Abstract
Protein microarrays presenting spots of collagen and lipidated tissue factor (TF) allowed a determination of the critical surface concentration of TF required to trigger coagulation under flow. Whole blood supplemented with corn trypsin inhibitor (to inhibit factor XIIa) was perfused over microarrays for 5 minutes. Immunofluorescence staining of platelet glycoprotein GPIbα and fibrin(ogen) revealed a critical TF concentration (EC50) of 3.6, 8.4, and 10.2 molecules-TF/μm2 at wall shear rates of 100, 500, and 1000 s−1, respectively. For collagen arrays where only the center lane of spots (in the direction of flow) contained TF, a downstream distance of 14 mm was required for the thrombus to widen enough to reach across a 300-micrometer gap to the adjacent TF-free lanes of collagen spots, in agreement with numerical simulation. To investigate the effect of low levels of circulating TF, whole blood (± 100 fM added TF) was tested under static and flow conditions. After 5 minutes, the addition of 100 fM TF to whole blood had negligible effect under static conditions, but caused a 2.5-fold increase in fibrin formation under flow. This report defines the threshold concentrations of surface TF required to trigger coagulation under flow.
Introduction
Plaque rupture reveals tissue factor (TF) to flowing blood, resulting in coronary thrombosis and occlusion with consequent acute myocardial infarction. Despite the prevalence of this event, the critical concentration of surface tissue factor required to cause clotting at various hemodynamic conditions remains poorly defined. In addition, the existence, source(s), and functional activity of circulating levels of tissue factor are not fully resolved in health or disease. The function of circulating TF in concert with wall-derived TF may depend on prevailing flow conditions.
TF in a lipid surface serves as a cofactor for factor VIIa (present at ∼ 1% of the 10-nM factor VII concentration) resulting in approximately 105-fold enhancement of factor Xa formation.1 Platelet deposition may reduce access of factor X to the TF/VIIa complex formed on the damaged wall.2,3 Elevated TF antigen and activity are detectable in human atherosclerotic lesions and are expressed by various cell types.4 Bonderman et al5 determined, using ex vivo plaque disruption/scraping, that the average TF site density underneath plaques is 33 pg TF/cm2, corresponding to approximately 6 molecules-TF/μm2. Drake et al6 found that in human cardiac and skeletal muscle the TF levels were 7 and 119 ng TF/mg protein, respectively. Tissue factor pathway inhibitor (TFPI) is also elevated in atherosclerotic vessels in comparison with 10 to 20 ng TFPI/cm2 in healthy vessels.7
Blood-borne tissue factor antigen was first reported in a system using 5-minute ex vivo perfusion of human blood over collagen-coated slides,8 a system in which fibrin deposition was blocked by inhibited factor VIIa (FVIIai). Collagen-activated platelets are highly procoagulant and may present factor VIIa cofactor activity susceptible to antagonism by antibodies or FVIIai.9-11 A recent study of 91 individuals using the Luminex assay (Austin, TX) indicated that most healthy individuals had less than 2 pM TF in plasma,12 a value lower than the average 4 pM TF obtained from a literature survey of plasma TF levels in healthy individuals measured by enzyme-linked immunosorbent assay (ELISA). Addition of increasing amounts of subpicomolar levels of lipidated TF to corn trypsin inhibitor (CTI)–treated whole blood indicates that active TF in healthy individuals is subpicomolar, estimated to between less than 20 fM13 and less than 200 fM.14 Recently, rapid splicing of TF pre-mRNA and expression of TF antigen have been reported in sonicated membranes obtained from activated platelets.15 Under flow conditions, the transfer of tissue factor may be of importance via leukocyte delivery to platelets via CD1516 or capture of microparticles presenting TF and PSGL-117-19 or derived from platelets.10
Mathematic simulations of the hemostatic response have also taken into account the importance of tissue factor site density. Kuharsky and Fogelson3 developed a full transport-reaction coagulation model that takes into account surface-dependent reactions, transport of factors and cells due to flow, and populations of resting and activated platelets. In the Kuharsky-Fogelson model, an increase of the TF surface concentration from 2 to 8 fmol/cm2 (12 to 50 molecules-TF/μm2) was predicted to cause a 4- to 5-order of magnitude increase in local thrombin production under flow from 100 to 1500 s−1.
In prior work, we used a matrix protein microarray assay20 to spatially control surface composition by presenting collagen microspots to flowing platelet-rich plasma or whole blood. In the present study, we tested the effect of lipidated tissue factor in printed collagen microspots under defined laminar flow conditions. We also added low-level exogenous TF to whole blood prior to perfusion in this assay. We determined the threshold for TF to trigger coagulation under flow. While subpicomolar levels of lipidated TF had little effect under no-flow conditions at 5 minutes, this low level dramatically enhanced thrombosis in the presence of flow.
Methods
Materials
Mouse monoclonal antihuman CD42b (GPIbα Research Diagnostics, Flanders, NJ), FITC-conjugated rabbit antihuman fibrin/fibrinogen, FITC-conjugated Zenon Alexa Fluor 647 Mouse IgG1 Labeling Kit (Invitrogen, Carlsbad, CA), corn trypsin inhibitor (Haematologic Technologies, Essex Junction, VT), human serum albumin (Golden West Biologicals, Temecula, CA), native collagen fibrils (type I) from equine tendons suspended in isotonic glucose solution of pH 2.7 (Chrono-Log; Havertown, PA), bovine serum albumin and sodium citrate (Sigma-Aldrich, St Louis, MO), HEPES (N-(2-hydroxyethyl)piperazine-N′-2-ethanesulfonic acid; Fisher Scientific, Pittsburgh PA), lipidated recombinant human tissue factor (baculovirus expressed, amino acids 1-263; 43 kDa), and phosphate-buffered saline (PBS) without calcium chloride or manganese chloride (Invitrogen) were stored according to the manufacturers' instructions. IMUBIND Tissue Factor ELISA Test Kit (American Diagnostica, Stamford, CT) was used according to the manufacturer's instructions to determine the concentration of available tissue factor accessible to antibody in the intact lipidated TF preparation. The amount of available TF antigen of the stock solution was determined to be 18.2 nM.
Human blood was collected from healthy donors via venipuncture and anticoagulated with sodium citrate (9 parts blood to 1 part sodium citrate). Prior to perfusion, the plasma was treated with CTI (50 μg/mL) to block factor XIIa and associated intrinsic pathway initiation21 and then was recalcified with CaCl2 to a final calcium concentration of 20 mM. Phlebotomy was conducted in accordance with the Declaration of Helsinki and under University of Pennsylvania Institutional Review Board approval.
Matrix protein microarray perfusion assay
Collagen matrix microarrays were prepared as previously described.20 Briefly, an OmniGrid Accent (Genomic Solutions, Ann Arbor, MI) robotic contact microarrayer was used for arraying with a 1 × 1 pin protocol with an ArrayIt Stealth Micro Spotting Pin SMP-4 (Telechem International, Sunnyvale, CA). Prior to printing, plain glass slides (25 × 75 × 1.0 mm, SuperFrost Plus; Fisher Scientific) were incubated with 1 M NaOH (15 minutes), rinsed extensively using distilled water, ethanol rinsed, and vacuum-dried. Collagen (1 mg/mL) and tissue factor (0 TF, or 1 pM to 1 nM TF) in 5% (vol/vol) glycerol were printed via robotic contact printing (50% relative humidity). For the tissue factor/collagen microarrays, 14 columns (aligned in the direction of flow) by 30 rows were printed (spot center-to-center distance = 500 μm). Printed TF concentrations in the 14 columns were 0, 1, 2.5, 5, 7.5, 10, 25, 50, 75, 100, 250, 500, 750, or 1000 pM (1000 pM = 25 molecules-TF/μm2 for the 1-nL printed features of 175-μm diameter). After printing, the slides were stored at 4°C until mounted on flow chambers.
A parallel-plate perfusion chamber was used as previously described.22 The wall shear stress, τw (dyne/cm2) was calculated by τw = 6Qμ/B2W where Q is the volumetric flow rate (cm3/s), μ (Poise) is the viscosity of the fluid, B is the separation between the plates (0.02 cm), and W (1.11 cm) is the flow chamber width. The viscosity of whole blood was taken as 0.04 Poise at 37°C.23 Perfusion of 1% (wt/vol) human serum albumin (HSA) in HEPES-buffered saline (HBS, 20 mM HEPES, 150 mM NaCl, pH 7.4) at a wall shear rate of 25 s−1 for 5 minutes was used to rinse the glycerol from the microspots and to prevent nonspecific binding. Recalcified whole blood was then immediately perfused for 5 minutes at 37°C over the slide at various shear rates by withdrawal using a syringe pump (Harvard Apparatus, Holliston, MA).
Immunostaining and microarray scanning
After perfusion, the dismounted slides were immediately rinsed in 3% (wt/vol) BSA, fixed with 2% paraformaldehyde in PBS for 20 minutes, and rinsed with NH4Cl to quench any unreacted aldehyde. Anti-CD42b was fluorescently labeled using Zenon Alexa Fluor 647 Mouse IgG1 Labeling Kit according to the manufacturer's instructions. The labeled anti-CD42b and FITC conjugated antifibrin/fibrinogen was diluted 1:50 with 3% BSA solution. The slide was incubated with the antibodies for 1 hour and rinsed with 3% BSA. The slides were imaged using a cooled CCD microarray scanner (Alpha Array 8000; Alpha Innotech, San Leandro, CA) with Cy5 and FITC filter sets. The raw images were quantified for fluorescent intensity using ArrayVision 6.3 (Imaging Research, St Catharines, ON). The fluorescent intensity was background subtracted for each spot using background estimates from 30 regions around the array. For surface tissue factor EC50 analysis at each shear rate, a 4 parameter logistic equation, y = A + [(B − A)/(1 + 10(C−x)D)] was fit with the Levenburg-Marquardt algorithm (XLfit) with A indicating minimum y value; B, maximum y value; C, log (EC50); and D, slope factor. In calibration experiments, a maximum in fibrin production was reached at 12.5 to 25 molecules-TF/μm2 (corresponding to ∼ 1.3 × 109 FI signal for fibrin), with a slight decline in fibrin formation when higher levels of TF (> 25 molecules-TF/μm2) were used. The decrease in fibrin deposition at ultrahigh levels of TF more than 25/μm2 may be related to fibrin shielding of collagen and reduced platelet GPVI signaling in the microthrombus spots.
Numerical simulation of TF microspot experiment
The steady-state 3-dimensional mass conservation equation was solved using commercial finite element method software (COMSOL Multiphysics, Burlington, MA). The geometry consisted of a volume 1 mm in width, 14 mm in length, and 50 μm in height. Microspots with a diameter of 200 μm were spaced 0.5 mm apart along the y-axis at the center of the plane (physical properties and boundary conditions summarized in Table 1). A previously reported platelet thrombin flux24 (U/platelet-second) was multiplied by 500 platelets20 and divided by the microspot area to calculate the thrombin flux for each microspot. The thrombin conservation equation was coupled to the velocity profile for flow between 2 parallel plates: vy(z) = γw(z2/B − z), where vy(z) is the velocity (cm/s) in the direction of flow as a function of height from the bottom plate, γw is the wall shear rate (1/s), and B is the gap height (cm) between the 2 plates.
Pseudo first-order kinetics were assumed for ATIII inhibition of thrombin because the concentration of ATIII (5 μM) is much greater than the highest concentration achieved within the simulated thrombi (∼ 100 nM). The rate constant for thrombin inhibition was calculated by multiplying the second-order rate constant from Hockin et al25 by the ATIII plasma concentration. The grid consisted of 58 625 prism elements. Mesh independence was evaluated by comparing the concentration profiles for grids consisting of 23 996, 58 625, and 93 800. There was no greater than 1.5% difference in the magnitude of the concentration between the 3 grid sizes at any position within the simulation geometry. The steady-state mass conservation equation was solved in 12 minutes using a GMRES solver (2007, COMSOL) on a PC workstation (2.2 GHz processor, 12 GB RAM).
Results
Surface TF titration
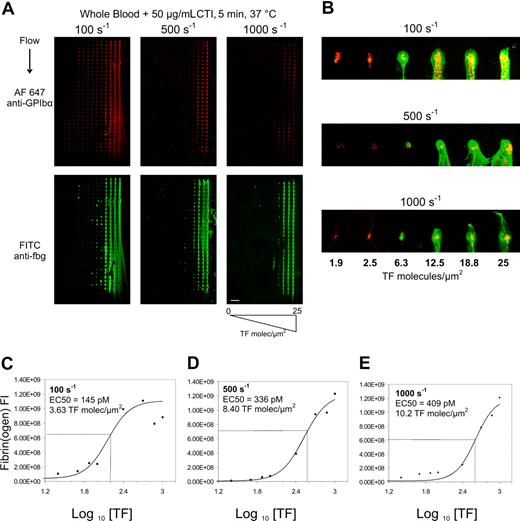
To determine the level of surface TF needed to cause combined platelet and fibrin deposition under physiological hemodynamic flows, microarrays presenting collagen and increasing levels of tissue factor from 1 pM to 1000 pM TF (corresponding to 0.025 to 25 molecules-TF/μm2) were mounted in parallel plate flow chambers. Recalcified CTI-treated whole blood was perfused over 3 separate arrays at 3 different wall shear rates (γw = 100, 500, or 1000 s−1) (Figure 1A). On pure collagen lacking TF (left-most column of each array in Figure 1A), platelet deposition was greatest at the venous shear rate of 100 s−1 and was reduced at arterial shear rates of 500 and 1000 s−1, as expected for surfaces lacking von Willebrand factor.
Titration of surface tissue factor required to trigger fibrin formation. Immunostaining of platelet GPIbα (top) and fibrin(ogen) (bottom) after 5-minute whole blood perfusion over collagen/TF arrays at wall shear rates of 100, 500, and 1000 s−1 (A). Surface tissue factor concentration in each feature increased from left to right from 0 to 25 molecules-TF/μm2 (scale bar = 1 mm). Increased magnification of the first row of each array for each shear rate for surface TF concentration ranging from 1.9 to 25 molecules-TF/μm2 (scale bar = 300 μm; yellow indicates colocalized GPIbα and fibrin(ogen)) (B). Column averages of fluorescent intensity (FI) of the fibrin(ogen) staining are plotted against the TF surface concentration for 100 s−1 (C), 500 s−1 (D), and 1000 s−1 (E) for 3 independent experiments (3 × 30 spots per plotted data point). Data were fit to a 4-parameter logistic equation to determine the TF EC50 values for each shear rate.
Titration of surface tissue factor required to trigger fibrin formation. Immunostaining of platelet GPIbα (top) and fibrin(ogen) (bottom) after 5-minute whole blood perfusion over collagen/TF arrays at wall shear rates of 100, 500, and 1000 s−1 (A). Surface tissue factor concentration in each feature increased from left to right from 0 to 25 molecules-TF/μm2 (scale bar = 1 mm). Increased magnification of the first row of each array for each shear rate for surface TF concentration ranging from 1.9 to 25 molecules-TF/μm2 (scale bar = 300 μm; yellow indicates colocalized GPIbα and fibrin(ogen)) (B). Column averages of fluorescent intensity (FI) of the fibrin(ogen) staining are plotted against the TF surface concentration for 100 s−1 (C), 500 s−1 (D), and 1000 s−1 (E) for 3 independent experiments (3 × 30 spots per plotted data point). Data were fit to a 4-parameter logistic equation to determine the TF EC50 values for each shear rate.
At each wall shear rate, a marked increase in fibrin(ogen) staining was detected at the highest concentrations of TF tested. At 100 s−1, as surface TF levels increased across the microarray, platelet deposition also increased at subcritical levels of TF (< 2 molecules-TF/μm2) indicating that thrombin production can enhance platelet deposition prior to fibrin formation. At fewer than 2 molecules-TF/μm2, there was essentially no effect of surface TF on either platelet deposition or fibrin formation at 5 minutes at arterial wall shear rates (500 or 1000 s−1). While collagen alone or collagen with low TF (< 2 molecules-TF/μm2) was insufficient to drive fibrin formation after 5 minutes of blood exposure, substantial quantities of platelets were deposited at each shear rate. At a venous wall shear rate of 100 s−1, moderate to high levels of TF ranging from 1.9 to 25 molecules-TF/μm2 caused increasing deposition of platelets and fibrin, indicating that thrombin production facilitated platelet incorporation into the growing thrombi (up to 300 to 500 platelets/spot). The impact of thrombin production at 1.9 to 25 molecules-TF/μm2 on platelet deposition was less pronounced at the highest arterial shear rate of 1000 s−1 (Figure 1B). Consistent with prior studies with platelet-rich plasma perfusion,20 essentially no platelet deposition was detected on the glass microarray surface lacking printed collagen features.
In the narrow range of 1.9 to 25 molecules-TF/μm2, fibrin formation switched from negligible to maximal production at all shear rates tested (Figure 1A-E). The calculated effective concentration to produce half maximal fibrin deposition (EC50) was 3.63 molecules-TF/μm2 at 100 s−1, 8.40 molecules-TF/μm2 at 500 s−1, and 10.2 molecules-TF/μm2 at 1000 s−1 (Figure 1C-E). At 100 s−1, fibrin formation at 5 minutes appeared to reach a maximal extent as surface TF increased to levels more than 12.5 molecules-TF/μm2. Based on fluorescence immunostaining, there was a more than 10-fold increase in fibrin density at the highest concentration of TF tested. At fewer than 1.9 molecules-TF/μm2, there was little dependence of fibrin(ogen) staining on surface TF concentration, consistent with detection of platelet-bound fibrinogen. To test the role of washout of the lipidated TF from the collagen microspot, we compared thrombosis on the TF-laden collagen microarrays with and without 5-minutes prerinse at 25 s−1. We found that prerinse had no effect on platelet deposition and caused a modest 29% right shift of the EC50 for fibrin formation (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Transport of species with and transverse to flow
Transport of soluble reactive species in the flow stream can remove species from the local site of formation as well as propagate thrombosis beyond the site of wall disruption. We developed a specialized printed array to investigate transport effects of soluble species in the direction of flow (by convection) and transverse to the direction of flow (by diffusion/dispersion). This array had one column of collagen and 1000 pM TF (25 molecules-TF/μm2) surrounded by collagen spots. Thus, there was one “active” lane of spots surrounded by “inactive” lanes. Whole blood was perfused at γw = 100 s−1 for 5 minutes at 37°C. The fibrin-rich thrombus extended well past the array length indicating that fibrin polymerization could continue well downstream of the location of printed features. The fibrin tail was notably depleted of platelets (Figure 2A). However, the fibrin remained highly localized over and downstream of the TF-laden lane of spots with a width comparable with the original collagen/TF features (Figure 2A,B). A substantial distance of 14 mm downstream was needed to achieve a maximum fibrin width of 350 μm from the centerline (250 micrometers from the outer radius of the feature). Even at a venous shear rate, it is difficult for a clot to propagate via diffusion or platelet deposition in a direction transverse to the flow, from a site of high surface TF to a nearby neighboring site of collagen lacking TF. Numerical simulation of constant thrombin release from a center lane of microspots (Table 1) followed by inhibition with anti–thrombin III predicted that active thrombin can be detected downstream of the printed collagen/TF features, however diffusive/dispersive penetration of thrombin in a direction transverse to the flow is severely restricted by the flow (Figure 2C). Diffusive penetration of thrombin into the flow field normal to the surface was also equally restricted above the surface (not shown).
Convection-diffusion processes in coagulation. Immunostaining of platelet GPIbα and fibrin(ogen) after 5-minute whole blood perfusion over “center lane” array at 100 s−1. (A) Bottom arrow indicates column where collagen and 25 molecules-TF/μm2 spots were printed (scale bar = 1 mm). Magnified images are of the regions indicated by the white squares (scale bar = 300 μm). Identical results observed in 3 separate experiments. Growth of fibrin width from center of “center lane” plotted against distance downstream from first spot (B). Steady-state numerical simulation of thrombin at 10 μm above the surface for the center lane experiment shown in panel A using constant flux of thrombin from each microarray feature into a flow field containing ATIII (see Table 1 for parameters) (C). Magnified images are the same areas as those in panel A.
Convection-diffusion processes in coagulation. Immunostaining of platelet GPIbα and fibrin(ogen) after 5-minute whole blood perfusion over “center lane” array at 100 s−1. (A) Bottom arrow indicates column where collagen and 25 molecules-TF/μm2 spots were printed (scale bar = 1 mm). Magnified images are of the regions indicated by the white squares (scale bar = 300 μm). Identical results observed in 3 separate experiments. Growth of fibrin width from center of “center lane” plotted against distance downstream from first spot (B). Steady-state numerical simulation of thrombin at 10 μm above the surface for the center lane experiment shown in panel A using constant flux of thrombin from each microarray feature into a flow field containing ATIII (see Table 1 for parameters) (C). Magnified images are the same areas as those in panel A.
Effect of 100 fM added TF: role of flow
In healthy or diseased individuals, low levels of circulating active TF may have a consequence on the propagation of the coagulation response. Since less is known about the interaction of circulating TF with wall-derived TF under flow conditions, we tested the functional significance of adding 100 fM lipidated TF to CTI-treated whole blood under static and flow conditions. Butenas et al13 found that under rocking conditions the addition of approximately 100 fM lipidated TF to CTI-treated whole blood reduced the visual clot time (appearance of clumps) by approximately 50% within approximately 20 minutes, although thrombin-antithrombin or fibrin production was not measured. Using thrombin-antithrombin (TAT) ELISA or the fluorogenic thrombin substrate boc-VPR-MCA in CTI-treated whole blood in static well plate assay, we found that adding 100 fM TF produced no detectable thrombin within 5 minutes, but reduced the clotting time from greater than 60 minutes to between 20 and 40 minutes (Figure S2). Thus, addition of 100 fM lipidated TF to CTI-treated whole blood was a minimal perturbation of blood under static conditions with respect to thrombin production at 5 minutes after TF addition. Next, CTI-treated whole blood with and without added 100 fM TF was introduced into 2 independent microarray flow chambers. For the static assay, whole blood was perfused into the chamber at 50 s−1. Once the chamber was filled with blood, perfusion was stopped and the chamber was incubated for 5 minutes at 37°C. Under static conditions, increasing surface TF to more than 2 molecules-TF/μm2 caused a marked enhancement of platelet and fibrin deposition at 5 minutes (Figure 3A,B dotted line). Interestingly, adding 100 fM lipidated TF to whole blood caused a slight reduction in platelet and fibrin deposition on the printed microarray under the no-flow condition. In the absence of flow, only platelets initially near the surface can interact with the microarray, and a low buildup of platelets on the collagen was expected. The slight decrease in platelet deposition by added TF under no-flow conditions shown in Figure 3B was unexpected. Under no-flow conditions, cell settling is the dominant mechanism of platelet delivery to the surface. Subtle changes in red blood cell or platelet motions under gravity-driven settling could be altered by the activity of the added TF. In addition, we note that the platelet structures formed under static conditions were not nearly as dense or stable as those formed under flow. Thus, platelet incorporation by settling to the surface may lead to more friable structures (analogous to structural differences due to diffusion-limited vs ballistic aggregation) with potentially reduced interaction with collagen. For CTI–whole blood perfusion (no added TF), the presence of flow substantially enhanced platelet delivery and accumulation on the microarray, at all prevailing surface TF levels (Figure 3B solid line). In the presence of flow, adding 100 fM TF slightly enhanced platelet deposition by approximately 30% to 40% at all surface TF levels (Figure 3B). Thus, addition of 100 fM TF did not enhance platelet deposition on collagen or collagen/TF microspots in the absence of flow, but did enhance platelet deposition on collagen or collagen/TF microspots in the presence of flow at 5 minutes. The effect of 100 fM lipidated TF was apparent under flow at fewer than 2 molecules-TF/μm2 as indicated by the number of activated platelets captured to the surface and by the fibrinogen staining of those captured platelets (Figure 3A,B). Platelet deposition was largely independent of surface TF up to approximately 8 molecules-TF/μm2. However, under flow conditions at more than 10 molecules-TF/μm2, the intensity of fibrin deposition was markedly enhanced by 2.5-fold by the addition of 100 fM circulating TF. Such elevation in fibrin deposition was not observed by adding 100 fM soluble TF under static incubation, even in the presence of 25 molecules-TF/μm2 on the surface.
Amplification of surface TF by circulating 100 fM TF requires flow. The effect of exogenously added lipidated TF (100 fM) was studied in the presence and absence of venous wall shear rate of 100 s−1. Immunostaining for platelet GPIbα and fibrin(ogen) after 5-minute whole blood perfusion (with or without 100 fM TF) over TF titration arrays (A). Column averages of fluorescent intensity of the GPIbα staining (top) and fibrin(ogen) staining (bottom) are plotted against the TF surface concentration for static (dashed line) or venous (solid line) flow for perfusion of CTI whole blood without (○) or with (●) 100 fM added lipidated TF for 3 independent experiments (3 × 30 spots per plotted data point) (B).
Amplification of surface TF by circulating 100 fM TF requires flow. The effect of exogenously added lipidated TF (100 fM) was studied in the presence and absence of venous wall shear rate of 100 s−1. Immunostaining for platelet GPIbα and fibrin(ogen) after 5-minute whole blood perfusion (with or without 100 fM TF) over TF titration arrays (A). Column averages of fluorescent intensity of the GPIbα staining (top) and fibrin(ogen) staining (bottom) are plotted against the TF surface concentration for static (dashed line) or venous (solid line) flow for perfusion of CTI whole blood without (○) or with (●) 100 fM added lipidated TF for 3 independent experiments (3 × 30 spots per plotted data point) (B).
Discussion
We report that surface tissue factor functions over a very narrow concentration range between 2 and 20 molecules-TF/μm2 to trigger rapid platelet and fibrin deposition on collagen between venous levels (γw = 100 s−1) and arterial levels of flow (γw = 500 or 1000 s−1). At fewer than 2 molecules-TF/μm2, tissue factor has relatively minor effect on fibrin formation or platelet deposition over 5 minutes. At more than 20 molecules-TF/μm2, thrombin and fibrin production appeared maximal. The level of 6 molecules-TF/μm2 in human atherosclerotic carotid artery plaques5 is well within the range defined in the microarray experiments to produce an intense and rapid coagulation response. A level of 0.05 U/mL thrombin (∼ 0.5 nM) is known to be sufficient to cause threshold activation and allow reliable detection of more than 10% activated platelets within a platelet sample.26
The concept of a threshold TF concentration under flow conditions has been anticipated theoretically.3 In addition, patch size thresholds have been investigated under no-flow conditions where a very strong patch size threshold exists for plasma-based systems, but not in whole blood at 24°C (see Figure 2F of Kastrup et al27 ). We have extended these theoretic and experimental studies to define the critical surface TF needed to trigger whole blood clotting on collagen within 5 minutes under physiological flow conditions. In recent numerical simulations, Kuharsky and Fogelson3 predicted a very similar narrow concentration regime for surface tissue factor to trigger clotting under flow. In the Fogelson model and in our experimental results, the EC50 to cause 50% maximal fibrin formation was only moderately sensitive to flow over the physiological regime. We observed that the surface TF EC50 right shifts only 2.8-fold (from 3.6 to 10.2 molecules-TF/μm2) as wall shear rate increased 10-fold from venous to arterial levels (Figure 1C-E). Convection and ATIII limit fibrin formation to zones above and downstream of focal locations of surface tissue factor (Figure 2).
Low circulating levels of 100 fM tissue factor when added to flowing CTI-treated whole blood did not substantially left shift the dose response curve for fibrin formation triggered by surface tissue factor. Rather, the results shown in Figure 3B indicated that 100 fM circulating tissue factor served to amplify the response initiated by the surface TF, allowing for higher levels of fibrin to be formed under flow. The enhancing effect of adding 100 fM circulating TF required flow and was not seen under stationary conditions. Convective delivery of circulating TF may cause its accumulation at the thrombotic site as well as facilitate Xa production at later stages of the clotting process.
A recent study has shown that citrate/recalcification protocols may alter the function of plasma coagulation and platelet aggregation responses.28 In this recent study, the use of citrate had essentially no effect on platelet activation response to collagen. Collagen was the surface protein used in the current study, and the use of citrate/recalcification is not expected to have any effect on the platelet responses to collagen in the flow experiments shown in Figures 1,Figure 2–3. In addition, Mann et al28 showed that the use of citrate had no effect on the clotting time of CTI-treated whole blood (no added TF) as measured by the authors using thromboelastography (R*) (CTI [citrate] whole blood, R* = 66 ± 19 minutes vs CTI [no citrate]whole blood, R* = 66.1 ± 18.7 minutes). Thus, we conclude that recalcified, citrated CTI-treated whole blood treated with a minimal perturbation of 100 fM tissue factor displays kinetics of fibrin production and formation that are very weakly affected by the use of citrate.
Estimates of functional or antigenic blood-borne TF concentrations range from femtomolar to low picomolar levels.6,12,13,29 The purpose of the experiments shown in Figure 3 was not intended to resolve the functional role of blood-borne tissue factor in healthy individuals. Rather, the intent of the experimental design shown in Figure 3 was to understand the conditions by which a low level of circulating TF (100 fM) may demonstrate functionality during a coagulation event. We conclude that fibrin formation in flow systems presenting surface TF may be useful for detecting the effects of low-level circulating TF. The role of blood-borne tissue factor remains an area of intense investigation and various phenomena including platelet synthesis or release, de-encryption, secreted splice variants, or microparticle capture may be usefully examined under hemodynamically relevant conditions.
Flow chambers coated with various proteins (fibrinogen, collagen, von Willebrand factor [VWF], selectins, etc) have been used for decades to study blood function.24,30-32 However, most of these studies typically use anticoagulated blood or isolated blood cells to study events related to adhesion, independent of the in situ production of thrombin and fibrin. Since the advent of CTI to antagonize contact activation in vitro,21,33 we are not aware of any in vitro flow studies that have included the effects of surface TF on platelet and fibrin deposition over collagen. The collagen/lipidated TF microarray assay allows a single variable to be titrated in a single flow experiment. An additional aspect of this assay is that it provides an in vitro mimic of focal “patches” of subendothelial proteins exposed to blood, analogous to thrombosis triggered via laser injury in mouse models,18 albeit an in vitro assay lacking endothelial functionalities such as nitric oxide, prostacyclin, or thrombomodulin. While lipidated TF can be washed out of the collagen microspot to a limited extent (∼ 30%) over the 5-minute time frame of this in vitro experiment, it is important to note that TF washout from damaged vessels also occurs in vivo, but is poorly quantified with respect to rates and sensitivity to flow.
The printed microarray mounted in parallel-plate flow chambers allows the spatial control of platelet adhesion with collagen and thrombin/fibrin production triggered by lipidated TF under physiological flow. This approach may also be particularly useful for evaluating the pharmacological efficacy of antiadhesion agents, antiplatelet agents, anticoagulants, and thrombolytic or clot imaging agents under a range of flow and procoagulant surface stimuli.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge helpful discussions with Dr L. F. Brass (University of Pennsylvania).
U.O. was supported by a National Institutes of Health (NIH) Cardiovascular Training Predoctoral Fellowship. K.B.N. was supported by an NIH F32 Postdoctoral Award. The authors acknowledge research support by NIH R01-HL-56621 and R33-HL-87317 (S.L.D.).
National Institutes of Health
Authorship
Contribution: U.O. conducted all flow experiments; K.B.N. conducted numerical simulation; W.S.D. and M.S.C. conducted well plate experiments; S.L.D. oversaw all aspects of study and wrote the paper with contributions from all authors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Scott L. Diamond, Institute for Medicine and Engineering, Department of Chemical and Biomolecular Engineering, University of Pennsylvania, Philadelphia, PA 19104; e-mail: sld@seas.upenn.edu.




 C=0; and microspots: JThrombin=1.11×10−10 mol/m2-s.
C=0; and microspots: JThrombin=1.11×10−10 mol/m2-s.