Abstract
The anti-cytomegalovirus (CMV) activity and safety of oral maribavir in CMV-seropositive allogeneic stem-cell transplant recipients were evaluated in a randomized, double-blind, placebo-controlled, dose-ranging study. After engraftment, 111 patients were randomized to receive CMV prophylaxis with maribavir (100 mg twice daily, 400 mg once daily, or 400 mg twice daily) or placebo. Within the first 100 days after transplantation, the incidence of CMV infection based on CMV pp65 antigenemia was lower in each of the respective maribavir groups (15%, P = .046; 19%, P = .116; 15%, P = .053) compared with placebo (39%). Similarly, the incidence of CMV infection based on plasma CMV DNA was lower in each of the respective maribavir groups (7%, P = .001; 11%, P = .007; 19%, P = .038) compared with placebo (46%). Anti-CMV therapy was also used less often in patients receiving each respective dose of maribavir (15%, P = .001; 30%, P = .051; 15%, P = .002) compared with placebo (57%). There were 3 cases of CMV disease in placebo patients but none in the maribavir patients. Adverse events, mostly taste disturbance, nausea, and vomiting, were more frequent with maribavir. Maribavir had no adverse effect on neutrophil or platelet counts. These results show that maribavir can reduce the incidence of CMV infection and, unlike ganciclovir, does not cause myelosuppression. This trial is registered at www.ClinicalTrials.gov as #NCT00223925.
Introduction
Before the availability of effective prophylaxis, cytomegalovirus (CMV) disease was a common cause of morbidity and mortality after allogeneic stem cell transplantation.1 Currently, CMV disease can be prevented in most allogeneic stem cell transplant recipients by ganciclovir. Preventive strategies using ganciclovir include (1) the initiation of preemptive therapy only in patients who become positive for CMV antigen or CMV DNA in the blood after transplantation or (2) universal prophylaxis initiated in all at-risk patients at the time of engraftment and continued until day 100 after transplantation.2-5 Although both of these strategies are effective in preventing CMV disease, they are limited by the frequent neutropenia caused by ganciclovir. In addition, because of the low bioavailability of oral ganciclovir capsules, ganciclovir is frequently administered intravenously through a central venous catheter, which can be inconvenient, costly, and associated with line-related infections.6 Valganciclovir, the oral prodrug of ganciclovir, has much greater bioavailability than the oral ganciclovir capsules but also causes neutropenia and is not approved for use in stem cell transplant recipients.7,8 Second-line prophylactic agents, such as foscarnet and cidofovir, are limited by renal toxicity and other adverse events.9,10 Finally, even in the preemptive therapy era, CMV-seropositive patients who receive an unrelated donor or T cell–depleted graft continue to have a higher mortality rate compared with seronegative recipients with a seronegative donor.11 Thus, there is clearly a need for a more effective and safer antiviral agent that can be given prophylactically to stem cell transplant recipients
Maribavir is an antiviral drug that inhibits the UL97 viral protein kinase of human CMV and causes inhibition of viral encapsidation and nuclear egress of viral particles from infected cells.12,13 In vitro, maribavir is more potent than ganciclovir against CMV, including some CMV strains resistant to ganciclovir. Maribavir has good oral bioavailability in animals and humans. In phase 1 studies among HIV-infected patients, oral maribavir decreased CMV levels in semen.14 Except for a reversible taste disturbance and skin rash, maribavir was well tolerated and caused no obvious myelosuppression.14 Based on these favorable in vitro and preliminary clinical data, we conducted a phase 2 dose-ranging study to evaluate the safety, tolerability, and anti-CMV activity of oral maribavir in CMV-seropositive allogeneic stem cell transplant recipients.
Methods
Patients
Adult patients (≥ 18 years of age) who were seropositive for CMV immunoglobulin G antibody before transplantation and had received a first allogeneic stem cell transplant were eligible for the study. At the time the study drug was initiated, patients had to have evidence of post-transplantation engraftment (absolute neutrophil count ≥ 0.5 × 109/L [500/mm3] for at least 3 consecutive days), no detectable CMV infection (both a negative CMV pp65 antigenemia assay and a negative plasma CMV DNA polymerase chain reaction [PCR] assay on blood collected within 5 days before starting study drug), no previous posttransplantation anti-CMV therapy, and the ability to swallow tablets. Patients were excluded from the study if they had HIV infection, renal insufficiency (serum creatinine > 221 μmol/L [2.5 mg/dL]), hepatic dysfunction (serum alanine or aspartate aminotransferase levels of > 5 times the upper limit of the normal range or a serum direct bilirubin of > 17.1 μmol/L [1 mg/dL]), or severe vomiting, diarrhea, or other gastrointestinal illness precluding the administration of oral medications. Patients with graft-versus-host disease (GVHD) of the gastrointestinal tract were allowed to participate in the study if they could take oral medications. The study was approved by the Institutional Review Board at each transplantation center, and informed consent was obtained from each patient in accordance with the Declaration of Helsinki before enrollment into the study.
Study design
This was a multicenter, randomized, double-blind, placebo-controlled, dose-ranging study performed at 13 transplantation centers in the United States (ClinicalTrials.gov #NCT00223925). After posttransplantation engraftment, eligible patients were randomized to receive either maribavir or placebo in a 3:1 allocation ratio. Randomization was stratified by type of transplant (myeloablative or nonmyeloablative). Three different dosing regimens of oral maribavir were evaluated sequentially: 100 mg twice daily, 400 mg once daily, and 400 mg twice daily, respectively. Although a wide range of doses (300-2400 mg/day) had been evaluated in previous phase 1 studies, relatively low doses were selected for this study with the goal of identifying a regimen that would provide good tolerability yet retain potent anti-CMV activity for prophylaxis. For each dosing regimen, 36 patients were to be enrolled into the study (27 receiving maribavir, 9 receiving placebo). Thus, a total of 108 patients were to be evaluated (81 receiving maribavir, 27 receiving placebo). After enrollment into the first maribavir dose group (100 mg twice daily) was completed, an independent safety committee reviewed safety data before proceeding to the next higher dose of maribavir (400 mg once daily). A similar review was conducted before studying the highest dose of maribavir (400 mg twice daily).
Treatment
Study drug was started after engraftment between 14 and 30 days after transplantation and continued for a maximum of 12 weeks. Outpatients used a diary to record compliance with the dosing regimen. During administration of the study drug, concomitant use of other anti-CMV prophylaxis or therapy was prohibited. Because maribavir is not active against herpes simplex virus (HSV) or varicella-zoster virus (VZV), the use of acyclovir, valacyclovir, or famciclovir at low doses was allowed for prophylaxis of these infections. These antivirals could also be used for treatment of documented HSV or VZV infection at the discretion of the investigator.
While receiving study drug, patients had weekly surveillance testing for CMV infection. Tests for both CMV pp65 antigenemia and plasma CMV DNA were performed, as described in “Laboratory procedures.” If CMV infection was detected or a diagnosis of CMV disease was made, the study drug was discontinued. Patients were then treated with either ganciclovir or another antiviral, at the discretion of the investigator. Study drug was also stopped if patients withdrew from the study or died early after transplantation and could also be discontinued when an adverse event attributable to the study drug or relapse of underlying malignancy occurred. Patients who completed 12 weeks of study drug had follow-up assessments after stopping the study drug at 1, 4, and 8 weeks for CMV infection, CMV disease, or use of anti-CMV therapy.
Laboratory procedures
The CMV serologic status of patients and stem cell donors was determined by latex agglutination, or enzyme immunoassay. A central laboratory (Viromed Laboratories, Minnetonka, MN) performed weekly surveillance testing for both CMV pp65 antigenemia (CMV Brite Turbo Kit; Biotest Diagnostics, Denville, NJ) and plasma CMV DNA (COBAS Amplicor CMV Monitor Assay; Roche Diagnostics, Basel, Switzerland). In addition, the local laboratory at each study site could perform similar tests for CMV pp65 antigenemia and plasma CMV DNA as well as viral cultures at the discretion of the investigator for the diagnosis of CMV infection or disease. Results from either the central laboratory or a local laboratory could be used by the investigator to diagnose CMV infection or disease and, subsequently, discontinue study drug and initiate treatment. Before starting treatment for CMV infection or disease, a repeat blood sample for detection of CMV DNA by PCR was sent to the central laboratory, and viral cultures of the blood, urine, throat, and other available suspected sites of infection were performed by a local laboratory. Genotypic analyses were performed on CMV DNA extracts from selected frozen plasma samples from patients who had a positive CMV DNA PCR result either during or after dosing with maribavir to determine whether any genetic variants had arisen in the UL97 or UL27 CMV genes, including those previously associated with maribavir resistance.15,16
At 7 study sites, serial blood samples for determination of plasma maribavir concentrations were collected on day 7 and on day 28 (week 4) of the study period for evaluation of maribavir pharmacokinetics. On these 2 days, morning study drug was taken on an empty stomach, and blood was collected for plasma maribavir concentrations before dosing of the study drug and at 1, 2, 4, 6, and 8 hours after dosing of study drug. Plasma maribavir concentrations were determined by liquid chromatography tandem mass spectrometry.
Safety monitoring
Safety was assessed weekly by the recording of adverse events, changes in physical examination, and the results of electrocardiograms, standard hematologic and clinical chemistry tests, and urinalyses. An independent safety monitoring committee reviewed all available safety data in an unblinded fashion approximately every 4 weeks during the study. The diagnosis and grading of GVHD were performed by investigators using standard criteria.17
Statistical analysis
Determination of the sample size for the study was based on the anticipated incidence of CMV infection and safety considerations. Previous controlled clinical trials in CMV-seropositive allogeneic stem cell transplant recipients suggested that the incidence of CMV infection determined by CMV pp65 antigenemia is approximately 60% in patients receiving placebo and 20% in patients receiving prophylactic ganciclovir.2,18 Therefore, assuming a 60% incidence of CMV infection in a placebo group, detection of a reduction in the incidence of CMV infection to 18% in patients receiving prophylactic maribavir requires 25 patients per treatment group based on a significance level of .05 with 80% power. If a 10% dropout rate per treatment group is assumed, 27 patients need to be enrolled into each study group. Thus, enrollment of 108 patients (4 groups of 27 patients each) was planned. Assuming an incidence of maribavir-related adverse events of 5%, 10%, 15%, or 20% in this study population, the probability of observing an event in at least 1 of the 27 patients receiving maribavir in each dosing group is .750, .942, .988, or .998, respectively. All patients receiving placebo were pooled into one group for efficacy and safety analyses.
The primary objective of the study was to evaluate the safety and tolerability of maribavir administered for up to 12 weeks in this population. Safety data were analyzed only for patients who received at least 1 dose of study drug. Safety endpoints included all adverse events, adverse events related to study drug, mortality, and changes in laboratory evaluations. Adverse events were defined as events that started or worsened during administration of study drug or within 7 days after the last dose of study drug. Adverse events related to study drug were defined as events considered by the investigator to be possibly, probably, or definitely related to study drug. Causes of death were also assessed by each investigator.
The primary efficacy end point was the incidence and time of onset of CMV infection or disease. CMV infection was defined as a positive pp65 antigenemia assay (≥ 1 positive cell per 100 000 leukocytes) or positive plasma CMV DNA by PCR (≥ 1000 DNA copies/mL). CMV disease was defined according to published criteria.19 Secondary efficacy endpoints included the incidence of CMV disease alone and the use of antiviral therapy for treatment of CMV infection. All randomized patients were included in the analyses of antiviral activity (intent-to-treat population). Analyses of the incidence and time to onset of CMV infection or disease were stratified according to transplant type (myeloablative or nonmyeloablative). The Cochran-Mantel-Haenszel test was used to compare the incidences of CMV infection or disease and the incidences of anti-CMV therapy across groups. Kaplan-Meier estimates of median time to onset of CMV infection or disease were determined for all study groups and compared using the stratified Log-rank test. All statistical tests were 2-sided with a significance level of alpha equal .05.
Results
Patient characteristics
A total of 111 patients were enrolled in the study and randomized to receive study drug. Twenty-eight patients received placebo, 28 received maribavir 100 mg twice daily, 28 received maribavir 400 mg once daily, and 26 received maribavir 400 mg twice daily. One patient assigned to maribavir 400 mg twice daily never received the drug. The 4 study groups were similar in terms of age, underlying disease, donor type, stem cell source, conditioning regimen, and time of initiation of study drug after transplantation (Table 1). There were more men than women in each study group, except for the maribavir 400 mg twice-daily group, which had 10 men and 17 women. Most stem cell donors in each study group were CMV-seropositive, except in the placebo group, which had more CMV-seronegative donors (61%) than CMV-seropositive donors (39%). The median number of days that patients received the study drug was 34 in the placebo group, 57 in the group given maribavir 100 mg twice daily, 57 in the group given maribavir 400 mg once daily, and 29 in the group given maribavir 400 mg twice daily. The most common reasons for premature discontinuation of the study drug were the development of CMV infection or disease, which was required by the protocol before initiation of preemptive therapy, and adverse events. The incidences of these events are described in the following sections.
Plasma maribavir concentrations
Figure 1 shows the mean plasma concentrations of maribavir on days 7 and 28 (week 4) of the study period. For each dosing regimen, the mean plasma concentrations of maribavir were similar on day 7 and during week 4 of the study period. Peak mean plasma concentrations of maribavir were more than 2.5 times higher following the 400-mg doses of maribavir compared with the 100-mg doses of maribavir. The greatest amount of drug exposure over 24 hours occurred in patients taking 400 mg of maribavir twice daily. There were no significant differences in the plasma maribavir concentrations between patients who developed CMV infection and patients who did not have CMV infection (data not shown).
Mean plasma concentration-time profiles of maribavir. Blood samples were taken up to 8 hours after dose. Symbols represent maribavir dose groups: 100 mg twice daily (●), 400 mg once daily (■), and 400 mg twice daily (▴). (A) Plasma concentration-time profile on day 7. The 100-mg twice-daily, 400-mg once-daily, and 400-mg twice-daily groups comprised 17, 11, and 10 patients, respectively. (B) Plasma concentration-time profile at week 4. The 100-mg twice-daily, 400-mg once-daily, and 400-mg twice-daily groups comprised 12, 9, and 3 patients, respectively.
Mean plasma concentration-time profiles of maribavir. Blood samples were taken up to 8 hours after dose. Symbols represent maribavir dose groups: 100 mg twice daily (●), 400 mg once daily (■), and 400 mg twice daily (▴). (A) Plasma concentration-time profile on day 7. The 100-mg twice-daily, 400-mg once-daily, and 400-mg twice-daily groups comprised 17, 11, and 10 patients, respectively. (B) Plasma concentration-time profile at week 4. The 100-mg twice-daily, 400-mg once-daily, and 400-mg twice-daily groups comprised 12, 9, and 3 patients, respectively.
CMV infection and disease
Table 2 compares the incidence of CMV infection or disease within 100 days after transplantation in the placebo group and the 3 maribavir groups. Analyses of CMV infection incorporate all available data, including results from both the central laboratory and any local laboratory testing. For each dosing regimen of maribavir, the incidence of CMV infection or disease was lower compared with the incidence in the placebo group. Furthermore, there were no notable differences in the incidence of CMV infection among the 3 dosing regimens of maribavir. CMV infection based on the development of CMV pp65 antigenemia occurred in 39% of the placebo patients compared with 15% to 19% of patients in each of the 3 maribavir groups. Similarly, CMV infection based on a plasma PCR positive for CMV DNA occurred in 46% of the placebo patients compared with 7% to 19% of patients in each of the 3 maribavir groups. Treatment for CMV infection or disease, reflecting all clinical and virologic data available to investigators, was used less often with all 3 dosing regimens of maribavir. Fifty-seven percent of the placebo patients received anti-CMV therapy compared with 15% to 30% of patients in each of the 3 maribavir groups.
There were 3 cases of CMV disease during the study, all of which occurred in the placebo group. There were no cases of CMV disease in patients receiving prophylactic maribavir. The 3 cases of CMV disease included 2 cases of CMV gastroenteritis on days 42 and 80 after transplantation, respectively (1 transplant from a CMV seronegative donor and 1 from a CMV-seropositive donor), and 1 case of CMV pneumonia on day 45 after transplantation (CMV-seronegative donor). No additional cases of late CMV disease (occurring after day 100 posttransplantation) were reported in any patient during the protocol-defined follow-up period (through a maximum of 8 weeks after the completion of 12 weeks of study drug therapy).
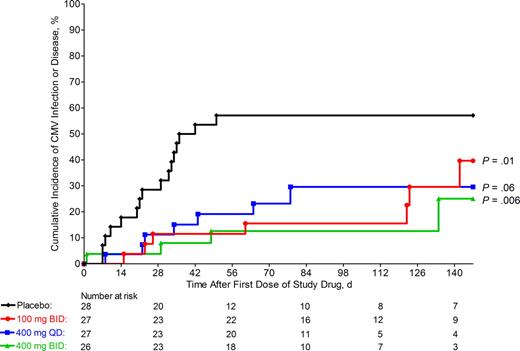
Figure 2 provides the Kaplan-Meier estimates of time to onset of CMV infection or disease in the placebo group and the 3 maribavir groups during the entire study. Compared with placebo, each of the 3 dosing regimens of maribavir was associated with a significant reduction and delay in the occurrence of CMV infection or disease. Furthermore, all 3 dosing regimens of maribavir appeared to be equally effective in reducing the incidence of CMV infection and delaying the time to onset of CMV infection. Among all maribavir groups, approximately half of all CMV infections occurred while the patient was receiving maribavir (pp65 antigenemia in 7 patients and CMV DNA by PCR in 5 patients). The remaining cases of CMV infection in these groups occurred after discontinuation of study drug therapy.
Incidence of CMV infection or disease. Kaplan-Meier curves of the cumulative incidence of CMV infection (as detected by either a positive pp65 antigenemia or positive plasma CMV DNA PCR assay, from either the central laboratory or local laboratory testing) or CMV disease in placebo and maribavir groups. P values vs placebo determined by Cox proportional hazards regression model. Symbols represent treatment groups: placebo (♦), 100 mg twice daily (●), 400 mg once daily (■), and 400 mg twice daily (▴).
Incidence of CMV infection or disease. Kaplan-Meier curves of the cumulative incidence of CMV infection (as detected by either a positive pp65 antigenemia or positive plasma CMV DNA PCR assay, from either the central laboratory or local laboratory testing) or CMV disease in placebo and maribavir groups. P values vs placebo determined by Cox proportional hazards regression model. Symbols represent treatment groups: placebo (♦), 100 mg twice daily (●), 400 mg once daily (■), and 400 mg twice daily (▴).
The incidence of CMV infection or disease was analyzed within patient subsets based on the randomization stratification variable of transplant type: myeloablative (70% of all enrolled patients) or nonmyeloablative (30% of all enrolled patients). For both subsets, the incidence of CMV infection or disease was numerically lower in each maribavir group compared with placebo (data not shown). Because of the smaller sample sizes in these subsets, statistical comparisons were not performed.
The incidence of CMV infection or disease was also analyzed within patient subsets based on the donor CMV serostatus (donors were CMV seropositive in 39% of the placebo group and 52%-61% of the maribavir groups). For both patients with CMV-seropositive donors and patients with CMV-seronegative donors, the incidence of CMV infection or disease was numerically lower in each maribavir group compared with placebo (data not shown). Furthermore, within the placebo group, the incidence of CMV infection was numerically higher in patients with CMV seropositive donors. Because of the smaller sample sizes in these subsets, statistical comparisons were not performed. However, these data suggest that the overall results were not influenced by any protective effect of CMV-seropositive donors.
Plasma samples for CMV genotypic analyses were available from 5 patients who received maribavir and subsequently had detectable CMV DNA by PCR (1 patient at 100 mg twice daily, 1 patient at 400 mg once daily, and 3 patients at 400 mg twice daily). No mutations known to be associated with resistance to maribavir were found in the UL97 protein kinase or UL27 polymerase genes. A virus culture positive for a CMV isolate was available from only 3 patients. Each of these patients received placebo, so further testing for susceptibility to antiviral drugs was not performed.
Other herpes virus infections
Prophylaxis for herpesvirus infections other than CMV followed standard practices at each study site. More than 80% of the study patients received prophylactic acyclovir or valacyclovir, with similar rates in each of the study groups. During administration of study drug, localized HSV infection occurred in 2 placebo patients, 1 patient receiving maribavir 100 mg twice daily, 3 patients receiving maribavir 400 mg once daily, and 1 patient receiving maribavir 400 mg twice daily. Similarly, localized VZV infection developed in 2 placebo patients, 1 patient receiving maribavir 100 mg twice daily, and 1 patient receiving maribavir 400 mg once daily.
Adverse events
Adverse events attributed by investigators to the study drug are summarized in Table 3. One patient randomized to maribavir (400 mg twice daily) never took the study drug and was excluded from the analysis. Adverse events related to study drug occurred more frequently with each of the 3 dosing regimens of maribavir and were most frequent with the highest dose of maribavir. Fifty-four percent of patients receiving 400 mg of maribavir twice daily had such adverse events, and 35% of the patients in this dose group discontinued the study drug because of a drug-related adverse event. Taste disturbance, nausea, and vomiting were the adverse events most often associated with maribavir. Taste disturbance, characterized as a bitter, metallic, or funny taste, occurred in 21%, 18%, and 31% of patients in each of the 3 maribavir groups but in none of the placebo patients. This was the only event for which the observed incidence rates were statistically higher compared with placebo. The onset of taste disturbance appeared to be dose related, with median onset time after starting maribavir of 22 days, 6 days, and 3 days for the maribavir 100-mg twice-daily, 400-mg once-daily, and 400-mg twice-daily doses, respectively. However, both the time of onset and duration of taste disturbance varied greatly among patients in each dose group. Many cases occurred intermittently after dosing. Overall, the median duration of the taste disturbance was 8 days, 15 days, and 20 days, respectively, for the maribavir 100-mg twice-daily, 400-mg once-daily, and 400-mg twice-daily doses. No dose-related trends were observed for the time of onset or duration of other gastrointestinal adverse events. In analyses of all adverse events, regardless of the investigators' assigned relationship to study drug, no additional adverse events were found to be associated with maribavir (data not shown).
Taste disturbance, nausea, and vomiting were also the most common drug-related adverse events causing discontinuation of study drug. Discontinuation of study drug resulting from taste disturbance occurred in 6 (7%) maribavir patients (4 receiving 400 mg twice daily). Discontinuation of study drug resulting from related adverse events of nausea or vomiting occurred in 1 (4%) placebo patient and 6 (7%) maribavir patients (3 receiving 400 mg twice daily).
Only 2 patients (1 patient receiving placebo, 1 patient receiving 100 mg of maribavir twice daily) had study drug discontinued because of myelosuppression. Furthermore, the incidence of neutropenia during administration of the study drug was similar among placebo and maribavir patients (Table 4). However, during the entire 100-day posttransplantation period, the incidence of neutropenia was generally higher in placebo patients than in maribavir-treated patients, but the differences did not reach statistical significance. The use of hemato-poietic growth factors during treatment with study drug was similar among all study groups. There were no significant differences in platelet counts between placebo and any of the maribavir groups.
Acute GVHD of grade 2 or greater severity developed somewhat more commonly during the study in placebo patients (46%) compared with those in the maribavir 100-mg twice-daily, 400-mg once-daily, and 400-mg twice-daily groups (14%, 29%, and 23%, respectively).
Mortality
Table 5 shows the number of deaths during the study. Twenty-one percent of the placebo patients died, compared with 14%, 11%, and 12% of the patients in maribavir 100-mg twice-daily, 400-mg once-daily, and 400-mg twice-daily groups. Relapse of leukemia and GVHD were the most common causes of death. There were no deaths attributed to study drug.
Discussion
This study showed that prophylactic maribavir was safe and well tolerated and effectively reduced CMV infection after allogeneic stem cell transplantation. CMV antigenemia and DNAemia were both reduced significantly, resulting in less use of preemptive therapy with ganciclovir. Maribavir was associated with an increased incidence of taste disturbance, nausea, and vomiting, but laboratory adverse effects were not more common than in placebo recipients.
Maribavir was effective in reducing the incidence of CMV antigenemia and plasma DNAemia by approximately 70%. Although this study did not use prophylactic ganciclovir as a comparator, these results are similar to what was observed in a previous randomized trial of prophylactic ganciclovir, which used a similar study design.20 Of note, there were no cases of CMV disease in any of the maribavir groups. However, because of the small sample size, this did not reach a level of statistical significance. There were no reports of late CMV disease beyond 100 days posttransplantation in this study, but the protocol-defined duration of follow-up extended only to a maximum of 5 months posttransplantation for those completing 12 weeks of study drug therapy. An ongoing phase 3 study of similar design has been powered to determine whether prophylactic maribavir leads to significant reduction of CMV disease and includes a longer follow-up period for evaluation of late CMV disease.
There was no increase in neutropenia or thrombocytopenia or any other laboratory toxicity among maribavir recipients compared with placebo. Indeed, when patients were analyzed from randomization until the end of the 100-day posttransplantation period, the incidence of neutropenia was higher in placebo patients than in patients receiving maribavir. Although these differences were not statistically significant, it is possible that this more frequent neutropenia in placebo patients was related to the higher incidence of CMV infection and subsequent greater use of myelosuppressive ganciclovir preemptive therapy. Many potential factors can contribute to neutropenia, including doses of immunosuppressive medications and other drugs, and comorbidities. Data from the larger, ongoing phase 3 study are needed to confirm this trend.
Clinical adverse events that occurred at a higher frequency among maribavir recipients included taste disturbance, nausea, and vomiting. Taste disturbance occurred in approximately 25% of patients, which is less than previously observed in studies of healthy volunteers and HIV-infected patients, where taste disturbance was seen in up to 80% of study participants.21 Taste disturbance was mild and transient in most cases. Only 6 patients discontinued study drug because of taste disturbance. Nausea was observed in up to 15% of maribavir recipients and was somewhat more frequent with the higher doses (Table 3). Vomiting and diarrhea were observed, but their incidences among maribavir patients were not dose-related and were not much different from placebo patients. Unlike a previous study in HIV-infected patients where there was an increased incidence of skin rashes associated with maribavir,14 skin rashes did not occur more frequently with maribavir in this study. There may be additional, less common, adverse events associated with greater usage of maribavir that we were unable to detect in this study.
Within the first 100 days after transplantation, CMV antigenemia and plasma DNAemia occurred in up to 20% of maribavir recipients. Approximately half of these cases occurred while patients were still receiving maribavir. The other cases occurred after maribavir had been discontinued for other reasons. Genotypic analysis from 5 patients who received maribavir and then developed plasma CMV DNAemia did not reveal any mutations in the UL97 and UL27 genes in CMV DNA that are known to be associated with resistance to maribavir. All 5 patients were successfully treated with preemptive antiviral therapy, usually intravenous ganciclovir.
The lowest dose of maribavir (100 mg twice daily) evaluated in this study appeared to be as effective as the higher doses for prevention of CMV infection and was better tolerated than the 400 mg twice daily dose of maribavir. Although it is not known which pharmacokinetic parameter or plasma level of maribavir is most important in determining prophylactic efficacy for CMV infection, pharmacokinetic analysis showed that 100 mg of maribavir twice daily and 400 mg of maribavir once daily provided similar trough plasma concentrations of maribavir (Figure 1). Higher maximal concentration (CMAX) and area under the curve (AUC) values for maribavir were achieved with 400 mg of maribavir twice daily, but this greater drug exposure was not associated with improved prophylactic antiviral activity and caused more frequent adverse events. In view of the importance of establishing a prophylactic regimen that is both effective and well tolerated, these results suggest that future studies of maribavir prophylaxis for prevention of CMV infection should consider using doses of 100 mg twice daily or 400 mg once daily.
These results provide the opportunity for reevaluating a prophylactic anti-CMV strategy in stem cell transplant recipients. Over the past decade, antigen- or PCR-based preemptive therapy with ganciclovir or foscarnet has replaced prophylactic strategies at many transplant centers. This shift was mainly driven by the toxicity profile of the available drugs (ie, ganciclovir and foscarnet). Although preemptive therapy is effective in preventing CMV disease,2,3 it is occasionally ineffective in cases of CMV disease with undetectable antigenemia or DNAemia before the onset of disease and does not protect against the indirect immunosuppressive effects of CMV.11 Despite the use of preemptive therapy, some epidemiologic studies have shown that high-risk CMV-seropositive recipients may have a survival disadvantage after stem cell transplantation compared with CMV-seronegative recipients with a CMV-seronegative donor.11 A prophylactic strategy using an effective and less toxic drug may overcome this disadvantage.
Maribavir was well tolerated, safe, and effective in preventing CMV infection after allogeneic stem cell transplantation, and it reduced the need for preemptive therapy with ganciclovir. The effectiveness of maribavir for prevention of CMV disease is being evaluated further in phase 3 studies.
An Inside Blood analysis of this article appears at the front of this issue.
Presented in abstract form at the 2006 American Society of Hematology annual meeting, December 9-12, 2006, Orlando, FL.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Ingrid Nielsen for coordinating the study at ViroPharma Inc, and the following study coordinators at each center: Kathy Bartoni and Bruck Habtemariam (UCLA Medical Center), Lynn Hallett (University of Minnesota Medical Center), Kathy Patane (City of Hope National Medical Center), Sharika Pierre-Louis (Memorial Sloan-Kettering Cancer Center), Samantha Barrios (Washington University School of Medicine), Sandy Li (Baylor University Medical Center), Mary Steigelman (Wayne State University & Karmanos Cancer Center), Joyce Brown (University of Texas M. D. Anderson Cancer Center), Carol Nielson (University of Utah Medical Center), Peggy Eren (Duke University Medical Center), Cecilia Petrowsky (Loyola University Medical Center), Carrie Grodman (Tufts-New England Medical Center), and Janice Huggler (Fred Hutchinson Cancer Research Center).
The safety monitoring committee consisted of the following members: Dr David L. Porter, University of Pennsylvania Medical Center, Philadelphia, PA; Dr Nina Singh, VA Medical Center, Pittsburgh, PA; Dr Thomas R. Spitzer, Massachusetts General Hospital, Boston, MA; and Audrey Evans, Statistician, Omnicare Clinical Research, King of Prussia, PA.
This work was supported by research funding from ViroPharma Inc, Exton, PA.
Authorship
Contribution: S.A.V. was responsible for study concept and design; D.J.W., J.-A.H.Y., V.P., G.A.P., R.V., E.V., G.J.A., R.F.C., F.P., N.C., J.K., K.S., and M.B. performed research; D.J.W., S.A.V., and M.B. analyzed data and drafted the manuscript.
Conflict-of-interest disclosure: S.A.V. is an employee of ViroPharma Incorporated. All other authors received research funding from ViroPharma Incorporated in association with this clinical trial. D.J.W., G.A.P., R.V., R.F.C., F.P., and M.B. have served as paid consultants for ViroPharma Incorporated. V.P. has received speaker fees from Novartis. G.J.A. has received research funding from Elan Pharmaceuticals and Ortho-McNeil and has received speaker fees from Pfizer Pharmaceuticals and Wyeth Pharmaceuticals. R.F.C. has received research funding from Roche. K.S. has served as a paid consultant for Genzyme. F.P. has served as a paid consultant for Pfizer and has received speaker fees from Pfizer, MGIpharma, Merck, and Schering-Plough. M.B. is a consultant and/or has received speaker fees or research funding from Roche Laboratories, AiCuris AG, and Vical Inc.
Correspondence: Dr Drew J. Winston, Department of Medicine, Room 42-121 CHS, UCLA Medical Center, Los Angeles, CA 90095; e-mail: dwinston@mednet.ucla.edu.