Abstract
We demonstrate that blockade of the MEK/ERK signaling module, using the small-molecule inhibitors PD184352 or PD325901 (PD), strikingly enhances arsenic trioxide (ATO)–induced cytotoxicity in human myeloma cell lines (HMCLs) and in tumor cells from patients with multiple myeloma (MM) through a caspase-dependent mechanism. In HMCLs retaining a functional p53, PD treatment greatly enhances the ATO-induced p53 accumulation and p73, a p53 paralog, cooperates with p53 in caspase activation and apoptosis induction. In HMCLs carrying a nonfunctional p53, cotreatment with PD strikingly elevates the (DR4 + DR5)/(DcR1 + DcR2) tumor necrosis factor (TNF)–related apoptosis-inducing ligand (TRAIL) receptors ratio and caspase-8 activation of ATO-treated cells. In MM cells, irrespective of p53 status, the combined PD/ATO treatment increases the level of the proapoptotic protein Bim (PD-mediated) and decreases antiapoptotic protein Mcl-1 (ATO-mediated). Moreover, Bim physically interacts with both DR4 and DR5 TRAIL receptors in PD/ATO-treated cells, and loss of Bim interferes with the activation of both extrinsic and intrinsic apoptotic pathways in response to PD/ATO. Finally, PD/ATO treatment induces tumor regression, prolongs survival, and is well tolerated in vivo in a human plasmacytoma xenograft model. These preclinical studies provide the framework for testing PD325901 and ATO combination therapy in clinical trials aimed to improve patient outcome in MM.
Introduction
Multiple myeloma (MM) is a clonal B-cell malignancy characterized by the accumulation of malignant plasma cells within the bone marrow (BM). Despite treatment with alkylating agents, anthracyclines, corticosteroids,1,2 and bortezomib3 as well as high-dose therapy and stem cell transplantation,4-6 MM remains an incurable disease because of the high resistance to apoptosis and both intrinsic and acquired drug resistance.7-12 Therefore, new therapeutic strategies are needed to improve patient outcome.
Preclinical in vitro and in vivo studies showed that arsenic trioxide (ATO) has antimyeloma effects both as a single agent13-16 and in combination with glutathione-depleting agents17-19 and/or other antimyeloma agents.15,20,21 Moreover, the combined results of 3 phase 2 studies in patients with relapsed MM refractory to conventional chemotherapy showed only modest efficacy of ATO as single agent,22-28 but combination therapies with ascorbic acid, melphalan, steroids, thalidomide, and bortezomib have shown promising results.29-34
We have previously demonstrated that PD184352 (PD), a highly selective inhibitor of MEK phosphorylation and activation, strikingly enhances ATO-mediated apoptosis in acute myelogenous leukemia (AML) via multiple intrinsic apoptotic pathways activation.35-37
MEK blockade efficiently and selectively sensitizes tumor cells to suboptimal doses of other apoptotic stimuli, including classic cytotoxic treatment (nucleoside analogs, microtubule-targeted drugs, γ-irradiation),38-43 biologicals (retinoids, interferons),44,45 steroids,46-48 and other signal transduction/apoptosis modulators (UCN-01, STI571, Bcl-2 antagonists, Bcl-2 antisense oligonucleotides).49-53 In this study, we tested the apoptotic activity of ATO combined with MEK inhibitors in MM cells, and we were able to demonstrate that PD enhances ATO-induced cytotoxicity both in vitro and in vivo in a human plasmacytoma xenograft model, through a multiple modulation of apoptotic regulatory proteins, including p53 family proteins, TRAIL receptors, several Bcl-2 family proteins, and caspases, that depend on the functionality of the p53 pathway.
Methods
Approval for the study was obtained from the Institutional Review Board of the Department of Clinical Sciences, University of Parma (Parma, Italy).
Reagents
ATO was purchased from Sigma-Aldrich (St Louis, MO). A 1 mM stock solution was obtained by dissolving ATO in phosphate-buffered saline. A 100 mM stock solution of either the MEK inhibitor PD184352 (2-[chloro-4-iodo-phenylamino]-N-cyclopropylmethoxy-3,4-difluoro-benzamide) or PD325901 (N-((R)-2,3-dihydroxy-propoxy)-3,4-difluoro-2-(2-fluoro-4-iodo-phenylamino)-benzamide), kindly provided to us by Dr J. S. Sebolt-Leopold (Cancer Molecular Sciences, Pfizer Global Research & Development, Ann Arbor, MI), were prepared in dimethyl sulfoxide (DMSO). These reagents are highly selective inhibitors of MEK phosphorylation and activation.54,55 The doses used by us of 1 μM for PD184352 or 50 nM for PD325901 in our in vitro experiments were those that proved effective in vitro in leukemic cells as documented both by ourselves and other authors.35,38,56 The doses of ATO hereby used were selected based on the evidence that ATO, from concentrations of 0.5 to 2 μM upward, has the capability to induce apoptosis. These in vitro concentrations are within the range found in the plasma of patients receiving ATO treatment for acute promyelocytic leukemia.57,58 For the other reagents, see Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Cell cultures
Cell lines.
Human myeloma cell lines (HMCLs) XG-6 and XG-1 were established from peripheral blood of patients with plasma cell leukemia in Dr Bataille's laboratory (Inserm, Nantes, France); OPM2, RPMI 8226, and JJN3 were purchased from DSM (Braunschweig, Germany).
MM cell and bone marrow stromal cell isolation.
Primary MM cells from MM patients, B lymphocytes from healthy subjects, and primary bone marrow stromal cells (BMSCs) from BM samples of healthy subjects were isolated and treated as described in Document S1. Approval for these studies was obtained from informed consent of all patients and healthy donors in accordance with the Declaration of Helsinki protocol.
Cord blood human progenitor cell purification and culture
Cord blood (CB) was obtained after informed consent from healthy full-term placentas according to institutional guidelines. Human CD34+ cells purification and culture were performed as previously described.59 For detailed information, see Document S1.
Colony-forming assays
Colony-forming assays were carried out by standard methods as previously described.59 For detailed information, see Document S1.
Apoptosis assays
Flow cytometric assays to evaluate cell cycle, sub-G1 DNA content, mitochondrial transmembrane potential (ΔΨm), and annexinV/PI staining were performed as previously described.35-37
siRNA transfections and molecular analysis
siRNA transfections, cell lysis in CHAP-lysis buffer, immunoblotting, and immunoprecipitation were carried out as described.35-37 siRNA and antibodies used in this study are described in the figure legends and in Document S1.
Animal studies
Five-week-old nonobese diabetic (NOD)/severe combined immunodeficiency (SCID) CB17-Prkdcscid/J (NOD-SCID) mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and maintained under the same specific pathogen-free conditions. All procedures involving animal models were performed in accordance with national and international laws and policies. The experimental protocol approved by the University of Parma Ethics Committee is described in Document S1.
Histology and immunohistochemistry
The expression of p-ERK and cleaved caspase-3 of neoplastic cells was determinated with immunohistochemical staining and performed as previously described.60 Slides were viewed with a Nikon Eclipse E800 microscope using a Nikon PLAN APO lens (Nikon, Tokyo, Japan) at 4×/0.2 WD 15.7, 10×/0.45 DIC L WD 4.0, and 20×/0.75 DIC M WD 1.0, and Bio Mount medium (Bio-Optica, Milan, Italy). Images were acquired using a DS Camera Control Unit DS-L2 (Nikon) and were processed with Adobe Photoshop version 7.0 software (Adobe Systems, San Jose, CA). For detailed information, see Document S1.
Statistical analysis
For multiple comparisons, statistical analysis was performed using analysis of variance for repeated measurements followed by a Tukey-Kramer, Hsu MCB, or Dunnett post tests. The Chou-Talalay method and Calcusyn software (Biosoft, Ferguson, MO) were used to assess synergistic or additive or antagonist effects of combined therapies. Survival curves were derived by the Kaplan-Meier method and compared using the log-rank test, followed by a Bonferroni correction for multiple comparisons.
Results
Inhibition of ERK signaling strongly enhances the apoptotic effect of ATO on MM cells
We first analyzed the pharmacologic interactions between PD and ATO using a fixed-ratio experimental design on 5 HMCLs with various p53 status: XG-6 and JJN3 cells express wild-type p53,61,62 XG-1 cells express both wild-type and mutated p53 alleles,62 and RPMI 8226 and OPM2 cells express mutated p53.62,63 We found that the combined treatment resulted in the synergistic (Combination Index < 1) induction of apoptosis in all tested HMCLs regardless of their p53 status (Figure 1A).
MEK inhibition potentiates the ATO-induced apoptosis in MM cells. (A) HMCLs seeded at 2.5 × 105 cells/mL were treated sequentially with escalating doses of PD (0.1-20 μM) for 3 hours and subsequently with ATO alone (0.125-10 μM) or in combination with PD at a 1:1 ratio (0.25/0.25, 0.5/0.5, 1.0/1.0, 1.5/1.5, 2.0/2.0 μM). After 48 hours, apoptosis was measured by annexin V labeling. Combination Index plots were then generated using the Calcusyn software. Combination Index values less than 1.0 indicate synergism, Combination Index values equal to 1.0 indicate additive effect, and Combination Index values more than 1.0 indicate an antagonistic effect. (B) CD138-purified plasma cells from 12 patients with MM were seeded at 2.5 × 105 cells/mL in the presence of DMSO (vehicle) or PD (1 μM) for 3 hours and then incubated with ATO (2 μM) for 24 hours. The apoptosis was measured as percentage of cells with hypodiploid DNA content. Results are expressed as the net apoptosis induction (percentage of apoptosis in treated cells − percentage of apoptosis in DMSO [vehicle-treated cells]) and represent the means plus or minus SD of the results obtained in 12 different patient samples (*P < .001, Dunnett test). (C) Normal bone marrow B cells from 3 healthy donors were treated as described in panel B. After 24 hours of treatment, apoptosis was then measured as the percentage of cells with hypodiploid DNA content. CTR indicates control; PD, PD184352 (1 μM); ATO, arsenic trioxide (2 μM). (D,E) Analysis of the effect of PD and ATO on hemopoietic progenitor colony formation studied either using total PBMCs (D) or purified CD34+ cells (E). A total of 2 × 105 PBMCs (D) or 2 × 102 CD34+ cells (E) have been grown in methylcellulose either in the absence (0) or in the presence of either PD (0.5, 1, or 2 μM) or ATO (0.5, 1, or 2 μM) or both drugs. (F) Study of the effect of PD and ATO on the growth of CD34+ cells grown under serum-free conditions allowing the selective growth of either erythroid (E), megakaryocytic (Mk), or granulocytic (G) cells. A total of 5 × 104 CD34+ cells have been grown in serum-free liquid suspension cultures under E, Mk, or G cell culture conditions either in the absence (C) or in the presence of PD (1 μM), ATO (1 μM), or PD + ATO (both at 1 μM), and the number of the cell progeny was evaluated at various days of culture. For panels D, E, and F, the results represent the means plus or minus SEM values observed in 3 separate experiments. C indicates control; PD, PD184352; ATO, arsenic trioxide.
MEK inhibition potentiates the ATO-induced apoptosis in MM cells. (A) HMCLs seeded at 2.5 × 105 cells/mL were treated sequentially with escalating doses of PD (0.1-20 μM) for 3 hours and subsequently with ATO alone (0.125-10 μM) or in combination with PD at a 1:1 ratio (0.25/0.25, 0.5/0.5, 1.0/1.0, 1.5/1.5, 2.0/2.0 μM). After 48 hours, apoptosis was measured by annexin V labeling. Combination Index plots were then generated using the Calcusyn software. Combination Index values less than 1.0 indicate synergism, Combination Index values equal to 1.0 indicate additive effect, and Combination Index values more than 1.0 indicate an antagonistic effect. (B) CD138-purified plasma cells from 12 patients with MM were seeded at 2.5 × 105 cells/mL in the presence of DMSO (vehicle) or PD (1 μM) for 3 hours and then incubated with ATO (2 μM) for 24 hours. The apoptosis was measured as percentage of cells with hypodiploid DNA content. Results are expressed as the net apoptosis induction (percentage of apoptosis in treated cells − percentage of apoptosis in DMSO [vehicle-treated cells]) and represent the means plus or minus SD of the results obtained in 12 different patient samples (*P < .001, Dunnett test). (C) Normal bone marrow B cells from 3 healthy donors were treated as described in panel B. After 24 hours of treatment, apoptosis was then measured as the percentage of cells with hypodiploid DNA content. CTR indicates control; PD, PD184352 (1 μM); ATO, arsenic trioxide (2 μM). (D,E) Analysis of the effect of PD and ATO on hemopoietic progenitor colony formation studied either using total PBMCs (D) or purified CD34+ cells (E). A total of 2 × 105 PBMCs (D) or 2 × 102 CD34+ cells (E) have been grown in methylcellulose either in the absence (0) or in the presence of either PD (0.5, 1, or 2 μM) or ATO (0.5, 1, or 2 μM) or both drugs. (F) Study of the effect of PD and ATO on the growth of CD34+ cells grown under serum-free conditions allowing the selective growth of either erythroid (E), megakaryocytic (Mk), or granulocytic (G) cells. A total of 5 × 104 CD34+ cells have been grown in serum-free liquid suspension cultures under E, Mk, or G cell culture conditions either in the absence (C) or in the presence of PD (1 μM), ATO (1 μM), or PD + ATO (both at 1 μM), and the number of the cell progeny was evaluated at various days of culture. For panels D, E, and F, the results represent the means plus or minus SEM values observed in 3 separate experiments. C indicates control; PD, PD184352; ATO, arsenic trioxide.
Fresh purified MM cells from 12 MM patients were treated with PD and/or ATO for 24 to 48 hours, and the apoptotic effect was monitored by sub-G1 DNA content, annexin V labeling, and ΔΨm. The characteristics of these patients are summarized in Table 1. Similarly to HMCLs, we found that the treatment with PD significantly enhanced the apoptosis of fresh purified MM cells induced by ATO (*P < .001) in 9 of 12 patients with MM analyzed (Figure 1B). Conversely, PD treatment attenuated (n = 1) or did not affect (n = 2) the ATO cytotoxicity in normal BM CD20+ B cells (Figure 1C).
To evaluate the effect of the MEK inhibitor (PD) and ATO on normal hematopoiesis, we have carried out 2 types of experiments: standard clonogenic assays and unilineage liquid suspension cultures.
Standard clonogenic assays were carried out either on total adult peripheral blood mononuclear cells (PBMCs) or on purified CB CD34+ cells. Using adult PBMCs, we observed that PD addition had only a very weak inhibitory effect on colony formation, whereas ATO elicited a moderately higher inhibitory effect; the combined addition of PD and ATO induced a slightly higher inhibitory effect compared with that observed using ATO alone (Figure 1D). Importantly, using the combined addition of PD and ATO at the highest dose (2 μM for both drugs), an inhibitory effect of approximately 40% on erythroid burst-forming units (BFU-E), granulocyte-macrophage colony-forming unit (CFU-GM), and colony-forming unit granulocyte-erythrocyte-macrophage-megakaryocyte (CFU-GEMM) formation was observed (Figure 1D). Similar results were observed in the colony-forming assays carried out using purified CB CD34+ cells, with the only exception that on these cells PD/ATO at the highest dose (2 μM) induced a more pronounced inhibitory effect on BFU-E colonies (∼50%) compared with that observed on BFU-E cultures generated from total PBMCs (Figure 1E).
The studies carried out in unilineage liquid suspension cultures showed that (1) PD elicited only a weak (∼20%) inhibitory effect on the growth of erythroid (E), megakaryocytic (Mk), and granulocytic cultures (G); (2) ATO moderately (∼30% of growth inhibition) inhibited E, G, and Mk cultures; and (3) PD/ATO exhibited an additive inhibitory effect (∼40% of growth inhibition) on the growth of E, G, and Mk cultures (Figure 1F).
Taken together, these findings demonstrate that the combined treatment with PD and ATO has moderate inhibitory effect on in vitro hematopoiesis, compatible with a clinical use of these 2 compounds administered in combination. Furthermore, it is important to point out that the major inhibitory effects of the combination on in vitro hematopoiesis are ATO dose dependent.
Neither growth factors nor adherence to BMSCs protects against PD/ATO-induced MM cell cytotoxicity
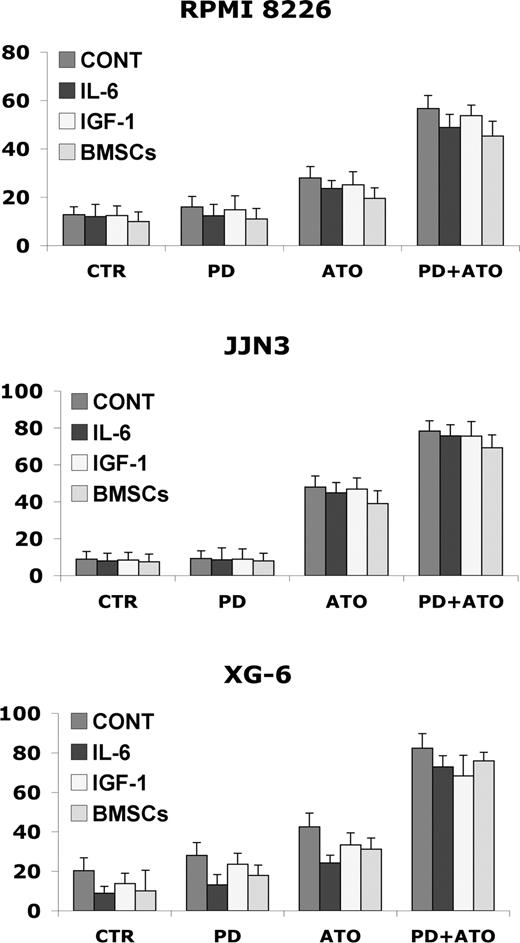
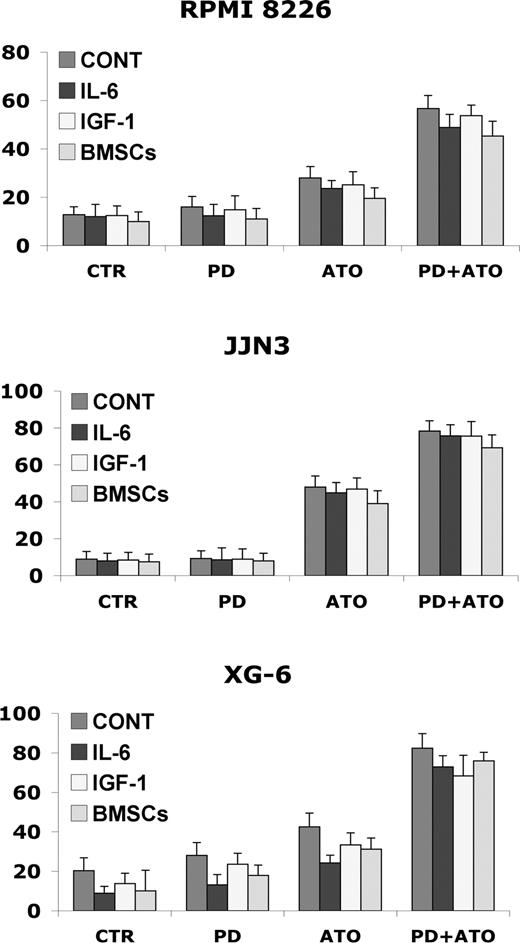
Interleukin-6 (IL-6) and insulin growth factor-1 (IGF-1), 2 major growth, survival, and drug-resistance factors for MM cells, activate a cascade of proliferative/antiapoptotic signaling pathways, including Ras/Raf/MAPK.64,65 We then examined whether the IL-6 and IGF-1 could abrogate the apoptotic effect of PD/ATO in vitro. Importantly, IL-6 (20 ng/mL) or IGF-1 (50 ng/mL) did not block PD/ATO-triggered apoptosis in HMCL cells (Figure 2). Interestingly, similar behaviors were also observed in fresh purified MM cells treated with PD/ATO in the presence or absence of growth factors (data not shown).
Combined treatment with PD184352 and ATO overcomes the protective effects of human IL-6, IGF-1, and BM microenvironment in MM cells. RPMI 8226, JJN3, and XG-6 cells were cultured with PD and/or ATO in the presence or absence of IL-6 (20 ng/mL), IGF-1 (50 ng/mL), or BMSCs. After 48 hours of treatment, the cells were harvested for annexin V labeling; data represent means plus or minus SD of triplicate cultures. CTR indicates control; PD, PD184352 (1 μM); ATO, arsenic trioxide (2 μM).
Combined treatment with PD184352 and ATO overcomes the protective effects of human IL-6, IGF-1, and BM microenvironment in MM cells. RPMI 8226, JJN3, and XG-6 cells were cultured with PD and/or ATO in the presence or absence of IL-6 (20 ng/mL), IGF-1 (50 ng/mL), or BMSCs. After 48 hours of treatment, the cells were harvested for annexin V labeling; data represent means plus or minus SD of triplicate cultures. CTR indicates control; PD, PD184352 (1 μM); ATO, arsenic trioxide (2 μM).
Because the BM microenvironment confers MM cell growth and drug resistance,64,65 we next studied whether PD/ATO induces MM cell apoptosis in the context of the BM microenvironment. Consistent with stimulation triggered by exogenous IL-6 or IGF-1, adherence of MM cells to BMSCs did not protect MM cells to PD/ATO-induced apoptosis (Figure 2). The cell death of BMSCs, assessed by annexin V labeling, was not significantly affected by PD/ATO treatment (data not shown). These data indicate that the combination PD/ATO triggers significant antitumor activity even against MM cells in the BM milieu.
Combined exposure to PD and ATO strongly induces caspase activation and mitochondrial depolarization in MM cells
Previous studies have shown that in cells expressing functional p53, caspase-9, and caspase-3 are activated by ATO, whereas in cells lacking functional p53, caspase-8 and caspase-3 are the primary caspases activated by ATO.15
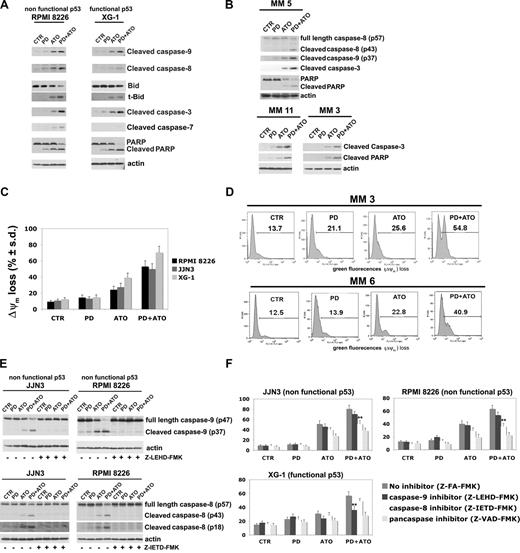
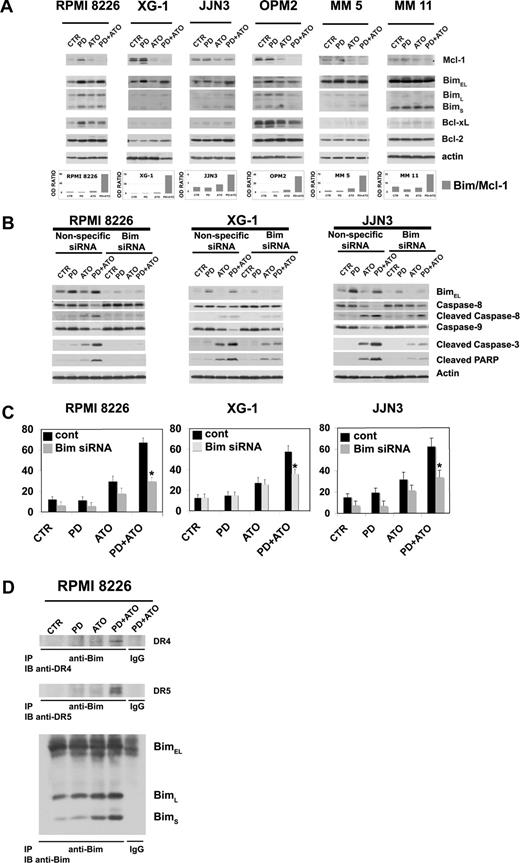
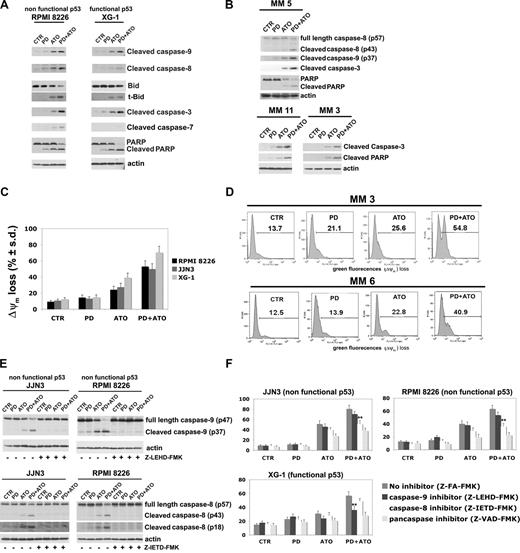
Therefore, we first compared the effect of the combined treatment on caspase activation and PARP degradation in HMCLs expressing nonfunctional p53 or functional p53: the RPMI 8226 (nonfunctional p53) and XG-1 (functional p53) cells were treated with PD and/or ATO for 24 hours: the treatment with PD strongly potentiated the ATO-induced activation of caspase-9, -8, -7, and -3 in RPMI 8226 (nonfunctional p53) cells and caspase-9 and -3 in XG-1 (functional p53) (Figure 3A). In addition, a modest activation of caspase-8 was observed in the PD/ATO-treated XG-1 (functional p53) (Figure 3A). Consistent with this latter finding, the combined treatment induced a more robust cleavage of Bid, a substrate of active caspase-8, in RPMI 8226 (nonfunctional p53) compared with XG-1 (functional p53; Figure 3A). The PD/ATO treatment induced PARP fragmentation in both HMCLs. Similar results were observed in PD/ATO-treated OPM2 (nonfunctional p53) and XG-6 (functional p53), respectively (data not shown). Caspase activation and PARP cleavage were also observed in fresh purified MM cells treated with PD/ATO (Figure 3B, representative data). In addition, the caspase-8–mediated proteolytic activation of Bid, a key protein involved in the cross-talk between the intrinsic and extrinsic apoptotic pathways,66,67 closely correlated with the caspase-9 activation and loss of ΔΨM observed in PD/ATO treated RPMI 8226 (nonfunctional p53) and JJN3 (nonfunctional p53; Figure 3A,C,E). Notably, in JJN3 (wild-type p53), conversely to XG-6 (wild-type) and XG-1 (hemizygous p53), neither apoptotic doses of ATO nor ATO in combination with PD were able to induce p53 accumulation/biologic function (see next paragraph), thereby indicating that JJN3 cells have functional defect(s) in the p53 pathway. Similarly to HMCLs, loss of ΔΨM was also observed in PD/ATO-treated primary MM cells (Figure 3D, representative data).
Coadministration of PD184352 and ATO activates the caspase cascade in MM. (A) HMCLs were exposed to 1 μM PD and/ or ATO 2 μM for 24 hours; cell lysates were analyzed by immunoblotting analysis using rabbit polyclonal anticleaved caspase-9, -8, -7, -3, Bid, and PARP, all provided by Cell Signaling Technology (CST, Danvers, MA). Blots were subsequently reprobed with goat polyclonal antiactin (sc-1616, Santa Cruz Biotechnology, Santa Cruz, CA) to ensure equivalent loading and transfer of protein. (B) CD138-purified plasma cells from 3 representative patients with MM were seeded at 2.5 × 105 cells/mL in the presence of DMSO (vehicle) or PD (1 μM) for 3 hours and then incubated with ATO (2 μM) for 24 hours, after which cells were lysed and subjected to Western blot analysis to monitor the expression of cleaved caspase-9, -8, -3, PARP, and cleaved PARP. (C) HMCLs were cultured with PD and/or ATO for 24 hours, after which the percentage of apoptotic cells displaying loss of mitochondrial membrane potential (ΔΨm) was monitored by flow cytometry. Values represent mean plus or minus SD for 3 separate experiments performed in triplicate. (D) CD138-purified plasma cells from 2 representative patients with MM were cultured with PD and/or ATO for 24 hours, after which the percentage of apoptotic cells displaying loss of ΔΨm was monitored by flow cytometry. (E) HMCLs cells were treated with PD and/or ATO in the presence or absence of 30 μM of caspase inhibitors for 24 hours, after which cells were lysed and subjected to Western blot analysis to monitor the expression of caspase-9 and -8 using rabbit polyclonal anticaspase-9 and -8 (Cell Signaling Technology); blots were subsequently reprobed for actin expression to ensure equivalent loading and transfer of protein. (F) HMCLs were cultured with PD and/or ATO in the presence or absence of 30 μM caspase inhibitors for 48 hours, after which the percentage of apoptotic cells was determined by the annexin V method (**P < .001 vs PD/ATO Z-FA-FMK; Dunnett test). CTR indicates control; PD, PD184352 (1 μM); ATO, arsenic trioxide (2 μM); t-Bid, truncated Bid; Z-FA-FMK, peptide control; Z-LEHD-FMK, selective caspase-9 inhibitor; Z-IETD-FMK, selective caspase-8 inhibitor; Z-VAD-FMK, pancaspase inhibitor.
Coadministration of PD184352 and ATO activates the caspase cascade in MM. (A) HMCLs were exposed to 1 μM PD and/ or ATO 2 μM for 24 hours; cell lysates were analyzed by immunoblotting analysis using rabbit polyclonal anticleaved caspase-9, -8, -7, -3, Bid, and PARP, all provided by Cell Signaling Technology (CST, Danvers, MA). Blots were subsequently reprobed with goat polyclonal antiactin (sc-1616, Santa Cruz Biotechnology, Santa Cruz, CA) to ensure equivalent loading and transfer of protein. (B) CD138-purified plasma cells from 3 representative patients with MM were seeded at 2.5 × 105 cells/mL in the presence of DMSO (vehicle) or PD (1 μM) for 3 hours and then incubated with ATO (2 μM) for 24 hours, after which cells were lysed and subjected to Western blot analysis to monitor the expression of cleaved caspase-9, -8, -3, PARP, and cleaved PARP. (C) HMCLs were cultured with PD and/or ATO for 24 hours, after which the percentage of apoptotic cells displaying loss of mitochondrial membrane potential (ΔΨm) was monitored by flow cytometry. Values represent mean plus or minus SD for 3 separate experiments performed in triplicate. (D) CD138-purified plasma cells from 2 representative patients with MM were cultured with PD and/or ATO for 24 hours, after which the percentage of apoptotic cells displaying loss of ΔΨm was monitored by flow cytometry. (E) HMCLs cells were treated with PD and/or ATO in the presence or absence of 30 μM of caspase inhibitors for 24 hours, after which cells were lysed and subjected to Western blot analysis to monitor the expression of caspase-9 and -8 using rabbit polyclonal anticaspase-9 and -8 (Cell Signaling Technology); blots were subsequently reprobed for actin expression to ensure equivalent loading and transfer of protein. (F) HMCLs were cultured with PD and/or ATO in the presence or absence of 30 μM caspase inhibitors for 48 hours, after which the percentage of apoptotic cells was determined by the annexin V method (**P < .001 vs PD/ATO Z-FA-FMK; Dunnett test). CTR indicates control; PD, PD184352 (1 μM); ATO, arsenic trioxide (2 μM); t-Bid, truncated Bid; Z-FA-FMK, peptide control; Z-LEHD-FMK, selective caspase-9 inhibitor; Z-IETD-FMK, selective caspase-8 inhibitor; Z-VAD-FMK, pancaspase inhibitor.
Because caspases are proteolytically cleaved and activated during PD/ATO-induced apoptosis in MM cells, we next attempted to discern which caspases were indispensable for apoptotic signaling in MM. We therefore evaluated the effect of various caspase inhibitors (the caspase-9 inhibitor Z-LEHD-FMK, the caspase-8 inhibitor Z-IETD-FMK, and the pancaspase inhibitor Z-VAD-FMK) on PD/ATO-induced apoptosis in MM cells: Z-LEHD-FMK or Z-IETD-FMK completely blocked caspase-9 or -8 activation, respectively (Figure 3E), and the latter significantly reduced the PD/ATO-induced apoptosis in RPMI 8226 (nonfunctional p53) and JJN3 (nonfunctional p53) cells, whereas only Z-LEHD-FMK but not Z-IETD-FMK significantly affected the PD/ATO-induced cell death of the XG-1 (functional p53) cells (Figure 3F). Moreover, the pancaspase inhibitor Z-VAD-FMK protected MM cells from PD/ATO-induced apoptosis, confirming that caspase activity was indispensable in PD/ATO-induced apoptosis (Figure 3F). Similar results were observed in OPM2 (nonfunctional p53) and XG-6 (functional p53), respectively (data not shown). Taken together, these results indicate that the extrinsic, caspase-8–mediated, apoptotic pathway is strongly activated and plays an important role in PD/ATO-induced apoptosis in MM cells carrying a nonfunctional p53, whereas the intrinsic, caspase-9–mediated, mitochondrial apoptotic pathway is primarily activated in MM cells retaining a functional p53.
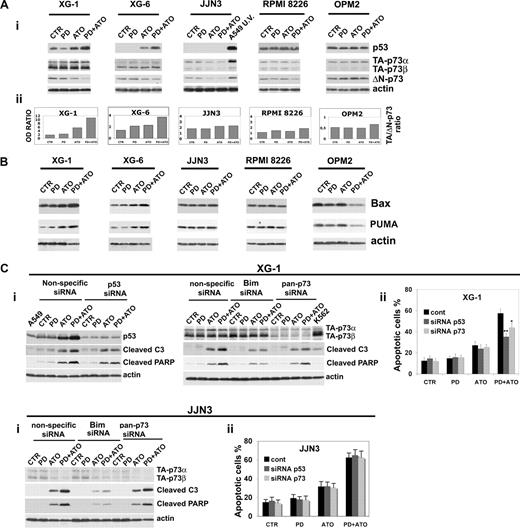
Modulation of levels of p53 family proteins by PD/ATO on MM cells
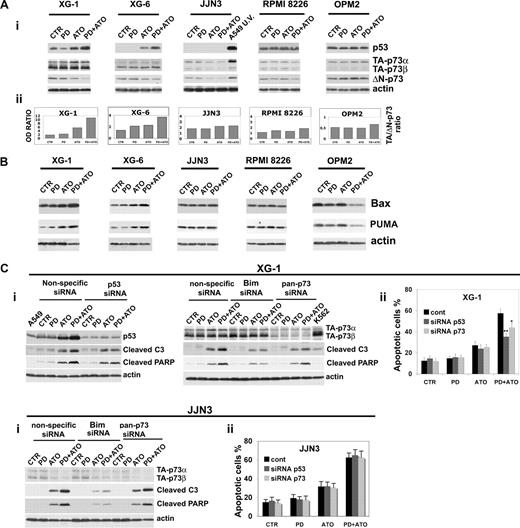
We recently reported that the disruption of the MEK/MAPK pathway potentiates the antileukemic activity of ATO in AML blasts through the activation of p73 and p53 proapoptotic pathways.35,37,68 Therefore, we studied whether p53 and the p53-related gene p73 are molecular targets of the combined treatment in MM cells. PD treatment greatly enhanced the ATO-induced p53 accumulation in XG-1 (functional p53) and XG-6 (functional p53) but not in JJN3 (nonfunctional p53), RPMI 8226 (nonfunctional p53), and OPM2 (nonfunctional p53; Figure 4Ai). Notably, the lack of p53 accumulation in response to apoptotic doses of ATO alone or in combination with PD observed in JJN3 carrying a wt p53 indicates that this cell line has functional defect(s) in the p53 pathway. Given the well-recognized ability of p73 to transactivate p53 target genes irrespective of p53 status,69-72 we investigated the role of p73 in PD and/or ATO-treated HMCLs presenting a wild-type or mutated p53. p73 exists as multiple transactivation competent (TA) proapoptotic and antiproliferative p73 COOH-terminal splicing isoforms (α, β, γ, δ, ϵ, ζ) of which the 2 major forms are p73α and p73β.73 In addition, dominant-negative (ΔN) variants are expressed from a second promoter, lack the amino-terminal transactivation domain, act as trans-repressors of p53- and p73-dependent transcription, and have antiapoptotic and proproliferative potential.74 The balance between TA and ΔNp73, or ratio is important in cancer and in the response to chemotherapy.75 TA-p73α, TA-p73β, and ΔN-p73 protein expression isoforms were evaluated in HMCLs after 24 hours of treatment, and the TA/ΔN-p73 ratio was calculated (Figure 4Ai,ii). We found that the combined treatment strongly elevated the TA/ΔN-p73 ratio induced by ATO in XG-1 (functional p53) and XG-6 (functional p53; 1.7- and 1.6-fold increase, respectively), whereas neither ATO nor PD/ATO significantly modulated the TA/ΔN-p73 ratio in JJN3 (nonfunctional p53), RPMI 8226 (nonfunctional p53), and OPM2 (nonfunctional p53; Figure 4Aii). Consistent with these results, we found that the proapoptotic p53/p73 target genes Bax and PUMA were up-regulated in PD/ATO-treated MM cells retaining a functional p53 (XG-1 and XG-6) but not in MM cells carrying a nonfunctional p53 (JJN3, RPMI 8226, and OPM2; Figure 4B).
Combined exposure of MM cells to PD184352 and ATO modulates the p53 family proteins. (Ai) HMCLs were seeded at 2.5 × 105 cells/mL in the presence of DMSO (vehicle) or PD (1 μM) for 3 hours and then were incubated for 24 hours with ATO (2 μM). Endogenous TA-p73α, TA-p73β, ΔN-p73, and p53 proteins were revealed by immunoblotting analysis using mouse monoclonal anti-p73 (clone 5B429) and mouse monoclonal anti-ΔN-p73 (clone 38C674), all provided by Imgenex (San Diego, CA) and mouse monoclonal anti-p53 (DO-1, sc-126) provided by Santa Cruz Biotechnology. Antiactin immunoblotting was performed as loading control. (Aii) TA-p73α, TA-p73β, ΔN-p73, and β-actin bands were subjected to densitometric scanning using the TINA 2 software, and the TA-(p73α + p73β)/ΔN-p73 ratio was calculated. (B) MM cells were cultured as described in panel A, and the expression of Bax and PUMA was revealed after 48 hours of treatment by immunoblotting using rabbit polyclonal anti-Bax and rabbit polyclonal anti-PUMA, all provided by Cell Signaling Technology. (Ci) Transfection of p53 (003329, Dharmacon RNA Technologies, Lafayette, CO) or pan-p73 (003331, Dharmacon RNA Technologies) siRNA, but not the unrelated nonspecific control siRNA (001206, Dharmacon RNA Technologies), led to a decrease in p53 or p73 protein expression in HMCLs without affecting the levels of the unrelated protein actin: 48 hours after siRNA transfection, the HMCLs were treated with PD and/or ATO for 24 hours and then lysed for Western blot analysis to monitor the expression of p53, p73, cleaved caspase-3, and cleaved PARP. (Cii) The percentages of sub-G1 apoptotic cells were monitored after 48 hours of treatment. Values are mean plus or minus SD of 4 independent experiments (*P < .05; **P < .01 vs PD/ATO cont; Dunnett test). CTR indicates control; PD, PD184352 (1 μM); ATO, arsenic trioxide (2 μM); cont, nonspecific control siRNA; C3, caspase-3.
Combined exposure of MM cells to PD184352 and ATO modulates the p53 family proteins. (Ai) HMCLs were seeded at 2.5 × 105 cells/mL in the presence of DMSO (vehicle) or PD (1 μM) for 3 hours and then were incubated for 24 hours with ATO (2 μM). Endogenous TA-p73α, TA-p73β, ΔN-p73, and p53 proteins were revealed by immunoblotting analysis using mouse monoclonal anti-p73 (clone 5B429) and mouse monoclonal anti-ΔN-p73 (clone 38C674), all provided by Imgenex (San Diego, CA) and mouse monoclonal anti-p53 (DO-1, sc-126) provided by Santa Cruz Biotechnology. Antiactin immunoblotting was performed as loading control. (Aii) TA-p73α, TA-p73β, ΔN-p73, and β-actin bands were subjected to densitometric scanning using the TINA 2 software, and the TA-(p73α + p73β)/ΔN-p73 ratio was calculated. (B) MM cells were cultured as described in panel A, and the expression of Bax and PUMA was revealed after 48 hours of treatment by immunoblotting using rabbit polyclonal anti-Bax and rabbit polyclonal anti-PUMA, all provided by Cell Signaling Technology. (Ci) Transfection of p53 (003329, Dharmacon RNA Technologies, Lafayette, CO) or pan-p73 (003331, Dharmacon RNA Technologies) siRNA, but not the unrelated nonspecific control siRNA (001206, Dharmacon RNA Technologies), led to a decrease in p53 or p73 protein expression in HMCLs without affecting the levels of the unrelated protein actin: 48 hours after siRNA transfection, the HMCLs were treated with PD and/or ATO for 24 hours and then lysed for Western blot analysis to monitor the expression of p53, p73, cleaved caspase-3, and cleaved PARP. (Cii) The percentages of sub-G1 apoptotic cells were monitored after 48 hours of treatment. Values are mean plus or minus SD of 4 independent experiments (*P < .05; **P < .01 vs PD/ATO cont; Dunnett test). CTR indicates control; PD, PD184352 (1 μM); ATO, arsenic trioxide (2 μM); cont, nonspecific control siRNA; C3, caspase-3.
Finally, to determine the biologic relevance of p53 and p73 pathways in mediating PD/ATO-apoptosis, the endogenous p53 or p73 transcripts were selectively knocked down by specific double-stranded RNA oligonucleotides (siRNA): the selective down-regulation of p53 significantly inhibited the caspase-3 activation, PARP fragmentation, and apoptosis in PD/ATO-treated XG-1 (functional p53) and XG-6 (functional p53) but not in JJN3 (nonfunctional p53) cell line that lack a functional p53 pathway (Figure 4Ci,ii; and data not shown). We subsequently silenced p73 gene expression and found that caspase-3 activity, PARP fragmentation, and apoptosis were significantly blocked in p73 knocked-down PD/ATO-treated XG-1 (functional p53) and XG-6 (functional p53) but not in the JJN3 (nonfunctional p53) or RPMI 8226 (nonfunctional p53; Figure 4Ci,ii; and data not shown).
Taken together, these results suggest a model in which p73 cooperates with p53 to promote apoptotic cell death in PD/ATO-treated HMCLs that retain a functional p53 pathway.
Modulation of levels of APO2/TRAIL receptors by PD/ATO on MM cells
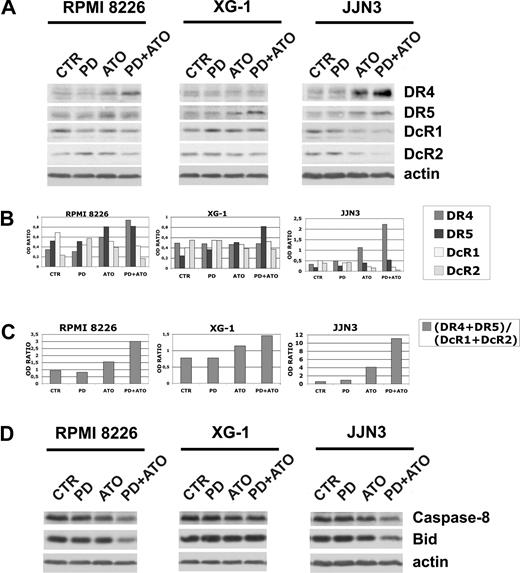
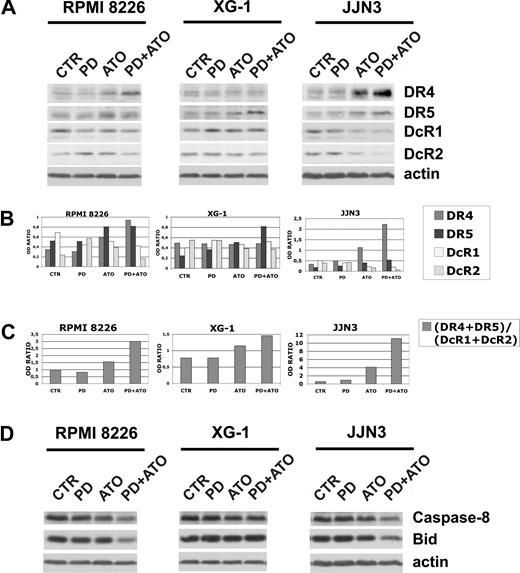
It has been reported that ATO and certain chemotherapeutic drugs induce APO2/TRAIL receptors and thereby can potentially engage both the extrinsic and the intrinsic apoptotic pathways.15,76-78 We then hypothesized that treatment with PD may also enhance the ATO-mediated induction of APO2/TRAIL receptors in MM cells. The cells were treated with PD and/or ATO for 24 hours and the levels of DR4 and DR5 agonist APO2/TRAIL surface receptors and DcR1 and DcR2 APO2/TRAIL decoy receptors were analyzed by Western blotting (Figure 5A). Densitometric analyses were then performed to evaluate the (DR4 + DR5)/(DcR1 + DcR2) ratio (Figure 5B,C). ATO treatment positively modulated the (DR4 + DR5)/(DcR1 + DcR2) ratio through the up-regulation of DR4 and DR5 and the concomitant down-regulation of DcR1 in RPMI 8226 (nonfunctional p53) or the up-regulation of DR4 and DR5 and the concomitant down-regulation of DcR1/DcR2 receptors in JJN3 (nonfunctional p53; Figure 5A,B). Interestingly, we found that, in RPMI 8226 (nonfunctional p53) and JJN3 (nonfunctional p53), but not in XG-1 (functional p53), the cotreatment with PD markedly elevated the (DR4 + DR5)/(DcR1 + DcR2) ratio (2.0- and 2.7-fold increase, respectively), procaspase-8 degradation/activation and Bid fragmentation of ATO-treated cells (Figure 5C,D). Collectively, our data indicate that the combined treatment strongly activates the extrinsic pathway by up-regulation of TRAIL receptors in the PD/ATO-treated HMCLs that do not possess a functional p53 pathway.
Expression of TRAIL receptors in HMCLs treated with PD184352 and ATO. (A) HMCLs were seeded at 2.5 × 105 cells/mL in the presence of DMSO (vehicle) or PD (1 μM) for 3 hours and then were incubated for 24 hours with ATO (2 μM), after which cells were lysed and subjected to Western blot analysis to monitor the expression of TRAIL receptors using goat polyclonal anti-DR4 (Santa Cruz Biotechnology), goat polyclonal anti-DR5 (Santa Cruz Biotechnology), rabbit polyclonal anti-DcR1 (Cell Signaling Technology), and rabbit polyclonal anti-DcR2 (Imgenex). β-Actin levels are shown for confirmation of equal protein loading. (B) TRAIL receptors and β-actin bands were subjected to densitometric scanning using the TINA 2 software, and the ratio DR4/β-actin, DR5/β-actin, DcR1/β-actin, or DcR2/β-actin was calculated. (C) DR4, DR5, DcR1, DcR2, and β-actin bands were subjected to densitometric scanning using the TINA 2 software, and the (DR4 + DR5)/(DcR1 + DcR2) ratio was calculated. (D) The same blots were subsequently stripped and reprobed for procaspase-8 and Bid. CTR indicates control; PD, PD184352 (1 μM); ATO, arsenic trioxide (2 μM).
Expression of TRAIL receptors in HMCLs treated with PD184352 and ATO. (A) HMCLs were seeded at 2.5 × 105 cells/mL in the presence of DMSO (vehicle) or PD (1 μM) for 3 hours and then were incubated for 24 hours with ATO (2 μM), after which cells were lysed and subjected to Western blot analysis to monitor the expression of TRAIL receptors using goat polyclonal anti-DR4 (Santa Cruz Biotechnology), goat polyclonal anti-DR5 (Santa Cruz Biotechnology), rabbit polyclonal anti-DcR1 (Cell Signaling Technology), and rabbit polyclonal anti-DcR2 (Imgenex). β-Actin levels are shown for confirmation of equal protein loading. (B) TRAIL receptors and β-actin bands were subjected to densitometric scanning using the TINA 2 software, and the ratio DR4/β-actin, DR5/β-actin, DcR1/β-actin, or DcR2/β-actin was calculated. (C) DR4, DR5, DcR1, DcR2, and β-actin bands were subjected to densitometric scanning using the TINA 2 software, and the (DR4 + DR5)/(DcR1 + DcR2) ratio was calculated. (D) The same blots were subsequently stripped and reprobed for procaspase-8 and Bid. CTR indicates control; PD, PD184352 (1 μM); ATO, arsenic trioxide (2 μM).
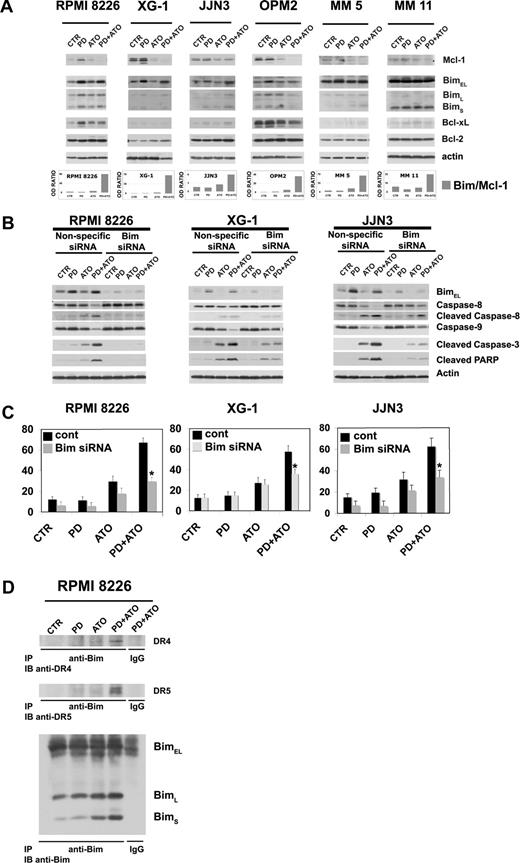
Combined exposure of MM cells to PD and ATO is associated with up-regulation of Bim and down-regulation of Mcl-1
Among the Bcl-2 family, antiapoptotic Mcl-1 and proapoptotic Bim play a pivotal role in controlling MM cell survival and apoptosis.79-81 First, we analyzed their expression in PD/ATO-treated cells. MM cells were treated with PD and/or ATO for 48 hours, and the levels of Bcl-2 family proteins were analyzed by Western blotting. ATO treatment decreased the level of antiapoptotic Mcl-1, whereas PD increased the level of the proapoptotic Bim, and the combined treatment provoked the simultaneous up-regulation of Bim and down-regulation of Mcl-1 in the MM cells retaining or not a functional p53 (Figure 6A). Similarly, an elevated ratio of Bim to Mcl-1 was also observed in fresh purified MM cells treated with PD/ATO (Figure 6A, representative data). In addition, in our experiments we did not observe a relevant modulation of Bcl-xL or Bcl-2 protein levels in PD/ATO vs ATO-treated MM cells except for OPM2 HMCL (Figure 6A).
Combined exposure of MM cells to PD184352 and ATO changes the balance between Bim and Mcl-1. (A) HMCLs or CD138-purified plasma cells from 2 representative patients were seeded at 2.5 × 105 cells/mL in the presence of DMSO (vehicle) or PD (1 μM) for 3 hours and then were incubated for 48 hours with ATO (2 μM). Endogenous Mcl-1, Bim, Bcl-xL, and Bcl-2 proteins were revealed by immunoblotting analysis using rabbit polyclonal anti-Mcl-1, rabbit polyclonal anti-Bim, and rabbit polyclonal anti-Bcl-xL, all provided by Cell Signaling Technology, and mouse monoclonal anti–Bcl-2 clone 124 (Upstate Biotechnology, Charlottesville, VA). Antiactin immunoblotting was performed as loading control. Bim, Mcl-1, and β-actin bands were subjected to densitometric scanning using the TINA 2 software, and the Bim/Mcl-1 ratio was calculated. (B) Transfection of Bim (004383, Dharmacon RNA Technologies), but not the unrelated nonspecific control siRNA (001206, Dharmacon RNA Technologies), led to a decrease in Bim protein expression in HMCLs without affecting the levels of the unrelated protein actin: 48 hours after siRNA transfection, the HMCLs were treated with PD and/or ATO for 24 hours and then lysed for Western blot analysis to monitor the expression of Bim, caspase-8, -9, -3 activation, and PARP fragmentation. Antiactin immunoblotting was performed as loading control. (C) HMCLs were treated as described in panel B, and the percentages of sub-G1 apoptotic cells were monitored after 48 hours of treatment. Values are mean plus or minus SD of 4 independent experiments (*P < .001 vs PD/ATO cont; Dunnett test). (D) RPMI 8226 cells were pretreated with either DMSO or PD (1 μM) for 3 hours and then treated with ATO (2 μM) for 12 hours, after which cells were lysed in CHAPS lysis buffer and subjected to immunoprecipitation (IP) using rabbit polyclonal anti-Bim (Cell Signaling Technology) or control antibody and then immunoblotted (IB) with either goat polyclonal anti-DR4 (Santa Cruz Biotechnology) or goat polyclonal anti-DR5 (Santa Cruz Biotechnology) or rabbit polyclonal anti-Bim antibodies (Cell Signaling Technology). CTR indicates control; PD, PD184352 (1 μM); ATO, arsenic trioxide (2 μM); cont, nonspecific control siRNA.
Combined exposure of MM cells to PD184352 and ATO changes the balance between Bim and Mcl-1. (A) HMCLs or CD138-purified plasma cells from 2 representative patients were seeded at 2.5 × 105 cells/mL in the presence of DMSO (vehicle) or PD (1 μM) for 3 hours and then were incubated for 48 hours with ATO (2 μM). Endogenous Mcl-1, Bim, Bcl-xL, and Bcl-2 proteins were revealed by immunoblotting analysis using rabbit polyclonal anti-Mcl-1, rabbit polyclonal anti-Bim, and rabbit polyclonal anti-Bcl-xL, all provided by Cell Signaling Technology, and mouse monoclonal anti–Bcl-2 clone 124 (Upstate Biotechnology, Charlottesville, VA). Antiactin immunoblotting was performed as loading control. Bim, Mcl-1, and β-actin bands were subjected to densitometric scanning using the TINA 2 software, and the Bim/Mcl-1 ratio was calculated. (B) Transfection of Bim (004383, Dharmacon RNA Technologies), but not the unrelated nonspecific control siRNA (001206, Dharmacon RNA Technologies), led to a decrease in Bim protein expression in HMCLs without affecting the levels of the unrelated protein actin: 48 hours after siRNA transfection, the HMCLs were treated with PD and/or ATO for 24 hours and then lysed for Western blot analysis to monitor the expression of Bim, caspase-8, -9, -3 activation, and PARP fragmentation. Antiactin immunoblotting was performed as loading control. (C) HMCLs were treated as described in panel B, and the percentages of sub-G1 apoptotic cells were monitored after 48 hours of treatment. Values are mean plus or minus SD of 4 independent experiments (*P < .001 vs PD/ATO cont; Dunnett test). (D) RPMI 8226 cells were pretreated with either DMSO or PD (1 μM) for 3 hours and then treated with ATO (2 μM) for 12 hours, after which cells were lysed in CHAPS lysis buffer and subjected to immunoprecipitation (IP) using rabbit polyclonal anti-Bim (Cell Signaling Technology) or control antibody and then immunoblotted (IB) with either goat polyclonal anti-DR4 (Santa Cruz Biotechnology) or goat polyclonal anti-DR5 (Santa Cruz Biotechnology) or rabbit polyclonal anti-Bim antibodies (Cell Signaling Technology). CTR indicates control; PD, PD184352 (1 μM); ATO, arsenic trioxide (2 μM); cont, nonspecific control siRNA.
To determine the biologic relevance of Bim modulation in response to PD/ATO treatment, we silenced the expression of endogenous Bim transcripts by using specific siRNA. The selective down-regulation of Bim strongly inhibited caspase-8, -9, and-3 activation and PARP cleavage in response to the combined treatment in RPMI 8226 (nonfunctional p53), XG-1 (functional p53), and JJN3 (nonfunctional p53; Figure 6B). Consistent with these results, we found that apoptosis was significantly reduced in Bim knocked-down PD/ATO-treated responsive RPMI 8226 (nonfunctional p53), XG-1 (functional p53), and JJN3 (nonfunctional p53; Figure 6C). Because abrogation of Bim expression affected both the intrinsic mitochondrial programmed cell death and the extrinsic caspase-8 mediated pathways, more evident in RPMI 8226 (nonfunctional p53) and JJN3 (nonfunctional p53) in which PD/ATO strikingly activates caspase-8 (Figure 6B), we tested by coimmunoprecipitation/Western blot analysis whether Bim is recruited to TRAIL death-inducing signaling complex. As depicted in Figure 6D, immunoprecipitates using an antibody directed against Bim from lysates of PD and/or ATO-treated RPMI 8226 (nonfunctional p53) cells showed association of DR4 and DR5 TRAIL receptors with Bim in PD/ATO-treated cells (Figure 6D).
Altogether, these results suggest that Bim is critical for PD/ATO-induced killing of MM cells, and we demonstrate that Bim interacts with DR4 and DR5 TRAIL receptors and contributes to the PD/ATO-mediated activation of the extrinsic pathway.
Combined exposure to PD and ATO suppresses human MM cell growth in vivo
To determine whether the PD plus ATO efficacy observed in vitro for MM cells was recapitulated in vivo, mice harboring human plasmacytoma (RPMI 8226) xenograft tumor were treated with the MEK inhibitor PD325901 and/or ATO. PD325901 is a derivative of PD184352 that has improved oral bioavailability and induces a longer duration of target suppression.56,82 We demonstrated that the effects of PD325901 alone or in combination with ATO on MM cells in vitro are qualitatively identical to those of PD184352, including the marked caspase-3 activation observed in PD/ATO-treated MM cells but occur at 20-fold lower concentration of PD325901 (50 nM) compared with PD184352 (1 μM; Figure S1).
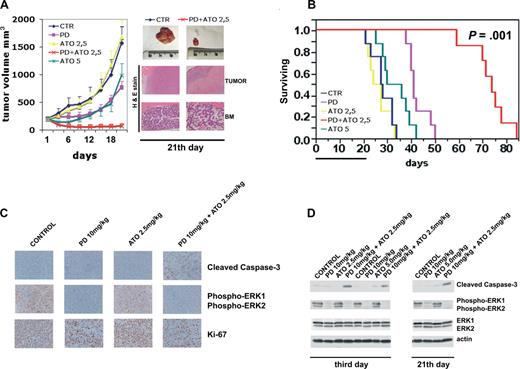
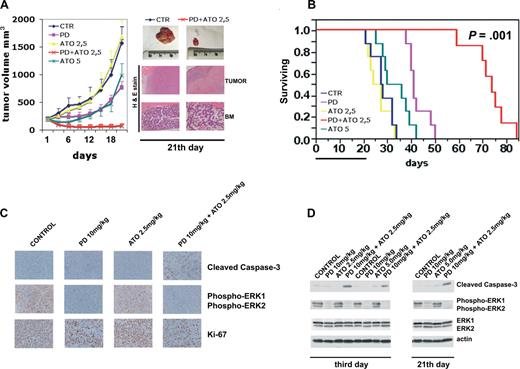
We tested the efficacy of PD/ATO in a murine model in which RPMI 8226 cells (107 cells/mouse) were injected subcutaneously into NOD-SCID mice. A similar plasmacytoma xenograft mouse model has been used in a broad range of preclinical compounds in MM.83-89 When the tumors reached approximately 200 mm3, mice were randomized (n = 13/group) to receive vehicle or PD325901 at 10 mg/kg administered by oral gavage or ATO (2.5 or 5.0 mg/kg) injected intraperitoneally or PD/ATO on a 5-days-a-week schedule for 3 consecutive weeks. Treatment of RPMI 8226 MM-tumor-bearing mice with PD325901 (10 mg/kg) significantly reduced MM-tumor growth compared with control (P < .01; Figure 7A). ATO at low dose (2.5 mg/kg) had minimal effect on the growth of tumors, which increased as in control mice (Figure 7A). Importantly, when PD (10 mg/kg) was combined with ATO (2.5 mg/kg), there was a significant reduction in tumor growth relative to untreated or PD-treated mice (P < .001 for PD/ATO vs control, and P < .01 for PD/ATO vs PD; Figure 7A). As an additional control, we also treated mice with a high dose of ATO (5 mg/kg). ATO (5 mg/kg) significantly (P < .05) reduced MM-tumor growth compared with control, but the extent of tumor growth inhibition was significantly (P < .01) lower in mice treated with a high-dose of ATO (5 mg/kg) versus mice treated with combination PD plus low-dose ATO (2.5 mg/kg; Figure 7A).
Combination of PD325901 plus ATO inhibits human plasmacytoma growth in immunodeficient NOD-SCID mice. (A) When tumor size reached 200 mm3, mice were randomly assigned (n = 10/group) to receive vehicle alone, PD325901 (orally), ATO (intraperitoneally), or PD325901 plus ATO at the indicated doses on a 5-days-a-week schedule for 21 days. Results are tumor volume (mm3), mean plus or minus SD, plotted against time. A significant delay in tumor growth in PD325901 plus ATO-treated mice was noted compared with vehicle-treated control mice (P < .001 Hsu MCB test). Inset shows tumors resected from control (vehicle) and PD325901 (10 mg/kg) plus ATO (2.5 mg/kg)–treated mice after 21 days of treatment (end point). RPMI 8226-derived tumors and BM from representative untreated or PD/ATO-treated mice were analyzed by hematoxylin and eosin staining (original magnification ×4 for tumor and ×10 for BM). (B) Kaplan-Meier survival curve was evaluated from the first day of treatment until death (mice were killed when tumors reached 2 cm3 in volume) using JMP version 7.0 statistical software (SAS Institute, Cary, NC). Survival was significantly prolonged in PD/ATO-treated animals versus control (P = .001 after Bonferroni correction). The black bar on the abscissa represents the 21-day period of treatment. (C) After 3 days of treatment, mice from each treatment group were humanely killed, and the tumors were removed for assay. RPMI 8226-derived tumors were analyzed by immunostaining for cleaved caspase-3 (Cell Signaling Technology), phospho-ERK (Cell Signaling Technology), and Ki-67 (NCL-L-Ki67-MM1, Novocastra, Newcastle, United Kingdom; original magnification ×20). The microphotographs shown are representative of similar observations in 3 different mice receiving the same treatment. (D) Tumor tissues from mice treated for 3 and 21 days were harvested and processed, and lysates were analyzed by immunoblotting analysis using rabbit polyclonal anticleaved caspase-3, rabbit polyclonal antiphospho-p44/42 ERK (Thr202/Tyr204), and rabbit polyclonal anti-p44/42 ERK, all provided by Cell Signaling Technology. Antiactin immunoblotting was performed as loading control. CTR indicates vehicle; PD, PD325901; ATO, arsenic trioxide.
Combination of PD325901 plus ATO inhibits human plasmacytoma growth in immunodeficient NOD-SCID mice. (A) When tumor size reached 200 mm3, mice were randomly assigned (n = 10/group) to receive vehicle alone, PD325901 (orally), ATO (intraperitoneally), or PD325901 plus ATO at the indicated doses on a 5-days-a-week schedule for 21 days. Results are tumor volume (mm3), mean plus or minus SD, plotted against time. A significant delay in tumor growth in PD325901 plus ATO-treated mice was noted compared with vehicle-treated control mice (P < .001 Hsu MCB test). Inset shows tumors resected from control (vehicle) and PD325901 (10 mg/kg) plus ATO (2.5 mg/kg)–treated mice after 21 days of treatment (end point). RPMI 8226-derived tumors and BM from representative untreated or PD/ATO-treated mice were analyzed by hematoxylin and eosin staining (original magnification ×4 for tumor and ×10 for BM). (B) Kaplan-Meier survival curve was evaluated from the first day of treatment until death (mice were killed when tumors reached 2 cm3 in volume) using JMP version 7.0 statistical software (SAS Institute, Cary, NC). Survival was significantly prolonged in PD/ATO-treated animals versus control (P = .001 after Bonferroni correction). The black bar on the abscissa represents the 21-day period of treatment. (C) After 3 days of treatment, mice from each treatment group were humanely killed, and the tumors were removed for assay. RPMI 8226-derived tumors were analyzed by immunostaining for cleaved caspase-3 (Cell Signaling Technology), phospho-ERK (Cell Signaling Technology), and Ki-67 (NCL-L-Ki67-MM1, Novocastra, Newcastle, United Kingdom; original magnification ×20). The microphotographs shown are representative of similar observations in 3 different mice receiving the same treatment. (D) Tumor tissues from mice treated for 3 and 21 days were harvested and processed, and lysates were analyzed by immunoblotting analysis using rabbit polyclonal anticleaved caspase-3, rabbit polyclonal antiphospho-p44/42 ERK (Thr202/Tyr204), and rabbit polyclonal anti-p44/42 ERK, all provided by Cell Signaling Technology. Antiactin immunoblotting was performed as loading control. CTR indicates vehicle; PD, PD325901; ATO, arsenic trioxide.
Histopathologic examination of the tumors from representative animals (Figure 7A inset) further supported the interpretation of tumor reduction in the PD/ATO-treated mice compared with the untreated controls. Tumors from the PD/ATO-treated mice showed large areas of tumor necrosis. Notably, after 21 days of PD/ATO treatment, mice showed only moderate bone marrow hypoplasia with respect to untreated animals (Figure 7A inset). Furthermore, the combination PD plus ATO was well tolerated without significant weight loss even after 3 weeks of treatment (data not shown).
As depicted in Figure 7B, survival was significantly prolonged in PD/ATO-treated animals versus either treatment alone (mean survival time of PD, 10 mg/kg plus ATO, 2.5 mg/kg: 74 days, 95% confidence interval [CI], 59-78 days; PD 10 mg/kg: 41 days, 95% CI, 38-48 days, P = .003 for PD/ATO vs PD; ATO 5.0 mg/kg: 36 days, 95% CI, 25-39, P = .001 for PD/ATO vs ATO 5.0 mg/kg; ATO 2.5 mg/kg: 27 days, 95% CI, 21-32, P = .001 for PD/ATO vs ATO 2.5 mg/kg; untreated: 28 days, 95% CI, 21-32 days, P = .001 for PD/ATO vs untreated).
We next investigated the in vivo effects of the drug combination on proliferation and apoptosis; whole tumor-cell tissues and tumor lysates from mice treated for 3 days (n = 3/group) were subjected to immunohistochemical staining and immunoblotting to assess in vivo phosphorylation of ERK, the proliferative antigen, Ki-67, and cleaved caspase-3. Tumor tissues from PD325901 (10 mg/kg) treatments resulted in profound p-ERK inhibition compared with tumor tissues from vehicle control or ATO-treated animals (Figure 7C,D). In agreement with these data, a significant decrement in the number of Ki-67-positive plasma cells was noted in tumor sections from PD-treated mice relative to tumors from mice receiving either vehicle control or ATO (2.5 mg/kg) treatment alone (Figure 7C), thereby confirming the tumor growth retardation observed in PD-treated mice. Either PD (10 mg/kg) or ATO alone at low-dose (2.5 mg/kg) did not increase caspase activation compared with tumors from control cohorts. However, the combination PD/ATO dramatically activated caspase-3 in tumors (Figure 7C,D). Similar results were observed after 21 days of treatment (end point; Figure 7D, representative data).
Taken together, these findings suggest that combining PD with ATO induces both cytostatic and cytotoxic responses in vivo, resulting in regression of tumors, prolongs survival in vivo, and is well tolerated in vivo.
Discussion
The present results show that the combined treatment with MEK inhibitors and ATO results in the synergistic (Combination Index < 1.0) induction of apoptosis in 5 HMCLs analyzed, irrespective of their p53 status. The combined treatment is also a highly potent inducer of apoptosis and mitochondrial damage in the majority of the primary MM cell samples ex vivo analyzed (9 of 12). Notably, 8 of 9 responders had stage III (Table 1), suggesting that the combination PD/ATO is effective on MM cells from aggressive late-stage disease. Importantly, growth factors or a coculture system with BMSCs failed to confer resistance to this combination regimen.
In this study, we have also demonstrated that PD/ATO-induced apoptosis occurs through multiple molecular mechanisms that may engage the extrinsic/receptor-mediated- and/or the intrinsic/mitochondria-associated pathways depending on the functionality of p53 in MM cells. In particular, we found that cotreatment with PD strikingly elevated the (DR4 + DR5)/(DcR1 + DcR2) TRAIL receptors ratio, caspase-8 activation, Bid fragmentation, mitochondrial depolarization, and caspase-9 activation of ATO-treated HMCLs that do not have a functional p53 pathway.
In HMCLs carrying a functional p53, we demonstrated that the extrinsic pathway was partially activated by PD/ATO and that PD greatly enhanced the ATO-induced p53 accumulation, Bax and Puma expression, mitochondrial depolarization, caspase-9/-3 activation, and apoptosis.
Furthermore, p73 was activated and had biologic relevance in PD/ATO-treated MM cells showing a partial activation of the extrinsic pathway (HMCLs carrying a functional p53), but not in MM cells in which PD/ATO highly activates the extrinsic pathway (HMCLs expressing nonfunctional p53). On the contrary, in AML blasts, we found that p73 is relevant for PD/ATO-induced apoptosis irrespective of p53 status.35,37 In this regard, tissue-specific tumor suppressor functions for the p53 family proteins have been recently demonstrated.90
In MM cells, a large variety of stimuli (growth factor withdrawal, cytotoxic agents) induce the disappearance of Mcl-1 that is associated with the onset of apoptosis. In contrast, regardless of the apoptotic stimulus, Bcl-2 expression remains unchanged.79,80 In addition, knockdown of Mcl-1 triggered a rapid induction of apoptosis in MM cells, which was observed neither with Bcl-2 nor with Bcl-xL antisenses.91 Mcl-1 down-regulation is important to make MM cells susceptible to BH3-only proteins and therefore to mitochondrial disruption.79 Bim belongs to the category of “BH3-only activators,” and myeloma cell death has been demonstrated to be totally dependent on Mcl-1/Bim complex disruption.79,80 Interestingly, in our study, we found that the combined treatment PD/ATO increased the level of the proapoptotic Bim (PD-mediated) and decreased its neutralizing antiapoptotic protein Mcl-1 (ATO-mediated), and Bim knockdown by siRNA significantly diminished caspase-3 cleavage/activation, PARP degradation, and apoptosis in PD/ATO-treated MM cells retaining, or not, a functional p53. These observations are in line with recent studies demonstrating the pivotal role of Bim in synergistic interactions between UCN-01 and MEK inhibitors in MM cells.81 Thus, the accumulation of Bim seems to represent a common mechanism by which MEK inhibitors can potentiate the effects of both UCN-01 and ATO in MM cells. Furthermore, a very recent study showed that Bim is necessary for ATO-induced apoptosis in MM.92
In addition, the concomitant up-regulation of Bim and its antagonists Mcl-1 and/or Bcl-xL protein levels under PD regimen could at least in part justify the absence of pronounced apoptosis observed in PD-treated cells (Figure 6A). Notably, the MEK inhibitor PD184352, at the concentration used in this study (1 μM), was able to completely block ERK1/2 phosphorylation in all HMCLs used (Figure S1, representative data).
The mechanism(s) by which Bim exerts its effects remains the subject of debate: Bim can trigger activation of Bax and Bak directly93-95 or indirectly by inactivating the prosurvival family members that guard Bax and Bak.96 In addition, the involvement of Bim and Mcl-1 in the TRAIL-mediated mitochondrial cascade has been recently reported.97 In our study, we demonstrated that in MM cells Bim plays an important role in both the intrinsic mitochondrial programmed cell death and the extrinsic caspase-8–mediated pathways. The functional linkage between Bim and the extrinsic pathway has been demonstrated in our experiments by the ability of Bim knockdown to significantly diminish caspase-8 cleavage/activation in PD/ATO-treated MM cells. In addition, a physical interaction between Bim and DR4/DR5 TRAIL receptors in PD/ATO-treated MM cells carrying a nonfunctional p53 was demonstrated by coimmunoprecipitation and Western blot studies. Taken together, these findings identify Bim as a prominent player in TRAIL-mediated, caspase-8 activation pathway in PD/ATO-treated MM cells, and the functional role(s) for Bim in TRAIL/caspase-8 activation pathway is now under intensive investigation in our laboratory.
A very recent study showed that AZD6244 (ARRY-142886), a novel and specific MEK inhibitor, targets both MM cells and osteoclasts in the BM milieu and enhances cytotoxicity of both conventional (dexamethasone) and novel (perifosine, lenalidomide, and bortezomib) therapies, thereby confirming the importance of targeting ERK pathway in MM.88 However, at concentrations close to the IC50 for ERK enzymatic activity, MEK inhibitors have cytostatic rather than cytotoxic effects, and higher doses are required to efficiently trigger apoptosis,38,81,98,99 thereby indicating that other parallel cytoprotective pathways should be targeted together, with MEK/ERK inhibition, to efficiently shift balance away from survival toward cell death.100 In this regard, we and others demonstrated the ability of MEK inhibitors to increase the cytoxicity of conventional chemotherapeutic drugs and/or other signal transduction/apoptosis modulators.35-37,49-53,68
Finally, the anti-MM activity of the combination PD (PD325901) plus ATO was also observed in vivo in a human MM xenograft mouse model. Immunohistochemistry and Western blot analysis of tumors from treated mice demonstrated that the potent anti-MM activity of PD/ATO in vivo was the result of either decreased proliferation or increased apoptosis.
The combination of PD and low-dose ATO prolonged survival compared with treatment with either drug alone and was well tolerated in vivo because no differences in body weight and general appearance were noted in mice during the treatment. Notably, after 3 weeks of treatment, only a moderate bone marrow hypoplasia in PD/ATO-treated versus untreated mice was observed.
The combination of PD and ATO showed a moderate inhibitory effect on normal hematopoiesis in vitro and had a minimal effect on normal bone marrow B cells in vitro. Both these in vivo and in vitro data indicate that the combination PD/ATO has a favorable therapeutic index in tumor cells versus normal cells.
Furthermore, the MEK inhibitor PD325901 used for our in vivo experiments, which has been shown to have improved pharmacologic properties over PD184352,56 is currently in phase 2 clinical trials to treat advanced breast cancer, colon cancer, and melanoma.101,102
Finally, the clinical observation that ATO therapy can be associated with limited efficacy and significant toxicity in MM, coupled with our present preclinical findings demonstrating that low doses of ATO together with PD325901 trigger a more potent anti-MM effect in vitro and in vivo without increased toxicity, suggests the promise of combination treatment strategies as a potential therapeutic avenue.
In conclusion, the current findings demonstrate a potent and specific antimyeloma activity of the combination of PD and ATO in vitro and in vivo and elucidate the multiple molecular mechanisms, most of them converging on Bim, by which MEK inhibition increases the therapeutic effect of ATO in MM cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Francesca Morandi, Dr Stefano Jottini, Dr Davide Martorana, and Davide Arienti for technical support.
This work was supported by grants from Associazione Italiana per la Ricerca sul Cancro (AIRC, Milan, Italy; A.B.), Fondazione Cassa di Risparmio di Parma (Cariparma, Parma, Italy; A.B.), the International Myeloma Foundation (IMF, Los Angeles, CA; N.G.), Ministero dell' Istruzione Università e Ricerca (MIUR, Rome, Italy; N.G.), and Programmi di Ricerca di Interesse Nazionale (PRIN, Rome, Italy; N.G.).
Authorship
Contribution: P.L. designed the research, designed the in vivo experimental protocol, performed the molecular biology experiments and statistical analysis, analyzed the data, and wrote the manuscript; N.G. provided multiple myeloma primary cells, performed coculture experiments, analyzed cell biology data, analyzed the paper, and contributed to the critical revision of the manuscript; L.M. performed cell culture experiments and apoptosis analysis, performed siRNA transfection experiments, and contributed to molecular biology experiments; G.L: carried out the in vivo experiments; M.R. contributed to in vivo experiments; A. Corradi supervised the immunohistochemical experiments; A.M.C. performed immunohistochemical analysis; L.S. contributed to cell culture experiments and apoptosis analysis; R.R. performed the in vitro hematopoieis experiments; A. Costanzo analyzed data, analyzed the paper, and contributed to the critical revision of the manuscript; U.T. supervised the in vitro hematopoieis experiments and analyzed the data; M.L. analyzed data, analyzed the paper, and contributed to the critical revision of the manuscript; V.R. analyzed data, analyzed the paper, and gave useful advice; and A.B. supervised all the experiments, analyzed all the data, and contributed to writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paolo Lunghi, Dipartimento di Scienze Cliniche, Via Gramsci 14, 43100 Parma, Italy; e-mail: p.lunghi@libero.it; or Antonio Bonati, Dipartimento di Scienze Cliniche, Unità OPERATIVA di Ematologia, Via Gramsci 14, 43100 Parma, Italy; e-mail: antbonny@unipr.it.

![Figure 1. MEK inhibition potentiates the ATO-induced apoptosis in MM cells. (A) HMCLs seeded at 2.5 × 105 cells/mL were treated sequentially with escalating doses of PD (0.1-20 μM) for 3 hours and subsequently with ATO alone (0.125-10 μM) or in combination with PD at a 1:1 ratio (0.25/0.25, 0.5/0.5, 1.0/1.0, 1.5/1.5, 2.0/2.0 μM). After 48 hours, apoptosis was measured by annexin V labeling. Combination Index plots were then generated using the Calcusyn software. Combination Index values less than 1.0 indicate synergism, Combination Index values equal to 1.0 indicate additive effect, and Combination Index values more than 1.0 indicate an antagonistic effect. (B) CD138-purified plasma cells from 12 patients with MM were seeded at 2.5 × 105 cells/mL in the presence of DMSO (vehicle) or PD (1 μM) for 3 hours and then incubated with ATO (2 μM) for 24 hours. The apoptosis was measured as percentage of cells with hypodiploid DNA content. Results are expressed as the net apoptosis induction (percentage of apoptosis in treated cells − percentage of apoptosis in DMSO [vehicle-treated cells]) and represent the means plus or minus SD of the results obtained in 12 different patient samples (*P < .001, Dunnett test). (C) Normal bone marrow B cells from 3 healthy donors were treated as described in panel B. After 24 hours of treatment, apoptosis was then measured as the percentage of cells with hypodiploid DNA content. CTR indicates control; PD, PD184352 (1 μM); ATO, arsenic trioxide (2 μM). (D,E) Analysis of the effect of PD and ATO on hemopoietic progenitor colony formation studied either using total PBMCs (D) or purified CD34+ cells (E). A total of 2 × 105 PBMCs (D) or 2 × 102 CD34+ cells (E) have been grown in methylcellulose either in the absence (0) or in the presence of either PD (0.5, 1, or 2 μM) or ATO (0.5, 1, or 2 μM) or both drugs. (F) Study of the effect of PD and ATO on the growth of CD34+ cells grown under serum-free conditions allowing the selective growth of either erythroid (E), megakaryocytic (Mk), or granulocytic (G) cells. A total of 5 × 104 CD34+ cells have been grown in serum-free liquid suspension cultures under E, Mk, or G cell culture conditions either in the absence (C) or in the presence of PD (1 μM), ATO (1 μM), or PD + ATO (both at 1 μM), and the number of the cell progeny was evaluated at various days of culture. For panels D, E, and F, the results represent the means plus or minus SEM values observed in 3 separate experiments. C indicates control; PD, PD184352; ATO, arsenic trioxide.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/6/10.1182_blood-2007-10-114348/7/m_zh80180824000001.jpeg?Expires=1769101762&Signature=X2KLAMc0y7Fv8Ng0B5gIL7jD~CvJmjxfT7RD2P9HwGI4gmyrMxO73SE-lgzHobyjoP02KjQeuSB15-GQhRBfL6Nu9RV0zoZ4tXZ7tyqoiY61NjHzJJHgxyopAZ5Av9DO486K0h3DUH6Pkn0rsYaPmigT5sMwKQOISrMxbcz~mZa9o9nyrTJyPR2ffVPgXLi01w1xXQPC6nOn03XVzzAGIX8gZ~n0vkQ5AUgDleWcSSnpWb2X-seK-kAo0jR~C9jnZCt7KoMst14jTOz2BtneEH6uaK7clxH19powq99iW1dW9YwTWxIJe33mPyh1iQPhH5dWrAPqVgnzmRCBBncvcA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 1. MEK inhibition potentiates the ATO-induced apoptosis in MM cells. (A) HMCLs seeded at 2.5 × 105 cells/mL were treated sequentially with escalating doses of PD (0.1-20 μM) for 3 hours and subsequently with ATO alone (0.125-10 μM) or in combination with PD at a 1:1 ratio (0.25/0.25, 0.5/0.5, 1.0/1.0, 1.5/1.5, 2.0/2.0 μM). After 48 hours, apoptosis was measured by annexin V labeling. Combination Index plots were then generated using the Calcusyn software. Combination Index values less than 1.0 indicate synergism, Combination Index values equal to 1.0 indicate additive effect, and Combination Index values more than 1.0 indicate an antagonistic effect. (B) CD138-purified plasma cells from 12 patients with MM were seeded at 2.5 × 105 cells/mL in the presence of DMSO (vehicle) or PD (1 μM) for 3 hours and then incubated with ATO (2 μM) for 24 hours. The apoptosis was measured as percentage of cells with hypodiploid DNA content. Results are expressed as the net apoptosis induction (percentage of apoptosis in treated cells − percentage of apoptosis in DMSO [vehicle-treated cells]) and represent the means plus or minus SD of the results obtained in 12 different patient samples (*P < .001, Dunnett test). (C) Normal bone marrow B cells from 3 healthy donors were treated as described in panel B. After 24 hours of treatment, apoptosis was then measured as the percentage of cells with hypodiploid DNA content. CTR indicates control; PD, PD184352 (1 μM); ATO, arsenic trioxide (2 μM). (D,E) Analysis of the effect of PD and ATO on hemopoietic progenitor colony formation studied either using total PBMCs (D) or purified CD34+ cells (E). A total of 2 × 105 PBMCs (D) or 2 × 102 CD34+ cells (E) have been grown in methylcellulose either in the absence (0) or in the presence of either PD (0.5, 1, or 2 μM) or ATO (0.5, 1, or 2 μM) or both drugs. (F) Study of the effect of PD and ATO on the growth of CD34+ cells grown under serum-free conditions allowing the selective growth of either erythroid (E), megakaryocytic (Mk), or granulocytic (G) cells. A total of 5 × 104 CD34+ cells have been grown in serum-free liquid suspension cultures under E, Mk, or G cell culture conditions either in the absence (C) or in the presence of PD (1 μM), ATO (1 μM), or PD + ATO (both at 1 μM), and the number of the cell progeny was evaluated at various days of culture. For panels D, E, and F, the results represent the means plus or minus SEM values observed in 3 separate experiments. C indicates control; PD, PD184352; ATO, arsenic trioxide.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/6/10.1182_blood-2007-10-114348/7/m_zh80180824000001.jpeg?Expires=1769217703&Signature=lwudvvJurA53jW1SmeSW6bv3jhhlM8oLR6-SyEGXaIDNgs90jWbeFVWuCgZ7DfulRQJB9a1aCc5BX9HnoW4WOoJC13NiVoQKPS4fuVDIylOT2rRakh-HtadMz7--0zBqNTvVGm99dsGg7XWUgxnx-vkr4kdfPE~WDu89Z2EXwHFu6wDMNEJWm3vyvHA00-AaglQs4JwrFrZXkE2nzJ7vUhkCfiousel8PUYkIS8RWMI03cEOz7W-RWw8p6XPKuzo-yLXBjIvX8hmcQkiXk70iGYLKANjP84wJUUAC-uBzjW7W-4GbAP42r1HmrA6C~pwgt5Jn98QtGe4VRAo0z9h4g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)