Abstract
Dihydrofolate reductase (DHFR) is the major target of methotrexate (MTX), a key component in childhood acute lymphoblastic leukemia (ALL) treatment. A total of 15 polymorphisms in DHFR promoter were analyzed, and 3 sites (C−1610G/T, C−680A, and A−317G) were identified as sufficient to define observed haplotypes (tag single nucleotide polymorphisms [tagSNPs]). These polymorphisms were investigated for association with treatment response in 277 children with ALL. Lower event-free survival (EFS) was associated with homozygosity for the allele A−317 and C−1610 (P = .03 and .02), and with the haplotype *1, defined by both C−1610 and A−317 alleles (P = .03). The haplotype *1 conferred higher transcriptional activity (P < .01 compared with haplotypes generating minimal luciferase expression). Quantitative mRNA analysis showed higher DHFR levels for particular haplotype *1 carriers (P < .01). The analysis combining haplotype *1 with thymidylate synthase (TS) and cyclin D1 (CCND1) genotypes previously shown to affect ALL outcome showed that the number of event-predisposing genotypes was associated with increasingly lower EFS (P < .001). In conclusion, DHFR promoter polymorphisms are associated with worse ALL outcome, likely due to a higher DHFR expression. Combined effects among genes of the folate cycle can further accentuate differences in the response to the treatment.
Introduction
Methotrexate (MTX), a folic acid antagonist, is an important component of the treatment of acute lymphoblastic leukemia (ALL). A major mechanism of MTX action involves competitive inhibition of dihydrofolate reductase (DHFR). This leads to the impaired regeneration of tetrahydrofolate from dihydrofolate, resulting in the lack of the folate coenzymes and impairment of purine and pyrimidine synthesis.1
A subset of patients can develop drug resistance or drug side effects, which may hamper the efficacy of treatment or require drug dose reduction and drug withdrawal.2 DHFR can play an important role in development of MTX resistance. In both experimental and clinical settings, altered levels of DHFR and/or decreased DHFR-MTX complex formation are found in relapsed patients and in cells manifesting MTX-resistant phenotype.3–5 Changes in the level of DHFR expression and consequently in the sensitivity to MTX can also be due to genetic polymorphisms, particularly those located in the regulatory elements. Here, we studied the impact of the promoter polymorphisms in the DHFR gene. The association of individual polymorphisms and derived haplotypes, with ALL treatment outcome and DHFR expression, was assessed. The recently described 19-bp insertion/deletion (indel) polymorphism was also included in haplotype analysis. The 19-bp indel is located within intron 1 and was shown to influence disorders dependent on folate levels.6 We have previously suggested that simultaneous effect of several genes are important to predict ALL outcome.7 Given that DHFR, thymidylate synthase (TS), and cyclin D1 (CCND1) all belong to the same subpathway of MTX action,1 we also examined their combined effect.
Methods
Study population and endpoints in the analysis
The study participants are 277 white children diagnosed with ALL at Hospital Sainte-Justine (HSJ; Montreal, QC) between January 1989 and December 2003. The patients underwent treatment with Dana-Farber Cancer Institute ALL Consortium protocols DFCI 87-01, 91-01, 95-01,8–10 or 2000-01. The MTX dose did not differ across the protocols; patients received a high MTX dose (4 g/m2) during induction phase and once-weekly doses of 30 to 45 mg/m2 during the consolidation (standard-risk patients) and maintenance (all patients) phases.
Association of genotypes/haplotypes with ALL outcome was assessed in all patients by event-free survival (EFS), disease-free survival (DFS), and overall survival (OS) analysis. Children who relapsed, had an induction failure, or died were defined to have had an event. Toxicity on bone marrow and liver function was based on the results of weekly laboratory tests collected during consolidation and maintenance treatment, as previously described.11 Toxicity was graded using the common criteria for adverse events of the National Cancer Institute.11 The toxicity data were available for 174 patients who were assessed for the relationship between genotypes and EFS probabilities. The mean number of weeks assessed per patient was 80.
Genotyping
A total of 7 DNA fragments of the DHFR gene that contain 15 promoter polymorphisms and one that contains a 19-bp indel variation were amplified by polymerase chain reaction (PCR) using 15 to 25 ng of genomic DNA from 48 controls. All control individuals were white; 29 women and 19 men had a mean age of 48 years. The sequence of forward and reverse primer pairs used for amplification of each fragment along with the size of the PCR products, relationship between polymorphisms, and the DNA fragments and relationship to HapMap data are outlined in Table 1. PCR reactions were performed in a final volume of 50 μL using 0.4 μM of each of the primers, 200 μM each of dNTPs, 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, and 1 U of Taq polymerase (Platinum; Invitrogen, Carlsbad, CA). For amplification of fragment 1, 2% dimethylsulfoxide (DMSO) is added to the reaction; for the 19-bp indel polymorphism, 2 mM MgCl2 (instead of 1.5 mM) is added to the reaction.
Fragments 1 and 2 and the 19-bp indel were amplified for 40 cycles composed of 45 seconds at 94°C, 45 seconds at 56°C (fragment 1) or 60°C (fragment 2, and 19-bp indel), and 45 seconds at 72°C. Fragments 3, 4, 5, 6, and 7 were amplified for 34 cycles: 14 cycles consisting of 30 seconds at 94°C, 30 seconds at 62°C to 55°C (−0.5°C/cycle) and 30 seconds at 72°C, and 20 cycles consisting of 30 seconds at 94°C, 30 seconds at 55°C, and 30 seconds at 72°C. The genotyping was performed by allele specific oligonucleotide (ASO) hybridization as described previously.14 The sequence of ASO probes specific for tested variants is given in Table 1. The presence of alleles of DHFR 19-bp indel polymorphism was visualized by 4% agarose gel electrophoresis. Genotyping of minimal number of promoter polymorphisms needed to define all observed haplotypes (tag single nucleotide polymorphisms [tagSNPs]) was subsequently performed in patients with ALL. Loci −1610 and −680 fail to amplify in 2 individuals.
The subset of samples was genotyped in duplicate to ensure genotype reproducibility. The genotyping for TS repeat and CCND1 A870G polymorphism were as previously described.7,15 Patient samples were collected at remission after informed consent was obtained in accordance with the Declaration of Helsinki. The Hospital Ethical Committee of Hospital Sainte-Justine approved the study protocol.
Statistics
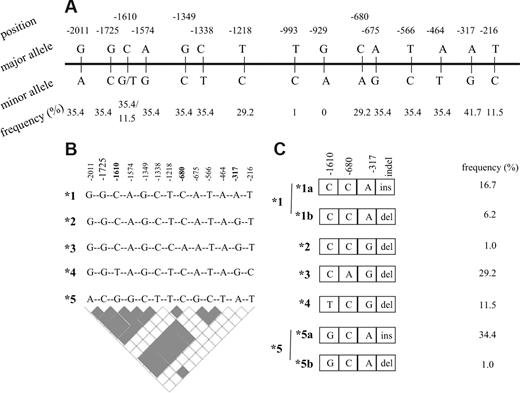
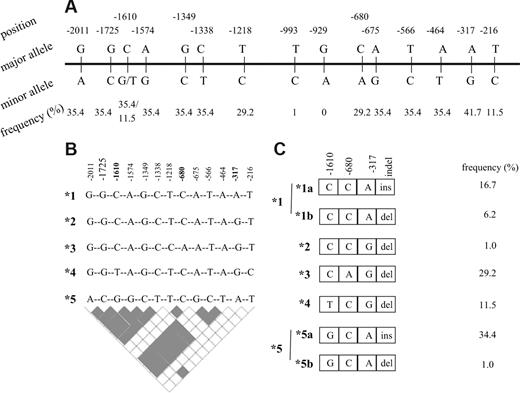
A total of 15 DHFR polymorphisms (Figure 1A) were previously identified in the 2-kb segment, upstream of the transcription initiation site.16 The estimates of linkage disequilibrium (LD), maximum likelihood estimates of frequencies of underlying haplotypes in controls and patients (event and nonevent groups), and haplotype phase were obtained by PHASE software, version 2.0 (Seattle, WA).17,18 The frequency estimates were used to calculate the number of each haplotype from the total number of chromosomes in each group of patients. Carriers of a given haplotype were calculated from all haplotype pairs containing respective haplotype. The tagSNPs was selected based on LD information (r2 = 1; Figure 1B). In 2 individuals, haplotypes were not inferred due to the missing data.
DHFR polymorphisms and derived haplotypes. (A) Promoter polymorphisms with the frequency of minor alleles. (B) Haplotypes arbitrarily named from *1 to *5 derived from 13 promoter polymorphisms with minor allele frequency higher than 1%. tagSNPs that are sufficient to infer all 5 haplotypes are given in bold. Pairwise estimates of linkage disequilibrium are given the bottom panel (▩ indicates r2 = 1; □ indicates r2 < 1). (C) Haplotypes derived from 3 tag promoter SNPs and the 19-bp indel polymorphism. The frequency of controls is given next to the haplotypes.
DHFR polymorphisms and derived haplotypes. (A) Promoter polymorphisms with the frequency of minor alleles. (B) Haplotypes arbitrarily named from *1 to *5 derived from 13 promoter polymorphisms with minor allele frequency higher than 1%. tagSNPs that are sufficient to infer all 5 haplotypes are given in bold. Pairwise estimates of linkage disequilibrium are given the bottom panel (▩ indicates r2 = 1; □ indicates r2 < 1). (C) Haplotypes derived from 3 tag promoter SNPs and the 19-bp indel polymorphism. The frequency of controls is given next to the haplotypes.
Survival differences, estimated by Kaplan-Meier analysis for patients with different genotypes, were assessed using a log-rank test. Times to event were measured as the time between diagnosis and the event of interest; for censored cases, it corresponded to time between diagnosis and the study endpoint (5 years after diagnosis [ie, 84 months]), or to the last observational period. Median EFS was 34 months among the 55 patients experiencing induction failure, induction death, relapse, or death in remission; median follow-up for censored patients was 84 months. Median DFS was 35 months among the 51 patients experiencing induction failure or relapse; median OS was 26 months among the 25 patients who experienced death due to any cause. The hazard ratio (HR; with a 95% CI) for genetic variants was estimated by the Cox regression analysis, with and without inclusion of the common prognostic factors8,19 categorized according to relapse risk prediction (boys or girls; age younger than 1 year, 1-9.99 years, and older than 10 years; white blood cell [WBC] count below or above 50 × 109/L; B- or T-cell type; DNA index above or below 1.16; and standard- or high-risk group). Differences between allele and haplotype frequencies were estimated by the chi-squared test.
The rate of each toxicity parameter (grades 3 and 4 separately, or grades 3 and 4 combined if toxicity grade 4 occurred rarely) was calculated for each individual as previously described.11 The toxicity rates were then compared between patients with and without a given genotype(s) using the Mann-Whitney and Kruskal-Wallis tests.
For the analysis of survival probabilities and drug-associated toxicities in the function of 3 genes, the number of event predisposing genotypes of DHFR, TS, and CCND1 genes were analyzed. The event-predisposing genotype for DHFR was haplotype *1; for TS, it was homozygosity for triple repeat allele (3R); and for CCND1, it was homozygosity for A870 variant.7,15
All analyses assessing the impact of genotypes/haplotypes were performed by SPSS statistical package (Chicago, IL), version 13.0.
Gene reporter assay and quantitative RT-PCR
Haplotype-specific fragments corresponding to 2115 bp of the proximal promoter region of DHFR were amplified from genomic DNA of individuals with known genotype and cloned into the promoterless pGL3-basic firefly luciferase reporter vector (Promega, Madison, WI). Constructs corresponding to the 4 major haplotypes were sequenced to confirm the presence of the expected polymorphic sites.
Approximately 6 to 8 × 104 cells (human placental Jeg-3, human cervical cancer HeLa, and human hepatoma HepG2 cell lines; American Type Culture Collection [ATCC], Manassas, VA) were plated out and grown in 96-well plates until reaching 80% to 90% of confluence. These cell lines were cotransfected with 100 ng of firefly luciferase plasmid containing corresponding allelic construct and 0.5 ng of SV40-driven renilla luciferase pRL-SV40 plasmid (plasmid ratio 200:1) to control transfection efficiency. Transfection was performed with lipofectamine 2000 according to the manufacturer's protocol (Invitrogen). Similar experiments were performed with empty, promoterless, pGL3-basic plasmid (Promega), presenting negative control. Cells were harvested 24 hours after transfection and luciferase reporter gene activity was measured with the Dual-Luciferase Reporter Assay System (Promega) in a Spectra Max 190 luminometer (Molecular Devices, Sunnyvale, CA). The renilla luciferase activity of the control pRL-SV40 was used to normalize the results of the firefly luciferase activity of the allelic constructs (relative luciferase activity was expressed as mean ± SD). Each experiment was performed in triplicate, and the difference between haplotypes in gene reporter activity was assessed by the Student unpaired t test.
The analysis of quantitative reverse transcription (RT)–PCR was performed on total RNA, originating from lymphoblastoid cell lines of CEPH families (n = 18), or from lymphocytes of anonymous volunteers (n = 32) following phytohemaglutinin stimulation. Total RNA (50 ng) from each sample was reverse transcribed using the cDNA Cycle Kit (Invitrogen). PCR was carried out in a final volume of 25 μL, using 5 ng of cDNA, 0.1 μM of each of the primers, and SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA). The real-time PCR was performed using ABI 7000 Sequence Detection System (Applied Biosystems) and consisted of denaturation at 95°C for 10 minutes and 40 cycles of amplification (15 seconds at 95°C and 1 minute at 60°C). All samples were assayed in triplicate for the DHFR and B2-microglobulin, against which relative DHFR gene expression was normalized. Sequences of respective forward and reverse primer pairs5 are given in Table 2. A calibration curve was run in parallel for each analysis using DNA extracted from a Hela cell line. The relative quantification of target gene expression was done using the comparative cycle threshold (CT) method.20,21 For all samples for which mRNA levels were quantified, DHFR genotype/haplotypes were determined as described in “Genotyping.” Depending on data distribution, relative expression between groups with and without given genotype/haplotype was compared by analysis of variance (ANOVA) or the Mann-Whitney test.
Results
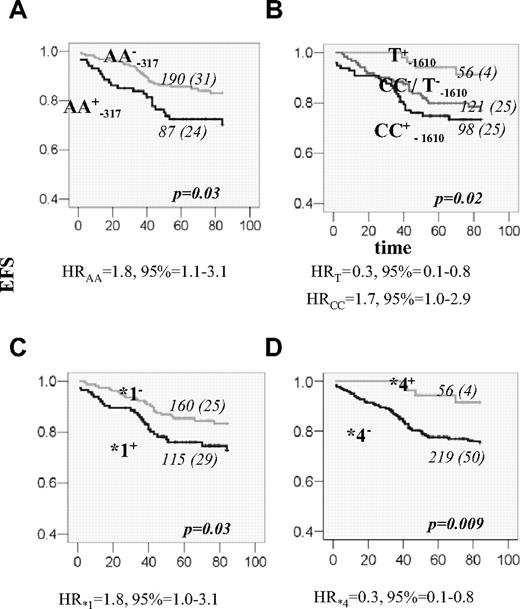
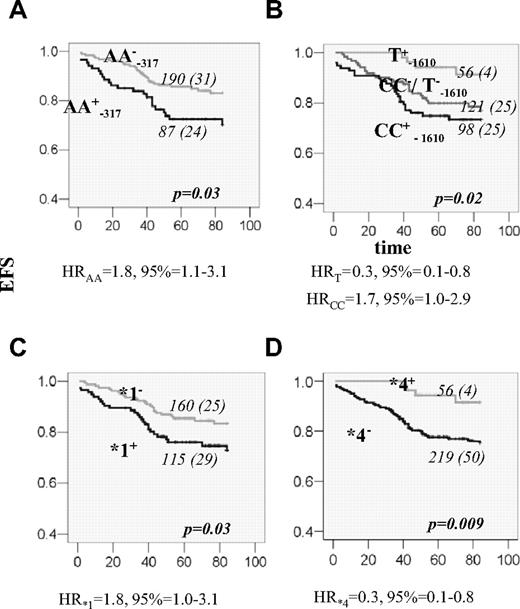
A total of 15 DHFR promoter polymorphisms were initially genotyped in 48 control volunteers to estimate allelic frequency and determine haplotype phase. Allelic frequency of 13 variant sites was higher than 1% and their genotype frequencies, ranging from 11% to 42%, were in agreement with the Hardy-Weinberg equilibrium (Figure 1A). All promoter variants were organized in 5 haplotypes (Figure 1B). A total of 3 tagSNPs (C−1610G/T, C−680A, and A−317G) were identified. The 19-bp indel polymorphism,6 with the frequency of del allele of 49%, diversifies haplotypes CCA (*1) and GCA (*5) into those without (*1a and *5a) and with deletion (*1b and *5b). The remaining haplotypes are all accompanied by the 19-bp del allele (organization in hapotypes and respective frequencies in controls are given in Figure 1C). Patients with ALL were subsequently genotyped for 3 promoter tagSNPs (C−1610G/T, C−680A, and A−317G) and the 19-bp indel polymorphism. The frequencies of genotypes and alleles are given in Table 3. No significant sex-dependent difference was seen. There was no difference in allele frequencies between patients and controls. Between patients with and without event there was a difference in the genotype and allele frequencies at the −317 and −1610 loci (Table 3). Individuals with the AA−317 genotype had poorer EFS compared with other genotype groups (P = .03; Figure 2A). For the C−1610G/T polymorphism, better EFS was seen for the T−1610 allele carriers and reduced EFS for CC−1610 individuals (P = .02; Figure 2B). Significant associations were retained in the Cox regression, when other prognostic factors were taken into account (Table 4; Model 1). Similar results for these 2 polymorphic sites were shown for DFS; similar trend was seen for OS, but with no significant association.
EFS for patients with ALL according to DHFR genotypes. (A) EFS curves for patients who are positive (+; black line) or negative (−; gray line) for DHFR AA−317 genotype. (B) EFS curves according to the genotypes of the polymorphism at the position −1610. The lower curve (black) represents EFS for homozygous CC patients (CC+); the line in the middle (dark gray) represents EFS for patients who have neither the CC genotype nor the T allele (CC−/T−). The upper curve (light gray) represents EFS for individuals that are carriers of the T allele (T+). (C) EFS for patients that are carriers (+; black line) or not (−; gray line) of DHFR haplotype *1. (D) EFS for patients that are carriers (+; gray line) or not (−; black line) of DHFR haplotype *4. The genotype and the number of patients in each curve, numbers of individuals with an event (in the parenthesis), as well as the P value, estimated by log-rank test for the survival differences between the patients groups, are indicated on each plot. Risk of event associated with the given genotype, expressed as a univariable HR with 95% CI, is indicated below the plots.
EFS for patients with ALL according to DHFR genotypes. (A) EFS curves for patients who are positive (+; black line) or negative (−; gray line) for DHFR AA−317 genotype. (B) EFS curves according to the genotypes of the polymorphism at the position −1610. The lower curve (black) represents EFS for homozygous CC patients (CC+); the line in the middle (dark gray) represents EFS for patients who have neither the CC genotype nor the T allele (CC−/T−). The upper curve (light gray) represents EFS for individuals that are carriers of the T allele (T+). (C) EFS for patients that are carriers (+; black line) or not (−; gray line) of DHFR haplotype *1. (D) EFS for patients that are carriers (+; gray line) or not (−; black line) of DHFR haplotype *4. The genotype and the number of patients in each curve, numbers of individuals with an event (in the parenthesis), as well as the P value, estimated by log-rank test for the survival differences between the patients groups, are indicated on each plot. Risk of event associated with the given genotype, expressed as a univariable HR with 95% CI, is indicated below the plots.
We further investigated if specific haplotypes, particularly those containing T−1610, C−1610, and A−317 alleles, are associated with the event. The best estimates of haplotype frequencies in the group with and without event are given in Table 5. Haplotype *4 is uniquely tagged by the T−1610 allele, and thus the protective effect of this haplotype was also seen (P = .005; Table 5). The A−317 and C−1660 alleles are both present in haplotype *1 (*1a and *1b), whereas A−317 alone is also present in haplotype *5 (*5a and *5b). The association with event seems to be confined to haplotype *1a and rare haplotype *5b (P = .04 and .02, respectively; Table 5). We further investigated if the EFSs were different in the carriers of any specific haplotype. However, the genotype combination that defines haplotype pairs *1a*5b can also define pair *1b*5a (Figure 1C). Given this ambiguity and the low power of the study for the low-frequency variants, we analyzed only the carriers of the common haplotypes (*1, *3, *4, and *5) whose pairs can be assigned unambiguously. Number and frequencies are given in Table 6. The effect in the individuals carrying haplotypes *1 and *4 could have been distinguished through the association with lower EFS and higher EFS, respectively (P = .03 and P = .009; Table 6; Figure 2C,D). Single DHFR polymorphisms or haplotypes were not associated with chemotherapy toxicity.
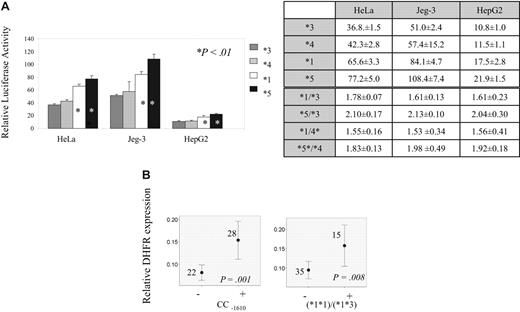
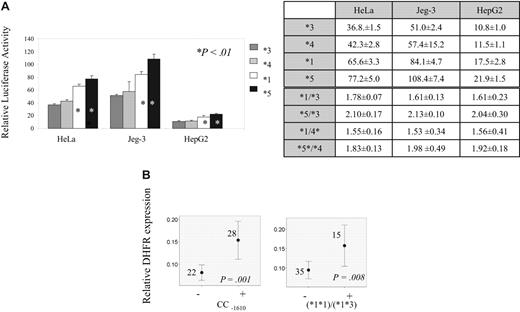
To define the functional significance of DHFR promoter variants and derived haplotypes, gene reporter assay and quantitative mRNA analysis were performed. Gene reporter assay using 3 different cell lines showed 1.5- to 2-fold higher transcription for haplotype *1 and haplotype *5 compared with remaining haplotypes (P < .01; Figure 3A). The results of quantitative mRNA analysis according to all genotypes and haplotype pairs are given in Table 7. Difference in mRNA levels are seen across genotypes of −1610 polymorphism (Table 7), with the highest levels in individuals with CC−1610 genotype (P = .001; Figure 3B). Among carriers of haplotype *1, individuals with haplotype pairs *1*1 and *1*3 had highest mRNA levels. (Figure 3B; P = .008 when these individuals were compared with those with all remaining haplotype pairs).
Transcription according to DHFR genotypes and haplotypes. (A) DHFR haplotype's promoter strength relative to blank (Gateway-adapted pGL3-basic vector). Haplotypes *3, *4, *1, and *5 are represented by dark gray, light gray, white, and black bars, respectively. The relative values are given for 3 different cell lines: HeLa, Jeg-3, and HepG2. *Haplotypes showing significant increase in transcription (P < .01, Student t test) when compared with haplotype 3 or to haplotype 4. Tables illustrate relative promoter activity (values and standard deviation) for each haplotype as well as ratio of relative promoter activity between haplotypes with high and low expression. (B) Relative mRNA levels according to DHFR genotypes and haplotypes. The mean value of expression with 95% CI, the number of individuals represented by each line, and P value obtained by Mann-Whitney test (CC −1610 genotype) and ANOVA (*1*1 and *1*3 haplotype pairs) for the difference of expression between carriers (+) and noncarriers (−) of indicated genotypes/haplotypes are indicated on the plots.
Transcription according to DHFR genotypes and haplotypes. (A) DHFR haplotype's promoter strength relative to blank (Gateway-adapted pGL3-basic vector). Haplotypes *3, *4, *1, and *5 are represented by dark gray, light gray, white, and black bars, respectively. The relative values are given for 3 different cell lines: HeLa, Jeg-3, and HepG2. *Haplotypes showing significant increase in transcription (P < .01, Student t test) when compared with haplotype 3 or to haplotype 4. Tables illustrate relative promoter activity (values and standard deviation) for each haplotype as well as ratio of relative promoter activity between haplotypes with high and low expression. (B) Relative mRNA levels according to DHFR genotypes and haplotypes. The mean value of expression with 95% CI, the number of individuals represented by each line, and P value obtained by Mann-Whitney test (CC −1610 genotype) and ANOVA (*1*1 and *1*3 haplotype pairs) for the difference of expression between carriers (+) and noncarriers (−) of indicated genotypes/haplotypes are indicated on the plots.
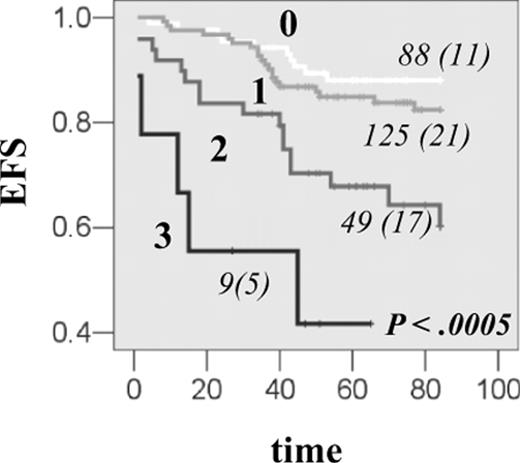
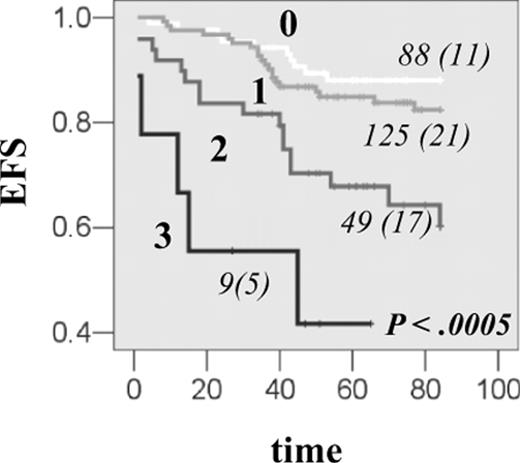
Finally, we addressed combined gene effect of DHFR with TS and CCND1, which belong to the same subpathway of MTX action. DHFR haplotype *1, which was associated with the increase in transcription in vitro and in vivo and with EFS reduction, was further investigated for the joint effect with CCND1 AA870 and TS 3R3R genotypes, which were previously shown to reduce EFS in the same group of patients.7,15 The reduction in EFS was proportional to the number of event-predisposing genotypes (P < .001; Figure 4A), and the risk of event was further increased in individuals with all 3 at-risk genotypes (HR = 8.0, 95% CI, 2.7-23.5). Significant association was retained in the Cox regression when other prognostic factors were taken into account (Table 5; Model 2 and 3). All 3 genes contributed to the reduction in EFS. There was no association between toxicity parameters investigated and the increasing number of event-predisposing genotypes.
Combined effect of DHFR, TS, and CCND1 genes on EFS in patients with ALL. The EFS curves for HSJ patients having 0, 1, 2, or 3 event-predisposing genotypes. Event-predisposing genotype for DHFR was haplotype *1; for TS, it was homozygosity for the 3R allele; and for CCND1, it was homozygosity for the A870 variant. The numbers of patients in each curve, numbers of individuals with an event (in parentheses), as well as the P value, are indicated on each plot.
Combined effect of DHFR, TS, and CCND1 genes on EFS in patients with ALL. The EFS curves for HSJ patients having 0, 1, 2, or 3 event-predisposing genotypes. Event-predisposing genotype for DHFR was haplotype *1; for TS, it was homozygosity for the 3R allele; and for CCND1, it was homozygosity for the A870 variant. The numbers of patients in each curve, numbers of individuals with an event (in parentheses), as well as the P value, are indicated on each plot.
Discussion
The antileukemia effect of methotrexate involves the inhibition of DHFR. Our study describes the role of DHFR promoter polymorphisms in disease outcome in children with ALL. We postulate that changes in the transcription levels associated with these polymorphisms result in different therapeutic responses to MTX. A total of 3 variants were found to influence ALL outcome: homozygosity for A−317 and C−1610 correlated with lower EFS, and presence of the T−1610 allele was associated with better EFS. These alleles are distributed across different haplotypes. Haplotype *1 contains both the A−317 and C−1610 alleles, and haplotype *5 contains −317A, whereas haplotype *4 is uniquely tagged by the −1610T allele. The carriers of haplotype *1 had reduced EFS, and those with haplotype *4 had better EFS, whereas no difference in EFS was seen for haplotype *5 carriers. The effect of haplotype *1 seems to be mostly due to the common *1a subtype, as suggested by haplotype frequency analysis. Haplotype *1 conferred higher transcription in gene reporter assay. Quantitative mRNA analysis showed that carriers of haplotype pairs *1*1 and *1*3* (but not *1*5) were associated with higher mRNA levels. No change in expression in relation to T−1610 allele (and haplotype *4 tagged by this variant) was observed, suggesting that the T−1610 effect that was seen in association study, might reflect the absence of respective DHFR risk allele. Higher transcription, associated with the haplotype *5, was seen in gene reporter assay. On the other hand, carriers of this haplotype did not have changed EFS or mRNA levels. The subtypes *5a and *5b have identical promoter polymorphism and cannot be distinguish in gene-reporter assay. Analysis of haplotype frequency suggested that rare *5b (but not common *5a) is associated with an event, probably explaining why the association with EFS or mRNA levels was not apparent when all haplotype *5 carriers were analyzed together. It is thus possible that further downstream mechanism might differentially regulate *5a and *5b haplotypes. For example, other not yet known polymorphisms, or an epigenetic mechanism, such as a recently described noncoding interfering DHFR transcript,12 might play a role. It is also possible that different cell-dependent regulation might explain differences in transcriptional activity of the *5 hapotype between cell lines used in the gene reporter assay and lymphocytes assayed in vivo. Further analysis would be needed to establish the potentially different function of the *5a and *5b subtypes. On the other hand, significance of *5b relative to other ALL event-predisposing variants would probably remain low due to its minor frequency.
Several attempts have been made to establish the role of DHFR expression in intrinsic MTX resistance in childhood ALL. Matherly et al3 provided the evidence for a key role of elevated DHFR levels, as found in subpopulations of blasts at disease presentation. Likewise, in T-cell childhood ALL, which has poorer prognosis, T lymphoblasts of newly diagnosed patients exhibited elevated DHFR, relative to DHFR of B cells,22 or to DHFR levels of ALL blasts from MTX-responsive patients.23 In addition, regardless of the leukemia phenotype, patients with WBC counts below 5 × 109/L whose DHFR expression was above a threshold level had a significantly shorter EFS.23 Surprisingly, a correlation between unfavorable EFS and DHFR expression below median was recently reported, as estimated in 40 newly diagnosed patients with ALL.20 Our finding supports the former studies that suggest a correlation between higher DHFR levels and MTX resistance. It is interesting to note that change in polymorphism or haplotype-dependent DHFR transcription can be due to the presence of different transcription factor binding sites at corresponding loci. Predicted binding sites as well as their loss or gain relative to the alleles of haplotype *1 are illustrated in Tables 8 and 9 (MatInspector; Genomatix, Munich, Germany; http://www.genomatix.de).24
Combined effect between TS and other gene variants have been previously observed by us and others.15,25,26 Here we showed similar effect among DHFR, TS, and CCND1 genes. TS is an important target for the glutamylated MTX,27 and the 3R variant associated with increased expression is described in the TS gene.28,29 Several studies have suggested that CCND1 overexpression can affect sensitivity to MTX.1,30.31CCND1 A870G polymorphism modulates the ratio of CCND1 mRNA isoforms, and the transcript associated with the A allele results in the protein with a longer half-life.32–34 CCND1 AA870 and TS 3R3R were previously associated with worse ALL outcomes.7,15,19 Presence of all 3 at-risk genotypes further reduced EFS. All of them contributed to the EFS reduction. There was no association between toxicity and the number of event-predisposing genotypes, most likely due to the fact that only the CCND1 at-risk genotype,11 but not TS11 and DHFR (this study), was individually associated with a lower frequency of toxic events.
In conclusion, we report the association of DHFR promoter variants with the poorer ALL outcome, likely due to the increase in DHFR expression. The study provides information that will further increase the knowledge how to use host genetic variations to tailor therapy of antileukemia drugs.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are thankful to all patients and their parents who consented to participate in genetics study related to leukemia. We are grateful to our colleagues Stephen E. Sallan, Lewis B. Silverman, Donna Neuberg, Jeffery L. Kutok, and Ivan Brukner for critical reading of the manuscript.
This work was supported by grants from the Canadian Institutes of Health Research, Leukemia Lymphoma Society of Canada, Research Center of CHU Sainte-Justine, Charles Bruneau Foundation, and Centre d'Excellence en Oncologie Pédiatrique et en Soins Palliatifs. M.K. is a scholar of the Fonds de la Recherche en Santé du Québec. Polymorphism discovery and gene reporter assay were carried out within the projects of Genome Quebec/Genome Canada: Regulatory genomics and Gene regulators in disease.
Authorship
Contribution: S.D., G.S.-O., V.G., and M.A. performed experiments; S.D. G.S.-O., V.G., and M.K. performed the data analysis; D.S., D.L., A.M., and M.K. contributed to the interpretation of data; M.K. designed the research and drafted the article; and D.S., D.L., and A.M. revised the manuscript critically.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Maja Krajinovic, Centre de Recherche, CHU Sainte-Justine, 3175 Chemin de la Côte-Ste-Catherine, Montréal, QC, H3T 1C5 Canada; e-mail: maja.krajinovic@umontreal.ca.