Abstract
The search for novel inhibitors of the platelet αIIbβ3 receptor continues with the dual goals of better defining structure-function relationships and developing second generation oral agents. We previously reported on a novel small compound (Compound 1; RUC-1) identified by high throughput screening that inhibits human αIIbβ3. RUC-1 did not inhibit αVβ3, suggesting that it interacts with αIIb, and molecular docking studies supported this speculation. RUC-1 also induced less extensive changes in αIIbβ3 conformation than existing small molecule inhibitors, which may be therapeutically desirable. We have now studied RUC-1’s effects on murine and rat platelets, which are less sensitive than human to inhibition by RGD peptides due to differences in the αIIb sequences contributing to the binding pocket. We found that RUC-1 (100 μM) was much less potent in inhibiting platelet aggregation of murine and rat platelets than human platelets or the platelets of a mouse expressing a hybrid receptor composed of human αIIb and murine β3 (hαIIb/mβ3) (mouse, 6±6%, n=4; rat, 0±15%, n=3; human, 97±2% n=3; hαIIb/mβ3 99±1%, n=4). RUC-1 also inhibited fibrinogen binding to murine platelets expressing the hybrid hαIIb/mβ3 receptor (94± 2%, n=4), but not a hybrid receptor composed of murine αIIb and human β3 (mαIIb/hβ3; 0%, n=4). Molecular docking studies of RUC-1 were consistent with the functional data with RUC-1 binding entirely within the β-propeller. αIIb In vivo studies of RUC-1 administered intraperitoneally (IP) at a dose of 26.5 mg/kg demonstrated antithrombotic effects in the FeCl3 carotid artery model in mice expressing hαIIb/mβ3 (Figure 1A), but did not protect WT mice from thrombotic occlusion at the same dose (Figure 1B). Collectively, these data support RUC-1’s specificity for αIIb, provide new insights into the αIIb ligand-binding pocket, and establish RUC-1’s anti-thrombotic effects in vivo. In addition, the hαIIb/mβ3 mice provide a convenient model for testing low molecular weight αIIbβ3 antagonist drugs such as RUC-1 for toxicity and therapeutic potential.
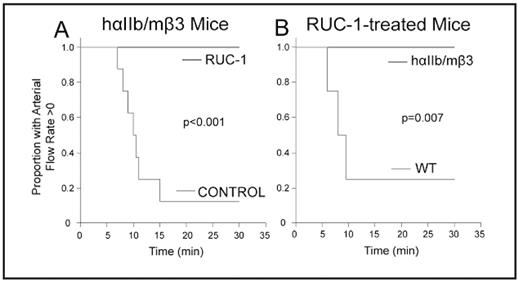
RUC-1 protects hαIIb/mβ3 mice, but not WT mice from FeCl3-induced carotid artery thrombotic occlusion. A. Mice expressing hαIIb/mβ3 receptor were injected IP with vehicle control (n=4), an inactive congener of RUC-1 (RUC-1-piperidine; n=4; 26.5 mg/kg), or RUC-1 (n=7; 26.5 mg/kg) 25 min before carotid artery injury with 20% FeCl3 for 3 min. Blood flow through the carotid artery was monitored for 30 min with a Doppler flow probe. Kaplan-Meier analysis was calculated from the time the FeCl3 was applied to the artery until complete occlusion. The control curve contains the combined data from the mice treated with vehicle and RUC-1-piperidine. B. WT mice (n=4) were given RUC-1 (26.5 mg/kg) and their data are compared to those reported in panel A for mice expressing hαIIb/mβ3.
RUC-1 protects hαIIb/mβ3 mice, but not WT mice from FeCl3-induced carotid artery thrombotic occlusion. A. Mice expressing hαIIb/mβ3 receptor were injected IP with vehicle control (n=4), an inactive congener of RUC-1 (RUC-1-piperidine; n=4; 26.5 mg/kg), or RUC-1 (n=7; 26.5 mg/kg) 25 min before carotid artery injury with 20% FeCl3 for 3 min. Blood flow through the carotid artery was monitored for 30 min with a Doppler flow probe. Kaplan-Meier analysis was calculated from the time the FeCl3 was applied to the artery until complete occlusion. The control curve contains the combined data from the mice treated with vehicle and RUC-1-piperidine. B. WT mice (n=4) were given RUC-1 (26.5 mg/kg) and their data are compared to those reported in panel A for mice expressing hαIIb/mβ3.
Disclosures: Blue:The Rockefeller University: Patents & Royalties. Coller:Centocor, Inc.: Patents & Royalties; The Rockefeller University: Patents & Royalties.
Author notes
Corresponding author