Abstract
This trial determined the safety and efficacy of the combination regimen clarithromycin (Biaxin), lenalidomide (Revlimid), and dexamethasone (BiRD) as first-line therapy for multiple myeloma. Patients received BiRD in 28-day cycles. Dexamethasone (40 mg) was given orally once weekly, clarithromycin (500 mg) was given orally twice daily, and lenalidomide (25 mg) was given orally daily on days 1 to 21. Objective response was defined by standard criteria (ie, decrease in serum monoclonal protein [M-protein] by at least 50%, and a decrease in urine M-protein by at least 90%). Of the 72 patients enrolled, 65 had an objective response (90.3%). A combined stringent and conventional complete response rate of 38.9% was achieved, and 73.6% of the patients achieved at least a 90% decrease in M-protein levels. This regimen did not interfere with hematopoietic stem-cell harvest. Fifty-two patients who did not go on to receive transplants received continued therapy (complete response, 37%; very good partial response, 33%). The major adverse events were thromboembolic events, corticosteroid-related morbidity, and cytopenias. BiRD is an effective regimen with manageable side effects in the treatment of symptomatic, newly diagnosed multiple myeloma. This trial was registered at www.clinicaltrials.gov as #NCT00151203.
Introduction
Multiple myeloma (MM) is a fatal neoplasm characterized by the uncontrolled proliferation of monoclonal plasma cells. Historically, the standard chemotherapy regimen for patients with advanced MM was the combination of oral melphalan and prednisone.1,2 Recently, the addition of thalidomide to this regimen has improved response rates and overall survival, especially in older patients.3,4 Initial therapy for younger patients has evolved into a 2-phase approach that consists of induction therapy designed to produce meaningful responses and to reduce myeloma-related comorbidities, followed by consolidation therapy with high-dose melphalan and subsequent autologous stem-cell rescue. At the outset of the 2-phase model, transplantation candidates would receive dexamethasone-based regimens as induction therapy, most notably thalidomide and dexamethasone (Thal/Dex).5-9 Thal/Dex yields superior responses compared with dexamethasone monotherapy, or vincristine/Adriamycin/dexamethasone combination.10 In fact, Thal/Dex produces response rates of between 63% and 76%.7,10-13 The most serious adverse events in the Thal/Dex studies are peri-pheral neuropathy, thromboembolic events (TEs; such as deep-vein thrombosis, pulmonary embolism, or myocardial infarction), somnolence, constipation, and hypersensitivity. Despite these adverse events, Thal/Dex has become the standard induction therapy because of its high objective response rate (ORR) and compatibility with hematopoietic stem-cell harvest and autologous transplantation.13
Previously, our group reported that the BLT-D combination (clarithromycin [Biaxin], low-dose thalidomide, and dexamethasone) yielded an ORR of 93% and a complete response (CR) rate of 13%.14,15 Responses were even observed in patients who had previously been resistant to Thal/Dex. Clarithromycin appears to optimize the pharmacologic effect of glucocorticoids by increasing the area under the curve and the maximum concentration levels of certain corticosteroids.16-20 Clarithromycin has immunomodulatory properties, partially mediated by the suppression of interleukin-6 and other inflammatory cytokines.21 There may also be a direct antineoplastic effect mediated by clarithromycin.21,22
Lenalidomide (Revlimid; Celgene Corporation, Summit, NJ), an immunomodulatory drug effective in treating relapsed or refractory MM, is more potent and better tolerated than thalidomide.23-25 In a phase 2 trial of lenalidomide/dexamethasone (Rev/Dex) for initial therapy, 31 (91%) of 34 patients achieved an objective response (decrease in M-protein by ≥ 50%).26 However, 47% of patients experienced grade 3 or higher nonhematologic toxicities, mainly attributable to dexamethasone. As an extension of the BLT-D studies, it was hypothesized that the addition of clarithromycin to Rev/Dex might enhance efficacy and permit reduction of the corticosteroid dose.
The goal of this phase 2 clinical trial was to assess the response rate and toxicity of a combination regimen of clarithromycin (Biaxin), lenalidomide (Revlimid), and dexamethasone (BiRD) for the treatment of newly diagnosed, symptomatic MM.
Methods
Eligibility
After informed consent was obtained, patients were eligible to participate if they had symptomatic MM of Durie-Salmon stage II or III. Measurable disease was defined as M-protein of at least 10 g/L, or serum-free light chains of at least 1 g/L, or urinary M-protein excretion of at least 0.2 g/24 hours, and/or measurable plasmacytomas. Other eligibility criteria included a Karnofsky performance status of at least 70%, no prior treatment, or less than one cycle of first-line therapy, and a negative pregnancy test within 7 days before commencing treatment in women of childbearing potential.
Laboratory markers required for patient eligibility included an absolute neutrophil count of at least 109/L (1000 cells/mm3), a platelet count of at least 75.0 × 109/L (75 000/mm3), serum glutamic oxaloacetic transaminase/aspartate aminotransferase of less than 3.0× the upper limit of normal (ULN), serum glutamic pyruvic transaminase/alanine aminotransferase of less than 3.0× the ULN, serum creatinine of less than 221 μmol/L (2.5 mg/dL), and serum total bilirubin of less than 34.2 μmol/L (2.0 mg/dL).
Patients with nonsecretory MM were excluded, as were patients with a history of TEs or other conditions currently requiring anticoagulation with warfarin. Also excluded were pregnant or lactating women; patients known to be HIV-positive; those with active infections; and patients with a known hypersensitivity to clarithromycin, lenalidomide, dexamethasone, or thalidomide. The study was approved by the Institutional Review Board of the Weill Medical College of Cornell University, New York Presbyterian Hospital–Cornell Medical Center, in accordance with federal regulations and the Declaration of Helsinki.
Treatment schedule
All patients received clarithromycin, lenalidomide, and dexamethasone in 28-day cycles. Dexamethasone (40 mg) was given orally on days 1, 2, 3, 8, 15, and 22 during cycle 1 and weekly on days 1, 8, 15, and 22 of each subsequent cycle. Clarithromycin (500 mg) was given orally twice daily, beginning on day 2 of cycle 1. Lenalidomide (25 mg) was given orally daily on days 3 to 21 of cycle 1 and on days 1 to 21 of subsequent cycles. Dose adjustments were permitted according to the toxicity criteria described in Table S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Prophylactic treatments included aspirin 81 mg once daily, omeprazole 20 mg once daily, and one double-strength tablet of trimethoprim/sulfamethoxazole twice daily, 3 times a week.
Cytogenetics
Overnight, unstimulated bone marrow cultures were performed, and slides were G-banded according to standard protocols. Twenty cells were analyzed for each case. No specific growth factors were used for conventional cytogenetic analysis.
Fluorescence in situ hybridization (FISH) was performed using the LSI FGFR3 (Spectrum Orange [SO])–IGH (Spectrum Green [SG]) dual-color dual-fusion probes, CCND1/MYEOV (SO)–IGH (SG) dual-color dual-fusion probes, LSI 13q (SG), and p53 (SO) probes (Abbott Molecular Inc, Des Plaines, IL) to rule out the translocations t(4;14)(p16;q32) and t(11;14)(q13;q32) and the deletions of 13q14 region and p53 gene, respectively. FISH was performed according to the manufacturer's protocols with minor modifications. Two hundred interphase nuclei were evaluated for each probe.
Adverse events
The National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 was used to grade adverse events. Data from all patients who received any combination of the study drugs are included in the safety analysis. The frequency of patients experiencing a specific adverse event was tabulated.
Response and study end points
The end points of this trial were response, time to first and maximum response, and adverse events. Response criteria were adopted from the International Myeloma Working Group criteria.27 In addition, the modified European Bone Marrow Transplantation Registry/International Bone Marrow Transplantation Registry criteria of Bladé et al28 were used for comparison purposes only.
Dose reductions, relative dose intensity, and dose delivered
This was an open-ended protocol, and no maintenance program was prospectively designed. This approach allowed us to determine the duration of therapy necessary for maximum response and the maximum duration of therapy tolerated because all dose reductions were recorded. Dose modifications were prescribed in accordance with the detailed data in Table S1. Briefly, for clarithromycin, there were 2 levels of dose reduction for at least grade 3 toxicity: level 1 reduced the dose from 500 mg twice daily to 250 mg twice daily, and level 2 was discontinuation of the drug. Dexamethasone also had 2 levels of dose reduction for at least grade 2 toxicity. Level 1 reduced dexamethasone from 40 mg on days 1, 8, 15, and 22, to 20 mg on days 1, 8, 15, and 22. Level 2 was a reduction to 20 mg on days 1 and 15 only. Lenalidomide had 4 levels of dose reduction for at least grade 3 toxicity. For neutropenia, level 1 was lenalidomide 25 mg on days 1 to 21, with the addition of granulocyte colony-stimulating factor 5 μg/kg per day. For all other toxicities, dose reduction started at level 2, which was 15 mg on days 1 to 21. Level 3 was 10 mg and level 4 was 5 mg, both on days 1 to 21. Both the dose delivered and the patient compliance were prospectively calculated by patient diaries and amount of drug returned, and dose delivered was also confirmed by physician and patient records. The relative dose intensity for lenalidomide, dexamethasone, and clarithromycin was calculated as a function of total dose intended per 28-day cycle, divided by the actual dose received at a given cycle. An index of relative dose intensity was calculated per cycle, and we report the mean, median, and SD.
Statistical design
A Simon 2-stage optimal design was used to test the null hypothesis that the objective CR rate is less than 10% versus the alternative hypothesis that the CR rate is greater than 30%.29 With a significance level of 0.05 and a power equal to 80%, a maximum of 29 evaluable patients were required to assess the objective CR rate. Overall, a total of 35 patients were required to ensure that 29 evaluable patients would be available for the primary efficacy analysis. After the enrollment of 35 patients, the sample size was increased to 70 evaluable patients to allow for a 95% confidence interval (CI) for the CR rate to extend no more than plus or minus 11% around the observed CR rate.
Statistical analysis methods
The primary efficacy analyses were performed according to the intent-to-treat (ITT) principle, which included all enrolled patients. Progression-free survival time was estimated as the time from the enrollment date to the date of progression or to last known date without evidence of progression. The duration of response (eg, stringent CR [sCR], CR, partial response [PR], very good PR [VGPR]) was defined as the time from achieving a response to the date of progressive disease or to last known remission date without evidence of relapse. Progression-free survival time was analyzed using the Kaplan-Meier method, and 95% CIs were constructed using the formula by Greenwood. Fisher exact test and Student t test (or Wilcoxon rank-sum test) were used for associating categories of response with potential risk factors such as sex, disease stage, C-reactive protein (CRP), β-2 microglobulin (β2M), lactate dehydrogenase (LDH), and albumin (alb). All P values are 2 sided with statistical significance evaluated at the 0.05 α level. The precision of the obtained estimates was assessed by calculating 95% CIs. All analyses were performed in SAS version 9.1 (SAS Institute, Cary, NC) and Stata version 8.0 (Stata Corporation, College Station, TX).
Results
Patient characteristics
A total of 72 patients were enrolled from December 21, 2004, through November 6, 2006. All patients were assessed for response. Three patients withdrew from treatment before completing the first cycle of therapy (2 patients withdrew consent for personal reasons, 1 because of early toxicity–cytopenia). The response analysis is based on ITT and therefore includes data from these 3 patients. Patient characteristics are listed in Table 1. The median age was 63 years (range, 36-83 years). The sex and isotype distribution is typical for population studies in advanced MM. A total of 54% of patients had Durie-Salmon stage III, and 54% had intermediate- or high-risk disease as defined by the International Staging System (ISS) (Table 1). The average baseline values for serum β2M, alb, LDH, and CRP are shown in Table 2.
Baseline conventional cytogenetic and FISH analyses were performed on 66 of 72 patients. Conventional cytogenetic analysis identified 8 patients (12%) with abnormal karyotypes (Table S2). Six of these patients had multiple chromosomal abnormalities, whereas 2 patients showed a single abnormality.
Molecular cytogenetic analysis showed abnormalities in 48 patients (72%) (Table S2). Five patients (7.6%) showed FGFR3-IGH gene rearrangements, whereas 13 patients (20%) showed CCND1-IGH gene rearrangements. Deletion of the 13q14 region was found in 23 patients (35%), whereas trisomy 11 was found in 25 patients (38%). Tetrasomy 11 was identified in 7 patients (11%). More than one abnormality was found in 25 patients (38%) (Table S2). Five patients (7.5%) showed 13q14 deletion and t(11;14) translocation, whereas 3 (4.5%) patients showed 13q14 deletion and t(4;14) translocation. Deletion 13q14 and trisomy 11 were found together in 11 patients (16.6%). One patient showed deletion of 13q14 region as well as loss of wild-type IGH allele. Unusual signal patterns for CCND1 and FGFR3 were also observed in this sample. These may represent complex chromosomal rearrangements. Three additional patients also showed loss or partial deletion of the wild-type IGH allele.
Response to therapy
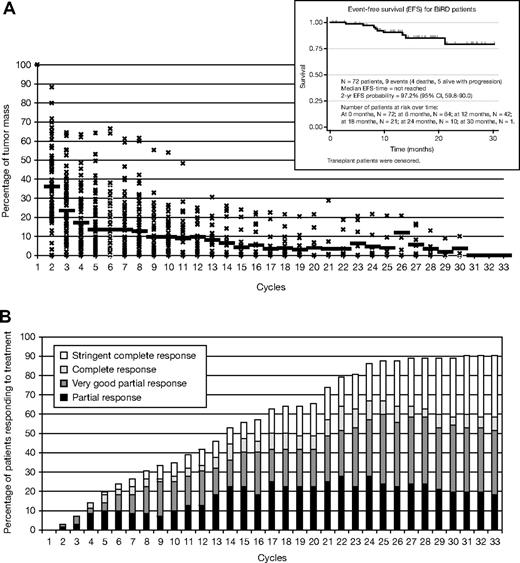
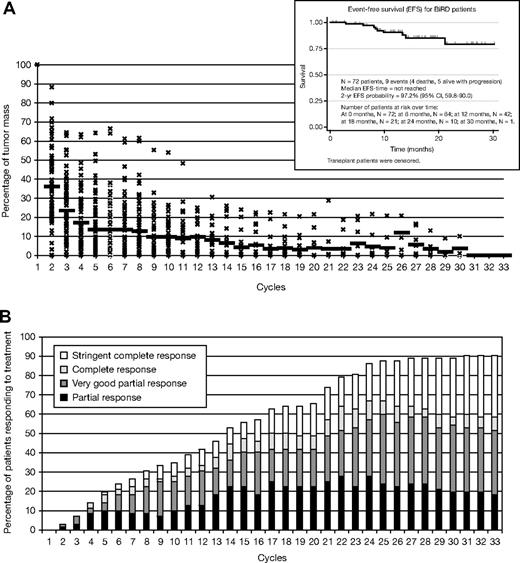
Mean time on treatment was 368 days (range, 29-944 days) with a mean duration of response of 333 days (range, 15-920). A total of 65 (90.3%) of 72 patients achieved an objective response (at least PR) to therapy (Table 3). Of the 72 patients, 22 (30.6%) achieved an sCR (including complete absence of M-protein by immunofixation, normal free light chain ratio, and a marrow biopsy negative by immunohistochemistry) and 6 (8.3%) achieved a CR, giving a total CR plus sCR of 38.9%. One third (25 of 72) achieved a VGPR, 12 (16.7%) achieved a PR, and 4 (5.6%) a minor response (> 25% drop in M-protein level) (Table 3); 3 patients could not be evaluated for response because they received less than 1 full cycle of therapy. Mean time to first response, as defined by a greater than 50% decrease of M-protein, was 53.9 days (range, 21-816 days), whereas mean time to maximum response was 209 days (range, 27-850 days) (Figure 1B). A total of 73.6% of patients achieved a greater than 90% reduction (sCR + CR + VGPR) in M-protein (mean time to VGPR, 158 days; range, 28-786 days). At the end of cycle 1, 51 (71%) of 72 patients had a greater than 50% decrease in M-protein (Figure 1A-B). Six patients (8%) had a 90% drop in M-protein level, whereas another 3 (4%) had a 100% decrease in M-protein. Five patients (7%) had progression of disease, in whom 3 were manifested only by the asymptomatic reappearance of the original M-protein by serum immunofixation. None of these 3 patients have evidence of either end-organ dysfunction or changes in the bone marrow or positron emission tomographic or computed tomographic scan suggestive of disease activity. Two patients had clear progression of disease that required second-line therapy with bortezomib. The median event-free survival has not been reached (Figure 1A, inset), and the actuarial 2-year event-free survival has reached 97.2%.
Patterns of response in 72 patients on BiRD per treatment cycle. (A) Tumor mass reduction and event-free survival (inset) and (B) cumulative response; mean time on treatment, 368 days (range, 29-944 days); mean duration of response, 333 days (range, 15-920 days). Mean time to first response, 53.9 days (range, 21-816 days); mean time to maximum response, 209 days (range, 27-850 days). A total of 74% of patients achieved a greater than 90% reduction in M-protein level (mean time to very good partial response, 158 days; range, 28-786 days). Fifty-one (71%) of 72 patients had a more than 50% decrease in M-protein level at the end of the first cycle.
Patterns of response in 72 patients on BiRD per treatment cycle. (A) Tumor mass reduction and event-free survival (inset) and (B) cumulative response; mean time on treatment, 368 days (range, 29-944 days); mean duration of response, 333 days (range, 15-920 days). Mean time to first response, 53.9 days (range, 21-816 days); mean time to maximum response, 209 days (range, 27-850 days). A total of 74% of patients achieved a greater than 90% reduction in M-protein level (mean time to very good partial response, 158 days; range, 28-786 days). Fifty-one (71%) of 72 patients had a more than 50% decrease in M-protein level at the end of the first cycle.
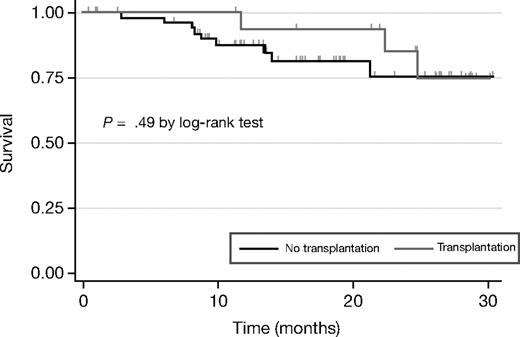
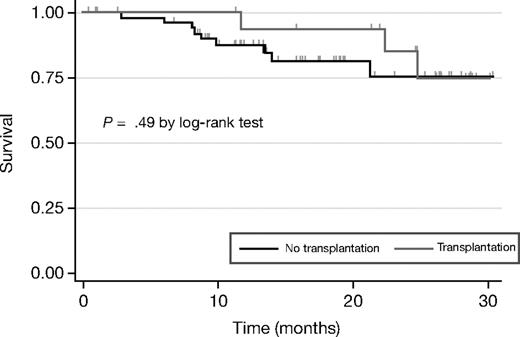
BiRD treatment did not impede stem-cell mobilization or consolidation therapy with autologous transplantation. The criteria for high-dose chemotherapy were the following: aged younger than 65 years, Karnofsky performance status more than 60, diffusing capacity of the lung for carbon monoxide more than 50%, ejection fraction more than 50%, responsive disease (≥ PR), normal liver function tests, HIV negativity, and no active viral or bacterial infections. As of June 30, 2007, 18 patients underwent stem-cell mobilization and harvest (Table 4). Mean time to stem-cell mobilization was 353 days (range, 137-651 days), whereas mean time to transplantation was 382 days (range, 137-651 days). Of these, all patients successfully completed high-dose chemotherapy, except one patient with baseline thrombocytopenia, who failed to engraft and ultimately died of bone marrow failure. Two patients relapsed or had disease progression after transplantation. The median event-free survival time has not been reached, with an actuarial 2-year event-free survival of 85.2% (95% CI, 51.9-96.2; Figure 2).
Event-free survival by transplantation status. The response analysis is based on the intent-to-treat population, and includes data from the 3 patients who withdrew from treatment before completing the first cycle of therapy (2 patients withdrew consent for personal reasons, 1 because of early toxicity–cytopenia). The median event-free survival times have not been reached for the transplantation or nontransplantation groups, with an actuarial 2-year event-free survival of 85.2% and 75.2%.
Event-free survival by transplantation status. The response analysis is based on the intent-to-treat population, and includes data from the 3 patients who withdrew from treatment before completing the first cycle of therapy (2 patients withdrew consent for personal reasons, 1 because of early toxicity–cytopenia). The median event-free survival times have not been reached for the transplantation or nontransplantation groups, with an actuarial 2-year event-free survival of 85.2% and 75.2%.
Fifty-two patients did not go on to receive a stem-cell transplant and instead received continued therapy (mean cycles, 14; range, 1-33 cycles), achieving a CR of 37% and VGPR of 33%. Of these 52 patients, 36 patients were ineligible to receive a transplant because of advanced age, and 7 refused transplantation because of personal reasons. The remaining 9 patients are still responding to therapy. Nine events occurred in this group (4 deaths and 5 relapses or disease progression). The median event-free survival time for the nontransplantation group has not been reached, with an actuarial 2-year event-free survival of 75.2% (95% CI, 54.6%-87.4%; Figure 2).
The results of a comprehensive review of potential risk factors, including stage (Durie-Salmon [P = .76/P = .62] and ISS [P = .98/P = .66]), alb (P = .41), β2M (P = .70/P = .98), LDH (P = .38/P = .95), CRP (P = .92/P = .97), free light chain ratio (P = .85/P = .70), cytogenetic abnormalities (all P > .05), and history of prior monoclonal gammopathy of unknown significance (P = .80/P = .27) did not predict the response type or duration of response or the event-free survival, respectively.
Adverse events and deaths
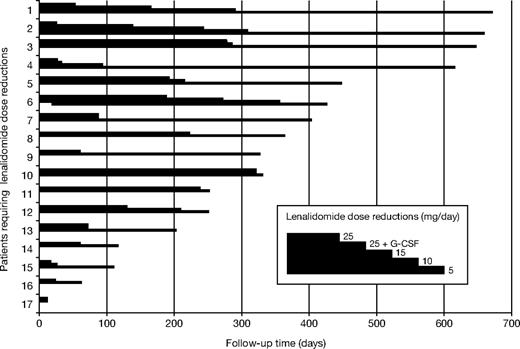
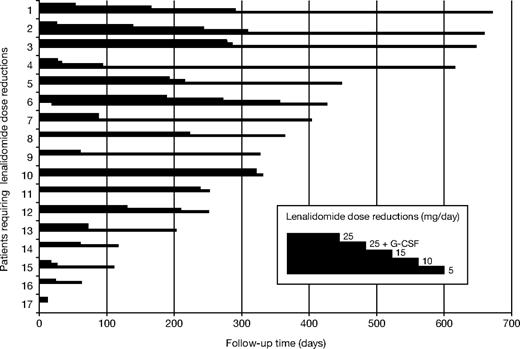
Most adverse events were manageable. The most common grade 3 or higher hematologic toxicities were neutropenia (19.4%), anemia (13.8%), and thrombocytopenia (22.2%; Table 5). These cytopenias necessitated 14% of patients to receive red blood cell transfusions and 6% to receive platelet transfusions. Because of myelosuppression, 45% of patients received erythropoietin and 17% received granulocyte colony-stimulating factor. All but 3 lenalidomide dose reductions were due to cytopenias (2 patients had a rash, and 1 had anorexia). Seventeen patients (23.6%) required at least 1 lenalidomide dose reduction. Of those 17 patients, 7 required a further dose reduction to level 2, 4 patients were reduced to level 3, and 1 patient was reduced to level 4 (Figure 3). The mean time to the first lenalidomide dose reduction was 110 days (range, 13-279 days), mean time to level 2 was 169 days (range, 27-286 days), mean time to level 3 was 246 days (range, 95-356 days), and the level 4 dose reduction occurred after 309 days of treatment. Mean time to follow-up for patients on BiRD who did not require dose reductions was 101 days. Of the 14 patients who had at least grade 3 myelosuppression and required dose reduction, 8 had a baseline creatinine clearance of 40 mL/minute or less. Kaplan-Meier survival analysis with the log-rank test showed a significant association between renal insufficiency and time to myelosuppression (hazard ratio, 8.4; 95% CI, 2.9-24.7; P < .001).30
Cumulative dose reduction (versus time on lenalidomide) because of toxicity in 17 of 72 patients. Patient 13 was dose-reduced to 15 mg lenalidomide because of grade 3 gastrointestinal toxicity. Patients 7 and 10 were dose-reduced to 15 mg lenalidomide because of grade 3 skin toxicity (rash). The remaining patients were dose-reduced because of myelosuppression according to the guidelines in Table S1. G-CSF indicates granulocyte colony-stimulating factor.
Cumulative dose reduction (versus time on lenalidomide) because of toxicity in 17 of 72 patients. Patient 13 was dose-reduced to 15 mg lenalidomide because of grade 3 gastrointestinal toxicity. Patients 7 and 10 were dose-reduced to 15 mg lenalidomide because of grade 3 skin toxicity (rash). The remaining patients were dose-reduced because of myelosuppression according to the guidelines in Table S1. G-CSF indicates granulocyte colony-stimulating factor.
All grade 3 or higher adverse events are summarized in Table 5. The most common grade 3 or higher nonhematologic adverse events were myopathy (11.1%), rash (5.6%), diverticular abscess (5.6%), hypocalcemia (4.2%), neuromood (4.2%), tremor (4.2%), and thrombosis (9.7%). TEs occurred in 9 patients (12.5%) of which 5 events (55.5%) occurred in the context of aspirin interruption or poor compliance.28
Dexamethasone and clarithromycin dose reductions were due to nonhematologic adverse events. Sixteen patients (34%) were dose-reduced to level 1 on dexamethasone, and 4 of these patients (9%) were further reduced to level 2. The mean time to dexamethasone dose reduction level 1 was 183 days (range, 27-290 days), and mean time to level 2 was 160 days (range, 103-222 days). Six patients (13%) were dose-reduced on clarithromycin and 2 (4%) discontinued the drug. Mean time to clarithromycin dose reduction level 1 was 178 days (range, 53-331 days), and mean time to clarithromycin discontinuation was 300 days (range, 290-309 days).
Maintenance, relative dose intensity, and long-term administration
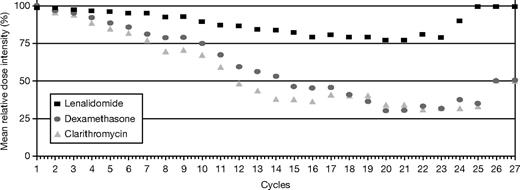
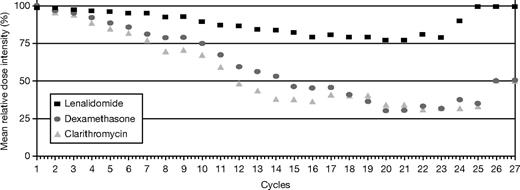
The relative dose intensity (RDI) for lenalidomide, dexamethasone, and clarithromycin is shown in Figure 4. Analysis of RDI per cycle shows that lenalidomide mean RDI was maintained above 75% throughout therapy, once patients achieved a maximum response. At least 80% of patients maintained the intended lenalidomide dose, and 75% maintained the intended dose for dexamethasone and clarithromycin.
Relative dose intensity (dose intended/dose delivered/time) of lenalidomide, clarithromycin, and dexamethasone. Lenalidomide mean relative dose intensity was maintained above 75% throughout therapy, once patients achieved a maximum response. At least 80% of patients maintained the intended lenalidomide dose, and 75% maintained the intended dose for dexamethasone and clarithromycin.
Relative dose intensity (dose intended/dose delivered/time) of lenalidomide, clarithromycin, and dexamethasone. Lenalidomide mean relative dose intensity was maintained above 75% throughout therapy, once patients achieved a maximum response. At least 80% of patients maintained the intended lenalidomide dose, and 75% maintained the intended dose for dexamethasone and clarithromycin.
Discussion
The novel combination of clarithromycin, lenalidomide, and dexamethasone (BiRD) in this phase 2 trial for treatment-naive, symptomatic MM showed a 90.3% ORR, with a combined sCR and CR rate of 38.9%. The combination therapy was well tolerated; 69 of 72 patients achieved evaluable milestones, and there were only 3 early withdrawals from the study. Profound and meaningful responses were readily seen with 73.6% of patients achieving at least a VGPR. Prompt responses were documented with 70% of patients achieving at least a PR within the first cycle of therapy. Furthermore, mean time to achieve a first response was 43 days. The major grade 3 or higher toxicities were cytopenias, TEs, myopathy, rash, and diverticular abscess. This regimen did not interfere with stem cell harvest or autologous transplantation. Furthermore, patients who were not deemed suitable for high-dose chemotherapy continued on the study therapy with modest dose reductions and similar response rates to those who underwent high-dose chemotherapy and autologous transplantation.
Rajkumar et al26 published results of a phase 2 trial of Rev/Dex done concomitantly with BiRD. In their population of 34 evaluable patients (47% Durie-Salmon stage III, 12% ISS stage III), they observed an ORR (CR + near-CR [nCR]/VGPR + PR) of 91%, comparable to the ITT ORR (90.3%) reported here for similar-risk patients receiving BiRD (44% Durie-Salmon stage III, 18% ISS stage III). However, a comparison of the subset response data reveals intriguing differences between these 2 regimens. Rev/Dex resulted in a 6% CR rate and 32% nCR/VGPR rate, for a combined CR + nCR/VGPR rate of 38%. In contrast, CR, nCR, and VGPR rates for BiRD were 38.9%, 13.9%, and 20.8%, respectively (Table 6).26,31 When the more stringent New Unified Response Criteria (NURC)27 are used, the combined sCR + CR + VGPR rate exceeded 70% for the BiRD regimen. Furthermore, most BiRD-achieved CRs fulfilled the NURC for sCR (22 of 28), indicating the regimen's association with complete normalization of the bone marrow immunohistochemistry and free light chain ratio.
The incorporation of clarithromycin into the BiRD regimen allowed for a higher complete remission response (RR) and significant reduction of the dexamethasone dosage compared with Rev/Dex. When used as a single agent in advanced myeloma, clarithromycin yields mixed results.17,32,33 Durie et al17 reported 6 CRs and 7 PRs from 23 evaluable patients, whereas Stewart et al34 found single-agent clarithromycin to have no efficacy in a phase 2 pilot study of 23 patients. However, the Stewart et al34 study included patients with chemotherapy-insensitive disease (smoldering, plateau-phase, refractory myeloma) and only 5 treatment-naive patients. The applicability of these results to the BiRD study is therefore limited, because all BiRD patients were treatment naive, and clarithromycin was given in combination with dexamethasone. The persistence of some corticosteroid-related complications with the BiRD regimen suggests that the addition of clarithromycin to weekly dexamethasone enhances the corticosteroid effect and may therefore increase both the ORR and toxicity.14-20
Recently, investigators from the Eastern Cooperative Oncology Group randomly assigned patients with newly diagnosed disease to receive lenalidomide in combination with standard-intensity corticosteroids (40 mg, days 1-4, 9-12, and 17-20) versus our schedule of dexamethasone once a week (40 mg).14,15,19,20,35,36 That study confirms that the higher doses of dexamethasone in standard Rev/Dex lead to increased adverse events, and these morbidities can be reduced significantly by limiting the dose intensity of corticosteroids. The extent to which the reduction of dexamethasone dose intensity affects RRs remains to be determined; however, it appears to improve survival.35,36 Therefore, it remains possible that the high RRs observed with the BiRD regimen are attributable to the use of low-dose dexamethasone and the prolonged use of lenalidomide rather than to the addition of clarithromycin to the regimen.
High-dose chemotherapy in single- or tandem-treatment programs yielded a CR/VGPR rate of approximately 50% to 60%.37 Conversely, older patients receiving oral melphalan, prednisone, and thalidomide achieve a CR of 15.5% and a VGPR ranging from 8.5% to 50%.38,39 More than 70% of patients on BiRD who did not undergo consolidation with high-dose therapy and peripheral blood stem-cell transplantation achieved more than a 90% reduction in baseline M-protein (> VGPR). Their baseline characteristics did not differ from the overall study population; however, their older age and comorbidities led them to decline high-dose chemotherapy (data not shown). As shown by data in Figure 1, long-term therapy with lenalidomide is feasible and can contribute to the robustness of the response. Similar results have been reported by a recent update of the data by Rajkumar et al26 and Lacy et al31 (Table 6). The results obtained with BiRD and Rev/Dex regimens suggest that lenalidomide-based therapy can be used long term in selected patient populations, when high-dose chemotherapy is declined. The prolongation of lenalidomide-based therapy may lead to more complete responses. Given that the mean relative dose intensity was maintained above 75% throughout the treatment, most patients can receive long-term lenalidomide without the fear or risk of cumulative toxicity. Furthermore, dose reductions for maintenance programs appear not to be justified. Maintenance programs beyond maximum response should be prospectively evaluated.
Hematologic toxicity seen in this trial was modest and well controlled with growth factor support. Myelosuppression was significantly associated with renal dysfunction because most patients who required growth factor support or dose reduction had a creatinine clearance of less than 40%.30 The major nonhematologic adverse side effect of the BiRD therapy was myopathy, which was likely associated with lenalidomide in combination with cortico-steroid use. TEs were associated with either poor compliance or interruption of aspirin prophylaxis. These results underscore the critical importance of thromboprophylaxis both during and for at least 10 days after BiRD treatment.40-42
The results of this study support further exploration of BiRD for induction therapy, because it produces high RRs and may increase disease-free and overall survival when used in conjunction with autologous transplantation. In addition, prolonged use of BiRD with judicious corticosteroid dose modifications may be a reasonable option for the treatment of older patients ineligible for aggressive consolidation strategies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs Stanley Ostrow and Roy Berger for patient care and referral, Drs Ruthee-Lu Bayer (North Shore University Hospital, Manhasset, NY) and Adam Cohen (Memorial Sloan-Kettering Cancer Center, Long Island, NY) for providing follow-up transplantation information, and Mr Larry Lyons and Mr Udi Gelbshtein with special gratitude for data coordination assistance.
This work was supported in part by The Leukemia and Lymphoma Society SCOR grant, Hermione Foundation, and an National Cancer Institute K23 award (CA109260-01).
Authorship
Contribution: R.N. conceived the treatment program, wrote and implemented the protocol, coordinated data and database, coordinated input from all the authors, conceived the figures, and wrote the manuscript; D.S.J. generated the database, coordinated translational efforts and marrow samples and generated figures; P.J.C. performed biostatistical analysis; J.R.F. and J.J. coordinated data and database; T.N. performed further biostatistical analysis and generated dose reduction and dose intensity analysis; S.E. provided immunohistochemistry analysis of marrow; R.N.P. provided significant input on trial design and expert patient care; F.Z., K.P., T.M., J.T., and M.W.S. provided significant contribution in patient care; A.L. contributed clinical research nursing and patient care; R.L. provided expert analysis of immunoprotein analysis; H.J.C. helped with writing the manuscript and provided expert care of patients; T.S. and J.H. provided analysis of stem-cell collection and expert patient care; J.P.L. coordinated the clinical research office; M.M. oversaw biostatistical analysis; S.C.-K. coordinated translational research and gave direction in science; S.M. contributed with cytogenetic analysis; M.C. contributed in original idea generation and treatment program concept, reviewed the manuscript, and contributed with expert patient care.
Conflict-of-interest disclosure: R.N., M.C., J.P.L., and M.W.S. have served as consultants and participate as lecturers in the speaker's bureau for Celgene. R.N. received research support and drug for the implementation of this trial. The other authors declare no competing financial interests.
Correspondence: Ruben Niesvizky, Multiple Myeloma Service, Division of Hematology and Medical Oncology, Weill-Cornell Medical College, New York Presbyterian Hospital-Cornell Medical Center, 525 East 68th Street, Payson Pavilion, 3rd Floor, New York, NY 10021; e-mail: run9001@med.cornell.edu.