Abstract
Normally factor (F) VIII is not expressed in megakaryocytes, but when human FVIII was transgenically expressed in murine megakaryocytes, it was stored in platelet α-granules and released at sites of injury. This platelet FVIII (pFVIII) is effective in correcting hemostasis, even in the presence of circulating inhibitors, so it offers a potential gene therapy strategy for hemophilia A. To understand clot development by pFVIII, we have examined clot response to laser injury in both cremaster arterioles and venules in FVIIInull mice either infused with FVIII or transgenic for pFVIII. In both sets of vessels, pFVIII is at least as effective as infused FVIII. However, there are temporal and spatial differences in fibrin and platelet accumulation within clots depending on how FVIII is delivered. These differences may be related to the temporal and spatial distribution of the α-granular–released FVIII within the developing clot, and may explain the increased frequency and size of embolic events seen with pFVIII. These observations may not only have implications for the use of pFVIII in gene therapy for hemophilia A, but may also have physiologic consequences, explaining why many procoagulant factors are delivered both in the plasma and in platelet α-granules.
Introduction
We and others have shown that human B–domainless factor (F) VIII can be expressed in murine megakaryocytes, stored in platelet α-granules independent of von Willebrand factor (VWF), and released at sites of injury in hemophilia A FVIIInull mice.1-3 Correction of bleeding occurred in FVIIInull mice transgenic for platelet-specific FVIII (pFVIII) expression, in spite of there being no detectable plasma FVIII level. In addition, pFVIII is protected from circulating inhibitors,3,4 a significant problem in the hemophilia A population.5 Several editorials have, therefore, suggested that this pFVIII gene therapy strategy may be of benefit in hemophilia A patients with problematic inhibitors who do not respond to present-day therapies to eliminate such inhibitors.6,7
Aside from a megakaryocyte-specific gene therapy, all other FVIII-based gene therapy strategies for hemophilia A correct plasma FVIII levels.8 We believe that the details of how FVIII released from activated platelets affects clot development must be examined to be assured that there are no untoward side effects before clinical application. Several clotting models have been examined in FVIIInull mice, which suggest that pFVIII is at least as efficacious as plasma FVIII. The tail exsanguination model, which involves both arterials and venules of various sizes and which extends over 16 hours, suggested very high relative efficacy for pFVIII, but we have shown that this model may be too sensitive to pFVIII, likely due to hypovolemia with subsequent blood stasis in the tail.1 A cuticular bleed model, which also involves both arterial and venule injury and which extends over 8 hours, suggested less relative efficacy.1 Since both the tail exsanguination and cuticular bleed models extend over many hours, translation of outcome to plasma FVIII equivalency was not done, especially given the short half-life of infused human FVIII in mice.9 In a third injury model, involving clot development after carotid artery FeCl3 injury, a direct comparison of platelet-delivered and infused human FVIII could be done because studies can be completed in 30 minutes. These studies suggested that platelet-delivered FVIII was greater than or equal to 3 times more effective than infused FVIII.1 Jugular injury studies using FeCl3 are technically difficult, so we did not extend those studies to look at whether pFVIII was equally effective on the venous side.
Since platelets are thought to be more important in arterial than venule injuries,10 a strategy that improves arterial clotting more than venous clotting may be prothrombotic. At this point, none of the published studies provide insights into the potential thrombotic risks to patients of correcting hemophilia A by this novel delivery strategy. In contrast, a great deal of information is known about plasma correction of FVIII levels, and only at high FVIII levels—several-fold greater than normal—are there concerns of prothrombotic risks, and these risks are of a venous thrombosis nature (reviewed in Franchini and Veneri11 ).
In situ studies of clot development have provided new insights into the temporal and spatial details of clot development. One of the more informative in situ models has been the cremaster arteriole laser injury model developed by the Furies.12,13 This model allows a detailed analysis of the contribution of various factors to a developing clot. After laser injury in this model, clot development is mostly instigated by tissue factor release and thrombin generation rather than collagen exposure and platelet adhesion, which are more important after FeCl3 carotid artery injury.14 Using this laser injury model, we found that there is a major defect in clot formation in FVIIInull mice after either arterial or venule injury, and that infused human FVIII can ameliorate this defect in a dose-dependent fashion. In this model, pFVIII/FVIIInull mice with a platelet content of human FVIII level equal to a 9% plasma antigen equivalency had improved clot formation compared with FVIIInull mice. However, the temporal and relative quantitative accumulation of platelets and fibrin in pFVIII/FVIIInull mice differed from FVIIInull mice treated with FVIII infusions. We believe that at least part of this difference involves the timing and spatial distribution of platelet degranulation within the clot, resulting in clot formation in the pFVIII/FVIIInull mice that embolize more readily than that seen after infused FVIII. The physiologic implications of these findings as well as their implications in the care of patients with hemophilia A are discussed.
Methods
Characterization of mice studied
The FVIIInull mice were previously described and had exon 16 deleted from the murine F8 gene.15 The pFVIII transgenic mouse had also been described1 and involves a construct in which a cDNA for the human B–domainless FVIII16 is driven by the glycoprotein Ibα promoter.17,18 All of the FVIIInull and pFVIII/FVIIInull mice studied were littermates on a C57BL/6J background, and were compared with wild-type (WT) C57BL/6J mice of the same age. Genotype was determined by polymerase chain reaction analysis of DNA isolated from tail clippings as described.1 Male mice studied were 8 to 14 weeks of age and weighed 20 to 28 g. Experimental approval was obtained from the Children's Hospital of Philadelphia Animal Care and Use Committee.
Antibodies used
Rat anti–mouse CD41 antibody (clone MWReg30) and rat anti–mouse CD62P antibody (clone RB 40.34) were purchased from BD Pharmingen (Franklin Lakes, NJ). Fab fragments from the anti-CD41 antibody were produced using the ImmunoPure Fab Preparation kit (Pierce Biotechnologies, Rockford, IL). Both anti-CD41 Fab fragments and anti-CD62P antibody were conjugated with Alexa488 by use of the Alexa Fluor Protein labeling kit (Molecular Probes, Eugene, OR). For the embolization studies, a separate batch of anti-CD41 Fab fragments was similarly labeled with Alexa647. A murine anti–human fibrin antibody,19 which was shown to cross-react with murine fibrin,20 was kindly provided by Dr Hartmut Weiler from the University of Wisconsin and was also conjugated with Alexa647. Based on prior empiric studies to define doses of antibody needed to clearly visualize clot development, antibodies were infused intravenously into mice at 0.2 mg/kg for the anti-CD41 Fab fragment, 0.34 mg/kg for the anti-fibrin antibody, and 0.45 mg/kg for the anti-CD62P antibody before the first laser injury.
Cremaster laser injury model
The intravital Video-microscopy system used to study clot formation within the cremaster muscle blood vessels has been previously described in detail.13 Briefly, the mouse was anesthetized using intraperitoneally injected pentobarbitol sodium (11 mg/kg; Abbott Laboratories, North Chicago, IL), and maintained under the anesthesia with the same anesthetic delivered via catheterized jugular vein as needed. To maintain isothermal conditions (37°C), the mouse was placed on a thermo-controlled rodent blanket (Gaymar Industries, Orchard Park, NY). The scrotum was incised, and cremaster muscle was isolated and pinned down to the intravital microscopy tray. During the course of the experiment, the exposed cremaster preparation was constantly superfused with prewarmed (37°C) bicarbonate-buffered saline. The microvessels were studied using an Olympus BX61WI microscope (Olympus, Tokyo, Japan or Center Valley, PA) with a 40×/0.8 numeric aperture (NA) water-immersion objective lens. Labeled antibodies were injected intravenously into the cannulated jugular vein 5 minutes before injury. Some FVIIInull mice also received 0% to 240% antigenic correction (0-10 U/20 g mice) of human FVIII (Advate, kindly provided by Baxter Laboratories, Westlake Village, CA) in less than 200 μL total volume 5 minutes before injury.
Laser injury was induced using an SRS NL100 Nitrogen Laser system (Photonic Instruments, St Charles, IL) at 65% energy level. Blood vessels used ranged in size from 20 to 40 μm. Arteriole and venule injuries were done at the edge of the vessel wall. A visual confirmation of small extravasations was made for each studied blood vessel as an assurance that the injury was made as well as an indicator of consistent injury. No more than 10 laser pulses were used to generate each injury. Colocalization studies were done by use of 2 fluorescent channels, 490 and 647, plus bright field. Data were collected over a course of 2.5 minutes at 5 frames per second (f/s; 750 frames per study). No more than 5 arteriole and 5 venule injuries were done in each mouse. In the embolization studies the use of multiple channels was omitted to increase the rate of image acquisition to approximately 62.5 f/s with a total of 15 000 frames collected over 4 minutes.
Data were analyzed by use of Slidebook software (Intelligent Imaging Innovations, Denver, CO). Background fluorescence was removed for colocalization studies by placing a high-sensitivity mask upstream of the growing clot, and the intensity of the signal collected subtracted from the intensity of the clot signal in a frame-by-frame fashion. For the embolization studies, a short individual frame collection was done before each injury and its fluorescent signal was subtracted as a background reading from the intensity of the subsequent study of this high-sensitivity mask after establishing an upstream injury. Passing emboli were then captured in an intensity-versus-time database and exported from Slidebook software into an Excel (Microsoft, Redmond, WA) spreadsheet for analysis.
Statistical analysis
Statistical analysis of the frequency, average size and total mass of emboli was done by a 2-tailed t test and was generated using Microsoft Excel 2004 for Macintosh (version 11.3.7). Values of P less than .05 were considered statistically significant.
Results
In situ arteriole and venule injury studies in FVIIInull mice
Platelet-delivered human FVIII is more effective in correcting the bleeding diathesis in FVIIInull mice than an infusion of an equal dose of human FVIII in the FeCl3 carotid artery injury model.1 However, this injury model involves denudation of a major artery's endothelial lining and is collagen-dependent.14 Clot development in this model might therefore be highly dependent on platelet activation and exaggerate the effectiveness of pFVIII. We now wish to study injuries that involve alternative clot-activation pathways and also involve smaller arteries and veins. We used the in situ laser injury model to further study the biologic effects of FVIII released from platelet α-granules in both an arteriole and a venule setting. Previous studies of laser-induced arteriole injury showed that clot development in this model is tissue-factor rather than collagen-dependent.14
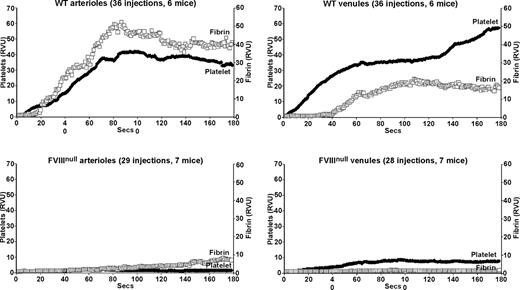
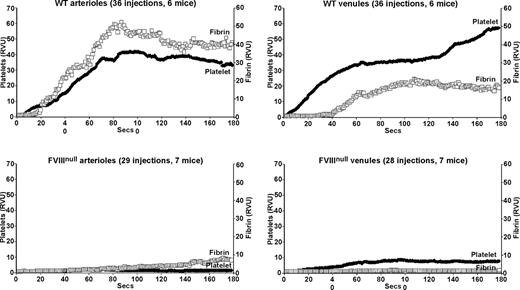
Studies of arteriolar laser injury in WT mice were similar to those previously published by others12,13 with an immediate accumulation of platelets and an approximately 15-second delay before significant fibrin accumulation (Figure 1 top left). Venular clot formation in WT mice was similar, but fibrin accumulation was more markedly delayed with limited fibrin clot formation before approximately 40 seconds (Figure 1 top right). Similar injuries in FVIIInull mice resulted in a virtual absence of a platelet plug in the arterioles and a marked delay and diminution in total fibrin clot formation (Figure 1 bottom left). In the venule system, there was an immediate, but diminished, platelet plug at the site of injury, but with less fibrin accumulation than in the arteriolar system (Figure 1 bottom right). The fibrin accumulation in the arterioles of the FVIIInull mice was likely secondary to tissue factor release. In these high flow–rate vessels,21-23 poor platelet plug formation might have been due to both limited thrombin generation and limited development of a stabilizing fibrin clot. Fibrin accumulation was less on the venule than on the arterial side in the FVIIInull mice, suggesting less tissue factor available to initiate thrombin generation; however, in spite of this lower amount of fibrin generation, platelet plug formation was noted, perhaps due to the lower flow rate in these vessels.
Platelet and fibrin accumulation in laser-induced arteriole and venule injuries in wildtype and untreated FVIIInull mice. Analysis of in situ laser injury in cremaster arterioles and venules. Average platelet accumulation was measured using an Alexa647-labeled anti-CD41 Fab fragment (RVU), and fibrin clot accumulation using an Alexa488-labeled anti-fibrin antibody (RVU). Studies of wildtype (WT) mice are shown on the top row and FVIIInull mice on the bottom row, with arterioles on the left and venules on the right for both rows. The number of individual injuries and total mice used are noted at the top of each graph. Measurements are in relative value units and held constant throughout these studies.
Platelet and fibrin accumulation in laser-induced arteriole and venule injuries in wildtype and untreated FVIIInull mice. Analysis of in situ laser injury in cremaster arterioles and venules. Average platelet accumulation was measured using an Alexa647-labeled anti-CD41 Fab fragment (RVU), and fibrin clot accumulation using an Alexa488-labeled anti-fibrin antibody (RVU). Studies of wildtype (WT) mice are shown on the top row and FVIIInull mice on the bottom row, with arterioles on the left and venules on the right for both rows. The number of individual injuries and total mice used are noted at the top of each graph. Measurements are in relative value units and held constant throughout these studies.
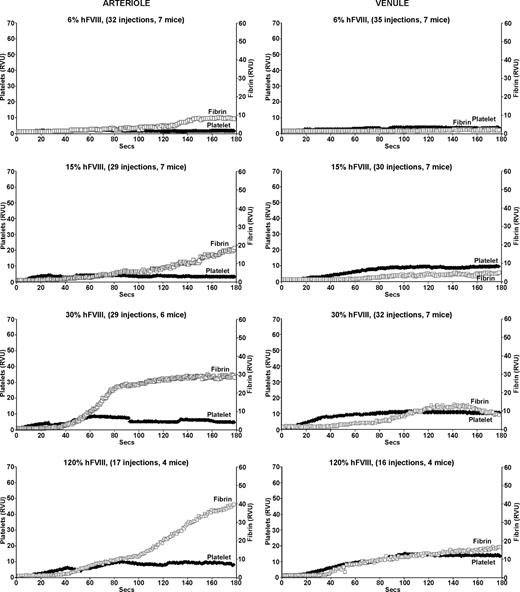
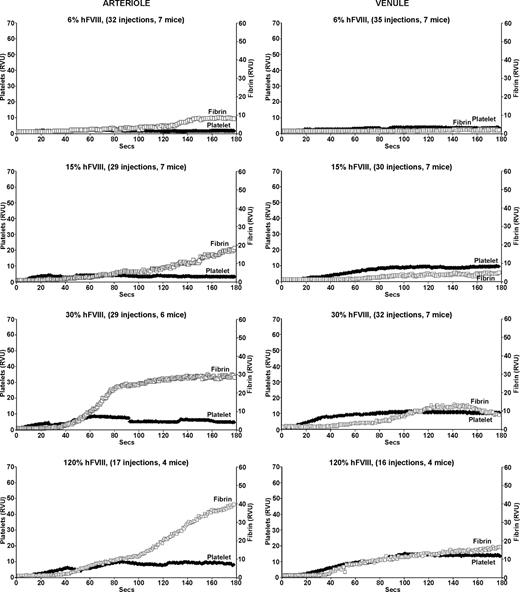
Infusion of increasing amounts of human FVIII improved clot formation in both arterioles and venules of the FVIIInull mice (Figure 2). At the lowest dose of human FVIII, which should result in a 6% antigenic correction or approximately 2% activity level, there was little change in either platelet or fibrin accumulation in both arterioles and venules (Figure 2 top row). Beginning at 15% antigenic correction (∼ 5% activity), there was improvement in fibrin clot formation in the arterioles both in terms of intensity and time to start of fibrin clot (Figure 2 second row left). However, while at higher corrections, the level of fibrin accumulation normalized on the arterial side, the time to begin fibrin clot formation did not, being more than twice as long as compared with WT mice, even at the highest amount of infused FVIII (Figure 2 bottom left). Platelet plug formation also improved, but even at the highest correction was never as vigorous as in WT mice arterioles. A further doubling of the infused human FVIII to 240% antigenic correction did not further improve fibrin or platelet accumulation (data not shown).
Platelet and fibrin accumulation in laser-induced arteriole and venule injuries in treated FVIIInull mice. Same as in Figure 1, but for FVIIInull mice receiving a human FVIII infusion 5 minutes before the first injury. All studies were completed within 1 hour of infusion. The estimated percentage of human FVIII antigenic correction is indicated at the top of each graph.
Platelet and fibrin accumulation in laser-induced arteriole and venule injuries in treated FVIIInull mice. Same as in Figure 1, but for FVIIInull mice receiving a human FVIII infusion 5 minutes before the first injury. All studies were completed within 1 hour of infusion. The estimated percentage of human FVIII antigenic correction is indicated at the top of each graph.
On the venule side, fibrin clot formation improved with increased correction. In this setting, time to onset of fibrin clot did return to normal at the higher correction, but the intensity of fibrin clot formation and platelet accumulation never returned to normal at all tested levels shown (Figure 2 right) even with doubling the highest dose (data not shown). The findings in Figure 2 are consistent with the known lower specific activity of human FVIII in mice,1 but also suggest that in vivo additional aspects of human FVIII's biology may be suboptimal in mice.
Details of clot formation in the pFVIII/FVIIInull mice
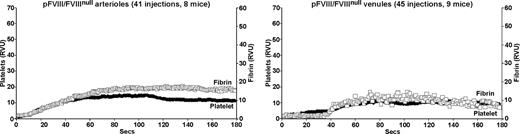
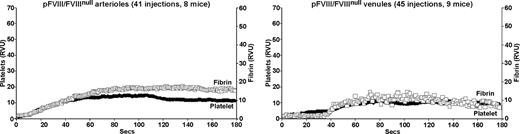
The pFVIII/FVIIInull mice have within their platelets a human FVIII equivalent of plasma 9% antigen and 3% activity.1 In the laser injury model, pFVIII improved both arterial and venule fibrin and platelet accumulation in the FVIIInull mice (Figure 3). Overall improvement in both vessels was comparable to that after a 15% human FVIII infusion (Figure 2 second row from top). However, details of the clots differed, especially as pFVIII fully corrected time to onset of clot formation in both arterioles and venules.
Platelet and fibrin accumulation in laser-induced arteriole and venule injuries in pFVIII/FVIIInull mice. Same as in Figure 1, but studies were done in pFVIII/FVIIInull mice that were littermates of the FVIIInull mice in Figures 1 and 2.
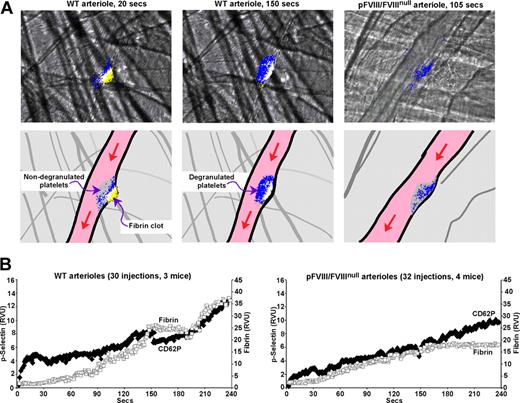
Prior studies in this injury system suggested that arteriolar platelet degranulation, measured using an anti-CD62P antibody that only detects degranulated platelets,24 occurs over the first several minutes after injury.12,24 We confirmed this finding using a similar approach on both the arteriole side (Figure 4A,B left) and venule side for WT mice (Figure 4B right). Degranulation begins at the base of the developing clot, especially at the upstream end, and extends throughout the thrombus by 2 minutes (Figure 4A,B and Video S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Importantly, significant accumulation of degranulated platelets occurs in the WT clots almost immediately in both the arterioles and venules (Figure 4B). Similarly, in the pFVIII/FVIIInull mice, degranulation begins immediately and at the base (Figure 4A,B and Video S2); however, the clots appear less organized and stable, and do not develop the more extensive and uniform degranulation seen in the WT mice. We believe that it is the rapid degranulation at the base of a developing clot that leads to rapid fibrin accumulation in both the arteriole and venule injuries in pFVIII/FVIIInull mice.
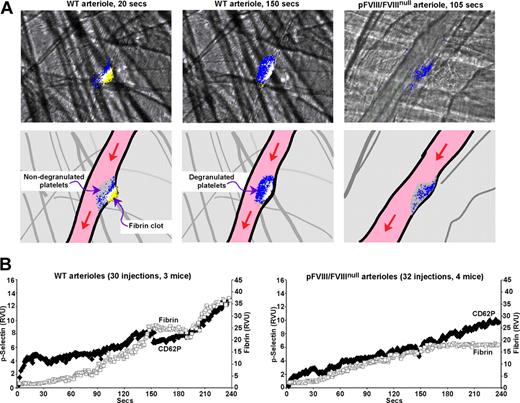
CD62P exposure in WT and pFVIII/FVIIInull mice. Studies as in Figures 1 through 3 with an Alexa647-labeled anti-fibrin antibody, but instead of measuring platelet accumulation using an anti-CD41 Fab fragment, CD62P exposure was measured using an Alexa647-labeled anti-CD62P antibody. (A) Top row shows representative frames from specific points in Videos S1 and S2 of fibrin (yellow) and p-selectin (blue) accumulation with overlap between fibrin and CD62P exposure in white. The left 2 panels present the growth of a clot from a WT arteriole injury shown at an early (20 seconds) and a late (150 seconds) time point after injury. The right panel presents growth of a clot from a pFVIII/FVIIInull arteriole injury at a late (105 seconds) timepoint. The bottom row is a set of cartoons depicting the details of the frames in the top row. Here, the whole platelet plug is shown in gray and the accumulation of fibrin in yellow, with CD62P exposure in blue and overlap in white. The entire Video for the WT injury is available as Video S1 and for the pFVIII/FVIIInull injury, Video S2. (B) Average data analysis for CD62P exposure and fibrin accumulation in WT arterioles (left) and pFVIII/FVIIInull arterioles (right) were carried out as in Figures 1 through 3 except that an Alexa647-labeled anti-CD62P antibody was used. Fibrin is depicted as □ and CD62P as ♦.
CD62P exposure in WT and pFVIII/FVIIInull mice. Studies as in Figures 1 through 3 with an Alexa647-labeled anti-fibrin antibody, but instead of measuring platelet accumulation using an anti-CD41 Fab fragment, CD62P exposure was measured using an Alexa647-labeled anti-CD62P antibody. (A) Top row shows representative frames from specific points in Videos S1 and S2 of fibrin (yellow) and p-selectin (blue) accumulation with overlap between fibrin and CD62P exposure in white. The left 2 panels present the growth of a clot from a WT arteriole injury shown at an early (20 seconds) and a late (150 seconds) time point after injury. The right panel presents growth of a clot from a pFVIII/FVIIInull arteriole injury at a late (105 seconds) timepoint. The bottom row is a set of cartoons depicting the details of the frames in the top row. Here, the whole platelet plug is shown in gray and the accumulation of fibrin in yellow, with CD62P exposure in blue and overlap in white. The entire Video for the WT injury is available as Video S1 and for the pFVIII/FVIIInull injury, Video S2. (B) Average data analysis for CD62P exposure and fibrin accumulation in WT arterioles (left) and pFVIII/FVIIInull arterioles (right) were carried out as in Figures 1 through 3 except that an Alexa647-labeled anti-CD62P antibody was used. Fibrin is depicted as □ and CD62P as ♦.
Embolic risk of platelet versus infused human FVIII
The platelet degranulation studies in Figure 4 suggest that pFVIII follows a temporal and spatial release pattern within a growing clot that likely differs from that seen by plasma FVIII. In addition, occasional Video Studies in the pFVIII/FVIIInull mice were associated with unusually large emboli (see Videos S3,S4). We therefore wondered whether platelet-released human FVIII was associated with an increased propensity to form emboli relative to infused human FVIII. To study this question, emboli downstream of a site of injury (Figure 5A) were measured in WT, pFVIII/FVIIInull, and FVIIInull mice. The FVIIInull mice were studied with or without a 15% antigenic correction, wherein overall FVIII levels are slightly higher than that within the pFVIII/FVIIInull platelets, but resulting in similar platelet and fibrin accumulation in the developing clot (Figure 2 row 2 versus Figure 3).
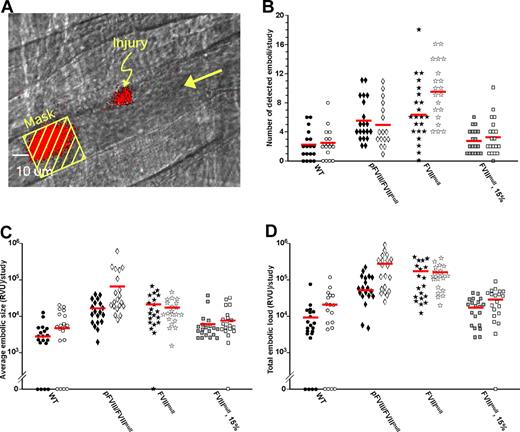
Embolization from WT, untreated and treated FVIIInull, and pFVIII/FVIIInull mice. (A) This panel from a venular WT injury illustrates how data were collected for these studies. Mice were injected with Alexa647-labeled anti-CD41 Fab fragments. A laser injury was made as indicated with a growing clot in red at that site, but data were collected at the indicated mask, where an embolus in red is passing by. The large arrow refers to the direction of blood flow. (B) Individual measurement of number of detected events for WT, untreated and treated FVIIInull, and pFVIII/FVIIInull mice are shown as well as the mean (red bar). Twenty separate injuries were analyzed for each study. Studies of arteriolar injuries are shown as filled symbols, while venular injuries are shown as empty symbols. Panels C and D are the same as panel B but show relative size of emboli and total amount of embolization, respectively.
Embolization from WT, untreated and treated FVIIInull, and pFVIII/FVIIInull mice. (A) This panel from a venular WT injury illustrates how data were collected for these studies. Mice were injected with Alexa647-labeled anti-CD41 Fab fragments. A laser injury was made as indicated with a growing clot in red at that site, but data were collected at the indicated mask, where an embolus in red is passing by. The large arrow refers to the direction of blood flow. (B) Individual measurement of number of detected events for WT, untreated and treated FVIIInull, and pFVIII/FVIIInull mice are shown as well as the mean (red bar). Twenty separate injuries were analyzed for each study. Studies of arteriolar injuries are shown as filled symbols, while venular injuries are shown as empty symbols. Panels C and D are the same as panel B but show relative size of emboli and total amount of embolization, respectively.
WT mice had the fewest detected emboli per study (2.3 ± 2.0, arteriole, and 2.3 ± 2.0, venule, Figure 5B). Untreated FVIIInull mice had a significant approximately 3-fold increase in embolization rate per study of 6.2 plus or minus 4.0, arteriole (P < .004 vs WT), and 9.6 plus or minus 2.0, venule (P < .001 vs WT; Figure 5B). Infusion of human FVIII largely corrected this increase in number of detected emboli (2.8 ± 1.6, arteriole, and 3.3 ± 2.5, venule, Figure 5B). Compared with the WT studies, the pFVIII/FVIIInull mice had a significant approximately 2.5-fold increase in both arteriole and venule embolization rate per study (5.6 ± 2.9, arteriole, P < .002, and 5.0 ± 2.8, venule, P < .002, Figure 5B).
Average size of the emboli per study was also measured (Figure 5C). Compared with WT mice, the FVIIInull mice had arteriole emboli approximately 6.5-fold larger and venule emboli approximately 2.4-fold larger. Both differences were significant (P < .002 and P < .003 vs WT, respectively). Infused human FVIII decreased the average size of emboli so that they were no longer significantly greater than in the WT mice (Figure 5C). Average embolic size was also increased in the pFVIII/FVIIInull mice where arteriole emboli were approximately 5.1-fold larger than in WT mice (P < .001; Figure 5C). The venule emboli in the pFVIII/FVIIInull mice were the largest of any of the conditions tested and were approximately 13-fold larger than those in the WT venule studies (P = .02) as well as approximately 5.6-fold larger than the untreated FVIIInull venule studies (P < .04).
Compared with the WT mice, total embolic mass was also significantly increased in the untreated FVIIInull mice by approximately 16-fold (P < .002) after arteriole injury and approximately 6.8-fold after venule injury (P < .001; Figure 5D). After infusion of human FVIII into FVIIInull mice, total embolic mass after injury was no longer statistically different from WT (Figure 5D). In the pFVIII/FVIIInull mice, total embolic mass was increased relative to WT mice, being approximately 8.2-fold higher (P < .001) after arteriole injury and approximately 12.6-fold higher (P < .001) after venule injury (Figure 5D). This total venule embolization mass was also approximately 1.9-fold greater than in the untreated FVIIInull mice (P < .04).
Discussion
To address the issue of arteriole versus venule efficacy for pFVIII, we used the previously described in situ laser-induced cremaster arteriole/venule injury system.12,13 Each injury study was completed in a matter of minutes and allowed a direct comparison of pFVIII with infused FVIII. Our studies of WT platelet plug and fibrin clot development on the arteriole side were similar to that previously published.12 Venule studies in WT mice showed a similar rapid growth of the platelet plug, but with a greater lag time of approximately 40 seconds before appreciable fibrin clot development. These differences may be related to greater tissue factor exposure on the arteriole side than on the venule side in this injury model. Degranulation within clots in both vascular systems begins briskly at the base of the clot, especially at its upstream end, and later involves the entire clot in WT mice.
FVIIInull mice have a severe defect in both platelet plug and fibrin clot formation in both the arteriole and venule models. Incremental increases in infusions of human FVIII improved outcome, yet even at greater than or equal to 120% antigenic levels, these mice had incomplete corrections. Especially lagging was the time to initial fibrin clot formation, most evident on the venous side, and also the magnitude of the final platelet plug size. These limitations may reflect species-related aspects of using human FVIII in a murine model of hemophilia A. Other models of bleeding in FVIIInull mice had not noted similar differences,1,15 but these studies had not looked at the details of clot development. In thromboelastography studies, infused human FVIII improved outcome in FVIIInull mice, but even with a 100% antigenic correction, maximum velocity was only approximately 84% of WT and amplitude of clot formation was also decreased.25 Prior studies have noted a shorter half-life and less specific activity for human FVIII in mice,1,9 but few studies have focused on the molecular bases of these observations. B-domainless murine FVIII has been expressed and shows marked resistance to thrombin inactivation relative to human FVIII.26 We and others have shown that pFVIII storage in murine α-granules occurs to a large extent independent of VWF,2,3 but whether that is due to decreased binding of human FVIII to murine VWF was not tested.
These limitations in clot development in the FVIIInull mice after human FVIII infusion must be kept in mind when interpreting the pFVIII/FVIIInull studies. We believe that our studies show that pFVIII improved both arteriole and venule clotting to approximately the same extent in this cremaster injury system. When compared with FVIIInull mice infused with human full-length FVIII, the pFVIII/FVIIInull mice, which express a B-domainless human FVIII, had a fibrin clot response approximately equal to animals receiving approximately twice as much infused human FVIII (15% antigen correction). Despite similarities between the clots in the pFVIII/FVIIInull mice and these infused FVIIInull mice, pFVIII clearly corrects the lag time in fibrin clot development, which was not seen after human FVIII infusion. We believe that this improvement is related to the rapid degranulation of platelet α-granules at the base of the developing clot, as demonstrated using an anti-CD62P antibody (Figure 4), making available a burst of FVIII that can supplement the tissue factor–driven thrombin formation that has already been shown to be present at the base of cremaster arteriole injury in this model.14 Whether this difference is related to pFVIII not necessarily being bound to VWF2,3 or to biologic differences between infused full-length FVIII and the B-domainless nature of the pFVIII. Studies are needed to compare the biology of these 2 different forms of FVIII in mice.
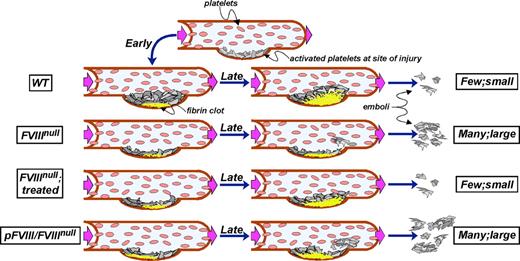
This rapid, localized FVIII release at the base of developing clots in the pFVIII/FVIIInull mice may also be associated with a temporal and spatial deficiency of FVIII in other areas of the developing clot and may explain the observed increased risk of embolization with pFVIII. We propose a model to explain these observations (Figure 6). As published in studies of this model of vascular injury,14 released tissue factor leads to a relatively rapid accumulation of a fibrin clot at the site of laser injury (Figure 6 WT mice). Thrombin generated during these initial events also leads to activation of the intrinsic coagulation pathway using available circulating FVIII and resulting in a fibrin scaffold that eventually extends throughout the platelet plug, anchoring the growing clot so that few large emboli form.
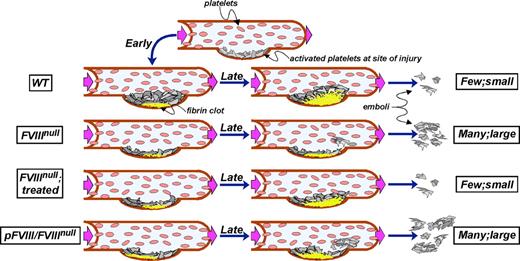
Model of thrombus development. The top row shows the initial accumulation of platelets at a site of injury in a vessel. Subsequent rows show early fibrin clot development on the left (at ∼20 seconds), later events (at ∼150 seconds) in the middle, and degree of embolization on the right in that vessel. From top to bottom, the rows are for WT, untreated and treated FVIIInull, and pFVIII/FVIIInull mice as indicated. WT mice rapidly develop a fibrin scaffold that leads to vigorous platelet plug growth and little embolization. Untreated FVIIInull mice barely develop a fibrin clot and any accumulated platelet plug is unstable and rapidly breaks off. Treated FVIIInull mice develop a spatially and temporally correct clot, but of a limited size so that platelet plug development remains limited, but embolization approaches that seen in WT mice. The pFVIII/FVIIInull mice develop a rapid fibrin clot at the base, but it may be spatially more upstream than normal. Furthermore the core and top of the clot remain deficient in available FVIII, and the developing platelet plug is not well scaffolded. Frequent and relative large emboli then occur.
Model of thrombus development. The top row shows the initial accumulation of platelets at a site of injury in a vessel. Subsequent rows show early fibrin clot development on the left (at ∼20 seconds), later events (at ∼150 seconds) in the middle, and degree of embolization on the right in that vessel. From top to bottom, the rows are for WT, untreated and treated FVIIInull, and pFVIII/FVIIInull mice as indicated. WT mice rapidly develop a fibrin scaffold that leads to vigorous platelet plug growth and little embolization. Untreated FVIIInull mice barely develop a fibrin clot and any accumulated platelet plug is unstable and rapidly breaks off. Treated FVIIInull mice develop a spatially and temporally correct clot, but of a limited size so that platelet plug development remains limited, but embolization approaches that seen in WT mice. The pFVIII/FVIIInull mice develop a rapid fibrin clot at the base, but it may be spatially more upstream than normal. Furthermore the core and top of the clot remain deficient in available FVIII, and the developing platelet plug is not well scaffolded. Frequent and relative large emboli then occur.
In the FVIIInull mice, the growing fibrin clot is limited to the base where tissue factor-generated thrombin is available, but does not extend through the remaining clot (Figure 6 FVIIInull mice). Therefore, almost none of the growing platelet plug is properly scaffolded, and the unstable platelet plugs easily break off, resulting in many detectable emboli. Infused FVIII corrects this defect with a return of a supporting fibrin scaffold and a decrease in the incidence and size of emboli (Figure 6 FVIIInull mice, infused). In contrast, available FVIII released from platelets is initially concentrated at the base of the clot, especially at its upstream end (Figure 6 pFVIII/FVIIInull mice). The rest of the clot remains sufficiently devoid of FVIII that a proper fibrin clot cannot develop, and frequent and large emboli occur.
This tendency to embolize is most prominent in the venules and raises the possibility that a platelet-directed form of gene therapy may lead to significant venous embolization. However, there are several caveats to this concern. These findings are based on a single murine model wherein human FVIII is being used to correct a bleeding diathesis in a murine model. The known shorter half-life of FVIII in mice, the decreased specific activity of human FVIII in mice, and difference in thrombin sensitivity as well as other cross-species differences that have yet to be explored may contribute to the limited efficacy of human FVIII in this model. Arterial flow and shear rates are also higher in mice,21-23 and this may contribute to embolization as well. We tried to take into account these species differences by comparing outcome between infused versus platelet-delivered human FVIII, but outcome may still be due to species differences that may not be of clinical relevance in the care of hemophilia A patients. In addition, the infused human FVIII was full-length, while the released human FVIII from platelets was B-domainless. In prior studies,16 both forms of human FVIII have very similar biology, but this sameness was not tested in a murine system. Another caveat is that while our transgenic mice expressed the highest level of pFVIII achieved to date,1,3 whether the risk of embolization would be decreased if higher levels of pFVIII were achieved needs to be addressed. Whether platelet expression of inactivation resistant FVIII27 or FVIII variants with higher specific activity would eliminate excessive embolization need also be examined. Finally, similar studies examining risk of embolization need to be carried out in a large animal model of FVIII to see whether this concern is valid in a more clinically relevant model.
These findings have implications beyond their impact on our understanding of the clinical utility of platelet-delivered FVIII. Certainly, they point out that human FVIII studies in a murine model need to address the noted species differences. This is especially true if variants of FVIII being considered for human use are tested in murine models. A recent study addressed the ability of several variants to improve outcome in FVIIInull mice after adenoviral liver-based gene therapy. Surprisingly, it appeared that the inactivation resistant FVIII (IR8) was not as effective at correcting a tail-bleeding study as a B domain–truncated variant with 6 putative asparagine glycosylation sites N6-FVIII variant that increased intracellular processing.28,29 Inclusion of a second mutation F309S, which also increased intracellular processing,30 countered the increased efficacy of N6-FVIII. These unexpected outcomes in efficacy as well as differences in immunologic reactivity of the various variants may be related to unexamined aspects of human FVIII biology in a murine system. Thus, conclusions drawn from such murine studies on variant human FVIII must be tempered until the underlying biology is better studied.
In addition, several procoagulant factors and ligands are expressed and/or stored within platelet α-granules as well as being present within the plasma. This list includes, but is not limited to FV, VWF and fibrinogen.31 What is the biologic value in having platelet as well as circulating stores of these proteins? Patients with inherited deficiencies of total α-granular content have Gray Platelet Syndrome with a minor bleeding diathesis, but the mechanistic basis for this bleeding diathesis has not been well understood and is complicated by the great variety of proteins released from within granules as well as found on the granular surface.32,33 The only specific study to address the importance of α-granule versus plasma source of a procoagulant factor involved murine studies of the 2 pools of FV, which suggested that platelet FV is not required for normal hemostasis after minor trauma.34 We believe that the presented pFVIII studies provide additional insights into why a procoagulant protein might be localized to 2 pools: we propose that the presence of procoagulant proteins within platelet α-granules allows rapid availability of these factors at the base of a developing clot, enhancing initial clot formation and stability. Full clot development then requires plasma-derived factors and ligands.
In summary, we show that pFVIII contributes to both arteriole and venule clot formation in an in situ laser injury model. pFVIII release appears to occur rapidly within the developing clot in both arterioles and venules, but this mode of delivery appears to have spatial and temporal differences in FVIII availability as compared with plasma-derived FVIII, leading to clot instability and embolization. Whether increased risk of embolization can be prevented by achieving higher pFVIII levels or by the use of variants of FVIII with altered biologic activity needs further study. Further, examination of the biology of pFVIII in larger mammalian models of FVIII deficiency that better simulate the clinical course of hemophilia A is needed to determine whether this concern of clot stability is only limited to the murine model. Studies presented here also provide some insights into the biology of naturally occurring procoagulant factors and ligands that are present both within platelet α-granules and in the plasma, suggesting that the platelet pool may provide a rapid source of the procoagulant at the base of the developing clot needed for initial clot growth, while the plasma pool leads to later clot stability.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Bruce Furie at Harvard University for his support in establishing the in situ microscopy system locally; Dr Hartmut Weiler from the University of Wisconsin for providing the anti-fibrin antibody, and Baxter Laboratories for the human FVIII; and Drs Michele Lambert, M. Anna Kowalska, and Lubica Rauova from our institutions for critical review of the manuscript.
Support for these studies was provided by National Heart, Lung and Blood Institute PO1 HL64190.
National Institutes of Health
Authorship
Contribution: M.N. was the primary individual to perform and evaluate the presented studies and to help with manuscript preparation; J.G. assisted in experimentation, focusing on the breeding and genotyping of the mice; and M.P. directed the proposed research and assisted in data analysis and in the preparation of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mortimer Poncz, MD, Children's Hospital of Philadelphia, 3615 Civic Center Boulevard, ARC, Room 317, Philadelphia, PA 19104; e-mail: poncz@email.chop.edu.