Abstract
Resistance toward apoptotic stimuli mediated by overexpression of antiapoptotic factors or extracellular survival signals is considered to be responsible for accumulation of malignant B cells in chronic lymphocytic leukemia (CLL). TOSO was identified as overexpressed candidate gene in CLL, applying unit-transformation assays of publicly available microarray datasets. Based on CLL samples from 106 patients, TOSO was identified to exhibit elevated relative expression (RE) of 6.8 compared with healthy donor B cells using quantitative real-time polymerase chain reaction (PCR; P = .004). High levels of TOSO expression in CLL correlated with high leukocyte count, advanced Binet stage, previous need for chemotherapy, and unmutated IgVH status. CD38+ CLL subsets harboring proliferative activity showed enhanced TOSO expression. We evaluated functional mechanisms of aberrant TOSO expression and identified TOSO expression significantly induced by B-cell receptor (BCR) stimulation compared with control cells (RE; 8.25 vs 4.86; P = .01). In contrast, CD40L signaling significantly reduced TOSO expression (RE, 2.60; P = .01). In summary, we show that the antiapoptotic factor TOSO is associated with progressive disease and enhanced in the proliferative CD38+ CLL subset. Both association with unmutated IgVH and the specific induction of TOSO via the BCR suggest autoreactive BCR signaling as a key mediator of apoptosis resistance in CLL.
Introduction
Chronic lymphocytic leukemia (CLL) is the most common leukemic disorder in the Western hemisphere and is characterized by an accumulation of mature B cells in the blood, bone marrow, and secondary lymphoid organs.1 Malignant cells accumulate due to the failure of CLL cells to undergo apoptosis.2 Various mechanisms of apoptosis resistance in CLL have been described, such as overexpression of bcl2 mediated by depletion of inhibitory miR-15 and miR-16.3 Regarding the extrinsic apoptosis pathway, CLL cells exhibit resistance toward Fas-mediated apoptosis. Although CLL cells express Fas and DISC-related molecules,4 they remain resistant toward Fas-induced apoptosis. Inducing Fas expression failed to increase Fas-mediated apoptosis in CLL.5 In the context of a gene therapy trial, CD40 ligation was demonstrated to increase Fas susceptibility of CLL cells.6,7 However, mechanisms of resistance toward Fas-mediated apoptosis remain to be elucidated
Reanalysis of previously published microarray data of malignant B cells by unit expression transformation assay revealed that TOSO, also known as Fas-inhibitory molecule 3 (FAIM3), is overexpressed in CLL. TOSO was first identified as Fas-inhibitory molecule and named after a “vitalizing” Japanese liqueur.8 In a murine model, inhibition of Fas-mediated apoptosis was shown to be mediated by binding of the C-terminal domain of TOSO with FADD.9 Nevertheless, the function of the transmembrane-protein TOSO has not yet been fully described, therefore neither a ligand of the TOSO extracellular domain nor regulatory mechanisms of TOSO expression could be identified.
Here we describe CLL-specific expression and regulation of TOSO as a novel marker overexpressed on CLL cells and propagate TOSO as a new antiapoptotic factor in CLL pathogenesis, triggered by BCR signaling and further regulated by stroma interaction via the CD40 molecule.
Methods
Patients and cells
After informed consent was given, blood was obtained from patients fulfilling diagnostic criteria for CLL. Only patients without prior therapy or patients who had a period of at least 6 months since their last chemotherapy were included in this study. Fresh CLL samples were enriched by applying B-RosetteSep (StemCell Technologies, Vancouver, BC) and Ficoll-Hypaque (Seromed, Berlin, Germany) density gradient purification, resulting in purity of more than 98% of CD19+/CD5+ CLL cells. CLL cells were characterized for CD19, CD5, CD23, FMC7, CD38, ZAP70, and sIgM expression on a fluorescence-activated cell sorting (FACS) Canto flow cytometer (BD PharMingen, Heidelberg, Germany). Healthy donor B-cell subsets were analyzed by flow cytometry applying anti–IgD-FITC, –CD5-PE, –CD19-PE-Cy5.5, and –CD27-APC. IgVH hypermutational status of CLL patients was analyzed as previously published.10 Control cells were isolated from healthy blood donors using untouched depletion (naive B-cell isolation Kit II; Miltenyi, Bergisch-Gladbach, Germany), by positive selection with anti–CD19-MACS beads (Miltenyi), and subsequent positive selection with anti-CD27 beads, CD5-PE (BD PharMingen), and anti-PE beads (Miltenyi) was performed, resulting in at least 90% purity of B cells. Hodgkin cell lines L1236, L428, KMH2, and L540cn were used for detection of TOSO. Primary lymphoma RNA derived from lymph nodes of patients with diffuse large B-cell lymphoma, follicular lymphoma, marginal zone lymphoma, and T-cell lymphoma was isolated from snap frozen samples stored in liquid nitrogen. This study was approved by the ethics committee of the University of Cologne (approval no. 01-163). Blood samples were given with informed consent in accordance with the Declaration of Helsinki.
Real-time polymerase chain reaction
Quantification of TOSO mRNA was performed by LightCycler Taqman Master (Roche, Penzberg, Germany). Universal probe no. 64 (Roche) with forward primer 5′-GCC-CAG-CTA-CAA-CCA-CCA-3′ and reverse primer 5′-TGT-GAG-CCA-TAG-TCC-AGT-GC-3′ was used for TOSO amplification. Beta-2-microglobulin was applied as housekeeping gene standard by universal probe no. 42 (Roche) with forward primer 5′-TTC-TGG-CCT-GGA-GGC-TAT-C-3′ and reverse primer 5′-TCA-GGA-AAT-TTG-ACT-TTC-CAT-TC-3′. All experiments were performed in replicates and crossing points were determined by second derivative maximum method. Relative quantification analysis was done using Exor3 software package (Roche).
TOSO protein expression analysis
The rat monoclonal antibody 6B10 was generated by immunizing rats with recombinant full-length human TOSO protein. Subsequently anti-TOSO hybridoma cell lines were generated, and purified rat IgG1 antihuman TOSO antibody 6B10 was evaluated for specificity on recombinantly expressed TOSO. Briefly, TOSO was cloned to pTRCHisTOPO vector (Invitrogen, Carlsbad, CA) and pGFP-C2 (Clontech, Palo Alto, CA) and expressed in TOP10 Escherichia coli or HEK293 cells, respectively. Cellular lysates were processed with radioimmunoprecipitation assay (RIPA) buffer, sonicated, and further blotted onto nitrocellulose membranes. Western blot analysis and density measurements were performed on Odyssey infrared imaging system platform (LI-COR Biosciences, Lincoln, NE), The specificity of 6B10 antibody was further controlled by TOSO-specific inhibitory siRNA (CCGGCCAGTTCTTCCAAATTCGTAACTCGAGTTACGAATTTGGAAGAACTGGTTTTTTG). Briefly, HEK293 cells were cotransfected with TOSO-pGFP-C2 and 50 nM siRNA by calcium phosphate transfection. 6B10 antibody was used for flow cytometry detection of surface-expressed TOSO protein; visualization was performed using anti–rat-PE secondary antibody (BD PharMingen). Costaining was performed using CD38-FITC, CD19-PerCP-Cy5, CD5-APC, and CD3-PE-Cy7 on a FACS Canto cytometer. Apoptosis was determined by flow cytometry using annexin V–FITC/7AAD staining (BD PharMingen). Specific staining by flow cytometry was defined for cells harboring fluorescence above 99% of isotypic control antibody staining. For ZAP-70 and CD38, a 20% threshold of positively stained cells was applied for definition of positivity for these prognostic markers.
Immunohistochemistry
Immunohistochemistry was performed on paraffin-embedded tissues. Samples were deparaffinized and rehydrated, and heat-induced epitope retrieval was performed by microwave method applying 0.1% sodium citrate. Anti-TOSO 6B10 monoclonal antibody served as primary antibody; subsequent staining was performed by biotinylated secondary antibody and HRP-streptavidin. 3-Amino-9-ethylcarbazole (AEC) staining was used as chromogen. All pictures were taken using a Zeiss Axiophot microscope (Zeiss, Oberkochen, Germany) with the objective lenses 40×/0.70 NA PL Fluotar or 100×/1.32 oil NA PL Fluotar. For digital photography, a JVC KY-F75 U (JVC Germany, Friedberg, Germany) was used, with the acquisition software Diskus Version 4.60.342 (Diskus, Koenigswinter, Germany). No further image processing was performed.
Stimulation of cells
Cells were cultivated in IMDM (Invitrogen) supplemented with 10% heat-inactivated fetal calf serum (FCS), 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin and incubated at 37°C in a 5% CO2 humidified atmosphere. BCR stimulation was performed as previously described.11 Briefly, anti–IgM-polyacrylamide immunobead reagent (Irvine Scientific, Santa Ana, CA) was added to isolated CLL cells and incubated for 3 hours.11 CD40 ligand (CD40L) stimulation was performed using irradiated human CD40L-transduced NIH-3T3 fibroblasts as feeder layer cocultivation system.12 For induction of Fas-mediated apoptosis, 0.5 μg/mL of the agonistic monoclonal antibody CH11 (MBL, Nagoya, Japan) was applied.
Statistics
To compare the data from different arrays deposited in public available databases, a z-transformation was applied to each array data set. The parameters of the z-transformation were chosen in such a way that the distribution of transcripts that were not expressed (unexpressed genes) is mapped to a standard normal distribution (unit transformation). SPSS (Chicago, IL), Excel (Microsoft, Redmond, WA), and Sigma-Plot (StataCorp, College Station, TX) were applied for statistical analysis of expression data; in particular, Student t test and paired t test were applied.
Results
Expression pattern of TOSO in CLL, healthy lymphocyte subsets, and other lymphoma entities
Analyzing publicly available microarray datasets previously published by Rosenwald et al13 applying a unit-transformation algorithm, a novel procedure for standardization of microarray data as a variant of the z-transformation (Figures S1–S5 and accompanying text, available on the Blood website; see the Supplemental Materials link at the top of the online article), revealed an elevated expression of TOSO in CLL. Further investigations using real-time polymerase chain reaction (PCR)-based quantification assay significantly demonstrated a CLL-specific increased TOSO expression (Table 1; Figure 1A). Freshly isolated CLL cells of 106 patients were analyzed for TOSO mRNA expression. As controls, B-cell subsets isolated from peripheral blood of healthy blood donors and human tonsils were analyzed. In CD19+ sorted peripheral B cells, the relative expression (RE) of TOSO was 2.9 (n = 10; 95% confidence interval (CI), 2.2 to 3.6), CD19+ selected B cells from human tonsils revealed an RE of 2.2 (n = 6; 95% CI, 1.82 to 2.57), and in untouched depletion sorted naive B cells from peripheral blood TOSO showed an RE of 2.6 (n = 5; 95% CI, 1.85 to 3.34). Sorting for CD27+ memory B cells from peripheral blood, the RE for TOSO was 0.4 (n = 3) and 0.76 for CD5+ B cells (n = 5).
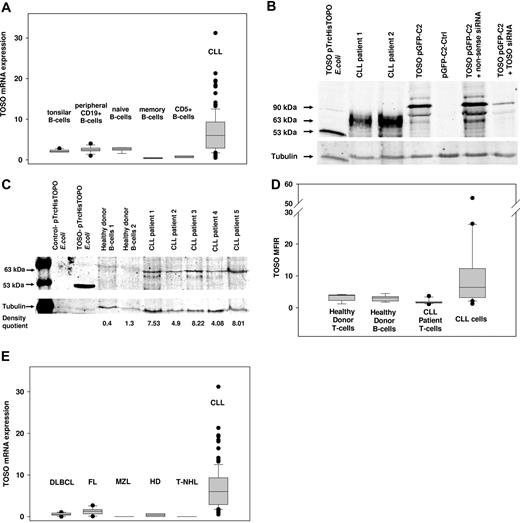
TOSO mRNA and protein expression detected by RT-PCR, immunoblotting, and flow cytometry. (A) TOSO mRNA expression in CD19+ peripheral B cells (n = 10), naive B peripheral B cells (n = 5), memory B cells (n = 3), and CD5+ B cells (n = 5) compared with purified CLL cells (n = 106). (B) TOSO is detected at 63 kDa due to posttranslational modification. Western blot using TOSO-specific 6B10 monoclonal antibody is shown for TOSO-pTRCHisTOPO recombinantly expressed in E coli, for patient CLL cells, and for TOSO-pGFP-C2 being transduced to HEK293 cells; cotransfection of TOSO-pGFP-C2 with TOSO-specific siRNA was performed for control of 6B10 antibody specificity. (C) TOSO protein expression is elevated in CLL cells compared with healthy donor CD19+ B cells; immunoblotting was performed using 6B10 monoclonal antibody; nontransformed TOP10 E coli served as negative control, and recombinantly expressed TOSO-pTRCHisTOPO served as positive control. Densitometry quotient of TOSO/tubulin is indicated accordingly. (D) Flow cytometry assessment of TOSO surface expression comparing healthy donor T and B cells to CLL patient T cells and malignant CLL B cells. (E) TOSO mRNA expression compared with other lymphoma entities: diffuse large B-cell lymphoma (DLBCL, n = 6), follicular lymphoma (FL, n = 6), marginal zone lymphoma (MZL, n = 2), T-cell non-Hodgkin lymphoma (T-NHL, n = 2), and Hodgkin lymphoma–derived cell lines (n = 4). Panel A, D, and E box plots show median, lst and 3rd quartiles, and whiskers (error bars) defined by, at most, 1.5× interquartile range (IQR).
TOSO mRNA and protein expression detected by RT-PCR, immunoblotting, and flow cytometry. (A) TOSO mRNA expression in CD19+ peripheral B cells (n = 10), naive B peripheral B cells (n = 5), memory B cells (n = 3), and CD5+ B cells (n = 5) compared with purified CLL cells (n = 106). (B) TOSO is detected at 63 kDa due to posttranslational modification. Western blot using TOSO-specific 6B10 monoclonal antibody is shown for TOSO-pTRCHisTOPO recombinantly expressed in E coli, for patient CLL cells, and for TOSO-pGFP-C2 being transduced to HEK293 cells; cotransfection of TOSO-pGFP-C2 with TOSO-specific siRNA was performed for control of 6B10 antibody specificity. (C) TOSO protein expression is elevated in CLL cells compared with healthy donor CD19+ B cells; immunoblotting was performed using 6B10 monoclonal antibody; nontransformed TOP10 E coli served as negative control, and recombinantly expressed TOSO-pTRCHisTOPO served as positive control. Densitometry quotient of TOSO/tubulin is indicated accordingly. (D) Flow cytometry assessment of TOSO surface expression comparing healthy donor T and B cells to CLL patient T cells and malignant CLL B cells. (E) TOSO mRNA expression compared with other lymphoma entities: diffuse large B-cell lymphoma (DLBCL, n = 6), follicular lymphoma (FL, n = 6), marginal zone lymphoma (MZL, n = 2), T-cell non-Hodgkin lymphoma (T-NHL, n = 2), and Hodgkin lymphoma–derived cell lines (n = 4). Panel A, D, and E box plots show median, lst and 3rd quartiles, and whiskers (error bars) defined by, at most, 1.5× interquartile range (IQR).
In contrast, in CLL cells, the mean RE of TOSO was 6.8 (n = 106; 95% CI, 5.8 to 7.5) and thus significantly higher compared with control cells (P = .004). However, TOSO expression varied up to RE values of 31.2, indicating heterogeneous intensity of TOSO expression within the CLL patient group.
Protein expression of TOSO was examined by immunoblotting using 6B10 rat monoclonal antibody. Specificity of antibody reactivity was controlled by recombinant TOSO protein or ectopic overexpression of TOSO (Figure 1B). TOSO molecular size of the unprocessed precursor was predicted for 43.1 kDa, however in patient samples TOSO was detected as a 63-kDa protein. Ectopic overexpressed TOSO-GFP fusion protein in HEK293 cells was also detected at 90 kDa (GFP is 27 kDa) and TOSO-MycHis-tagged protein at 50 kDa in TOP10 E coli (MycHis-Tag is 7 kDa), suggesting an additional posttranslational modification of TOSO that might underlie the enlarged protein size detected by immunoblotting. Specific knockdown of TOSO expression by siRNA completely correlated with decreased antibody reactivity and significantly demonstrated the antibody specificity (Figure 1B). Using 6B10 antibody semiquantitative immunoblotting revealed overexpression of TOSO in CLL cells (n = 13) compared with healthy donor CD19+ B cells (n = 6; Figure 1C). Applying flow cytometry analysis, TOSO cell surface expression could be detected specifically in CLL patients (n = 16) compared with healthy controls (n = 5), revealing 11.27 MFIR in CLL versus 2.95 MFIR in healthy donor B cells (P = .02; Figure 1D).
Analyses of TOSO expression in further B-cell lymphomas including diffuse large B-cell lymphoma, follicular lymphoma, marginal zone lymphoma, Hodgkin lymphoma, and T-cell lymphoma revealed no TOSO overexpression in these lymphoma samples in contrast to CLL samples (Figure 1E). Samples derived from lymph nodes of patients with diffuse large B-cell lymphoma revealed low TOSO relative expression (RE) of 0.45 (n = 6); follicular lymphoma samples, an RE of 1.27 (n = 6); and marginal zone lymphoma, an RE of 0.36 (n = 2). T-cell lymphomas (n = 2) and Hodgkin cell lines (n = 4) did not show a detectable TOSO expression (Figure 1E).
Immunohistochemistry
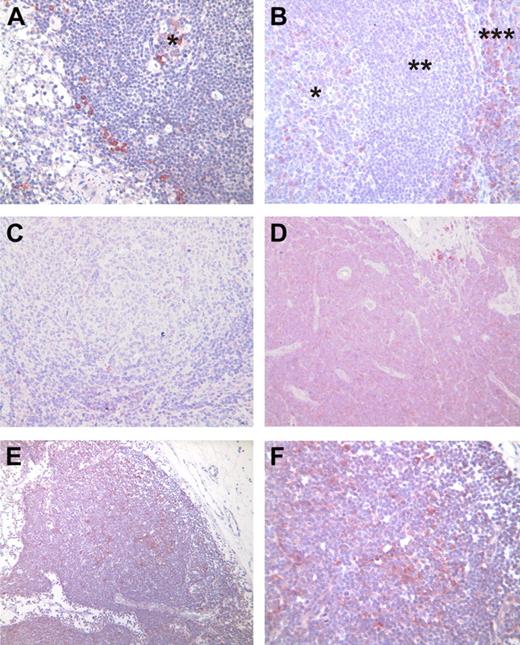
We performed immunohistochemical analysis of TOSO expression in lymph nodes derived from healthy donors, patients with follicular lymphoma, and patients with CLL. In healthy control lymph nodes and tonsils, specific TOSO expression was seen in plasmocytic cells within the germinal center and in the marginal zone (Figure 2A-B). In corresponding samples analyzed here by reverse transcriptase (RT)–PCR, concordant TOSO expression results could be demonstrated. Follicular lymphoma samples did not exhibit TOSO expression (Figure 2C). In addition, other lymphoma entities such as DLBCL, mucosa-associated lymphoid tissue (MALT) lymphoma, and mantle cell lymphoma (MCL) did not reveal detectable TOSO expression by immunohistochemistry (Figure S5). In lymph nodes derived from CLL patients, enhanced TOSO staining was detected (Figure 2D-F). Here, cases with generally elevated TOSO expression (Figure 2D) could be separated from cases with distinct clusters of tumor cells highly expressing TOSO (Figure 2E,F).
TOSO protein expression in lymph nodes from healthy donors, patients with follicular lymphoma, and patients with CLL. (A) Inguinal lymph node with small, regressive germinal center (*). Single TOSO-positive cells are seen in the germinal center as well as within the sinus. Cytomorphologically, these cells are assigned to plasmocytic cells. (B) Cervical lymph node with prominent germinal center (*), mantle zone (**), and small marginal zone (***). Single TOSO-positive plasmocytoid cells within the germinal center; ample TOSO positive cells in the marginal zone. (C) Lymph node infiltrated by a low-grade follicular lymphoma. Within the neoplastic follicle, no TOSO expression is visible. (D-F) Two examples of lymph node infiltration by CLL. (D) TOSO expression is seen as a distinct staining in almost all neoplastic B cells. (E) Distinct clusters of strongly positive cells are visible within the infiltrate. These clusters are not necessarily limited to proliferation centers within the infiltrates. (F) Magnification of panel E. (A-E) Magnification ×200; (F) magnification ×400. Immunohistochemistry was performed using 6B10 antibody on paraffin-embedded sections.
TOSO protein expression in lymph nodes from healthy donors, patients with follicular lymphoma, and patients with CLL. (A) Inguinal lymph node with small, regressive germinal center (*). Single TOSO-positive cells are seen in the germinal center as well as within the sinus. Cytomorphologically, these cells are assigned to plasmocytic cells. (B) Cervical lymph node with prominent germinal center (*), mantle zone (**), and small marginal zone (***). Single TOSO-positive plasmocytoid cells within the germinal center; ample TOSO positive cells in the marginal zone. (C) Lymph node infiltrated by a low-grade follicular lymphoma. Within the neoplastic follicle, no TOSO expression is visible. (D-F) Two examples of lymph node infiltration by CLL. (D) TOSO expression is seen as a distinct staining in almost all neoplastic B cells. (E) Distinct clusters of strongly positive cells are visible within the infiltrate. These clusters are not necessarily limited to proliferation centers within the infiltrates. (F) Magnification of panel E. (A-E) Magnification ×200; (F) magnification ×400. Immunohistochemistry was performed using 6B10 antibody on paraffin-embedded sections.
TOSO expression in CLL patient subgroups
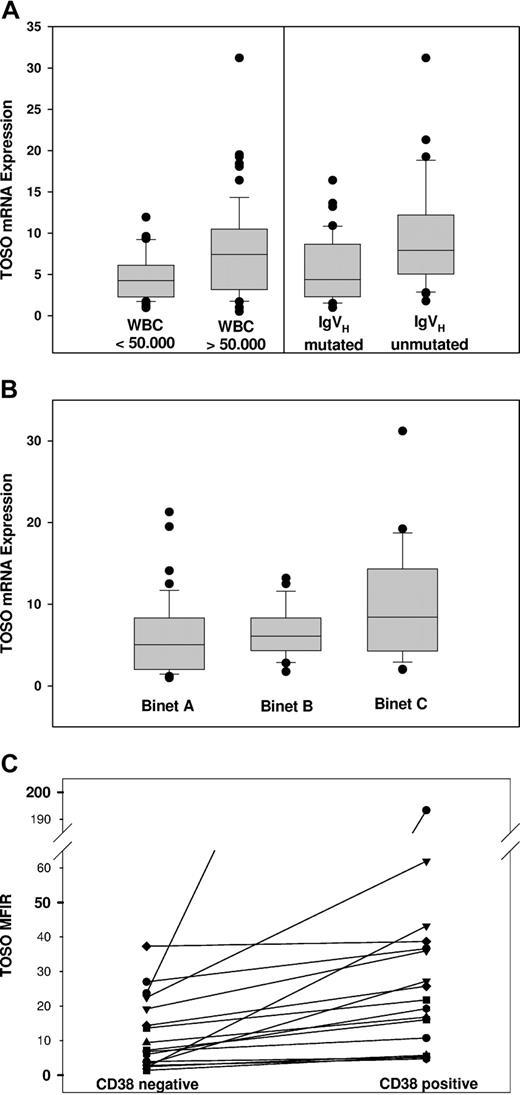
We analyzed TOSO expression based on RT-PCR data with regard to Binet stage, white blood cell count (WBC), lymphocyte doubling time, serum thymidine kinase levels, cytogenetics, IgVH mutational status, and CD38 and ZAP70 expression status (Table 1). For correlation with these clinical parameters, too few freshly isolated CLL samples were available for detection of TOSO expression on protein level by flow cytometry. No significant correlation of TOSO mRNA relative expression values with lymphocyte doubling time, thymidine kinase, cytogenetics, and CD38 and ZAP70 expression was observed. However, TOSO expression was significantly increased to an RE of 8.9 in patients harboring highly elevated white blood cell count above 50 × 109/L (50 000/μL) versus an RE of 5.6 in patients with WBC below 50 × 109/L (50 000/μL; P = .02; Figure 3A). Furthermore, IgVH-unmutated CLL patients were shown to exhibit significantly enhanced TOSO expression with an RE of 8.6 versus 5.7 in IgVH-mutated CLL patients (P = .013) (Figure 3A).
TOSO expression in distinct clinically and molecularly defined CLL subgroups. (A) Significantly increased TOSO expression of patients harboring elevated white blood cell (WBC) counts of more than 50 × 109/L (50 000/μL) (P = .02) and unmutated immunoglobulin heavy chain gene with elevated TOSO mRNA (P = .013). (B) Increased TOSO expression in Binet stage C patients (P = .015). (C) MFIR flow cytometric assessment of TOSO surface expression comparing CD38− and CD38+ CLL cells of the same patient (n = 19). Box plots in panels A and B show median, 1st and 3rd quartiles, and whiskers (error bars) defined by, at most, 1.5× IQR.
TOSO expression in distinct clinically and molecularly defined CLL subgroups. (A) Significantly increased TOSO expression of patients harboring elevated white blood cell (WBC) counts of more than 50 × 109/L (50 000/μL) (P = .02) and unmutated immunoglobulin heavy chain gene with elevated TOSO mRNA (P = .013). (B) Increased TOSO expression in Binet stage C patients (P = .015). (C) MFIR flow cytometric assessment of TOSO surface expression comparing CD38− and CD38+ CLL cells of the same patient (n = 19). Box plots in panels A and B show median, 1st and 3rd quartiles, and whiskers (error bars) defined by, at most, 1.5× IQR.
Patients previously treated with chemotherapy exhibited highly significant increased TOSO expression with an RE of 8.02 versus 4.25 in treatment-naive patients (P = .001). In Binet stage C patients, mean TOSO expression was significantly elevated to an RE of 10.54 versus 6.0 in Binet A and 6.6 in Binet B stage patients (P = .015; Figure 3B).
Although overall CD38 expression did not reveal significant differences with respect to TOSO mRNA expression levels, we compared TOSO cell surface expression on CD38+ and CD38− CLL cells of the same patients, harboring at least 10% CD38+ CLL cells. CLL cells were costained with CD19-, CD5-, CD38-, and TOSO (6B10)-specific antibodies, and CD19+/CD5+/CD38− and CD19+/CD5+/CD38+ CLL cell subsets were analyzed by flow cytometry. CD38− CLL cells showed decreased MFIR of 7.1 versus 16.02 in CD38+ CLL cells (n = 19; P = .043; Figure 3C).
Regulation of TOSO expression
To study the regulation of TOSO in a functional context, we analyzed for a possible influence of tumor micromilieu on TOSO expression. To elucidate TOSO in the context of bone marrow stromal interaction, we cocultivated CLL cells on HS5 bone marrow stroma cell lines for 72 hours and analyzed for time-dependent TOSO regulation. Cocultured CLL cells showed similar TOSO RE of 7.7 versus 7.1 in native cells (n = 6; ns), indicating no significant influence of HS5 cocultivation. To reveal reliable TOSO expression data, in addition to RT-PCR we also performed flow cytometry to unravel possible contamination of CLL cells by RNA derived from HS5 feeder cells. Use of flow cytometry FSC/SSC and CD19+/CD5+ gating allowed detailed analysis of cocultured CLL cells under exclusion of contaminating bone marrow cells. Flow cytometry confirmed stable TOSO expression both in native and stroma cell cocultivated CLL cells (data not shown).
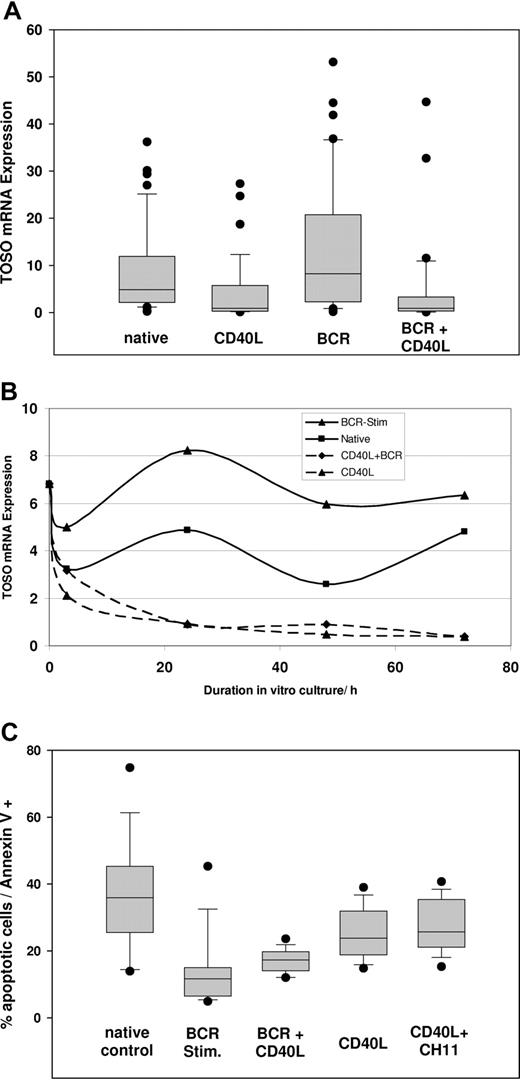
We further analyzed the specific regulation of TOSO by B-cell receptor (BCR) stimulation as a major pathway involved in pathogenesis of CLL. In addition, we addressed regulation of TOSO in the context of CD40 activation based on the previously observed Fas sensitization of CLL cells by CD40L stimulation and the general suppressed CD40L stimulus in CLL patients. TOSO expression was determined in 48 CLL patients by quantitative real-time PCR at baseline, 3, 24, 48, and 72 hours after onset of BCR or CD40L stimulation or combination of both stimuli. Time-dependent TOSO mRNA expression is depicted in Figure 4A-B. Native TOSO mRNA expression did not alter significantly during in vitro culture. Nevertheless a nonsignificant decrease of TOSO expression was seen from baseline value of 6.82 (95% CI, 5.74-7.88) to 2.6 (95% CI, 1.74-3.45; P > .05) after 48 hours. BCR stimulus significantly increased TOSO mRNA to an expression maximum of 8.25 in CLL cells compared with native control cells (RE 4.86; P = .013). Assessment of apoptotic CLL cells revealed a significant reduction of apoptotic cells by BCR stimulation to 11.7% annexin V+ CLL cells versus 35% annexin V+ apoptotic cells in the native control cells after 72 hours of incubation (P = .003; Figure 4C).
Regulation of TOSO and apoptosis resistance. (A) TOSO mRNA expression after 24-hour incubation with B-cell receptor (BCR) stimulation, recombinant CD40L-expressing feeder cell lines, and combined BCR/CD40L stimulus; significant increase of TOSO by BCR stimulus (P = .013) and suppression by CD40L (P = .007). (B) Time-dependent course of TOSO expression at time points of 3, 24, 48, and 72 hours. (C) Percentage of apoptosis in CLL cells assessed by annexin V/7-AAD flow cytometry. Significantly decreased apoptosis rate by BCR stimulation (P = .003); CD40L nonsignificantly reduces apoptosis. Slightly elevated apoptosis in CD40L-treated CLL cells by addition of the FAS-agonistic antibody CH11. (D) TOSO protein expression after 72-hour incubation with B-cell receptor stimulus (BCR), recombinant CD40L-expressing feeder cell lines, and combined BCR/CD40L stimulus; increase of TOSO by BCR stimulus and suppression by CD40L. Densitometry quotient of TOSO/β-actin is indicated accordingly. Box plots in panels A and C show median, 1st and 3rd quartiles, and whiskers (error bars) defined by, at most, 1.5× IQR.
Regulation of TOSO and apoptosis resistance. (A) TOSO mRNA expression after 24-hour incubation with B-cell receptor (BCR) stimulation, recombinant CD40L-expressing feeder cell lines, and combined BCR/CD40L stimulus; significant increase of TOSO by BCR stimulus (P = .013) and suppression by CD40L (P = .007). (B) Time-dependent course of TOSO expression at time points of 3, 24, 48, and 72 hours. (C) Percentage of apoptosis in CLL cells assessed by annexin V/7-AAD flow cytometry. Significantly decreased apoptosis rate by BCR stimulation (P = .003); CD40L nonsignificantly reduces apoptosis. Slightly elevated apoptosis in CD40L-treated CLL cells by addition of the FAS-agonistic antibody CH11. (D) TOSO protein expression after 72-hour incubation with B-cell receptor stimulus (BCR), recombinant CD40L-expressing feeder cell lines, and combined BCR/CD40L stimulus; increase of TOSO by BCR stimulus and suppression by CD40L. Densitometry quotient of TOSO/β-actin is indicated accordingly. Box plots in panels A and C show median, 1st and 3rd quartiles, and whiskers (error bars) defined by, at most, 1.5× IQR.
CD40L significantly reduced TOSO mRNA expression already after 24 hours to 0.92 compared with 4.86 in native controls (P = .007). A minimum TOSO RE of 0.475 after 48 hours compared with 2.6 in the native control was observed for CD40L-stimulated CLL cells. CD40L resulted in decreased apoptosis rates of 23.8% annexin V+ cells; nevertheless the apoptosis reduction was not shown to be significant (P = .11). Compared with BCR stimulus, CD40L induction showed a significantly higher rate of apoptotic cells (P = .048) (Figure 4C). Combining CD40L and BCR stimulation revealed a significant decrease of TOSO expression to an RE of 0.895 after 48 hours (P = .012) compared with native CLL cells. Comparing solely CD40L-stimulated CLL cells with cells stimulated with both CD40L and BCR revealed a decreased but nonsignificant drop in the rate of apoptotic cells.
To confirm mRNA-based analysis of TOSO regulation, stimulated CLL cells were processed for immunoblotting, showing significant increase of TOSO protein expression after 72 hours of BCR stimulation, whereas CD40L stimulation decreased TOSO expression. In accordance with mRNA expression data, TOSO suppression with still detectable TOSO expression was also seen after combined stimulation by CD40L and BCR (Figure 4D).
To evaluate the functional impact of TOSO down-regulation on the context of CD40L stimulation, we assessed the FAS-agonistic antibody CH11 to CD40L-stimulated primary CLL cells (n = 9) (Figure 4C). CH11 treatment induced a slightly elevated but nonsignificant increase of apoptosis after 72 hours. Although a significant change could not be demonstrated, Fas sensitization by CD40L was seen in a subset of 4 patients, leading to 20% mean increase of apoptotic cells.
Discussion
Resistance toward apoptosis is the main mechanism in CLL pathogenesis leading to accumulation of B cells especially resistant toward apoptosis induction by Fas via the extrinsic apoptosis pathway. Various mechanisms in apoptosis resistance in CLL have been described, however Fas resistance could not be completely explained. In Jurkat cells stably transfected with TOSO, it was demonstrated that Fas-induced activation of caspase-8 was significantly inhibited by the expression of TOSO.9 In addition to that, it was shown in CLL cells that PI3-kinase enables their survival by preventing caspase-8 activation.14 Furthermore, recent results suggest that caspase-8 processing is inhibited by TOSO through up-regulation of cFLIP. Besides protection of CLL cells from Fas-induced apoptosis, it was also elucidated that TOSO counteracts TNF-induced apoptosis.9 In CLL cells, 2 related members of the TNF family, that is, BAFF (B-cell activating factor of the TNF family) and APRIL (a proliferation-inducing ligand) were shown to be overexpressed and involved in resistance to apoptosis through an autocrine pathway.15
In our experiments, we could demonstrate that CLL cells down-regulate TOSO after being stimulated via their CD40 receptor by CD40L. In the context of a clinical trial using CD40L-transduced autologous CLL cells, Kater et al have shown that CLL cells initially resist CD95-mediated apoptosis within the first 3 days after CD40 ligation in vitro.16 This initial resistance to CD95-mediated apoptosis is associated with high-level expression of X-linked inhibitor of apoptosis protein (XIAP) by CLL cells. Thereafter, CLL cells become sensitive to apoptosis and express the proapoptotic protein B-cell leukemia 2 homology 3 (BH3) interacting domain death agonist (Bid). Although CD40 activation in our experimental setting was achieved only by CD40L-expressing feeder cells and not by CD40L-transduced autologous CLL cells, TOSO is down-regulated due to the strong survival signal through CD40 in vitro. The need for feeder layer–based CD40L stimulation might contribute to reduced apoptosis in CLL cells cocultivated on CD40L-expressing feeder layer cells.
Nevertheless, it was shown previously that CD40 triggering merely by coculture with CD40L-transfected cells is not sufficient to overcome resistance to CD95-mediated apoptosis despite a strong up-regulation of CD95 on the membrane of CLL cells.4 It can be speculated that TOSO down-regulation is an early event in the apoptotic cascade, but Bid expression is a conditio sine qua non for the full activation of apoptosis in CLL cells. In contrast to results obtained after CD40 activation, we could demonstrate in our study that BCR stimulation resulted in up-regulation of TOSO in CLL cells in vitro. Presence of both BCR and CD40L stimulus revealed a significant decrease of TOSO, indicating BCR stimulus as solitary inducer of TOSO expression in CLL inferior to CD40L. This could resemble the situation in vivo since there is increasing evidence that antigenic stimulation via the BCR is a major factor in the pathogenesis of CLL and CD40L signaling is reduced in CLL17,18 In our study, we showed that TOSO is not regulated by bone marrow stroma cells; moreover a specific antigenic stimulus via BCR signaling can be postulated as TOSO-inducing mechanism in CLL pathogenesis. Although it has to be kept in mind that BCR cross-linking induces both cell proliferation and apoptosis in healthy and malignant B cells,19 there is evidence that Akt and Mcl-1 are major components of a survival pathway that can be activated in CLL B cells by BCR engagement.20 Here, BCR engagement with bead-based cellular mimicking resulted in significantly decreased spontaneous apoptosis. Recently, we demonstrated BCR-induced expression of LPL as a prognostic and pathogenetic factor in CLL.21
An unmutated status of the IgVH gene was shown to exhibit enhanced autoreactivity and was shown to be the most reliable predictor of prognosis in CLL.22 Interestingly, we could show that IgVH-unmutated CLL cases exhibited significantly higher expression levels of TOSO. Furthermore, there is evidence from our CD38 expression data that TOSO expression is associated with the proliferative pool of the CLL cells. Along these lines, it is of interest that TOSO expression correlates with high leukocyte counts and advanced disease stage. Recently, Proto-Siqueira et al identified TOSO as a critical molecule in Fas-mediated apoptosis resistance in CLL cells by serial analysis of gene expression (SAGE). In accordance with our results, the authors also demonstrated an increased expression of TOSO in CLL cases with increased leukocyte counts. Furthermore, TOSO was positively correlated with high bcl-2 expression levels.23
In summary, we identified TOSO as a molecule specifically overexpressed in CLL and regulated by BCR activation. Studies are ongoing to assess further mechanisms by which TOSO mediates its antiapoptotic effects. TOSO will be prospectively evaluated as a prognostic marker for high-risk CLL. Furthermore, future studies will investigate whether TOSO can be targeted as a critical molecule of the proliferative pool in CLL, thus hopefully improving therapeutic outcome in this still incurable disease.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Reinhild Brinker for excellent technical support.
This study was supported by grants from the Else-Kröner-Fresenius-Stiftung, Bad Homburg, Germany (P17/06//A62/05), Köln Fortune, Köln, Germany (050808), the Deutsche José Carreras Leukämie Stiftung, München, Germany (DJCLS R06/16), the CLL Global Research Foundation, Houston, TX (CLLGRF), and the Marga and Walther Boll Stiftung, Köln, Germany (3640/0393/21).
Authorship
Contribution: C.P.P. and C.W. conceived and designed the present work; C.P.P., A.S., C.W., and N.K. performed the research; N.K., J.S., C.W., H.K., and S.H. contributed analytical tools; A.U. and C.P.P. conducted statistical analysis of the data; C.P.P., A.U., and C.-M.W. analyzed the data; C.P.P., M.H., and C.-M.W. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Clemens-Martin Wendtner, University of Cologne, Clinic I of Internal Medicine, D-50924 Cologne, Germany; e-mail: clemens.wendtner@uni-koeln.de.
References
Author notes
*C.P.P. and A.S. contributed equally to this work