Abstract
Acute myeloid leukemia (AML) is considered an oncologic emergency. Delaying induction chemotherapy until molecular testing results return, may benefit some patients but harm others. We examined the effect of time from AML diagnosis to treatment (TDT) on complete remission (CR) and overall survival (OS), using patient characteristics available at diagnosis. Regression models were applied to older (≥ 60 years) and younger (< 60 years) adults, controlling for age, baseline white blood cell count, secondary AML (sAML), and performance status. Median patient age was 60 years (range, 17-87 years), TDT 4 days (range, 1-78 days), and 45% had sAML. Cytogenetic risk distribution was: favorable, 8%; intermediate, 66%; unfavorable, 26%. CR rate was 67% and median OS was 68 weeks in patients younger than 60 years; 55% and 33 weeks in older patients, respectively. In univariate and multivariate analyses, longer TDT was associated with worse CR and OS in younger (univariate: P < .001 in both; multivariate: P < .001 and P = .001, respectively), but not older patients (univariate: P = .45, P = .19; multivariate: P = .63, P = .30, respectively). Results did not change with inclusion of cytogenetic data or in risk group subsets. AML therapy should be initiated immediately in younger patients. Delaying treatment does not seem harmful in older patients, allowing individualized approaches.
Introduction
Acute myeloid leukemia (AML) has traditionally been considered an oncologic emergency, with immediate initiation of therapy thought to be crucial to minimizing disease-related morbidity and mortality. In an ideal patient, such as one who is younger, with a core binding factor cytogenetic abnormality, and who is given standard anthracycline- and cytarabine-based induction chemotherapy, complete remission (CR) rates approach 85%, with long-term disease-free survival (DFS) rates of 60% or greater.1-4 However, in most patients, eg, those who are older or who have other cytogenetic abnormalities or secondary AML, outcome after such standard therapy is much worse, with CR rates less than 50%, treatment-related mortality rates that approach 25%, and minimal long-term DFS.1,5-12 Although indirect data support the use of intensive chemotherapy in this population, clearly most will derive little benefit from this approach13-15 ; thus, fewer than 40% of AML patients 65 years of age or older are treated with chemotherapy, and median survival among this population is 2.4 months.16
These data have motivated the widespread adoption of investigational therapies as initial treatment, particularly for older adults. For all age groups, waiting until cytogenetic or molecular results become known and offering individualized, investigational therapy may be beneficial, especially to higher-risk groups.17,18 However, use of cytogenetics to avoid administration of ineffective, possibly toxic therapy often entails waiting at least 1 week for results to become available. Thus, physicians must weigh the risks associated with giving “standard,” immediate therapy to patients in whom poor prognostic characteristics, such as adverse cytogenetics, auger a low CR rate, with the risk of waiting to initiate treatment and giving investigational therapy individualized according to the additional testing. The impact of delaying remission induction therapy on AML outcome has not been documented.
In this study, we examine, after accounting for other covariates readily available to clinicians at the time of diagnosis, the effect of time from AML diagnosis to treatment (TDT) on CR rates and overall survival (OS) in more than 1300 AML patients from 2 institutions.
Methods
Patients
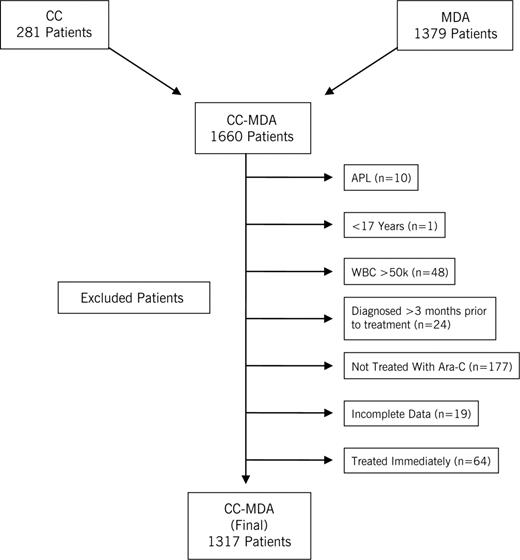
This study was approved by the institutional review boards of Cleveland Clinic (CC) and the M. D. Anderson Cancer Center (MDA), and informed consent was obtained in accordance with the Declaration of Helsinki. The joint CC-MDA database is composed of 1660 AML patients treated at both institutions from 1994 to 2005: 281 from CC and 1379 from MDA (Figure 1). An AML diagnosis was independently confirmed at each institution and classified according to French-American-British or World Health Organization systems for myeloid neoplasms.19,20 Cytogenetics were determined using metaphase karyotyping, based on analyses of 20 or more cells. Cytogenetic risk classification categories were defined approximating the Medical Research Council schema. Patients with complex cytogenetics or −7 abnormalities were placed into an “unfavorable” category, those with core binding factor abnormalities (t(8;21, inv(16), or t(16;16)) into a “favorable” category, and others into an “intermediate risk” category.7 Patients with insufficient metaphases to determine cytogenetic abnormalities were placed in the “intermediate risk” category, as outcome has been shown to be similar.21,22 After excluding patients with acute promyelocytic leukemia (n = 10), those younger than 17 years of (n = 1), with white blood cell counts (WBC) more than 50 000/mm3 (n = 48), diagnosed more than 3 months before therapy (n = 24), not treated with cytarabine (n = 177), who began therapy immediately on the date of diagnosis (n = 64, because of the potential for a selection bias, as they may have been perceived to be “sicker” by the treating physician), and with incomplete data (n = 19), the final dataset consisted of 1317 patients, 195 (15%) from CC, and 1122 (85%) from MDA, all of whom were treated with a remission induction regimen that included cytarabine.
Of the 195 patients treated at CC, data on cytarabine doses were available for 190, and for the 1122 patients treated at MDA, data were available on all 1122. For ease of interpretation, we divided regimens into those that contain conventional dose cytarabine (given at a dose of 100-200 mg/m2 continuous infusion daily × 7 days), those that contain mid-range doses of cytarabine given as a continuous infusion (500-1500 mg/m2 daily × 3-6 days), and those that contained mid- to high-dose cytarabine given as a bolus (1-3 g/m2 per day × 4-6 days). The conventional cytarabine dose group thus included 151 CC patients who received cytarabine combined with an anthracycline or anthracenedione, and 109 MDA patients. The continuous infusion cytarabine group included 32 CC patients treated with cytarabine at a dose of 500 mg/m2 daily times 6 days as part of a timed-sequential AML protocol plus mitoxantrone and etoposide, 378 MDA patients treated with cytarabine (1.5 g/m2 per day × 3-4 days by continuous infusion) plus idarubicin, and 80 MDA patients with the same cytarabine dose plus daunorubicin. The bolus mid- to high-dose cytarabine dose group included 1 CC patients who received cytarabine at 1.5 g/m2 daily times 6 days and 6 patients who received it at a dose of 3 g/m2 daily times 6 days. At MDA, 183 patients were treated with ara-C (1-2 g/m2 per day × 4-5 days) plus cytoxan plus topotecan, 155 patients with cytarabine 1 to 2 g/m2 daily times 5 days plus fludarabine plus idarubicin, 122 with cytarabine 1 to 2 g/m2 daily times 5 days plus fludarabine, 44 patients with cytarabine 1 to 2 g/m2 daily times 5 days plus clofarabine, 37 patients with cytarabine 1 to 2 g/m2 daily times 5 days plus topotecan, and 14 patients with cytarabine 1 to 2 g/m2 daily times 5 days plus miscellaneous agents.
Characteristics readily available at the time of diagnosis, and recognized as being predictive of outcome in multivariate analyses, included age, WBC, performance status (PS), and secondary AML (sAML, which includes patients with AML arising from an antecedent hematologic disorder, such as myelodysplastic syndromes, and therapy-related AML). The impact of cytarabine dose was also assessed. Cytogenetics were included in models a posteriori, as generally these results are not available when a decision is made about induction chemotherapy. The date of diagnosis was defined as the date an AML diagnosis was confirmed based on bone marrow findings at CC or MDA. This date almost invariably corresponded to the date of presentation to CC or MDA. CR was defined using International Working Group criteria.23 OS was calculated from the date of diagnosis.
Statistics
Patient characteristics and CR were summarized using frequency counts and descriptive statistics, which included the mean plus or minus SD, median, and range. OS was summarized using the method of Kaplan and Meier. In univariate analysis, the effect of TDT on CR was evaluated using χ2 tests and Cochran-Armitage trend tests; and the effect on OS was evaluated using the log-rank test and log-logistic regression models. Log-logistic models were used to analyze OS rather than proportional hazards models because examination of log survivor plots, and analysis of Martingale and Schoenfeld residuals indicated that the proportionality assumption was violated for several factors. Multivariate analysis of CR and OS was conducted using logistic and log-logistic regression models, respectively. A stepwise selection algorithm that used a P value equal to .05 cutoff for determining inclusion and removal of factors from models was used to identify independent predictors of each outcome. All models, however, included a term for treatment center to take into account any inherent differences between them.
Initially, analyses were conducted using all patients. Statistically significant interactions were found between patient age and TDT and type of AML; therefore, subsequent analyses were performed separately in older (≥ 60 years) and younger (< 60 years) adults. Initially, only TDT, pretreatment age, WBC, and type of AML (de novo vs sAML) and cytarabine dose were considered along with their pairwise interactions. PS, however, was also analyzed in the subset of patients for whom it was known (MDA patients). In addition, the impact of cytogenetics was evaluated once final models based on the information available at diagnosis were identified.
Transformations (eg, logarithm) and categorization, based primarily on recursive partitioning, of TDT, age, and WBC were considered. For TDT the original scale, uncoded and untransformed, provided the best fit to the data and was therefore used in all analyses. Among patients younger than 60 years, using the age categories younger than 40 years versus 40 years and older resulted in very little loss of information, in terms of the log-likelihood, compared with the uncoded form, and is therefore reported here in the analyses of both CR and OS. Similarly, among patients 60 years and older, using younger than 70 years versus 70 years and older, resulted in little information loss and is therefore reported in the analysis of OS. The uncoded/untransformed age, however, provided the best fit to the data with respect to CR. An interaction between the type of AML and WBC was found in the analysis of CR in patients younger than 60 years as well as those 60 years and older. The interaction was thus characterized with no loss of information by categorizing WBC as less than 8.5/mm3 versus more than 8.5/mm3 in patients younger than 60 years and less than 5.0/mm3 versus more than 5.0/mm3 in patients 60 years and older (in whom the 5.0/mm3 cutoff also provided a good fit to the data with respect to survival) and is therefore reported here.
To test the robustness of conclusions from the regression analyses, models were constructed by splitting the data randomly into “training” (60%) and “validation” (40%) sets. The impact of delaying therapy was the same in both sets, despite minor differences with respect to other factors. Thus, only the results from the regression analyses involving the combined 1317 patients are presented here.
Once final models were identified, further analyses were conducted to determine whether subsets of patients could be identified for whom treatment delay was especially harmful. These analyses were conducted by forming “risk groups” based on the final model (after excluding treatment delay if it was in the model), and then assessing the effect of treatment delay and the interaction between delay and risk group. For example, in the analysis of survival in adults younger than 60 years, etiology (de novo vs sAML), age (< 40 years vs ≥ 40 years), and interval from diagnosis to treatment were all seen to be independent predictors of outcome (see Table 4). Using a scoring algorithm that includes the number of poor prognostic features present based on age and etiology (sAML and age 40 years and older; each counts as 1 poor prognostic factor), 3 risk groups were generated: favorable, age younger than 40 years and de novo AML (0 poor prognostic factors); intermediate, age older than 40 years or secondary AML, but not both (1 poor prognostic factor); and unfavorable, age older than 40 years and sAML (2 poor prognostic factors). Three risk groups were similarly formed for survival in patients 60 years and older. The favorable risk group for survival in patients 60 years and older was defined as age younger than 70 years and WBC less than 5.0/mm3; intermediate, age older than 70 years or WBC more than 5.0/mm3, but not both; unfavorable, age older than 70 years and WBC more than 5.0/mm3. Risk groups for CR in patients younger than 60 years were based on age older than 40 years, sAML, and sAML with a WBC more than 8.5/mm3: favorable, no poor prognostic factors; intermediate, 1 poor prognostic factor; unfavorable, 2 or 3 poor prognostic factors. Three risk groups for CR in patients 60 years and older were based on patient age and sAML with WBC more than 5.0/mm3. Patients were considered to have a favorable or intermediate risk profile if they had de novo AML or sAML with a WBC less than 5.0/mm3. Favorable patients were those younger than 70 years; intermediate risk patients were those older than 70 years. The unfavorable group was composed of patients with sAML and a WBC more than 5.0/mm3, regardless of age. Once risk groups were defined, the effect of treatment delay was then examined by fitting a model with just risk group, treatment delay, and their interaction.
Results
Univariate analyses
The characteristics of the 1317 patients treated at CC and at MDA are presented in Table 1. Patients had a median age of 60 years (range, 17-87 years), a median pretreatment WBC of 5.0/mm3 (range, 0.3-48.6/mm3), and a median TDT of 4 days (25th and 75th percentiles were 2 and 8 days, respectively; range, 1-78 days). Twenty percent of patients were treated with conventional doses of cytarabine, 37% received mid-range doses given as continuous infusions, and 43% received mid- to high-dose bolus cytarabine. Within the full group, 653 patients were considered “younger” (age < 60 years) and 664 patients were considered “older” (age ≥ 60 years); 45% of patients had sAML. Cytogenetic risk distribution was similar to other large AML databases7,24 : favorable, 8%; intermediate, 66%; and unfavorable, 26%.
A CR was achieved in 801 patients (61%): 67% of those younger than 60 years and 55% of those 60 years and older. Median survival was 48 weeks, 68 weeks for those younger than 60 years and 33 weeks for those 60 years and older. In univariate analyses in patients younger than 60 years, increasing age and sAML predicted for lower CR and OS rates (P < .001 for both, respectively), although not increasing WBC (Tables 2,3). In patients 60 years and older, CR rates and OS were worse with increasing WBC (P = .008 and P = .03, respectively) and increasing age (P = .02 and P = .004, respectively), but not with sAML. PS was a significant prognostic factor for both response and survival regardless of the age group examined (CR in patients younger than 60 years of age, P = .03; response in patients 60 years and older, P < .001; OS in either age group, P < .001). Cytogenetics was also a significant prognostic factor for outcome regardless of age group (P < .001 in all cases). Cytarabine dose was not seen to impact outcome in either age group. When all patients were considered together, TDT predicted for CR and OS when treated continuously (P < .001 and P = .04, respectively), as did AML etiology, PS, and cytogenetics.
Multivariate analyses
In patients younger than 60 years, increasing age and sAML predicted for lower CR rates (P < .001 for both) and OS (P < .001 and P = .003, respectively), as did the combination of sAML and WBC more than 8.5/mm3 for CR rates (P = .007, Table 4). In patients 60 years and older, CR rates were again worse in patients with sAML and elevated WBC (> 5.0/mm3, P < .001) and also with increasing age (P = .02). OS was worse with increasing age (P = .004) and WBC more than 5.0/mm3 (P = .008) but not with sAML. CR rates and OS were not impacted by cytarabine dose for either age group.
Effect of TDT
In both univariate and multivariate analyses, longer TDT was associated with worse CR rates and OS in younger (P < .001 in all cases), but not older (P > .45 for CR and > .19 for OS), patients (Tables 2,Table 3–4). Notably, in younger adults, CR rates and OS appeared to decrease more dramatically with a TDT of 5 or more days. Incorporating PS and/or cytogenetics into these models did not alter these conclusions (Table 5). Restricting attention to patients for whom both cytogenetics and PS were known, cytogenetics were independent prognostic factors for both CR and OS in both age groups (P < .001 in all cases). Similarly, with the exception of CR in patients younger than 60 years, PS was also an independent predictor of outcome in both groups (P < .001 in all cases). In patients younger than 60 years, PS was not seen to impact CR when accounting for cytogenetics (P = .16). This is probably the result, at least in part, to the correlation between these 2 factors (P = .007). Adjusting for cytogenetics and PS, treatment delay was still seen to be an independent prognostic factor for poor outcome (CR and OS) in patients younger than 60 years (P = .003 and P = .009, respectively) and was not associated with outcome in older patients (P = .49 and .94, respectively).
Using the scoring algorithms described in “Statistics” to identify patients by risk group, in younger AML patients, 144 patients (22%) were classified as favorable, 314 (48%) as intermediate, and 195 (30%) as unfavorable with respect to survival. Similarly, 144 patients (22%) were classified as favorable with respect to CR, 303 (46%) as intermediate, and 206 (32%) as unfavorable. As would be expected, risk group was highly correlated with both outcomes (P < .001 in both cases; Table 6; Figure 2). Assignment by risk group did not affect the impact that TDT had on survival in any of these groups (P = .81 for the interaction); however, there was some suggestion that it did affect the impact TDT had on CR (P = .13 for the interaction), particularly in the unfavorable group (P = .007 for the effect of TDT; vs P = .06 in intermediate patients and P = .50 in favorable patients).
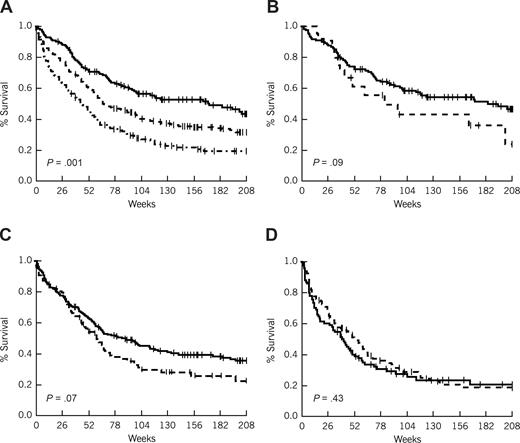
Survival in patients less than 60 years of age by risk group and interval from diagnosis to treatment. (A) Survival by risk group. ------- indicates favorable (age ≤ 40 and de novo AML); - - - -, intermediate (age ≤ 40 and secondary AML, or age > 40 and de novo AML); and - · - · -, unfavorable (age > 40 and secondary AML). (B) Survival by treatment lag (favorable risk patients). (C) Survival by treatment lag (intermediate risk patients). (D) Survival by treatment lag (unfavorable risk patients). (B-D) ------- indicates less than or equal to 5-day treatment delay; and - - - -, more than 5-day treatment delay.
Survival in patients less than 60 years of age by risk group and interval from diagnosis to treatment. (A) Survival by risk group. ------- indicates favorable (age ≤ 40 and de novo AML); - - - -, intermediate (age ≤ 40 and secondary AML, or age > 40 and de novo AML); and - · - · -, unfavorable (age > 40 and secondary AML). (B) Survival by treatment lag (favorable risk patients). (C) Survival by treatment lag (intermediate risk patients). (D) Survival by treatment lag (unfavorable risk patients). (B-D) ------- indicates less than or equal to 5-day treatment delay; and - - - -, more than 5-day treatment delay.
In older AML patients, 194 (29%) were classified as favorable, 331 (50%) as intermediate, and 139 (21%) as unfavorable with respect to survival; 284 (43%) patients were considered favorable with respect to CR, 232 (35%) were intermediate, and 148 (22%) were unfavorable. As with younger patients, risk group was highly correlated with outcome (P < .001 in both cases; Table 6; Figure 3); however, there was no indication of an impact on TDT.
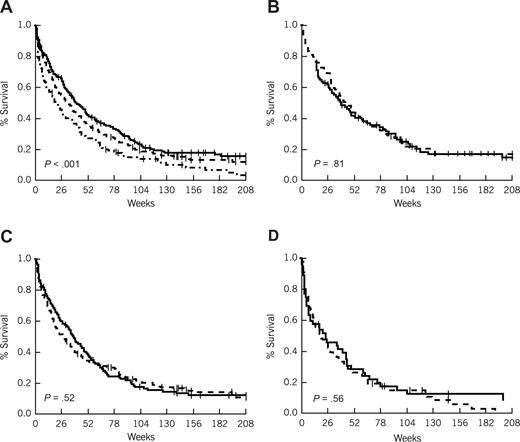
Survival in patients more than 60 years of age by risk group and interval from diagnosis to treatment. (A) Survival by risk group. ------- indicates favorable (age < 70 and WBC ≤ 5.0 K/μL); - - - -, intermediate (age < 70 and WBC > 5.0 K/μL, or age ≥ 70 and WBC ≤ 5.0 K/μL); and - · - · -, unfavorable (age ≥ 70 and WBC > 5.0 K/μL). (B) Survival by treatment lag (favorable risk patients). (C) Survival by treatment lag (intermediate risk patients). (D) Survival by treatment lag (unfavorable risk patients). (B-D) ------- indicates less than or equal to 5-day treatment delay; and - - - -, more than 5-day treatment delay.
Survival in patients more than 60 years of age by risk group and interval from diagnosis to treatment. (A) Survival by risk group. ------- indicates favorable (age < 70 and WBC ≤ 5.0 K/μL); - - - -, intermediate (age < 70 and WBC > 5.0 K/μL, or age ≥ 70 and WBC ≤ 5.0 K/μL); and - · - · -, unfavorable (age ≥ 70 and WBC > 5.0 K/μL). (B) Survival by treatment lag (favorable risk patients). (C) Survival by treatment lag (intermediate risk patients). (D) Survival by treatment lag (unfavorable risk patients). (B-D) ------- indicates less than or equal to 5-day treatment delay; and - - - -, more than 5-day treatment delay.
Figures 2 and 3 plot survival curves by risk group to show the impact of TDT in older and younger patients, respectively. For convenience, TDT has been dichotomized using a cutoff point of 5 days in both sets of curves. P values are given for the comparison of TDT within risk groups; however, they must be viewed cautiously, in part because the categorization results in a loss of information contained in the data when viewed as a continuous measure. For example, although it appears in Figure 2B,C (favorable and intermediate risk AML patients younger than 60 years) that there is only a trend toward a worse survival for a TDT of more than 5 days, and in Figure 2D (unfavorable risk AML patients younger than 60 years) that there is no significant difference, in multivariate analyses incorporating all patients TDT retained the same significant impact on survival regardless of risk group assignment.
Discussion
As knowledge of AML biology has grown, so has the realization of the heterogeneity of the disease. Indeed, it has been proposed that treatment of AML will eventually become “personalized,” that is, dependent on patients' distinctive molecular characteristics as embodied in array-based tests. Thus, it is natural to ask how to reconcile the desire to personalize treatment with the presumed need to begin treatment immediately, as a substantial proportion of these patients will die without immediate institution of medical therapy. Hence, there is a need to identify subsets of AML patients who require immediate cytotoxic therapy and to distinguish them from those who may benefit from delaying therapy until results of additional testing return.
In this study, we merged 2 databases of AML patients treated with cytarabine-containing therapy over the course of a decade in an attempt to identify these subsets. These patients were typical of other large AML studies, in the distribution of pretreatment covariates, the rates of CR and OS, and the effect of the covariates on these outcomes.25-29 One exception is the possible overrepresentation of patients with sAML compared with other databases, probably a result of a referral bias to leukemia specialty centers and to the underrepresentation of these patients in databases derived from cooperative group clinical trials, in which sAML patients often are excluded. Of note, we excluded from our analyses patients with WBC more than 50 000/mm3. Our databases indicated that treatment delay was exceptionally uncommon in such patients. Furthermore, we thought it would be unethical to delay treatment in them and wanted to avoid issues of selection bias that might arise in measuring their treatment delay.
Our data suggest that, in younger patients with WBC less than 50 000/mm3, AML should continue to be considered an oncologic emergency. Longer TDT, when analyzed continuously, predicted for lower CR rates and OS rates. This held true in univariate analyses; in multivariate analyses, when incorporating clinical factors readily available to every treating physician on presentation (age, performance status, WBC, and type of AML); in multivariate analyses incorporating the aforementioned factors and cytogenetics; and in patients identified as favorable, intermediate, or unfavorable using a scoring algorithm of clinical factors available at the time of diagnosis. Outcome appeared to worsen particularly with treatment delays of 5 days or greater, a delay that occurred in 41% of younger patients. Such delays are typical of patients referred to a tertiary care facility, especially if patients arrive on a weekend, when treatment may be delayed on average by 1 additional day.
An interesting side observation was the significant correlation between cytogenetic risk classification and performance status in younger adults. It is difficult to develop a plausible mechanism for how this would occur, unless we were to hypothesize that poor-risk cytogenetics affected blood counts more adversely than better-risk cytogenetics at presentation, and this resulted in a worse PS. Interestingly, in a separate publication exploring predictors of outcome of AML patients admitted to the intensive care unit,30 there was a hint that cytogenetics predicted for clinical improvement to resume aggressive therapy from the ICU stay, and survival at 2 and 6 months, perhaps pointing toward a correlation between cytogenetic risk category and short-term clinical outcomes.
In contrast, in patients 60 years of age or older, TDT did not appear to affect CR or OS rates. Subgroups of older adults in whom delay had an impact could not be identified. TDT was longer in older adults compared with younger adults, by less than 1 day on average. This difference was not statistically significant (P = .17), and it is difficult to determine whether this additional delay resulted from time to screen for investigational trials, to await the return of cytogenetic results, or from a sense of diminished urgency given the patient's advanced age. On the surface, these results differ from those of the Eastern Cooperative Oncology Group (ECOG) study,10 in which AML patients age older than 55 years were randomized to 1 of 3 remission induction regimens. Later, patients in the study were also randomized to granulocyte-macrophage colony-stimulating factor vs placebo; the latter randomization resulted in a median delay of 4 days in initiating cytotoxic therapy. Although results were not influenced by remission induction regimen or receipt of granulocyte-macrophage colony-stimulating factor, patients in whom therapy was delayed had a significantly lower CR rate (P = .04), although no difference in OS. However, the ECOG study did not exclude patients with hyperleukocytosis, did not allow patients with sAML to be enrolled, and did not report multivariate analyses of the effect of TDT.
Nonetheless, the discrepancy between our results and those of ECOG highlights the need to discuss limitations of our study. Principal among these is the retrospective nature of our analyses and hence the inability to know all the factors that entered into the decision to delay treatment, although we were able to identify major factors that have been recognized previously as having an impact on outcome.2,5,11 Furthermore, the frequent delay between suspicion of a diagnosis of AML at a referring hospital and confirmation of the diagnosis at a tertiary center implies that, in many cases, we may have underestimated delay. This possibility, although mostly unavoidable, may diminish the credibility of our results in younger patients, in whom actual delay was longer than our nominal TDT. In a subsequent analysis of 422 patients treated with cytarabine-based induction chemotherapy at CC, 126 (30%) had an AML diagnosis before arrival at CC. The median time from diagnosis at an out-side facility to date of admission at CC was 3 days (range, 1-99 days). Although more older patients were diagnosed with AML before arrival at CC, there was no significant difference in the time from diagnosis at an outside facility to admission date for patients younger than 60 years (n = 50) and older than 60 years (n = 76). Thus, the impact of an outside diagnosis does not appear to differentially affect older, compared with younger, adults. Finally, the simplest explanation for the lack of significant effect of TDT in older patients is that cytarabine-containing therapy itself is so ineffective in this population. As the nature of such treatment changes, TDT may become more significant in older patients.
Nonetheless, our findings in younger patients raise the question of whether it is prudent to delay therapy, to determine eligibility for studies that target a poor-risk factor molecular abnormality, such as the FMS-like tyrosine kinase 3 internal tandem duplication. As demonstrated in a preliminary fashion by Stone et al,31 92% of younger AML patients with this abnormality who are treated with a FMS-like tyrosine kinase 3 inhibitor combined with a cytotoxic induction regimen achieve a CR. Thus, the benefits of this drug in a selected population may offset the deleterious effects of delaying definitive therapy. It remains to be seen if the drug's benefits for an abnormality affecting up to 30% of patients are sufficient to justify delaying therapy in the 70% of patients in whom it may not be of benefit. In contrast, our results in older patients suggest that they may benefit from waiting for the results of additional testing to return, allowing enrollment into studies that account for cytogenetic findings or that target molecular abnormalities, such as the Southwest Oncology Group's study S0605, in which older patients with AML and a del(5q) cytogenetic abnormality are treated with single-agent lenalidomide. Ideally, the question of effect of delaying therapy should be explored prospectively in older patients being treated with both intensive and nonintensive types of therapy.
Presented in part at the American Society of Hematology Annual Meeting in Atlanta, GA, December 10, 2007, and published in abstract form.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Marc Earl for his assistance with obtaining treatment regimens for the subjects of this study.
This work was supported in part by the National Institutes of Health (U54RR19397-03; M.A.S.).
National Institutes of Health
Authorship
Contribution: M.A.S. designed and performed the research, analyzed data, and wrote the manuscript; P.E. analyzed the data and helped write the manuscript; M.E.K., A.S.A., E.A.C., S.F., and H.M.K. edited the manuscript and provided input into the study design; and E.E. designed and performed the research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mikkael A. Sekeres, Department of Hematologic Oncology and Blood Disorders, Cleveland Clinic Taussig Cancer Institute, Desk R35, 9500 Euclid Avenue, Cleveland, OH 44195; e-mail: sekerem@ccf.org.