti–p-Akt, -Akt, –p-S6R, and –α-tubulin antibodies. (D) BCWM.1 cells were treated similarly to that in panel A, and then whole cell lysates were immunoprecipitated overnight with anti-Akt antibody. Then the immunoprecipitates were subjected to in vitro kinase assay according to the manufacturer's protocol. Western blotting used -Akt and fusion protein -p-GSK3α/β antibodies. (E) Antibody-dependent cell-mediated cytotoxicity assay (ADCC). BCWM.1 cells were pretreated with either perifosine (10 μM), bortezomib (10 nM), and the combination overnight then washed then treated with rituximab (10 μg/mL) for 4 hours in the presence of the effector cells. Results are reported in terms of mean percentage of specific lysis characterized by measurement of release of calcein-AM with different effector:target ratios (E/T ratio). Data represent mean plus or minus SD of triplicate experiments (A,B,E).
The combination of perifosine with bortezomib-induced cytotoxicity is enhanced in combination with the anti-CD20 monoclonal antibody, rituximab. (A,B) BCWM.1 cells were cultured with the combination of perifosine (10 μM) and bortezomib (10 nM) for 4 to 6 hours and with rituximab (10 μg/mL) during the last hour. (A) NF-κB activity assay using Active Motif. NF-κBp65 transcription factor-binding to its consensus sequence on the plate-bound oligonucleotide was studied from nuclear extracts. WT and Mut are wild-type and mutated consensus competitor oligonucleotides, respectively. (B) Relative quantitative PCR of IκB gene. (C) Whole cell lysates were subjected to Western blotting using anti–p-Akt, -Akt, –p-S6R, and –α-tubulin antibodies. (D) BCWM.1 cells were treated similarly to that in panel A, and then whole cell lysates were immunoprecipitated overnight with anti-Akt antibody. Then the immunoprecipitates were subjected to in vitro kinase assay according to the manufacturer's protocol. Western blotting used -Akt and fusion protein -p-GSK3α/β antibodies. (E) Antibody-dependent cell-mediated cytotoxicity assay (ADCC). BCWM.1 cells were pretreated with either perifosine (10 μM), bortezomib (10 nM), and the combination overnight then washed then treated with rituximab (10 μg/mL) for 4 hours in the presence of the effector cells. Results are reported in terms of mean percentage of specific lysis characterized by measurement of release of calcein-AM with different effector:target ratios (E/T ratio). Data represent mean plus or minus SD of triplicate experiments (A,B,E).
Discussion
WM is a distinct B-cell lymphoproliferative disease.3 Little is known about signaling pathways implicated in WM pathogenesis, and the majority of therapeutic agents currently used to treat WM have been applied because of their activity in related lymphoproliferative diseases, such as chronic lymphocytic leukemia and multiple myeloma. It is therefore important to determine targeted agents that demonstrate strong single-agent cytotoxicity on WM tumor cells and then find the best combination to improve their efficacy and overcome potential resistance, and limit their toxicity. Therefore, there is a strong rationale for combining novel therapies that target intrinsic molecular pathways mediating WM cells resistance.
We previously demonstrated that WM is characterized by high Akt expression in WM tumor cells and that Akt inhibitor perifosine showed significant in vitro and in vivo activity on WM tumor cells lines and patients' BM CD19+ tumor cells, and a phase 2 clinical trial of perifosine is demonstrating exciting activity.25,26 Furthermore, the monoclonal antibody rituximab is one of the major therapies used in WM but leads to only approximately 35% response rate in patients with WM.3 Similarly, bortezomib has significant activity in clinical trials in WM.27,28 The purpose of this study was to investigate the effect of perifosine, bortezomib, and their combination on the NF-κB pathway, and determine the cytotoxic effect of these agents on WM cells as well as their activity in combination with rituximab.
We first showed that the baseline expression of phospho-NF-κBp65 was significantly higher in WM samples compared with CD19+ healthy controls. We then showed that bortezomib, perifosine, and their combination inhibit p65NF-κB nuclear translocation and transcriptional function by immunoblotting, ChIP analysis, and Active Motif assay. We also demonstrated that these 2 agents are synergistic in inducing cytotoxicity and growth inhibition in WM cells, and this effect was higher than that observed with any other combination of perifosine with other traditional chemotherapeutic agents. Moreover, the combination of bortezomib and perifosine was able to overcome resistance induced by coculture with mesenchymal cells as well as the addition of stimulatory cytokines, including IL-6 and CD40L. In addition, we showed that perifosine and bortezomib induced cytotoxicity in primary patient samples, but not in normal mononuclear cells or BM colonies, suggesting a favorable therapeutic index. We found that these drugs induce apoptosis in WM cells that their combination leads to extended cell death through both the intrinsic and extrinsic apoptotic pathways, resulting in caspase -8, -9, then -3 and PARP cleavage. Moreover, bortezomib, but not perifosine, induced G1 arrest in WM cells and inhibited proteins regulating cell-cycle analysis, including CDK2, CDK4, CDK6, p21waf1/cip1, and p27kip1.
The mechanism of synergistic cytotoxic effect by perifosine and bortezomib is most probably multifactorial and not uniquely linked to their inhibitory effect on NF-κB transcriptional activity, which is only slightly improved by their combination. We show here that combined inhibition of the PI3K/Akt and ERK MAPK pathways by the 2 drugs represents an important mechanism that can explain their synergistic cytotoxic activity. Indeed, these pathways are crucial survival signaling pathways in WM cells, and specific inhibition of these kinase cascades is sufficient to induce cell death in a synergistic manner. This probably involves modulation of the activity of other transcriptional factors downstream of these pathways in addition to NF-κB.
The importance of NF-κB pathway in growth and survival of tumor cells has been extensively demonstrated in MM.10 Although the role of NF-κB has not been shown in WM tumor cell pathogenesis, proteomic studies indicate that this pathway is active in WM and may determine the clinical activity of bortezomib in WM compared with other types of lymphomas that are not sensitive to bortezomib.4 The regulation of signaling pathways in malignant cells is complex, and therefore rationally designed combinations of novel agent are needed. Although perifosine and bortezomib appeared to have a clear synergistic activity on WM tumor cells in combination, we also found that their cytotoxic effect was reinforced by addition of a third agent, the monoclonal antibody rituximab that not only inhibits PI3K/Akt and NF-κB pathways but can also induce cytotoxicity through recruitment of NK effectors cells.
In conclusion, we demonstrated that the combination of perifosine and bortezomib and the differential effect of these 2 agents on the PI3K/Akt and ERK MAPK pathways led to a significant synergistic cytotoxic effect of these 2 agents in WM cells. This effect can further be improved by addition of rituximab. We confirmed that the activity of these drugs relies in part on inhibition of NF-κB, defined as an important therapeutic target in WM. Altogether, this study provides the framework for clinical studies of perifosine, bortezomib, and rituximab combination in treatment of WM.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by the National Cancer Institute (R21 1R21CA126119-01A1), International Waldenstrom Macroglobulinemia Foundation, the Leukemia and Lymphoma Research Foundation, and the Lymphoma Research Foundation. X.L. is supported by a grant from the Franco-American Fulbright Foundation.
National Institutes of Health
Authorship
Contribution: X.L., S.P.T., D.R.C., T.H., M.B., T.E.W., K.C.A., and I.M.G. designed research, analyzed data, and wrote the paper; X.L., J.E., X.J., M.F., A.S., A.S.M., H.T.N., J.R., A.A., Z.H., A.M.R., E.H., F.A., K.S., M.R.M., and N.B. performed research.
Conflict-of-interest disclosure: I.M.G. and K.C.A. declare research grant support from Keryx Inc; they have also received research support from Millennium Pharmaceuticals and have served on that company's Speaker's Bureau. The remaining authors declare no competing financial interests.
Correspondence: Irene M. Ghobrial, Medical Oncology, Dana-Farber Cancer Institute, 44 Binney Street, Mayer 548A, Boston, MA 02115; e-mail: irene_ghobrial@dfci.harvard.edu.
References
Author notes
*X.L. and J.E. contributed equally to this study.

![Figure 5. Growth factors and coculture with BM microenvironment cells do not protect WM cells against the combined perifosine and bortezomib-induced cytotoxicity. (A) BCWM.1 cells were cultured with the combination perifosine (5 μM and 10 μM) and bortezomib (10 nM) for 48 hours, in the presence or absence of BMSCs. Cell proliferation was assessed using the [3H]-thymidine uptake assay. (B) BCWM.1 cells were cultured with either perifosine (10 μM), bortezomib (5 nM and 10 nM), or the combination in presence of IL-6 (50 ng/mL) or CD40L (3 μg/mL) for 48 hours. Cytotoxicity was assessed by the MTT assay. (C) PBMCs from 3 healthy donors with either perifosine (10 μM), bortezomib (5 nM and 10 nM), and the combination for 48 hours. Cytotoxicity was assessed by MTT assay. (D) Colony-forming cell assay. Nonadherent mononuclear cells were cultured using methylcellulose semisolid technique cultured with either single agents or the combination. The plates were read at days 14 to 16. BFU-E indicates burst-forming units–erythroid; CFU-GM, colony-forming units–granulocyte/macrophage; CFU-M, colony-forming units–macrophage; CFU-GEMM, colony-forming units–granulocyte/erythroid/macrophage/megakaryocyte. Data represent mean plus or minus SD of triplicate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/10/10.1182_blood-2007-09-115170/6/m_zh80100819530005.jpeg?Expires=1770435964&Signature=OYCRxKEpRdQlTboxgasW2i64QpVdOyj5VCvjIvcrn8FmAGIZ31WXoQ24Ui1JlrFxpXDdpa-sAjZmMciwfJUmG10NI5hp9ayWp2RW0ltgM3JbEWPeke-A~5Zs62T0vDZ2xCqkdPTsAqrNrJ-zpTStK66l-d-4JszUNiO-ox7Y8PsmKIXS9hv5b91OFoygivpAWjpMee7mGriZY65232FPx1PrWEhSp8HR5IIXWzWlb8Q~fkjvO02vg556Ejm3jhmxSoUcj2ONIFVUpO-Bhhp3q~GyKXlaEMHOWj8cLVmxLo1iJYMWoZDrWI706vaV9Xj~JLTMdu3TchQJILhe0nXK9g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 5. Growth factors and coculture with BM microenvironment cells do not protect WM cells against the combined perifosine and bortezomib-induced cytotoxicity. (A) BCWM.1 cells were cultured with the combination perifosine (5 μM and 10 μM) and bortezomib (10 nM) for 48 hours, in the presence or absence of BMSCs. Cell proliferation was assessed using the [3H]-thymidine uptake assay. (B) BCWM.1 cells were cultured with either perifosine (10 μM), bortezomib (5 nM and 10 nM), or the combination in presence of IL-6 (50 ng/mL) or CD40L (3 μg/mL) for 48 hours. Cytotoxicity was assessed by the MTT assay. (C) PBMCs from 3 healthy donors with either perifosine (10 μM), bortezomib (5 nM and 10 nM), and the combination for 48 hours. Cytotoxicity was assessed by MTT assay. (D) Colony-forming cell assay. Nonadherent mononuclear cells were cultured using methylcellulose semisolid technique cultured with either single agents or the combination. The plates were read at days 14 to 16. BFU-E indicates burst-forming units–erythroid; CFU-GM, colony-forming units–granulocyte/macrophage; CFU-M, colony-forming units–macrophage; CFU-GEMM, colony-forming units–granulocyte/erythroid/macrophage/megakaryocyte. Data represent mean plus or minus SD of triplicate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/10/10.1182_blood-2007-09-115170/6/m_zh80100819530005.jpeg?Expires=1771212371&Signature=G3-4AlYHxk~VR4H3irx3iXCTd8JMhRi~YVKOngNE~uK-Wpii22m6kFSnAJ~Lw23qtpCAM1~yDKkSd6U4LNL5p4AdLtF1vhK9NBb-f9AzMlrlM5jijgAP6cugk-arOXyJXDzmm0wOejSOXf7-qR4kscfaDO1MfzoWysmbL61Ic3N7ZG79HV8-2CB0Kc1~i--GbZ7Bp4UYtkAmbpnpy3z0iaXSSTlmnXMoJAcQL9N8vf6~8SyTlVhzeYtkiAZlC4HwsCO8Ao9OIXm~OFCfMkspCBEbuYHvQIylceBZSsg2SUlwpYUA0mTgBfukDXP5CY5g79EEhqsF2xvBLssk6zQOiw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)