The role of B-cell receptor (BCR)–mediated survival signals in diffuse large B-cell lymphoma (DLBCL) remains undefined. Ligand-induced BCR signaling induces receptor oligomerization, Igα/β immunoreceptor tyrosine-based activation motif (ITAM) phosphorylation, and activation of the spleen tyrosine kinase (SYK), which initiates downstream events and amplifies the initial BCR signal. BCRs also transmit low-level tonic survival signals in the absence of receptor engagement. Herein, we assess the role of SYK-dependent tonic BCR survival signals in DLBCL cell lines and primary tumors and evaluate the efficacy of an ATP-competitive inhibitor of SYK, R406, in vitro. R406 induced apoptosis of the majority of examined DLBCL cell lines. In R406-sensitive DLBCL cell lines, R406 specifically inhibited both tonic- and ligand-induced BCR signaling (autophosphorylation of SYK525/526 and SYK-dependent phosphorylation of the B-cell linker protein [BLNK]). The majority of examined primary DLBCLs also exhibited tonic- and ligand-induced BCR signaling; in these primary tumors, BCR signaling was also inhibited by R406. Of note, BCR-dependent and R406-sensitive DLBCL cell lines were independently identified as “BCR-type” tumors by transcriptional profiling. Therefore, SYK-dependent tonic BCR signaling is an important and potentially targetable survival pathway in some, but not all, DLBCLs. In addition, R406-sensitive DLBCLs can be identified by their transcriptional profiles.
Introduction
Several lines of evidence suggest that many B-cell lymphomas depend on B-cell receptor (BCR)–mediated survival signals. Most B-cell lymphomas retain BCR expression and limit immunoglobulin (Ig) loci translocations to nonproductively rearranged Ig alleles.1 In addition, B-cell lymphomas with ongoing somatic hypermutation rarely exhibit loss of BCR expression.1 Furthermore, treatment with anti-idiotypic antibodies uncommonly leads to the emergence of BCR-negative lymphoma variants.1
BCR signaling induces receptor oligomerization and phosphorylation of Igα and β immunoreceptor tyrosine-based activation motifs (ITAMs) by SRC family kinases.2 ITAM phosphorylation results in the recruitment and activation of SYK, a protein tyrosine kinase (PTK) that initiates downstream events and amplifies the original BCR signal.2,–4 Although BCR signaling is generally thought to depend on ligand-induced aggregation, additional studies highlight the important role of “tonic” BCR maintenance or survival signals in the absence of receptor engagement.4,,–7 Lam et al5 first demonstrated that the inducible loss of murine BCR resulted in the death of peripheral B cells, highlighting the requirement for continued BCR expression in viable B cells. In follow-up studies, the selective excision of the Igα ITAM and ablation of Igα signaling led to the loss of mature B cells, further emphasizing the role of tonic BCR signaling in B-cell survival.6
Although the molecular mechanisms regulating tonic BCR signaling remain to be defined, recent studies highlight the central role of the SYK PTK and the balance between BCR-associated SYK activation and protein tyrosine phosphatase (PTP)–mediated SYK inhibition.3,4,8,–10 Under basal conditions, SYK activity is tightly controlled by PTPs.9 However, BCR signaling leads to the local production of reactive oxygen species (ROSs), which inhibit PTP activity.9,11 The likely role of PTPs in modulating SYK activity and tonic BCR signaling was initially revealed by studies in which SYK was activated by pervanadate/H2O2 without BCR crosslinking.3,4,7
In an earlier screen for genes that might contribute to the pathogenesis of diffuse large B-cell lymphoma (DLBCL), we identified and preliminarily characterized a lymphoid PTP termed PTP receptor-type O truncated (PTPROt).12 PTPROt is a member of the PTPRO family, a group of highly conserved receptor-type PTPs that are thought to function as tumor suppressor genes.10,12 We recently found that SYK is a major substrate of this tissue-specific and developmentally regulated PTP.10 The overexpression of PTPROt inhibited BCR-triggered SYK tyrosyl phosphorylation, activation of associated adaptor proteins such as BLNK, and downstream signaling events.10 Most importantly, PTPROt overexpression also inhibited DLBCL proliferation and induced apoptosis in the absence of BCR crosslinking.10 These observations support the hypothesis that PTPROt and SYK modulate tonic BCR signaling and tumor cell survival in certain DLBCLs.
DLBCLs are clinically and genetically heterogeneous disorders, suggesting that additional disease subtypes remain to be defined. Our group has applied consensus clustering methods to the transcriptional profiles of 2 large independent series of primary DLBCLs to identify the dominant substructure and classify these tumors in an unbiased manner.13 The consensus clusters obtained were highly reproducible and included 3 groups of DLBCLs termed B-cell receptor (BCR), oxidative phosphorylation (OxPhos), and host response (HR) tumors.13 BCR tumors have increased expression of multiple components of the BCR signaling cascade including SYK, prompting speculation that this subset of DLBCLs might have increased activity of and reliance on BCR-mediated survival signals. These BCR DLBCLs also have more abundant expression of BCL6 and exhibit more frequent translocations of the BCL6 locus and significantly greater repression of BCL6 targeted genes and sensitivity to BCL6 inhibitors.14
Given the role of tonic BCR signaling in normal B cells5,6 and the SYK-dependent survival of DLBCL cell lines in vitro,10 we postulated that SYK might be a promising rational treatment target in certain DLBCLs and used a recently described SYK inhibitor, R406, to test this hypothesis. R406 is an ATP-competitive SYK inhibitor that has been evaluated in models of allergen-induced airway hyper-responsiveness15 and rheumatoid arthritis.16 More recently, R406 was found to promote the differentiation of SYK-transformed pre-B cells into mature B cells in a murine leukemia model.17 From a clinical perspective, R406 is of particular interest because the oral compound has completed phase 1 testing and is available for disease-specific phase 2 trials. For these reasons, we have evaluated SYK-mediated tonic BCR signaling and targeted SYK inhibition with R406 in DLBCL.
Methods
Cell culture
The DLBCL cell lines DHL4, DHL6, DHL8, DHL10, Wsu-NHL, Karpas422 (K422), OCI LY1, LY4, LY7, LY18, LY19, Pfeiffer, and Toledo were cultured in RPMI 1640 medium (Mediatech, Herndon, VA) supplemented with 10% fetal calf serum and 2 mM glutamine. DLBCL cell lines LY3 and LY10 were cultured in Iscove modified Dulbecco medium (IMDM) (Invitrogen, Grand Island, NY) supplemented with 20% human serum (Gemini Bio-Products, West Sacramento, CA) and 2 mM glutamine. All the cells were maintained at 37°C in 5% CO2. The cell lines, which were all mycoplasma-free, were obtained from the following sources: Pfeiffer and Toledo, American Tissue Culture Collection (ATCC, Manassas, VA); DHL4, DHL6, DHL8, DHL10, Wsu-NHL, Karpas422, Ly19, Deutsche Sammiung von Mikroorganismen und Zellkulturen GmbH (DSMZ, Braun-schweig, Germany); and OCI Ly1, Ly3, Ly4, Ly7, Ly10, Ly18, Ontario Cancer Institute (University of Toronto, Toronto, ON).
Consensus cluster assignment
The DLBCL cell lines were assigned to consensus clusters13 using their transcriptional profiles and a recently described ensemble classifier14 which combines by majority voting the class assignments of 14 independent predictive algorithms (Document S1 and Table S2, available on the Blood web site; see the Supplemental Materials link at the top of the online article).
R406
The small molecule SYK inhibitor, R406,18 was a gift from Rigel (San Francisco, CA). R406 was dissolved in DMSO at a concentration of 10 mM and stored at −80°C. After thawing, the R406 stock solution was kept in a desiccator at room temperature for up to 1 week.
Analysis of cellular proliferation and apoptosis
DLBCL cell lines were treated with serial dilutions of R406 (0.3, 0.6, 1.25, 2.5, or 5 μM) or vehicle alone for 72 hours. Thereafter, cellular proliferation was determined by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay (Roche Diagnostics, Indianapolis, IN) using standard protocols. For each cell line, R406 EC50 was calculated using GraphPad Prism 4 software (GraphPad Software, San Diego, CA). DLBCL cell lines were also treated with 1 μM or 4 μM R406 or vehicle alone for 96 hours and analyzed thereafter for apoptosis using annexin V–FITC/propidium iodide (PI) staining (Annexin V–FITC apoptosis detection kit I; BD Biosciences, San Jose, CA). In these experiments, all cells were analyzed and the annexin V+, annexin V+/PI+, and PI+ cells were considered apoptotic. In companion experiments, 4 of the R406-sensitive DLBCL cell lines were treated with 4 μM R406 or vehicle alone for 96 hours, lysed, size-fractionated by polyacrylamide gel electrophoresis (PAGE), and immunoblotted for caspase 9, 8, and 3 (see “Immunoblotting”).
BCR crosslinking and R406 inhibition
Cells (5 × 106) in 0.5 mL RPMI were incubated with 1 μM R406 or vehicle alone in a 37°C water bath for 30 minutes and subsequently stimulated with goat anti–human IgM and IgG for 10 minutes. Thereafter, cells were lysed in NP-40 lysis buffer.
Immunoblotting
Samples were size-fractionated by sodium dodecyl sulfate (SDS)–PAGE and proteins were transferred to Immunobilon-PVDF membranes (Millipore, Billerica, MA). Blots were first incubated in blocking buffer (5% milk, 0.1% Tween in phosphate-buffered saline [PBS]) at room temperature for 1 hour and subsequently incubated with primary antibodies in precooled blocking buffer overnight at 4°C. After washing in 0.1% Tween/PBS, blots were incubated with horseradish peroxidase (HRP)–labeled secondary antibodies at room temperature for 1 hour, developed by enhanced chemiluminescence (GE Healthcare Biosciences, Piscataway, NJ), and visualized with Hyperfilm-ECL (GE Healthcare Biosciences). To reprobe with another antibody, the blots were incubated with stripping buffer (0.063 M Tris-HCL [pH 6.8], 2% SDS, 0.026 M DTT) at 50°C for 40 minutes, and analyzed as described. Primary antibodies included rabbit polyclonal anti–phospho-SYK-Tyr352, phospho-SYK-Tyr525/526, caspase-3, murine monoclonal anti–phospho-BLNK (pY84), caspase-8, caspase-9 (Cell Signaling Technology, Danvers, MA), and anti–(total)SYK and anti–(total)BLNK (Santa Cruz Biotechnology, Santa Cruz, CA). The secondary antibodies included donkey anti–rabbit IgG HRP-linked (Fab')2 fragment and sheep anti–mouse IgG HRP-linked (Fab')2 fragment (GE Healthcare Biosciences).
Intracellular phosphospecific flow cytometry
Intracellular phosphospecific flow cytometry was performed as previously described19 according to the manufacturer's instructions. In brief, 5 × 106 cells were resuspended in 1 mL cold PBS plus 1% FCS and left untreated or stimulated with goat anti–human IgG and IgM at 37°C for 10 minutes. Thereafter, cells were fixed, permeabilized, and stained with the following antibodies: PE-conjugated p-SYK (pY348), PE-conjugated p-SYK (pY352), PE-conjugated p-BLNK (pY84) (BD Biosciences) or isotype control antibodies, PE-conjugated mouse IgG1κ (for anti-SYK(pY352)) or PE-conjugated mouse IgG2bκ (for p-BLNK) (BD Biosciences). Flow cytometric analysis was performed using a Cytomics FC 500 MPL Beckman Coulter (Beckman Coulter, Fullerton, CA).
Flow cytometric analysis of surface Ig
The DLBCL cell lines were separately immunophenotyped with PE-conjugated mouse anti–human IgG and IgM monoclonal antibodies (BD Biosciences) using standard protocols and analyzed by flow cytometry with a Cytomics FC 500 MPL Beckman Coulter.
DLBCL viable tumor cell suspensions
Using an institutional review board–approved protocol through NCI SPORE CA97274-06, freshly obtained nodal primary DLBCL specimens were minced over a wire mesh screen, washed, filtered, and centrifuged over Ficoll Hypaque (Isolymph; Gallard-Schlesinger Industries, Garden City, NY) at 500g for 15 minutes to isolate viable mononuclear tumor cells. Thereafter, the viable tumor cell suspensions were washed in RPMI, resuspended in DMSO, and cryopreserved in liquid nitrogen. Prior to analysis, the tumor cell suspensions were thawed and viable cells were isolated from a Ficoll Hypaque monolayer. The primary DLBCL tumor cell suspensions were immunophenotyped for cell-surface IgG and IgM (see “Flow cytometric analysis of surface Ig”). In addition, these viable tumor cell suspensions were analyzed by intracellular phosphospecific flow cytometry for pSYK (Y352) and pBLNK (pY84) at baseline, and following anti–IgG/IgM crosslinking in the presence or absence of R406 (see “Intracellular phosphospecific flow cytometry”).
Results
R406 inhibits proliferation and induces apoptosis of DLBCL cell lines
We first assessed the effects of the SYK inhibitor, R406, on the cellular proliferation of a large panel of DLBCL cell lines. These DLBCLs were treated with serial dilutions of R406 or vehicle alone and proliferation was evaluated thereafter by MTT assay. In the majority of the DLBCL cell lines, R406 inhibited cellular proliferation at EC50s ranging from 0.8 μM to 8.1 μM (Table S1).
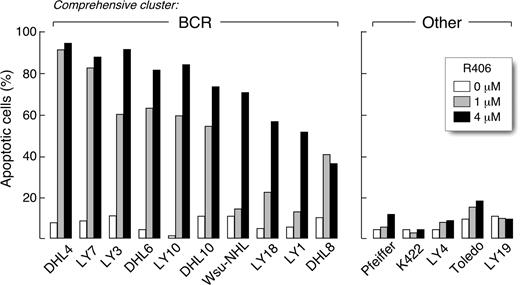
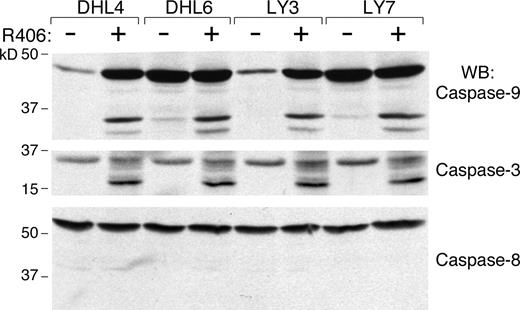
We next determined whether R406 was cytotoxic to DLBCLs using 2 doses of the SYK inhibitor, 1 μM and 4 μM, derived from the EC50 analysis (Table S2). The DLBCLs were cultured with R406 or vehicle alone and assessed for apoptosis by annexin V–FITC/propidium iodide (PI) staining. Ten of the DLBCL cell lines exhibited high levels of apoptosis following R406 treatment (Figure 1 left panel), whereas 5 lines did not undergo R406-associated apoptosis (Figure 1 right panel). To further characterize R406-induced apoptosis in sensitive DLBCL cell lines, we immunoblotted several of these lines following R406 or vehicle treatment and evaluated the cleavage of caspases 3, 8, and 9 (Figure 2). R406 induced cleavage of caspases 9 and 3, but not caspase 8, indicating involvement of the intrinsic apoptotic pathway20 (Figure 2).
The SYK inhibitor, R406, induces apoptosis in a subset of DLBCL cell lines. DLBCL cell lines were cultured with 1 μM or 4 μM of R406 or vehicle alone for 96 hours. Thereafter, cellular apoptosis was assessed using annexin V–FITC/propidium iodide (PI) staining. All of the R406-sensitive cell lines (left panel) were previously designated as “BCR-type” DLBCLs using the cell line transcriptional profiles and a recently described ensemble comprehensive cluster classifier.14 None of the R406-insensitive cell lines were identified as “BCR-type” (“Other,” right panel).
The SYK inhibitor, R406, induces apoptosis in a subset of DLBCL cell lines. DLBCL cell lines were cultured with 1 μM or 4 μM of R406 or vehicle alone for 96 hours. Thereafter, cellular apoptosis was assessed using annexin V–FITC/propidium iodide (PI) staining. All of the R406-sensitive cell lines (left panel) were previously designated as “BCR-type” DLBCLs using the cell line transcriptional profiles and a recently described ensemble comprehensive cluster classifier.14 None of the R406-insensitive cell lines were identified as “BCR-type” (“Other,” right panel).
R406 induces DLBCL apoptosis via the intrinsic apoptotic pathway. R406-sensitive DLBCL cell lines were cultured with 4 μM of R406 or vehicle alone for 96 hours. Thereafter, cells were lysed and lysates were size-fractionated and immunoblotted with antibodies directed against caspase-9 (top panel), caspase-3 (middle panel), and caspase-8 (bottom panel).
R406 induces DLBCL apoptosis via the intrinsic apoptotic pathway. R406-sensitive DLBCL cell lines were cultured with 4 μM of R406 or vehicle alone for 96 hours. Thereafter, cells were lysed and lysates were size-fractionated and immunoblotted with antibodies directed against caspase-9 (top panel), caspase-3 (middle panel), and caspase-8 (bottom panel).
Of interest, all of the R406-sensitive cell lines were previously designated “BCR-type” DLBCLs using the cell line transcriptional profiles and a recently described ensemble classifier14 (Figure 1 left panel). In contrast, none of the R406-insensitive cell lines were identified as “BCR-type” tumors (Figure 1 right panel). Taken together, these data suggest that transcriptional profile-defined “BCR-type” DLBCLs may be uniquely reliant on BCR-mediated survival signals.
BCR signaling is intact in R406-sensitive DLBCL cell lines
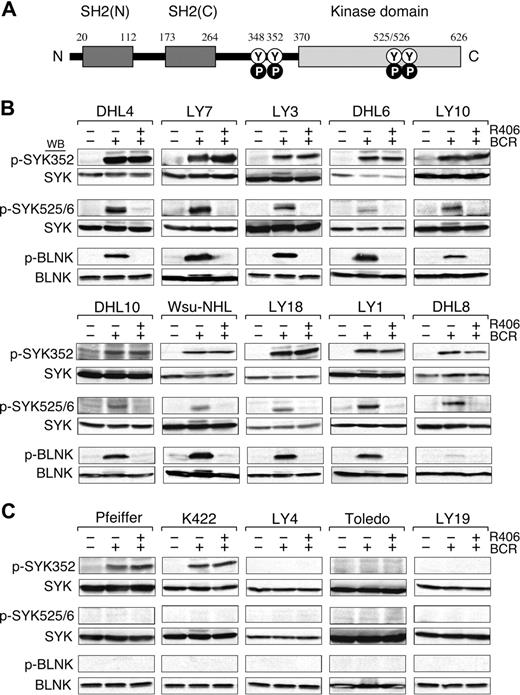
We next evaluated the integrity of the BCR signaling pathway in the DLBCL cell lines by crosslinking the B-cell receptor and analyzing downstream events including the initial tyrosyl phosphorylation of SYK352, the subsequent autophosphorylation of SYK525/526 (Figure 3A), and the SYK-dependent phosphorylation of B-cell linker protein (BLNK). In these studies, the DLBCL cell lines were stimulated with anti-IgM and anti-IgG antibodies, lysed, size-fractionated, and immunoblotted with phosphospecific antibodies directed against SYK352, SYK525/526, and BLNK (Figure 3B,C, top, middle, and bottom panels, respectively).
BCR signaling is intact in R406-sensitive DLBCL cell lines. (A) SYK domains and key tyrosine residues. The SYK tandem SH2 domains (black boxes), the linker region (aa 264-370), and the kinase domain (gray box) are shown. N indicates NH2-terminal; C, C-terminal; Y, tyrosine; P, phosphorylation. Following BCR engagement, LYN induces phosphorylation of SYKTyr348 and Tyr352 in the linker region. Thereafter, SYK undergoes autophosphorylation of SYKTyr525/526 and associated activation. (B-C) BCR signaling in R406-sensitive (B) and R406-resistant (C) DLBCL cell lines. The integrity of the BCR signaling pathway was assessed by crosslinking the B-cell receptor (+ BCR) and analyzing downstream events including the initial tyrosyl phosphorylation of SYK352, the subsequent autophosphorylation of SYK525/526 and the SYK-dependent phosphorylation of the B-cell linker protein (BLNK). In the same experiments, the specificity of R406 was assessed by incubating each of the DLBCL cell lines with the compound prior to BCR crosslinking (+ R406). Untreated cells or cells stimulated with anti-IgG/IgM in the presence or absence of R406 were then lysed and lysates size-fractionated, blotted, and analyzed with phospho-specific antibodies directed against SYK352 (top panel), SYK525/526 (middle panel), and BLNK (bottom panel). Thereafter, the blots were subsequently stripped and reprobed with anti-(total) SYK or anti-(total) BLNK antibodies as indicated.
BCR signaling is intact in R406-sensitive DLBCL cell lines. (A) SYK domains and key tyrosine residues. The SYK tandem SH2 domains (black boxes), the linker region (aa 264-370), and the kinase domain (gray box) are shown. N indicates NH2-terminal; C, C-terminal; Y, tyrosine; P, phosphorylation. Following BCR engagement, LYN induces phosphorylation of SYKTyr348 and Tyr352 in the linker region. Thereafter, SYK undergoes autophosphorylation of SYKTyr525/526 and associated activation. (B-C) BCR signaling in R406-sensitive (B) and R406-resistant (C) DLBCL cell lines. The integrity of the BCR signaling pathway was assessed by crosslinking the B-cell receptor (+ BCR) and analyzing downstream events including the initial tyrosyl phosphorylation of SYK352, the subsequent autophosphorylation of SYK525/526 and the SYK-dependent phosphorylation of the B-cell linker protein (BLNK). In the same experiments, the specificity of R406 was assessed by incubating each of the DLBCL cell lines with the compound prior to BCR crosslinking (+ R406). Untreated cells or cells stimulated with anti-IgG/IgM in the presence or absence of R406 were then lysed and lysates size-fractionated, blotted, and analyzed with phospho-specific antibodies directed against SYK352 (top panel), SYK525/526 (middle panel), and BLNK (bottom panel). Thereafter, the blots were subsequently stripped and reprobed with anti-(total) SYK or anti-(total) BLNK antibodies as indicated.
In all 10 R406-sensitive DLBCL cell lines, BCR crosslinking markedly increased SYK352 and SYK525/6 phosphorylation; 9 of 10 lines also had significantly increased BLNK tyrosyl phosphorylation following BCR engagement (Figure 3B). In contrast, only 2 of the 5 R406-resistant DLBCL cell lines exhibited increased SYK352 phosphorylation and none of these lines had more abundant SYK525/6 or BLNK phosphorylation after BCR crosslinking (Figure 3C). Taken together, these data indicate that the BCR signaling pathway is intact in the R406-sensitive/BCR-type DLBCL cell lines (Figure 3B) and inactive or nonfunctional in R406-resistant/non-BCR tumors (Figure 3C).
In these same experiments, we assessed the specificity of R406 by preincubating each of the DLBCL cell lines with the compound prior to BCR crosslinking. Consistent with the known specificity of R406 for the auto-activation domain (SYK525/526), R406 did not alter SYK352 phosphorylation following BCR engagement (Figure 3B top panel). However, R406 completely inhibited the phosphorylation of SYK525/526 and the SYK-dependent phosphorylation of BLNK in R406-sensitive DLBCLs following BCR crosslinking (Figure 3B middle and bottom panels). In contrast, R406 had no effect on downstream events in the resistant/non-BCR DLBCL cell lines (Figure 3C). Taken together, these biochemical data strongly support the hypothesis that R406 cytotoxicity is due to the specific inhibition of SYK525/526 autophosphorylation and activation in DLBCLs with an intact BCR signaling pathway.
R406-sensitive DLBCL cell lines exhibit tonic BCR signaling
Given the critical role of SYK in tonic BCR signaling, we sought a more sensitive method to assess low-level SYK and BLNK phosphorylation in the DLBCL cell lines in the absence of BCR crosslinking. For these studies, we used the recently described method of single-cell phospho-flow cytometry.19,21 With this approach, it is possible to precisely quantitate SYK activation by measuring phospho-SYK348, -SYK352, and -BLNK expression in DLBCLs before and after BCR engagement (Figure 4).
Tonic BCR signaling in R406-sensitive and -resistant DLBCL cell lines. Single-cell phospho-flow cytometry was used to assess low-level SYK348 and 352 and BLNK phosphorylation in the absence (green) or presence (red) of BCR crosslinking in R406-sensitive (A) and -resistant (B) DLBCL cell lines. Cells stained with an isotype-matched control Ig are shown in black. The x-axis denotes expression (log scale) and the y-axis indicates cell number.
Tonic BCR signaling in R406-sensitive and -resistant DLBCL cell lines. Single-cell phospho-flow cytometry was used to assess low-level SYK348 and 352 and BLNK phosphorylation in the absence (green) or presence (red) of BCR crosslinking in R406-sensitive (A) and -resistant (B) DLBCL cell lines. Cells stained with an isotype-matched control Ig are shown in black. The x-axis denotes expression (log scale) and the y-axis indicates cell number.
Of interest, all of the R406-sensitive/“BCR-type” DLBCL cell lines exhibited immunodetectable tonic phosphorylation of SYK348 and SYK352 and the majority of these lines also had lower but detectable baseline phospho-BLNK (Figure 4A). As expected, SYK348, SYK352, and BLNK phosphorylation markedly increased following BCR crosslinking in these DLBCLs (Figure 4A).
Consistent with the preceding biochemical analysis (Figure 3C), only 2 of 5 R406-insensitive/non-BCR DLBCL cell lines exhibited tonic phosphorylation of SYK348 and SYK352 and none of these lines had evidence of tonic phospho-BLNK (Figure 4B). In agreement with the biochemical studies (Figure 3C), only 2 of the R406-insensitive cell lines exhibited modest increased SYK348 or SYK352 phosphorylation and none had increased phospho-BLNK following BCR engagement (Figure 4B).
R406 inhibits tonic BCR signaling in DLBCL cell lines
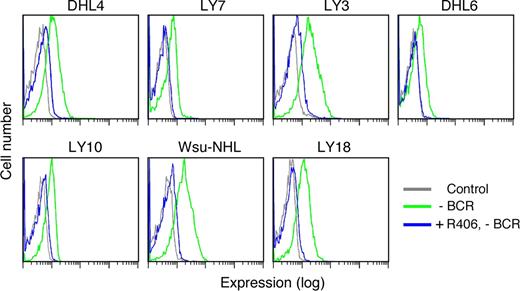
After documenting SYK and BLNK phosphorylation in R406-sensitive DLBCLs in the absence of Ig crosslinking (Figure 4A), we assessed the effects of R406 on tonic BCR signaling. Since R406 specifically inhibits SYK525/526 autophosphorylation and downstream signaling events including the phosphorylation of BLNK (Figure 3), we selected R406-sensitive DLBCL cell lines (Figures 1 to 3), treated the lines with R406 or vehicle alone, and evaluated tonic pBLNK levels in the absence of Ig crosslinking (Figure 5). As indicated, R406 treatment markedly reduced tonic BLNK phosphorylation (Figure 5).
R406 inhibits tonic BLNK tyrosine phosphorylation in DLBCL cell lines. DLBCL cell lines were treated with 4 μM of R406 (blue) or vehicle alone (green) at 37°C for 16 hours without crosslinking the BCR receptor (-BCR). Tonic BLNK phosphorylation in R406- or vehicle-treated cells was detected by single-cell phospho-flow cytometry. Gray lines represent cells stained with an isotype-matched control Ig.
R406 inhibits tonic BLNK tyrosine phosphorylation in DLBCL cell lines. DLBCL cell lines were treated with 4 μM of R406 (blue) or vehicle alone (green) at 37°C for 16 hours without crosslinking the BCR receptor (-BCR). Tonic BLNK phosphorylation in R406- or vehicle-treated cells was detected by single-cell phospho-flow cytometry. Gray lines represent cells stained with an isotype-matched control Ig.
R406-sensitive DLBCL cell lines express high levels of cell-surface Ig
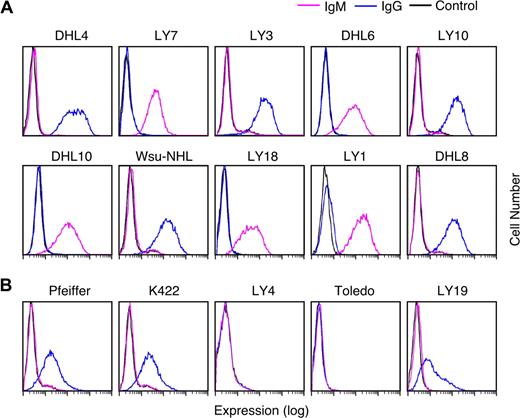
Given the documented association between R406 sensitivity and an intact BCR signaling cascade (Figures 3,Figure 4–5), we next asked whether R406-sensitive and resistant DLBCL cell lines differed in their expression of cell-surface Ig. For this analysis, the respective DLBCL cell lines were immunophenotyped with murine anti–human IgG and IgM and analyzed by flow cytometry. All R406-sensitive DLBCL cell lines expressed cell-surface IgM or high levels of cell-surface IgG (Figure 6A). In contrast, 2 of the R406-insensitive lines lacked surface Ig and 3 lines had low to moderate levels of surface IgG (Figure 6B).
Cell-surface immunoglobulin expression in R406-sensitive and -resistant DLBCL cell lines. Cell-surface IgG (blue) and IgM (purple) expression in R406-sensitive (A) and -resistant (B) DLBCL cell lines were evaluated using standard immunophenotyping procedures and flow cytometry. As indicated, 5 of the R406-sensitive DLBCL cell lines expressed high levels of cell-surface IgG and 5 lines expressed surface IgM. Two of the R406-resistant cell lines were surface Ig negative and 3 lines had low/moderate levels of cell-surface IgG. Cells stained with an isotype-matched control Ig are shown in black.
Cell-surface immunoglobulin expression in R406-sensitive and -resistant DLBCL cell lines. Cell-surface IgG (blue) and IgM (purple) expression in R406-sensitive (A) and -resistant (B) DLBCL cell lines were evaluated using standard immunophenotyping procedures and flow cytometry. As indicated, 5 of the R406-sensitive DLBCL cell lines expressed high levels of cell-surface IgG and 5 lines expressed surface IgM. Two of the R406-resistant cell lines were surface Ig negative and 3 lines had low/moderate levels of cell-surface IgG. Cells stained with an isotype-matched control Ig are shown in black.
Predictors of R406 sensitivity
We then compared the utility of the following parameters as predictors of R406 sensitivity in the DLBCL cell lines: cell-surface Ig expression; tonic phosphorylation of SYK352; BCR crosslink-induced phosphorylation of SYK525/526 and BLNK; R406 inhibition of SYK525/6 and BLNK phosphorylation following BCR engagement; and designation as a “BCR-type” DLBCL by transcriptional profiling (Table 1). All 10 R406-sensitive DLBCL cell lines exhibited markedly increased SYK525/526 tyrosyl phosphorylation following BCR crosslinking, whereas none of the R406-resistant cell lines responded in this manner (P < .001, Table 1). Similarly, 9 of 10 R406-sensitive lines and 0 of 5 resistant lines exhibited increased BLNK tyrosyl phosphorylation following BCR engagement (P = .002, Table 1). Furthermore, all of the 10 R406-sensitive DLBCL cell lines were previously and independently classified as BCR-type tumors whereas none of the R406-resistant cell lines fell into the BCR category (P < .001, Table 1). Taken together, these data indicate that R406-sensitive DLBCL cell lines have an intact BCR signaling pathway, depend on tonic BCR survival signals, and molecularly classify as “BCR-type” tumors (Table 1).
BCR signaling and SYK inhibition in primary DLBCLs
After demonstrating that R406 sensitivity was dependent on intact BCR signaling in DLBCL cell lines (Figures 1,3,Figure 4,Figure 5–6 and Table 1), we evaluated the same parameters in viable tumor cell suspensions from 10 primary DLBCLs. Given the limited numbers of tumor cells in each sample, we used standard and phosphoflow cytometry to evaluate cell-surface Ig, assess pSYK352 and pBLNK expression at baseline and following BCR crosslinking, and compare pBLNK levels after anti-Ig treatment in the presence or absence of R406.
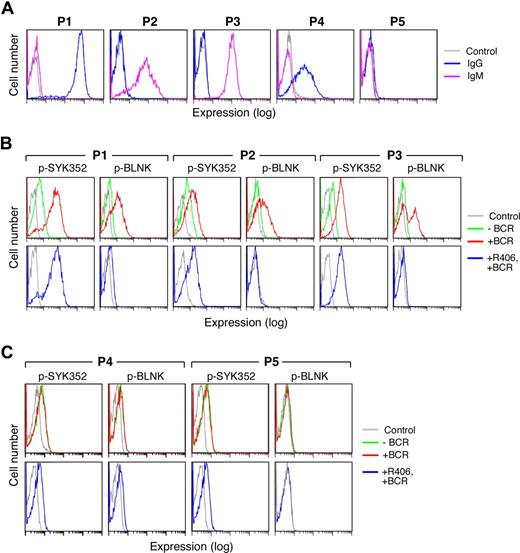
Five representative primary DLBCLs are shown in Figure 7. In the majority of examined primary DLBCLs, the tumor cells expressed sIgM or high-level sIgG (Figure 7A, P1-3) and clearly detectable pSYK352 at baseline (Figure 7B, top panel). In each of these viable tumor cell samples, BCR crosslinking markedly increased SYK352 and BLNK tyrosyl phosphorylation (Figure 7B top panel). Furthermore, R406 inhibited the SYK-dependent phosphorylation of BLNK following BCR engagement (Figure 7B bottom panel).
Surface Ig expression and BCR signaling in primary DLBCLs. Cryopreserved tumor cell suspensions were thawed and viable tumor cells were isolated from a Ficoll Hypaque monolayer. Thereafter, the tumor cell suspensions were over 90% viable by Trypan blue staining. Light microscopy, light scatter analysis at flow cytometry, and cell-surface Ig expression confirmed the presence of a predominant population of tumor cells. (A) Cell-surface Ig expression in primary DLBCLs. Cell-surface IgG (blue) and IgM (purple) were evaluated by flow cytometry. P1 expressed high levels of sIgG whereas P2 and P3 expressed sIgM. P4 expressed lower levels of sIgG and P5 lacked surface Ig expression. Cells stained with an isotype-matched control Ig are shown in gray. (B,C) BCR signaling in primary DLBCLs. Single-cell phospho-flow cytometry was used to assess pSYK352 and pBLNK expression in the absence (green) or presence (red) of BCR crosslinking or BCR crosslinking following R406 treatment (blue). Primary DLBCLs with intact BCR signaling (B) and ineffective BCR signaling (C) are shown. Cells stained with isotype-matched control Ig are shown in gray.
Surface Ig expression and BCR signaling in primary DLBCLs. Cryopreserved tumor cell suspensions were thawed and viable tumor cells were isolated from a Ficoll Hypaque monolayer. Thereafter, the tumor cell suspensions were over 90% viable by Trypan blue staining. Light microscopy, light scatter analysis at flow cytometry, and cell-surface Ig expression confirmed the presence of a predominant population of tumor cells. (A) Cell-surface Ig expression in primary DLBCLs. Cell-surface IgG (blue) and IgM (purple) were evaluated by flow cytometry. P1 expressed high levels of sIgG whereas P2 and P3 expressed sIgM. P4 expressed lower levels of sIgG and P5 lacked surface Ig expression. Cells stained with an isotype-matched control Ig are shown in gray. (B,C) BCR signaling in primary DLBCLs. Single-cell phospho-flow cytometry was used to assess pSYK352 and pBLNK expression in the absence (green) or presence (red) of BCR crosslinking or BCR crosslinking following R406 treatment (blue). Primary DLBCLs with intact BCR signaling (B) and ineffective BCR signaling (C) are shown. Cells stained with isotype-matched control Ig are shown in gray.
In marked contrast, other primary DLBCLs expressed only low-to-moderate levels of sIgG or lacked cell-surface Ig (Figure 7A, P4-P5). These viable tumor cell samples had no or low levels of detectable pSYK352 at baseline (Figure 7C top panel). In these primary DLBCLs, there was no change in pSYK352 and pBLNK levels following BCR crosslinking (Figure 7C top panel) and R406 had no observed effect (Figure 7C bottom panel). These analyses of primary DLBCLs (Figure 7), which are in agreement with those of DLBCL cell lines (Figures 3,4,6), indicate that primary DLBCLs also differ in their cell-surface Ig expression, tonic, and induced BCR signaling and sensitivity to R406.
Discussion
Herein, we report that the majority of examined DLBCL cell lines exhibit tonic BCR signaling as evidenced by basal phosphorylation of SYK348 and 352 and the SYK-dependent linker protein, BLNK. DLBCL cell lines with an intact BCR signaling pathway were highly sensitive to the ATP-competitive SYK inhibitor, R406, which blocked SYK525/6 autophosphorylation and downstream signaling and induced apoptosis. Of interest, the DLBCL cell lines with an intact BCR signaling pathway and sensitivity to the SYK inhibitor were independently identified as “BCR” tumors on the basis of their transcriptional profiles. These data suggest that tonic BCR signaling is an important and potentially targetable survival pathway in some, but not all, DLBCLs and that R406-sensitive DLBCLs can be identified by their transcriptional profiles. Of importance, we also detected tonic and induced BCR signaling and R406 responses in some, but not all, primary DLBCLs, indicating that the findings in DLBCL cell lines are directly applicable to primary tumors.
The precise mechanism for initiating and regulating tonic BCR signaling remains to be defined. Monroe et al4 have described a likely model, termed homeostatic equilibrium, in which these responses are regulated by the steady-state activity of BCR-associated PTKs and PTPs. In this model, a transient ligand-independent signal is generated by the stochastic interaction of positive regulators with the BCR complex and subsequent activation of BCR-associated PTKs, ITAM phosphorylation, assembly of Igα/β signaling complexes, and SYK phosphorylation.4 Thereafter, the stochastic signaling at individual BCR complexes is quickly terminated by multiple negative regulators, likely including PTPs such as PTPROt.4,10 In the proposed model, inhibition of the negative-regulatory arm would lead to a stabilized signal, which occurs in the absence of ligand-induced aggregation.4 This hypothesis is consistent with experimental data indicating that in B cells, chemically induced global PTP inactivation with pervanadate or H2O2 results in ligand-independent BCR signaling and phosphorylation of downstream substrates.4,7,8
Although the majority of examined DLBCL cell lines exhibited tonic BCR signaling and SYK-dependent survival, a subset of lines either had no detectable BCR signals or truncated ineffective signals that were not transmitted downstream of SYK (Figures 3C and 4B). These R406-insensitive DLBCL cell lines included ones with no cell-surface BCR expression or comparatively lower levels of cell-surface IgG and no BCR signaling beyond SYK itself. These data suggest that R406-insensitive lines likely rely on alternative survival pathways, consistent with their identification as “non-BCR” DLBCLs on the basis of their transcriptional profiles. Of importance, primary DLBCLs with no or low cell-surface Ig and no detectable BCR signals were also identified (Figure 7C).
In certain DLBCL cell lines and primary tumors, the absence of tonic and induced BCR signals can be attributed to the lack of cell-surface Ig. However, other mechanisms likely explain the ineffective BCR signaling in DLBCL cell lines and primary tumors with at least low or moderate surface Ig expression. In the DLBCL cell lines with these features (Pfeiffer, K422, and LY19), there were no mutations of the SYK coding sequence, prompting consideration of other alternatives. Of interest, only cell lines and primary tumors with relatively lower levels of cell-surface IgG had ineffective BCR signaling; in contrast, all DLBCL lines and primary tumors with high cell-surface IgG or (any) cell-surface IgM had intact BCR signaling.
Given the promising activity of R406 in in vitro studies and the availability of the oral SYK inhibitor for clinical trials, it is important to develop methods for identifying the tumors that are most likely to respond to inhibition of tonic BCR signaling. In an extensive panel of DLBCL cell lines, intracellular phospho-flow analysis of basal pSYK348 and 352 and p-BLNK was extremely accurate in identifying lines dependent on tonic BCR signaling and sensitive to R406-mediated cytotoxicity (Figure 4 and Table 1). In addition, DLBCL cell lines with evidence of tonic BCR signaling and R406 sensitivity typed as “BCR tumors,” highlighting the potential value of profile-defined comprehensive clusters13 with targetable subtype-specific survival pathways. In an initial series of primary DLBCLs, phospho-flow analysis also identified those tumors with intact BCR signaling and R406 responses. Of interest, the R406 concentrations that inhibit tonic BCR signaling and induce apoptosis in DLBCLs in vitro, 1 μM to 4 μM, are readily achievable in vivo (E. Grossbard, Rigel Pharmaceuticals, San Francisco, CA, personal written communication, July 2007).
Additional recent studies also suggest that SYK-dependent survival signals may play a role in B-cell malignancies, including DLBCL, mantle cell lymphoma, and follicular lymphoma.19,22,,–25 Treatment of 2 DLBCL lines (DHL4 and LY10) with compounds reported to inhibit SYK (piceatannol or curcumin) decreased the proliferation of these cells.23,24 Similarly, piceatannol inhibited the proliferation and induced apoptosis of a mantle cell lymphoma cell line (JEKO-1) with low-level SYK amplification.22 In addition, BCR-mediated signaling (Ig crosslinking in the presence of H2O2) occurred more rapidly and was more prolonged in tumor cells from primary follicular lymphoma samples than in infiltrating nonmalignant B cells.19
For all of these reasons, SYK is an attractive rational target in DLBCL and possibly other B-cell malignancies and oral R406 is a promising targeted treatment that is now being evaluated in a phase 1/2 clinical trial.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This research was funded by National Institutes of Health grants CA92625 and CA97274 and the Leukemia & Lymphoma Society of America grant 7391-07.
National Institutes of Health
Authorship
Contribution: L.C. designed research, performed research, contributed vital new reagents, analyzed data, and wrote the paper; S.M. designed research, performed research, analyzed data, and wrote part of the paper; P.J., T.E.W., and T.M.H. contributed vital new reagents and analyzed data; J.D., W.C., and J.L.K. performed research; M. A. S. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: M.A.S. consulted with Rigel. The authors declare no competing financial interests.
Correspondence: Margaret A. Shipp, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA; e-mail: margaret_shipp@dfci.harvard.edu.