Abstract
Proteasome inhibitors (PIs) are effective against multiple myeloma (MM), but the mechanisms of action and bases of individual susceptibility remain unclear. Recent work linked PI sensitivity to protein synthesis and proteasome activity, raising the question whether different levels of proteasome expression and workload underlie PI sensitivity in MM cells (MMCs). Exploiting human MM lines characterized by differential PI sensitivity, we report that highly sensitive MMCs express lower proteasome levels and higher proteasomal workload than relatively PI-resistant MMCs, resulting in the accumulation of polyubiquitinated proteins at the expense of free ubiquitin (proteasome stress). Manipulating proteasome expression or workload alters apoptotic sensitivity to PI, demonstrating a cause-effect relationship between proteasome stress and apoptotic responses in MMCs. Intracellular immunostaining in primary, patient-derived MMCs reveals that polyubiquitinated proteins hallmark neoplastic plasma cells, in positive correlation with immunoglobulin (Ig) content, both intra- and interpatient. Moreover, overall proteasome activity of primary MMCs inversely correlates with apoptotic sensitivity to PI. Altogether, our data indicate that the balance between proteasome workload and degradative capacity represents a critical determinant of apoptotic sensitivity of MMCs to PI, potentially providing a framework for identifying indicators of responsiveness and designing novel combination therapies.
Introduction
Multiple myeloma (MM) is a frequent and still incurable plasma cell malignancy, causing 2% of all cancer deaths. In recent years, treatment of MM has improved remarkably. For example, the proteasome inhibitor (PI) bortezomib (PS-341) proved effective even in the context of heavily pretreated, relapsed, and refractory MM,1-3 although more than 50% of patients fail to respond to second-line treatment.4 The molecular bases of different individual responsiveness to bortezomib remain unclear. Age (< 65 years) and extent of bone marrow plasma cell infiltration (< 50%) are the conventional factors for successful treatment identified so far.5-7 Identifying the molecular bases underlying PI sensitivity would provide the framework for their improved clinical application
Bortezomib targets the proteasome, a 2.4-MDa multicatalytic protease complex ubiquitously expressed in eukaryotic cells.1,8 Crucial for degrading proteins involved in cell cycle, angiogenesis, adhesion, cytokine production, and apoptosis,3,9,10 proteasome inhibition can affect tumor cell growth via direct and indirect mechanisms (eg, by blocking interactions with endothelial and bone cells).8,11 Proteasomes also dismantle damaged and misfolded/unfolded proteins, which are potentially harmful for the cell.8 As a result, proteasome impairment causes buildup of polyubiquitinated proteins and eventual cell death.3 Proteasomes also degrade a significant proportion of newly synthesized proteins in mammalian cells (rapidly degraded polypeptides [RDPs]).12 Thus, increased protein synthesis or other metabolic unbalances could increase proteasome workload.
We recently showed that plasma cell differentiation in vitro, ex vivo, and in vivo entails a dramatic decrease in proteasome expression and activity, correlating with increased sensitivity to PIs.13,14 Indeed, PIs reduce antibody (Ab) responses in vivo.14,15 Moreover, inducible expression of orphan Ig-μ chains sensitizes nonlymphoid tumor cells to PI-induced toxicity.13 In MM cells (MMCs), the levels of both Ig synthesis and retention correlate with apoptotic sensitivity to PIs, and manipulating Ig synthesis alters sensitivity.16,17 Altogether, these data suggest that the exquisite sensitivity of certain MMCs to PIs could stem from decreased proteasomal capacity, increased proteasomal workload, or both (ie, an adverse load-versus-capacity ratio).
In this study, we exploited MM lines with differential apoptotic sensitivity to PIs to address if proteasome expression and degradative workload vary among different clones, and defined their role in determining apoptotic sensitivity to PIs. Moreover, using primary patient-derived MMCs, we revealed correlations between proteasome stress and PI sensitivity. By establishing a causal relationship between proteasome stress and PI sensitivity in MM, our data may provide a framework for testing novel pharmacologic synergies aimed at exacerbating proteotoxicity, and for identifying prognostic indicators to predict responsiveness to bortezomib in patients with MM.
Methods
Cell cultures
Patient-derived MMCs were purified from bone marrow aspirates by CD138 immunomagnetic positive selection (Miltenyi Biotec, Bergisch Gladbach, Germany) after informed consent was obtained in accordance with the Declaration of Helsinki. Approval for use of primary samples was obtained from the Institutional Review Board of the Scientific Institute San Raffaele. Negative fractions were cultured for up to 4 weeks to purify stromal cells, as described.18 The mouse B lymphoma cell line I.29μ+, human MM lines (U266, RPMI8226, KMS.18 and MM.1S), and primary MMCs were cultured in RPMI media supplemented with 10% fetal calf serum (FCS), glutamax (1 mM), penicillin (100 U/mL), and streptomycin (100 μg/mL). HeLa cells were cultured in Dulbecco modified Eagle medium (DMEM) supplemented as RPMI.
Flow cytometric analyses of apoptosis
Cells were treated and harvested as indicated, stained with FITC-conjugated annexin V and propidium iodide as per manufacturer's instructions, and analyzed by FACScalibur (BD Biosciences, San Jose, CA). Patient-derived MMCs were seeded at 30 000 cells/well and treated for 24 hours as indicated. High-sensitivity assessment of apoptosis was obtained with LSRII (BD Biosciences).
Pulse-chase assays for intracellular protein degradation
I.29μ+ and MMCs were incubated for 15 minutes in starvation medium (2% FCS, methionine and cysteine-free) at 37°C, then pulse-labeled with 1.85 MBq/mL (50 μCi/mL) Pro-mix [35S] (Amersham Biosciences; now GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom) for the indicated time, washed twice in phosphate-buffered saline (PBS), resuspended, and chased in complete media containing 2.5 mM cold methionine with or without PI, with triplicates (106 cells each) per time point. After the chase, supernatants were recovered and cells were lysed in RiPA buffer. Lysates and supernatants from 10 000 cells were precipitated with 25% TCA. After 30 minutes on ice, samples were collected on glass fiber filters and analyzed in a liquid scintillation counter (Canberra Packard, Zurich, Switzerland).
RT-PCR analyses
Total cellular RNA was extracted with Trizol and reverse-transcribed with Superscript II reverse transcriptase (RT; Invitrogen, Carlsbad, CA). Splicing of XBP-1 mRNA was analyzed by polymerase chain reaction (PCR) as described,19 with primers flanking the 26b intron (5′-GGAGTTAAGACAGCGCTTGG; 5′-ACTGGGTCCAAGTTGTCCAG). PCR products from the spliced (s) and unspliced (u) XBP-1 mRNAs were resolved by electrophoresis on a 2.5% agarose gel and visualized by ethidium bromide. Levels of HSP70i were quantitated by real-time PCR (SybrGreen I; Roche, Indianapolis, IN) with the following primers: 5′-CAGGTGATCAACGACGGAGACA; 5′-GTCGATCGTCAGGATGGACACG, upon normalization by histone H3 (5′-GTGAAGAAACCTCATCGTTACAGGCCTGGT; and 5′-CTGCAAAGCACCAATAGCTGCACTCTGGAA).
Proteasome activity assays
Proteasome activity was assessed in MMC extracts using fluorogenic peptides as described.13,20-22 Briefly, cells were sonicated in ice-cold buffer (50 mM Tris/HCl [pH 7.5], 1 mM DTT, 0.25 M sucrose, 5 mM MgCl2, 0.5 mM EDTA, and 2 mM ATP), and extracts were prepared by centrifugation for 30 minutes at 10 000g and 15 minutes at 100 000g. Proteasome-specific peptidase activities were assayed by monitoring the production of 7-amino-4-methylcoumarin (amc) from the following fluorogenic peptides (Bachem, Bubendorf, Switzerland): 100 μM Suc-LLVY-amc (for chymotrypsin-like), 500 μM Bz-VGR-amc (for trypsin-like), and 100 μM Ac-YVAD-amc (for caspase-like activity) in 20 mM Tris-HCl (pH 7.5), 1 mM ATP, 2 mM MgCl2, and 0.2% bovine serum albumin (BSA). Reactions were started by adding an aliquot of cellular extract, and the fluorescence of released amc (excitation, 380 nm; emission, 460 nm) was monitored continuously at 37°C with a Carry Eclipse spectrofluorometer (Varian, Palo Alto, CA). Background activity (caused by nonproteasomal degradation) was determined by addition of the PI MG132 (for chymotryptic and caspase-like activities) and β-lactone (for trypsin-like activity) at a final concentration of 10 μM and 20 μM, respectively. Assays were calibrated using standard solutions of free fluorophores, and the reaction velocities were calculated from the slopes of the initial linear portions of the curves. Substrate consumption at the end of incubation never exceeded 1%.
Immunoblot analyses
Immunoblot analyses of proteasomal subunits and proteasome-related factors were performed upon cell lysis in RiPA buffer as described.23 To detect ubiquitin (Ub) and Ub-conjugates, cells were lysed in 150 mM NaCl, 1% NP-40, and 50 mM Tris HCl (pH 7.5). Extracts were resolved by electrophoresis, blotted, and probed with the following primary Abs: rabbit antisera against LMP2 (a kind gift of Dr K. Tanaka, Tokyo Metropolitan Institute of Medical Science, Tokyo, Japan); LMP7, MECL1, α3, α4, α5, α6 (BIOMOL International, Exeter, United Kingdom); X and Y (Boston Biochem, Cambridge, MA); mouse monoclonal against Z (a kind gift of Prof A. L. Goldberg, Harvard Medical School, Boston, MA); and Ub (sc-8017; Santa Cruz Biotechnology, Santa Cruz, CA). Densitometric analysis of bands was performed with a VersaDoc 1000 Imaging System (Bio-Rad, Hercules, CA).
Immunofluorescence
MMCs were seeded on poly-L-lysine–coated slides, fixed with 3.7% formaldehyde, and permeabilized with PBS 0.1% Triton X100. Cells were stained with monoclonal Abs against Ub (Fk2), LMP2 (LMP2/13), LMP7 (LMP7-1; BIOMOL International), or rabbit anti-κ or anti-λ antisera (Dako, Glostrup, Denmark), rinsed in PBS and stained with Alexa Fluor 488 goat anti–mouse IgG or Alexa Fluor 546 goat anti–rabbit IgG and Hoechst 33342 (Molecular Probes, Eugene, OR). Coverslips were observed on a DeltaVision workstation (Applied Precision, Issaquah, WA) with an Olympus IX70 camera (Center Valley, PA). Numerical apertures were 1.4 (100× lens) and 0.5 (20× lens). Higher magnification images of primary MMCs were deconvoluted with SoftWorx 3.5.0 (Applied Precision). Fk2 fluorescence in MMCs was quantified with the IN Cell Investigator software (GE Healthcare, Piscataway, NJ).
Generation of UbG76V-GFP–expressing MM lines
MM.1S and U266 cells were transduced using a lentiviral vector previously generated by us13 (1 μg/mL) encoding green fluorescent protein (GFP) destabilized by fusion to a mutated uncleavable ubiquitin moiety (UbG76V-GFP).24 As revealed by PI treatment and microscopy, the proportion of cells stably expressing the reporter was 100% and 60% in MM.1S and U266, respectively. Stable U266 expressants were sorted upon reversible proteasomal inhibition (FACSCalibur; BD Biosciences).
Statistic analyses
To compare average measures of proteasome activity and apoptosis, we adopted a 2-tailed Student t test. For quantified immunofluorescent signals, an intensity threshold (5000) was set to discriminate clonal plasma cells as Ig light chain (Ig-L) expressants, based on Ig-L intensity distribution. Pearson correlation coefficients (r) were calculated with Prism version 5.0 (GraphPad Software, La Jolla, CA) to study the correlation between Ig-L and Fk2 signals intra- and interpatient.
Results
Decreased proteasome expression in differentiating B lymphocytes is associated with increased proteasomal workload and higher apoptotic sensitivity to PI
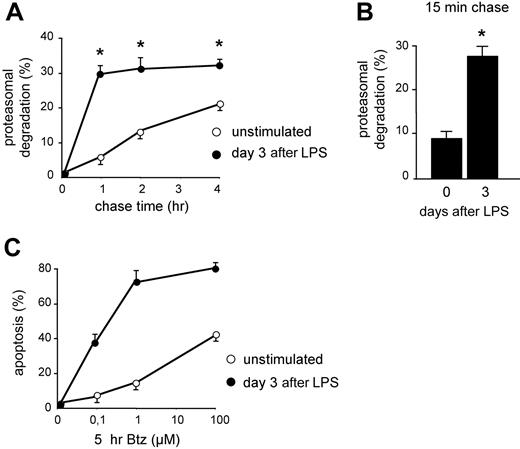
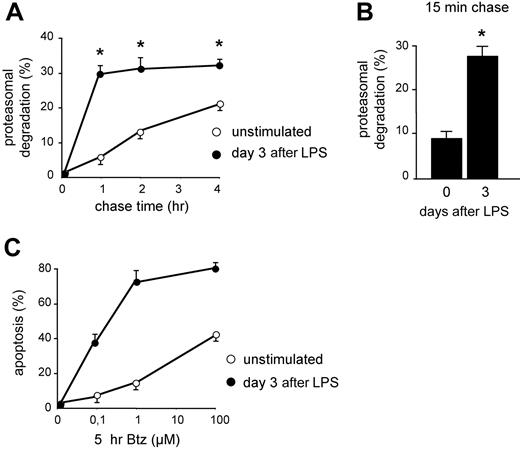
In previous studies, we have shown that B cells acquire apoptotic sensitivity to PI when stimulated to differentiate into Ig-secreting cells both in vitro and in vivo, correlating with reduced expression of functional proteasomes and reduced overall proteasome activity.13,14 To gain insight into the actual usage of the remaining proteasomes, we measured the disappearance of TCA-insoluble newly made proteins that could be inhibited by PI in LPS-stimulated I.29μ+ cells by pulse-chase assays. Whereas degradation in controls proceeded linearly for 4 hours of chase, LPS stimulation increased significantly the fraction of proteins degraded by proteasomes within 1 hour of chase, approaching 30% at day 3 (Figure 1A), on exit from the cell cycle and acquisition of an Ig-synthesizing phenotype.13 Therefore, the amount of proteins undergoing proteasomal degradation soon after synthesis was approximately 6 times higher in LPS-stimulated cells. This dramatic change could reflect the quantitative and qualitative proteomic and metabolic changes linked to B-cell differentiation.25 Whatever their origin,12 these RDPs represent an increased load for the diminishing pool of proteasomes. In line with the stabilization of certain proteasome substrates (eg, XBP-1, IkBα, and Bax) in differentiating I.29μ+ cells,13 after the initial phase of rapid degradation, the loss of TCA-insoluble radioactivity was slower in activated cells (Figure 1A), probably reflecting reduced availability of proteasomes, fewer and actively degrading RDPs.13 The enhanced synthesis of longer-lived proteins, such as endoplasmic reticulum (ER) chaperones, and the possible reuse of radioactive amino acids generated by degradation could contribute to a slower second-phase degradation in LPS-activated cells. Shorter chase times revealed that the first phase in LPS-activated cells was essentially completed in the first 15 minutes of chase, with 27.5% of RDPs already degraded (Figure 1B). These data demonstrate that Ig-synthesizing cells display higher proteasome-mediated degradation of short-lived polypeptides, imposing a higher workload on decreased proteasome levels.13 This correlated with increased apoptotic sensitivity to bortezomib (Figure 1C) and other PIs,13 supporting a causative role for an unfavorable proteasomal workload-versus-capacity ratio in predisposing to PI toxicity.
Increased proteasomal workload in differentiating B lymphocytes is associated with higher apoptotic sensitivity to PIs. (A) Increased rapid protein degradation in LPS-stimulated B cells. I.29μ+ cells stimulated with LPS for 0 or 3 days were pulsed for 30 minutes with 35S aminoacids and chased for the indicated times, with or without MG-132 (2 μM). The data indicate the percentage of TCA-insoluble radioactivity, the disappearance of which was inhibited by MG-132 at any given timepoint, relative to the total radioactivity present at the end of the pulse (*P < .05). (B) Increased RDPs in LPS-stimulated B cells. I.29μ+ cells cultured for 0 or 3 days with LPS were pulsed for 10 minutes and chased for 15 minutes, with or without MG-132. The bars show the percentage of radioactive polypeptides degraded by proteasomes during the chase (*P < .05). (C) Increased apoptotic sensitivity in LPS-stimulated I.29μ+ cells. Cells were stimulated for 3 days with LPS or left untreated, and then treated for 5 hours with the indicated concentrations of bortezomib (Btz). The proportion of apoptotic (annexin V+ propidium iodide−) cells was measured by FACS analysis. One of 3 representative experiments is shown.
Increased proteasomal workload in differentiating B lymphocytes is associated with higher apoptotic sensitivity to PIs. (A) Increased rapid protein degradation in LPS-stimulated B cells. I.29μ+ cells stimulated with LPS for 0 or 3 days were pulsed for 30 minutes with 35S aminoacids and chased for the indicated times, with or without MG-132 (2 μM). The data indicate the percentage of TCA-insoluble radioactivity, the disappearance of which was inhibited by MG-132 at any given timepoint, relative to the total radioactivity present at the end of the pulse (*P < .05). (B) Increased RDPs in LPS-stimulated B cells. I.29μ+ cells cultured for 0 or 3 days with LPS were pulsed for 10 minutes and chased for 15 minutes, with or without MG-132. The bars show the percentage of radioactive polypeptides degraded by proteasomes during the chase (*P < .05). (C) Increased apoptotic sensitivity in LPS-stimulated I.29μ+ cells. Cells were stimulated for 3 days with LPS or left untreated, and then treated for 5 hours with the indicated concentrations of bortezomib (Btz). The proportion of apoptotic (annexin V+ propidium iodide−) cells was measured by FACS analysis. One of 3 representative experiments is shown.
Decreased proteasome activity in PI-sensitive MMCs
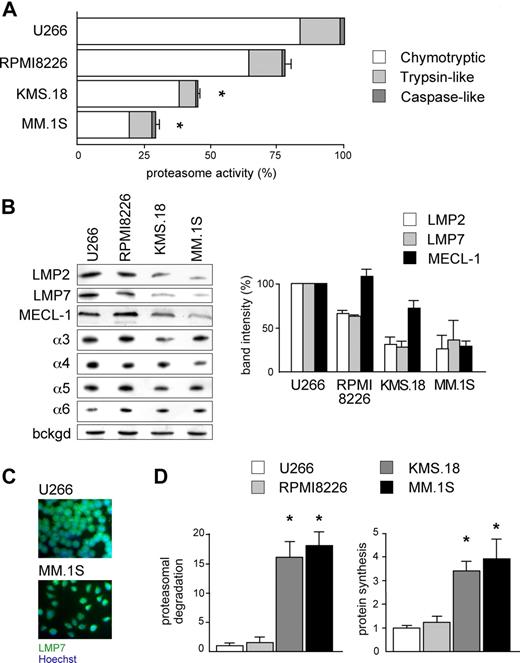
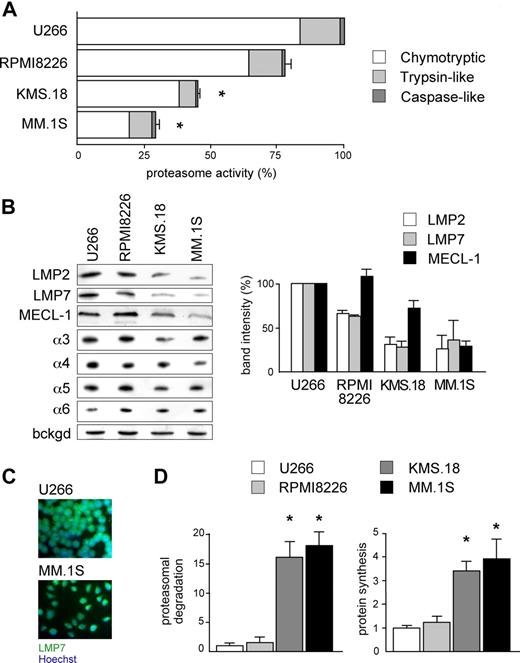
According to the load-versus-capacity model, cells equipped with fewer proteasomes, higher workload, or both should be more vulnerable to the toxic effects of bortezomib. Given the exquisite sensitivity of MMCs to bortezomib and the importance of this drug in MM therapy, we decided to challenge our model in MMCs. When exposed to bortezomib for 48 hours, the MM cell lines KMS.18 and MM.1S proved approximately 10 times more sensitive than U266 and RPMI8226, with the following EC50 values: approximately 4 nM for MM.1S cells, approximately 6 nM for KMS.18 cells, approximately 46 nM for RPMI8226 cells, and approximately 55 nM for U266 cells (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). When challenged with other PIs, similar differences were observed, with MM.1S cells showing 5 and 10 times higher sensitivity than RPMI8226 or U266 to epoxomicin and MG-132, respectively. Next, to determine overall proteasome capacity, we assessed 26S-specific peptidase activities in cellular extracts both per protein content (Figure 2A) and per cell (data not shown). In line with other reports on lymphoid cells,26 the chymotryptic activity accounted for 80%, and the caspase-like activity accounted for only 2% of all proteasome-specific activity. Whereas RPMI8226 had similar chymotryptic activity (79%), the highly sensitive lines KMS.18 and MM.1S displayed significantly lower chymotryptic activity than U266 (44.2% and 28%, respectively). Similarly, the trypsin-like activity of RPMI8226 cells was close (83%) to that of U266 cells, whereas it was significantly lower in KMS.18 and MM.1S cells (43% and 57% of U266, respectively). Finally, the minor caspase-like activity was also significantly lower than U266 in KMS.18 (44%) and slightly but consistently lower in MM.1S cells (90%; Figure 2A).
Reduced proteasome activity and increased workload in PI-sensitive MMCs. (A) Proteasome activity in the relatively sensitive KMS.18 and MM.1S and the relatively resistant U266 and RPMI8226 lines. Proteasome-specific chymotryptic, trypsin-like, and caspase-like activities were assessed in cell extracts and expressed on a per-protein basis. The histogram shows the relative quantification of all 3 activities within each line, and levels relative to the corresponding activity in U266 (*P < .001). The average of at least 4 independent experiments (± SD) is shown. (B) Proteasome β-catalytic and α subunits in MM lines. Extracts from U266, RPMI8226, KMS.18, and MM.1S cells were resolved by SDS-PAGE and blotted with Abs to different proteasome catalytic β and noncatalytic α subunits (1 of 3 representative experiments). Equal protein amounts were loaded in each lane, with a background stable band serving as a loading control (bckgd). The right panel shows the relative densitometric quantification of catalytic β subunits in 3 independent experiments (average ± SD), on correction by the band intensity of β-actin in the corresponding blot. (C) Immunofluorescence against LMP7 in U266 and MM.1S cells reveals higher immunoproteasome levels in the relatively less sensitive U266 cells. (D) Higher proteasome workload and protein synthesis in PI-sensitive MMC. U266, RPMI8226, KMS.18, and MM.1S cells were pulsed for 5 minutes with 35S amino acids and chased for 30 minutes, with or without PIs (lactacystin, bortezomib, and epoxomicin; 2 μM each). Proteasome-mediated degradation of newly synthesized proteins was calculated as the percentage of TCA-insoluble radioactivity, the disappearance of which during the chase was inhibited by PIs, relative to the total radioactivity present at the end of the pulse, as in Figure 1. The left panel shows proteasomal degradation in all MM lines, while the right panel quantifies the proteosynthetic activity of each line as the incorporation of hot amino acids into TCA-insoluble polypeptides at the end of the pulse. An average of 3 independent experiments (± SD) is shown. *P < .001.
Reduced proteasome activity and increased workload in PI-sensitive MMCs. (A) Proteasome activity in the relatively sensitive KMS.18 and MM.1S and the relatively resistant U266 and RPMI8226 lines. Proteasome-specific chymotryptic, trypsin-like, and caspase-like activities were assessed in cell extracts and expressed on a per-protein basis. The histogram shows the relative quantification of all 3 activities within each line, and levels relative to the corresponding activity in U266 (*P < .001). The average of at least 4 independent experiments (± SD) is shown. (B) Proteasome β-catalytic and α subunits in MM lines. Extracts from U266, RPMI8226, KMS.18, and MM.1S cells were resolved by SDS-PAGE and blotted with Abs to different proteasome catalytic β and noncatalytic α subunits (1 of 3 representative experiments). Equal protein amounts were loaded in each lane, with a background stable band serving as a loading control (bckgd). The right panel shows the relative densitometric quantification of catalytic β subunits in 3 independent experiments (average ± SD), on correction by the band intensity of β-actin in the corresponding blot. (C) Immunofluorescence against LMP7 in U266 and MM.1S cells reveals higher immunoproteasome levels in the relatively less sensitive U266 cells. (D) Higher proteasome workload and protein synthesis in PI-sensitive MMC. U266, RPMI8226, KMS.18, and MM.1S cells were pulsed for 5 minutes with 35S amino acids and chased for 30 minutes, with or without PIs (lactacystin, bortezomib, and epoxomicin; 2 μM each). Proteasome-mediated degradation of newly synthesized proteins was calculated as the percentage of TCA-insoluble radioactivity, the disappearance of which during the chase was inhibited by PIs, relative to the total radioactivity present at the end of the pulse, as in Figure 1. The left panel shows proteasomal degradation in all MM lines, while the right panel quantifies the proteosynthetic activity of each line as the incorporation of hot amino acids into TCA-insoluble polypeptides at the end of the pulse. An average of 3 independent experiments (± SD) is shown. *P < .001.
To confirm that MM.1S and KMS.18 cells are endowed with lower proteasome pools, we measured the levels of catalytic β-subunits by immunoblotting. Being subjected to autocatalytic cleavage upon assembly to generate the active form, the steady-state levels of mature β-subunits provide a measurement of the actual proteasome capacity.27 Immunoproteasomes are believed to represent the majority of cellular proteasomes and the main extralysosomal proteolytic system in lymphoid organs, in particular in the B-cell lineage.23,28,29 Indeed, while the steady-state levels of constitutive catalytic subunits do not change significantly between the lines (data not shown), we found that all 3 immunocatalytic subunits (LMP2, LMP7, and MECL-1) are significantly lower in MM.1S and KMS.18 cells than RPMI8226 and U266 cells (Figure 2B). Therefore, MM.1S and KMS.18 cells, the most PI-sensitive lines, express a smaller proteasome complement, accounting for reduced overall proteasome activity. Conversely, the steady-state levels of α subunits, which do not report on assembled, functional proteasomes,27 were similar in the 4 MM lines (Figure 2B). Immunofluorescence for LMP2 (not shown) or LMP7 confirmed higher general levels of immunoproteasomes in U266 compared with MM.1S cells (Figure 2C). Altogether, these findings reveal that different myelomas may be equipped with different levels of assembled, functional proteasomes, indicating a potential molecular mechanism contributing to variable susceptibility to PIs.
Increased proteasomal workload in PI-sensitive MMCs
We next asked whether MM lines also differ in the actual use of the proteasomal degradative route. In several independent pulse-chase assays, the amount of proteasomal degradation of newly synthesized proteins was significantly higher in KMS.18 and MM.1S than RPMI8226 or U266 cells (Figure 2D left panel). Moreover, in a 5 minute pulse, KMS.18 and MM.1S incorporated 3 to 4 times more radioactivity than RPMI8226 or U266 cells into TCA-insoluble polypeptides, indicative of higher protein synthesis (Figure 2D right panel), but secreted approximately half as much as U266 cells in a 30 minute chase (data not shown). The observation that PI-sensitive MMCs also rely more on proteasomal degradation suggests that proteasomal workload may contribute to determine the intrinsic sensitivity of a neoplastic clone to PI.
Critical proteasome stress in PI-sensitive MMCs
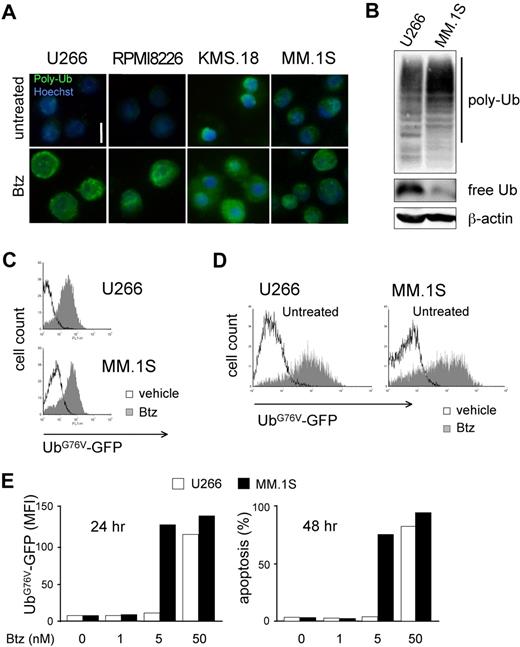
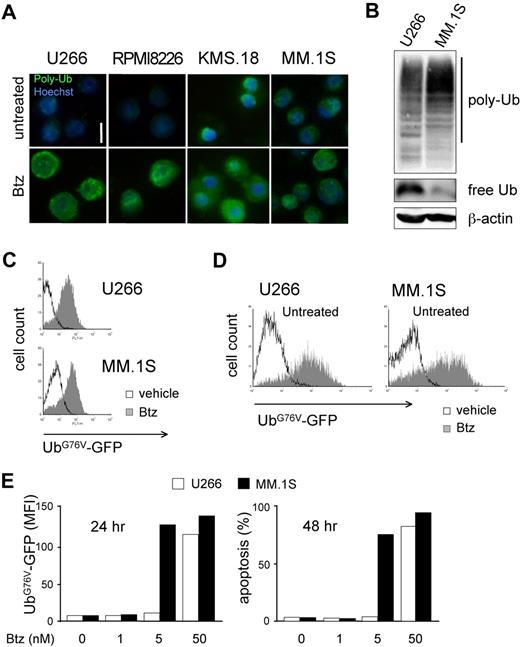
The combination of lower proteasome expression and higher workload in PI-sensitive MMCs led us to predict profound biologic differences between differentially sensitive MM lines. Thus, to address if PI-sensitive MMCs suffer from proteasome stress, we assessed accumulation of polyubiquitinated proteins. Immunofluorescent staining revealed the presence of polyubiquitinated proteins in basal conditions in KMS.18 and MM.1S cells with a discrete cytosolic pattern, exceeding the signal present in U266 or RPMI8226 cells (Figure 3A). Immunoblot analyses confirmed higher polyubiquitinated proteins in MM.1S compared with U266 cells, with parallel lower levels of free ubiquitin (Figure 3B). A 24-hour treatment with increasing doses of bortezomib causes further accumulation of polyubiquitinated proteins in all lines, reaching comparable levels at the EC50 dose calculated at 48 hours (Figure 3A bottom panels). To test if a critical level of proteasome overload precedes death, we exploited an unstable GFP fused to a mutated, uncleavable ubiquitin moiety driving polyubiquitination and rapid proteasome-mediated degradation (UbG76V-GFP), an established reporter of proteasome overload.13,30 By lentiviral transduction, we engineered MM lines to stably express UbG76V-GFP. Despite differential basal efficiency of the ubiquitin proteasome system in U266 and MM.1S cells (Figure 3A,B), basal UbG76V-GFP was comparable in the 2 cell lines and accumulated on pharmacologic proteasome blockade, as assessed by fluorescence-activated cell sorter (FACS; Figure 3C). Importantly, PI sensitivity of engineered MM lines was comparable to that of wild-type cells, with MM.1S proving approximately 10 times more responsive than U266 cells both by GFP accumulation and in apoptosis assays (data not shown). Treatment of UbG76V-GFP–expressing U266 and MM.1S cells with increasing doses of bortezomib demonstrated a critical proteasomal overload on 24-hour treatment at doses that differed by a factor of 10 between the 2 lines, 24 hours before cell death (Figure 3D,E). Altogether, these data further confirm a lower efficiency of the ubiquitin-proteasome system in PI-vulnerable MMCs. As a result, critical proteasomal overload levels precede PI-induced death.
Differential sensitivity to proteasome stress in MMCs. (A) Basal and PI-induced accumulation of polyubiquitinated proteins in MMCs. Immunofluorescent staining of polyubiquitinated proteins in U266, RPMI8226, KMS.18, and MM.1S cells. Top panels show untreated cells. Bottom panels show cells treated with bortezomib (Btz) for 24 hours at the corresponding EC50 dose (calculated at 48 hours). One of 3 independent experiments is shown. Size bar equals 10 μm. (B) Accumulation of polyubiquitinated proteins and lower levels of free ubiquitin in PI-sensitive MMCs. Extracts from U266 and MM.1S cells were blotted with antiubiquitin (Ub). Polyubiquitinated proteins are detected as a smear in a 10% gel, while free Ub is detected in an 18% gel. β-actin serves as a loading control. (C) MM.1S and U266 were engineered to stably express UbG76V-GFP, an established in vivo reporter of proteasomal overload.13,23,29 FACS analysis of basal UbG76V-GFP expression and its accumulation upon 4 hours of treatment with Btz (1 μM for U266 and 100 nM for MM.1S). (D,E) UbG76V-GFP accumulation upon 24 hours of treatment reveals critical proteotoxicity levels. UbG76V-GFP–engineered U266 and MM.1S were treated with increasing doses of Btz for up to 48 hours. (D) FACS analysis of GFP expression reveals massive accumulation of GFP upon 24 hours of treatment in both MM.1S and U266 with 5 and 50 nM Btz, respectively. (E) Mean fluorescence intensity (MFI) at different doses reveals a critical accumulation of the reporter at 24 hours (left panel), 1 day before onset of apotosis (right panel).
Differential sensitivity to proteasome stress in MMCs. (A) Basal and PI-induced accumulation of polyubiquitinated proteins in MMCs. Immunofluorescent staining of polyubiquitinated proteins in U266, RPMI8226, KMS.18, and MM.1S cells. Top panels show untreated cells. Bottom panels show cells treated with bortezomib (Btz) for 24 hours at the corresponding EC50 dose (calculated at 48 hours). One of 3 independent experiments is shown. Size bar equals 10 μm. (B) Accumulation of polyubiquitinated proteins and lower levels of free ubiquitin in PI-sensitive MMCs. Extracts from U266 and MM.1S cells were blotted with antiubiquitin (Ub). Polyubiquitinated proteins are detected as a smear in a 10% gel, while free Ub is detected in an 18% gel. β-actin serves as a loading control. (C) MM.1S and U266 were engineered to stably express UbG76V-GFP, an established in vivo reporter of proteasomal overload.13,23,29 FACS analysis of basal UbG76V-GFP expression and its accumulation upon 4 hours of treatment with Btz (1 μM for U266 and 100 nM for MM.1S). (D,E) UbG76V-GFP accumulation upon 24 hours of treatment reveals critical proteotoxicity levels. UbG76V-GFP–engineered U266 and MM.1S were treated with increasing doses of Btz for up to 48 hours. (D) FACS analysis of GFP expression reveals massive accumulation of GFP upon 24 hours of treatment in both MM.1S and U266 with 5 and 50 nM Btz, respectively. (E) Mean fluorescence intensity (MFI) at different doses reveals a critical accumulation of the reporter at 24 hours (left panel), 1 day before onset of apotosis (right panel).
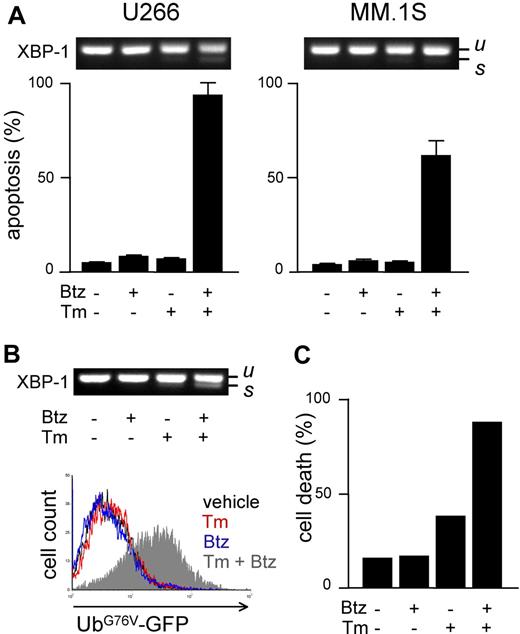
Increasing workload through ER stressors sensitizes MMCs to PIs
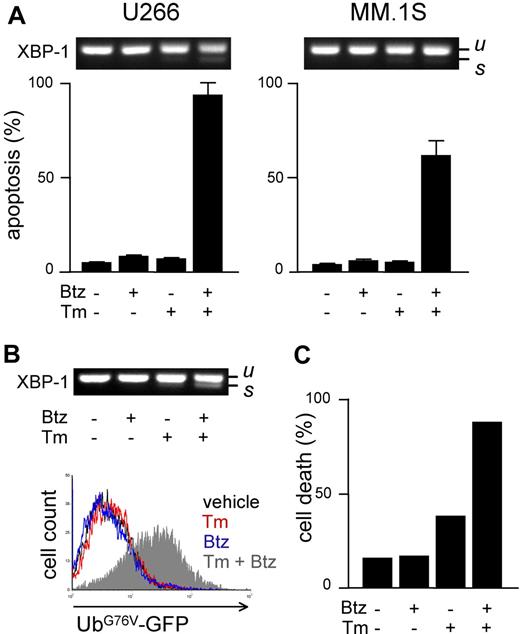
We next aimed at determining whether proteasome activity and workload are causally linked to apoptotic sensitivity to PIs in MMCs. To this aim, we manipulated either parameter before measuring PI sensitivity. To increase proteasome load, we used drugs known to induce protein misfolding in the ER (eg, the N-glycosylation inhibitor tunicamycin [Tm]) and to activate the unfolded protein response (UPR).31 To establish a solid assay, we selected a low dose of Tm (2.5 μg/mL) capable of inducing a UPR, as indicated by modest XBP-1 splicing after 24 hours of treatment (Figure 4A top panels), followed by negligible toxicity, as indicated by FACS analysis of apoptosis, at 48 hours (Figure 4A bottom panels). In combination with low, nontoxic doses of bortezomib, massive apoptosis ensued in both cell lines (Figure 4A), revealing strong synergism. Similar results were obtained with different PIs (MG-132 and epoxomicin) and ER stressors (thapsigargin and brefeldin A; data not shown). To establish if the capacity of ER stressors to sensitize MM lines to PIs is due to enhanced proteasome workload, we used UbG76V-GFP–engineered MM lines. In engineered U266 cells, Tm caused a modest UPR, which was greatly enhanced by addition of bortezomib (Figure 4B top panel). Importantly, combination of the 2 drugs caused proteasomal overload in striking synergism in 24 hours (Figure 4B bottom panel), followed by extensive cell death at 48 hours (Figure 4C). Similar results were obtained in engineered MM.1S cells (data not shown). Taken together, these findings suggest that proteasome workload is causally involved in determining PI sensitivity, possibly underlying the powerful synergy between PIs and ER stressors against MMCs.
Increasing proteasome workload through ER stress sensitizes MMCs to PIs. Pharmacologic ER stressors increase proteasome workload and PI-induced cytotoxicity in MMCs. (A) FACS analysis of apoptosis upon treatment with tunicamycin (Tm; 2.5 μg/mL) or bortezomib (Btz; 10 nM for U266 and 1 nM for MM.1S), alone or together for 48 hours. Top panels show the level of XBP-1 splicing after 24 hours (u and s for unspliced and spliced, respectively). (B) Twenty-four–hour treatment with Tm (2.5 μg/mL) and Btz (5 nM) synergistically causes ER stress (XBP-1 splicing; top panel) and accumulation of UbG76V-GFP (bottom overlay FACS histogram) in engineered U266 cells. (C) Forty-eight–hour treatment with Tm and Btz triggers synergistic death of engineered U266 cells. Cell death was assessed by modifications of physical parameters by FACS. One representative experiment is shown.
Increasing proteasome workload through ER stress sensitizes MMCs to PIs. Pharmacologic ER stressors increase proteasome workload and PI-induced cytotoxicity in MMCs. (A) FACS analysis of apoptosis upon treatment with tunicamycin (Tm; 2.5 μg/mL) or bortezomib (Btz; 10 nM for U266 and 1 nM for MM.1S), alone or together for 48 hours. Top panels show the level of XBP-1 splicing after 24 hours (u and s for unspliced and spliced, respectively). (B) Twenty-four–hour treatment with Tm (2.5 μg/mL) and Btz (5 nM) synergistically causes ER stress (XBP-1 splicing; top panel) and accumulation of UbG76V-GFP (bottom overlay FACS histogram) in engineered U266 cells. (C) Forty-eight–hour treatment with Tm and Btz triggers synergistic death of engineered U266 cells. Cell death was assessed by modifications of physical parameters by FACS. One representative experiment is shown.
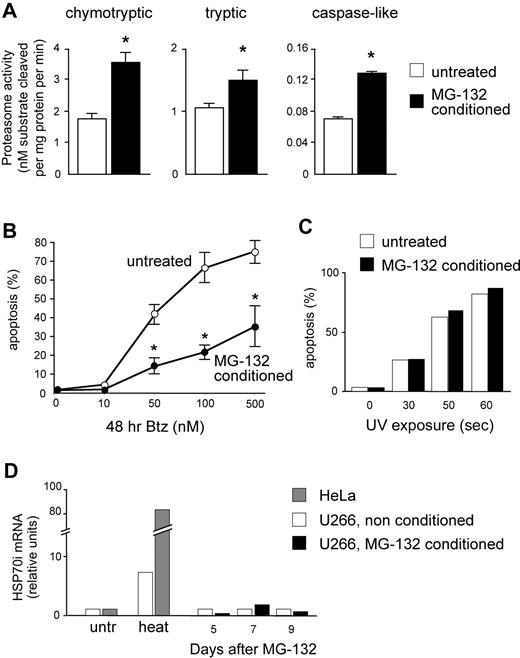
Raising proteasome capacity increases MMC resistance to PIs
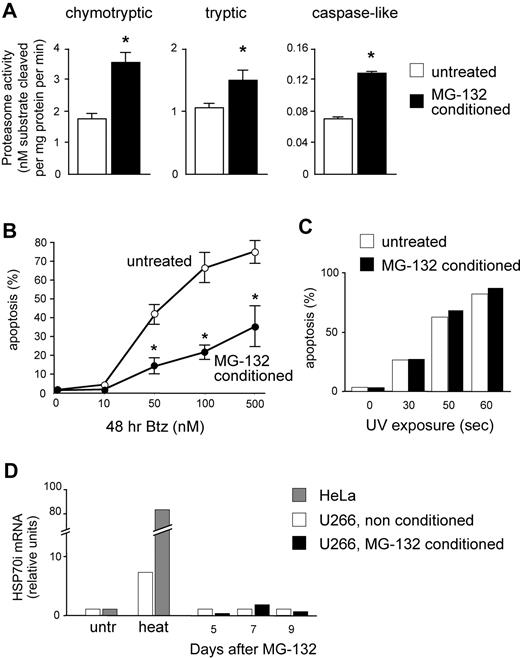
We then asked whether a causal link exists between proteasome activity and apoptotic sensitivity to PIs in MMCs. To this aim, we exploited the postulated capacity of mammalian cells to induce proteasome biogenesis in response to increasing proteolytic demands (proteasome stress response).32-34 Continuous proteasome inhibition was recently used to select PI-resistant cells expressing proteasome genes at higher levels in a Burkitt lymphoma cell line. This treatment took weeks and markedly induced apoptosis, thereby failing to establish a rigorous cause-effect relationship between PI sensitivity and proteasome activity because other detoxification mechanisms may be selected.35 We thus searched for low, nontoxic doses of reversible PIs capable of increasing proteasome expression in a shorter time, enabling us to avoid selection of resistant clones and better appraise the direct effect of increased proteasome biogenesis on the apoptotic sensitivity to bortezomib. Moreover, to counteract unlikely detoxification strategies, we used 2 distinct PIs of different classes to induce proteasome biogenesis (MG-132, a peptide aldehyde) and test PI sensitivity (bortezomib, a peptide boronate). U266 cells were treated with 1 nM MG-132 for 2 days, followed by 10 nM for 3 days, washed thoroughly, left untreated for 4 days, sampled for protein extracts, and assayed for apoptosis after 48 hours of treatment with increasing doses of bortezomib. Attesting to successful induction of proteasome biogenesis, MG-132–pretreated (conditioned) cells displayed significant increases of all 3 proteasome peptidase activities compared with vehicle-treated controls (Figure 5A). Importantly, conditioned MMCs proved remarkably more resistant to bortezomib than unconditioned controls both at 24 (data not shown) and 48 hours (Figure 5B), but not to UV-induced apoptosis (Figure 5C). Further attesting to specific protection via up-regulated proteasome expression, MG-132 treatment failed to induce a significant heat shock response (Figure 5D). The data strongly suggest that total proteasomal capacity contributes to determine PI susceptibility in MMCs.
Increasing proteasome expression specifically enhances resistance toward PIs. U266 cells were treated for 5 days with low, nontoxic doses of MG-132 (2 days at 1 nM followed by 3 days at 10 nM). Cells were then washed and cultured in fresh media for 4 more days, and then assayed for overall proteasome activity and apoptotic sensitivity to bortezomib. (A) Significant increases of proteasome-specific activities in MG-132–conditioned MMCs. Conditioning treatment resulted in doubled chymotryptic and caspase-like activities, and an approximately 50% increase in trypsin-like activity. *P < .05. (B) Conditioned cells show enhanced PI resistance. MG-132–conditioned and vehicle-treated cells were exposed to the indicated doses of bortezomib (Btz) for 48 hours, and apoptosis was assessed by FACS as the proportion of annexin V+ propidium iodide− cells. The line graph averages (± SD) 2 representative experiments. P < .05. (C) Conditioned cells are not protected from UV-induced apoptosis. MG-132–conditioned and vehicle-treated cells were exposed to UV rays for the indicated times, and apoptosis assessed after 24 hours as in panel B. One representative experiment is shown. (D) MG-132 conditioning treatment fails to induce a detectable heat shock response. MG-132–conditioned and vehicle-treated cells were sampled at the indicated days from the beginning of the treatment, and mRNA levels for inducible HSP70 was quantitated by real-time RT-PCR. A total of 1 hour of exposure to 43°C (heat) significantly increased HSP70i mRNA in U266 and HeLa cells. One representative experiment is shown.
Increasing proteasome expression specifically enhances resistance toward PIs. U266 cells were treated for 5 days with low, nontoxic doses of MG-132 (2 days at 1 nM followed by 3 days at 10 nM). Cells were then washed and cultured in fresh media for 4 more days, and then assayed for overall proteasome activity and apoptotic sensitivity to bortezomib. (A) Significant increases of proteasome-specific activities in MG-132–conditioned MMCs. Conditioning treatment resulted in doubled chymotryptic and caspase-like activities, and an approximately 50% increase in trypsin-like activity. *P < .05. (B) Conditioned cells show enhanced PI resistance. MG-132–conditioned and vehicle-treated cells were exposed to the indicated doses of bortezomib (Btz) for 48 hours, and apoptosis was assessed by FACS as the proportion of annexin V+ propidium iodide− cells. The line graph averages (± SD) 2 representative experiments. P < .05. (C) Conditioned cells are not protected from UV-induced apoptosis. MG-132–conditioned and vehicle-treated cells were exposed to UV rays for the indicated times, and apoptosis assessed after 24 hours as in panel B. One representative experiment is shown. (D) MG-132 conditioning treatment fails to induce a detectable heat shock response. MG-132–conditioned and vehicle-treated cells were sampled at the indicated days from the beginning of the treatment, and mRNA levels for inducible HSP70 was quantitated by real-time RT-PCR. A total of 1 hour of exposure to 43°C (heat) significantly increased HSP70i mRNA in U266 and HeLa cells. One representative experiment is shown.
Proteasome stress and PI sensitivity in patient-derived MMCs
The findings that proteasome workload and capacity contribute to determine the apoptotic sensitivity to PIs in MM lines prompted us to challenge our model in primary myelomas. We thus used MMCs from newly diagnosed patients to investigate the relationship ex vivo between Ig synthesis, proteasome stress, and PI sensitivity. We purified MMCs from bone marrow aspirates and combined immunofluorescence, FACS, and enzymatic assays with specific fluorogenic peptides13 to measure accumulation of polyubiquitinated proteins, Ig content, apoptotic sensitivity to bortezomib and proteasome activity. MMCs display spontaneous accumulation of polyubiquitinated proteins, while CD138− cells accumulate polyubiquitinated proteins only upon treatment with PIs (Figure 6A), and prove much more resistant (up to 300 times) to PI-induced apoptosis (not shown). Although variable in intensity, the presence of polyubiquitinated proteins coincides with plasma cell identity (certified by Ig expression; Figure 6A,B). Moreover, the fluorescence intensity associated to polyubiquitinated proteins positively correlates with that of Ig-L within the MMC population of each patient analyzed (Figure 6C), and among different patients (Figure 6D), suggesting a role for Ig synthesis and/or retention in determining proteasome workload.
Proteasome stress predicts sensitivity of primary MMCs to bortezomib. Primary, patient-derived MMCs were selected by immunomagnetic purification from bone marrow biopsies and divided in 3 pools. The first pool was seeded onto polylysinated slides and assayed for accumulation of polyubiquitinated proteins and Ig light chain (Ig-L) expression by immunofluorescence. The second pool was plated in multiwell plates, challenged with increasing doses of bortezomib for 24 hours, and apoptotic responses assessed by highly sensitive FACS analysis (LSRII) on labeling with conjugated annexin V and propidium iodide. The third pool was assayed in vitro for proteasome-specific chymotryptic activity by means of a specific fluorogenic peptide, as in Figure 3. (A) Spontaneous accumulation of polyubiquitinated proteins in primary MMCs. While in the CD138− fraction polyubiquitinated proteins accumulate only upon treatment with PIs (16 hours with 100 nM bortezomib [Btz] in the bottom left panel), CD138+ MMCs (hallmarked by Ig-L expression) show Fk2+ fluorescence in basal conditions (2 representative cases shown). Images were deconvoluted with DeltaVision and SoftWorx (see “Methods”); single z sections are shown. Size bars equal 5 μm. (B) Polyubiquitinated proteins are highly specific of Ig-L+ MMCs. Automated quantification of fluorescence in Ig-L+ and Ig-L− nucleated cells was performed using the IN Cell Investigator software. The box plot shows the average fluorescence intensity (FI), with 2.5 top and bottom percentiles; *P < .001. (C) Intrapatient positive correlation between polyubiquitinated accumulation and Ig-L content. Each dot corresponds to a single MM cell, and each plot corresponds to 1 patient. Scatters from 3 representative patients are shown. r > 0.6; P < .001. (D) Positive correlation of polyubiquitinated protein accumulation and Ig-L content among different patients. MFI indicates mean fluorescence intensity. r = 0.95; P < .05. (E) Primary MM endowed with high proteasome capacity is intrinsically less responsive to Btz. In 4 primary samples we measured overall proteasome activity in cell lysates by means of a fluorogenic peptide specifically probing the 26S chymotryptic activity (LLVY-amc).13,14 Proteasome-specific chymotryptic activity is expressed as nanomoles probe cleaved per minute per milligram of protein. Dose-response curves were generated by 24-hour treatments with Btz. To determine the intrinsic sensitivity of each MM clone, EC50 values were calculated using nonlinear regression with Prism version 5.0 software (GraphPad Software). (F) A model of proteasome-related determinants of apoptotic sensitivity to PI. Relatively PI-resistant MMCs are equipped with high proteasome pools, both in absolute terms, and relative to degradative demands. As a result, few proteins await degradation, and high levels of free ubiquitin are available to target new proteasome substrates. In contrast, PI-sensitive MMCs are equipped with poor proteasome levels, despite high metabolic demands. As a result, huge amounts of polyubiquitinated proteins accumulate upstream of overloaded proteasomes, and little ubiquitin is available for proteins to be degraded. Although cells are healthy, stresses that further challenge the ubiquitin-proteasome system will unveil a lower apoptotic threshold.
Proteasome stress predicts sensitivity of primary MMCs to bortezomib. Primary, patient-derived MMCs were selected by immunomagnetic purification from bone marrow biopsies and divided in 3 pools. The first pool was seeded onto polylysinated slides and assayed for accumulation of polyubiquitinated proteins and Ig light chain (Ig-L) expression by immunofluorescence. The second pool was plated in multiwell plates, challenged with increasing doses of bortezomib for 24 hours, and apoptotic responses assessed by highly sensitive FACS analysis (LSRII) on labeling with conjugated annexin V and propidium iodide. The third pool was assayed in vitro for proteasome-specific chymotryptic activity by means of a specific fluorogenic peptide, as in Figure 3. (A) Spontaneous accumulation of polyubiquitinated proteins in primary MMCs. While in the CD138− fraction polyubiquitinated proteins accumulate only upon treatment with PIs (16 hours with 100 nM bortezomib [Btz] in the bottom left panel), CD138+ MMCs (hallmarked by Ig-L expression) show Fk2+ fluorescence in basal conditions (2 representative cases shown). Images were deconvoluted with DeltaVision and SoftWorx (see “Methods”); single z sections are shown. Size bars equal 5 μm. (B) Polyubiquitinated proteins are highly specific of Ig-L+ MMCs. Automated quantification of fluorescence in Ig-L+ and Ig-L− nucleated cells was performed using the IN Cell Investigator software. The box plot shows the average fluorescence intensity (FI), with 2.5 top and bottom percentiles; *P < .001. (C) Intrapatient positive correlation between polyubiquitinated accumulation and Ig-L content. Each dot corresponds to a single MM cell, and each plot corresponds to 1 patient. Scatters from 3 representative patients are shown. r > 0.6; P < .001. (D) Positive correlation of polyubiquitinated protein accumulation and Ig-L content among different patients. MFI indicates mean fluorescence intensity. r = 0.95; P < .05. (E) Primary MM endowed with high proteasome capacity is intrinsically less responsive to Btz. In 4 primary samples we measured overall proteasome activity in cell lysates by means of a fluorogenic peptide specifically probing the 26S chymotryptic activity (LLVY-amc).13,14 Proteasome-specific chymotryptic activity is expressed as nanomoles probe cleaved per minute per milligram of protein. Dose-response curves were generated by 24-hour treatments with Btz. To determine the intrinsic sensitivity of each MM clone, EC50 values were calculated using nonlinear regression with Prism version 5.0 software (GraphPad Software). (F) A model of proteasome-related determinants of apoptotic sensitivity to PI. Relatively PI-resistant MMCs are equipped with high proteasome pools, both in absolute terms, and relative to degradative demands. As a result, few proteins await degradation, and high levels of free ubiquitin are available to target new proteasome substrates. In contrast, PI-sensitive MMCs are equipped with poor proteasome levels, despite high metabolic demands. As a result, huge amounts of polyubiquitinated proteins accumulate upstream of overloaded proteasomes, and little ubiquitin is available for proteins to be degraded. Although cells are healthy, stresses that further challenge the ubiquitin-proteasome system will unveil a lower apoptotic threshold.
Finally, in vitro assessment of overall proteasome activity per total protein content revealed that, similar to MM lines, proteasome capacity varies among patients (ranging from ∼ 0.2 to ∼ 0.6 nM fluorogenic probe cleaved/mg total protein/min), as did the intrinsic apoptotic sensitivity to PI (the EC50 ranging from ∼ 2 to ∼ 20 nM bortezomib). Importantly, a direct correlation was evident between EC50 and proteasome capacity (Figure 6E), strongly suggesting that the size of the proteasome compartment also contributes to determine PI sensitivity in primary MM.
Altogether, these data indicate a strong relationship between Ig synthesis and accumulation of polyubiquitinated proteins, a hallmark of proteasome stress. Single-cell assays allowing for the measuring of proteasome capacity and proteotoxic stress might prove useful to predict individual PI sensitivity and possibly customize MM therapy.
Discussion
To maintain homeostasis, cells activate adaptive strategies against stressful and potentially harmful conditions (eg, hypoxia, nutrient deprivation, and oxidative stress). On the other hand, stress duration and intensity can activate apoptotic escapes, turning these responses maladaptive.36 Due to deregulated growth, cancer cells generally experience more cytotoxic stress than normal counterparts, leading to constitutive activation of such responses,37-40 which may in turn confer a growth advantage and mediate resistance against cytotoxic insults, including drugs. Because physiologic apoptotic responses to genotoxic stress are often disabled in cancer, strategies to increase cytotoxic stress offer great therapeutic promise to achieve cancer cell death through intact apoptotic programs.37
Having established intriguing correlations between proteostasis and PI sensitivity in Ab secretors,13,14 we became interested in investigating whether proteotoxic stress may represent a therapeutic target against MM, the paradigmatic PI-responsive cancer. Not surprisingly, in view of their secretory origin, MMCs display an active UPR, the pathway activated by the accumulation of misfolded/unfolded peptides in the ER.17 In MM, increased expression of XBP-1s, a UPR component essential for plasma cell differentiation and function,41,42 correlates with bad prognosis and poor survival,43 and could directly contribute to MM pathogenesis.44 However, the role played by the UPR in MMCs, and how PIs affect it, is still controversial.45 Recently, the UPR required for differentiation and function of professional secretory cells has been distinguished from the ER stress response (ESR), in which selective branches of the UPR are activated upon acute ER stress. The ESR generally restores homeostasis, but under overwhelming stress it triggers apoptosis.31,46,47 The different toxicity of PIs could depend on basal stress conditions and ongoing adaptive responses, including the UPR.
Given the key role of proteasomes in degrading unfolded/misfolded proteins, we wondered if the exquisite sensitivity to bortezomib displayed by certain MMCs could be, at least partially, explained by the high requirement for proteasome function. To test this hypothesis, we first exploited an established model of plasma cell differentiation characterized by a dramatic loss of proteasome expression associated with acquisition of apoptotic sensitivity to PIs.13,14 We found that, in addition to the reduced proteasome capacity, Ab-secreting cells also display a previously unreported increase in proteasomal workload, presumably due to metabolic stress associated with Ig synthesis (Figure 1). An increased workload on fewer functional proteasomes generates an imbalance, predisposing to apoptosis.13 We then explored whether a similar imbalance underlies PI sensitivity in MMCs. We first verified that 4 MMCs with different sensitivities to bortezomib also have similar differential sensitivity to other PIs, implying that the effectiveness of these drugs resides in specifically targeting the proteasome. Next, by assessing the level of proteasome activity with enzymatic and immunoblotting assays, we discovered that the most vulnerable cells, KMS.18 and MM.1S, indeed have fewer active proteasomes (Figure 2). Collectively, our findings imply that different myelomas may be equipped with different levels of functional proteasomes, with reduced proteasome capacity correlating with high PI sensitivity.
Together with reduced proteasomal activity, KMS.18 and MM.1S also display increased RDP production (Figure 2D). This suggests that proteasomal workload is not constant, but can vary greatly, correlating with PI sensitivity in MMCs. Thus, proteasome expression can be low despite elevated functional demands. Importantly, PI-sensitive MMCs also display higher incorporation of radioactive amino acids into TCA-insoluble polypeptides, reflective of increased protein synthesis during the pulse. Thus, the work accomplished by each proteasome (as can be judged by dividing load by capacity) in PI-sensitive MMCs is almost 2 logs higher than in relatively PI-resistant MMCs. Moreover, our pulse-chase assays showed that PI-sensitive MMCs secreted fewer proteins, despite higher synthesis, as in a recent report showing that increased ER retention of Ig components could possibly cause PI sensitivity.17 Taken together, the data suggest that efficiency of protein synthesis is greatly reduced in KMS.18 and MM.1S cells, generating more side products requiring proteasomal degradation, but the increased demand is not matched by an upgrade of the proteasomal apparatus, leading to proteasome stress, in coincidence with exquisite PI sensitivity (Figure 3). Interestingly, the observed basal accumulation of polyubiquitinated proteins in PI-sensitive MMCs is compatible with normal cell functions and with degradation of an unstable Ub-GFP reporter, which proved useful to demonstrate critical proteotoxic levels and to unveil pharmacologic synergies (Figures 3,4).
The obvious next question is whether an imbalance between proteasome workload and capacity is causally linked to the higher susceptibility to PIs distinctive of certain myelomas. If this were the case, proteasome stress could be exploited to predict PI sensitivity. To address this question, we set out to modulate proteasome expression and functional workload to modify PI sensitivity. We successfully increased workload by means of ER stressors: tunicamycin, an inhibitor of N-linked glycosylation; thapsigargin, which disrupts ER calcium stores; and brefeldin A, a blocker of ER-Golgi transport. By increasing misfolded proteins in the ER, these drugs increase the requirement for proteasome degradation.31,48 As expected, the administration of nontoxic doses of these drugs induced a modest UPR, but in combination with nontoxic doses of PIs synergistically triggered ER stress, proteasome overload as revealed by accumulation of the unstable Ub-GFP reporter, and cell death (Figure 4). Our finding that ER stressors strongly sensitize MMCs to bortezomib-induced apoptosis is consistent with previous reports,17,45,49 and extends our earlier observation that inducible expression of orphan Ig-μ chains made nonlymphoid cells vulnerable to PIs, linking ER load to PI sensitivity.13
In MM lines, Ig synthetic levels correlated with PI sensitivity,16 raising the possibility that increased Ig side products may overload proteasomes and sensitize to PIs.16 Our data prove this hypothesis correct, and demonstrate that proteasome workload may represent an independent variable directly influencing PI sensitivity in MMCs. This framework may help to increase responsiveness to PIs, or to reduce PI doses and toxicity, and contribute to explain the synergistic effect of ER stressors and PIs against MMCs reported by others and confirmed herein.45,49 Furthermore, our findings validate MM lines engineered to express UbG76V-GFP for potential preclinical drug screening.
We next tested if proteasome capacity per se modulates PI sensitivity. Eukaryotic cells can respond to decreased proteasome function or increased proteasomal requirement by enhancing proteasome biogenesis, thereby defining a proteasome stress response.32-34 We thus augmented proteasome activity by growing MMCs in the presence of low doses of MG-132, a rapidly reversible PI, for a few days. Importantly, our approach increased proteasomes without any overt toxicity, avoiding selection of high proteasome-expressing clones. Together with higher proteasome capacity, conditioned MMCs acquire increased resistance to bortezomib, but not UV-induced apoptosis (Figure 5), confirming that the size of the proteasome complement is a key determinant of PI sensitivity.
Although a recent study on more heterogeneous cell line panels yielded opposite correlations between levels of proteasome subunits and PI sensitivity,50 our findings clearly show that in MM, lower levels of functional proteasomes not only correlate, but may be causally involved in determining high PI sensitivity.
Our data are in line with a recent report that forced overexpression of the rate-limiting catalytic β5 subunit increased proteasome activity and resistance to cytotoxic insults in fibroblasts.51 Clearly, the development of acquired resistance to bortezomib may involve other mechanisms, including overexpression of mutated proteasome target subunits, not necessarily affecting overall proteasome activity.52
Our results indicate that basal proteasome levels and function may vary greatly among different MMCs, with profound implications for the intrinsic capability of coping with cytotoxic stress, given the key role of the proteasome in integrating signals that control cell-cycle progression, apoptosis, and metabolism. We are aware that studying MMCs prescinding from their natural bone marrow milieu may present limits. However, we found that incubation with patient-derived purified stromal components does not alter proteasome activity in MM lines, which maintain the observed differences (Figure S2).
At present, it is not known whether differential proteasome expression in MMCs results from reduced subunit synthesis and/or proteasome assembly/stability.27 The molecular mechanism mediating the proteasome stress response has been identified in yeast, but remains elusive in mammalian cells. We have recently shown that differentiating B cells decrease their proteasomes despite increasing proteolytic demands,13,14 and now provide evidence that certain MMCs express inadequate proteasome levels. It could be that short-lived Ab-secreting cells and certain myelomas respond poorly to proteasome stress, becoming vulnerable to conditions challenging proteasome function. In this connection, it is worth mentioning that unlike U266 and RPMI8226 cells, MM.1S proved irresponsive to proteasome stress, failing to increase their proteasome content (not shown). Thus, B-cell differentiation and MMC may provide powerful experimental models to investigate the mechanisms regulating the proteasome stress response in mammalian cells. It remains to be seen whether and how long-lived plasma cells homing in the bone marrow maintain a suitable load-versus-capacity ratio.53
The identification of symptoms of proteasome stress in PI-vulnerable MMCs (Figure 3) implies that simple cell-based assays could be designed to predict individual responsiveness. A similar predictor might help design customized therapies, avoiding unnecessary exposure to bortezomib and unfavorable side effects. Although we did not correlate clinical responsiveness with cellular features, we assessed the intrinsic sensitivity to PIs in vitro in primary, patient-derived MMCs, and measured accumulation of polyubiquitinated proteins, Ig content, and overall proteasome capacity. We found that polyubiquitinated proteins mark specifically neoplastic plasma cells, and their levels correlate with Ig content within every tumor cell population and among different patients, suggesting a direct effect of Ig synthesis and/or retention on proteasome workload. Moreover, overall proteasome activity varies among primary tumors, showing an inverse correlation with intrinsic responsiveness to PIs as assessed ex vivo (Figure 6). These data prompt to validate the assessment of proteasome stress and capacity as potential predictors of individual responsiveness to bortezomib of both prognostic and therapeutic value.
In conclusion, our data strongly support a model in which the balance between the workload on the ubiquitin-proteasome system and overall proteasome capacity may be crucial in determining the intrinsic apoptotic sensitivity of MMCs to PIs (Figure 6F). Moreover, manipulating proteasome workload and capacity may disrupt adaptive responses, enhance cytotoxic stress, and trigger apoptosis of MMCs with high specificity. Our work provides the framework for future experiments aimed at identifying new targets and designing novel combination therapies against MM and potentially other “stressable” cancers.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are indebted to Miriam Ascagni, Holger Auner, Elisa Benasciutti, Elena Bois, Federico Caligaris-Cappio, Fabio Ciceri, Salvatore De Vita, Marina Ferrarini, Anna Fra, Alfred L. Goldberg, David Lomas, Elisabetta Mariani, Silvia Masciarelli, Giampaolo Merlini, Nir Netzer, Giulia Perrone, Luca Rampoldi, Aldo Roccaro, Keiji Tanaka, Larry Wrabetz, Jonathan Yewdell, and Enzo Zimarino for scientific discussion, reagents, and precious technical advice. We are thankful to Raffaella Brambati and Ana Fella for cheerful secretarial support. We apologize to our colleagues whose work was not cited because of space limitations.
This work was supported through grants from the Associazione Italiana per la Ricerca sul Cancro (AIRC, Milano, Italy), Cariplo (NoBEL project, Milano, Italy), Ministero della Sanità, Roma, Italy, MIUR (CoFin and Center of Excellence in Physiopathology of Cell Differentiation, Roma, Italy), and Telethon, Roma, Italy.
Authorship
Contribution: G.B., L.O., and P.C. designed, performed, and analyzed experiments and drafted the manuscript; N.P., F.F., F.C., A.O., E.P., and A.M. performed experiments and analyzed data; V.C., G.P., and N.G. provided clinical samples; K.C.A. provided general advice and critically reviewed the manuscript; and R.S. and S.C. designed and supervised research and wrote the manuscript.
Conflict-of-interest disclosure: K.C.A. holds a consultancy with and receives research funding from Millennium. All other authors declare no competing financial interests.
Correspondence: Simone Cenci, DiBiT 4A3 room 68, San Raffaele Scientific Institute, Via Olgettina 58, 20132 Milano, Italy; e-mail: cenci.simone@hsr.it.
References
Author notes
*G.B. and L.O. contributed equally to this work.
†R.S. and S.C. are equal senior authors.

![Figure 6. Proteasome stress predicts sensitivity of primary MMCs to bortezomib. Primary, patient-derived MMCs were selected by immunomagnetic purification from bone marrow biopsies and divided in 3 pools. The first pool was seeded onto polylysinated slides and assayed for accumulation of polyubiquitinated proteins and Ig light chain (Ig-L) expression by immunofluorescence. The second pool was plated in multiwell plates, challenged with increasing doses of bortezomib for 24 hours, and apoptotic responses assessed by highly sensitive FACS analysis (LSRII) on labeling with conjugated annexin V and propidium iodide. The third pool was assayed in vitro for proteasome-specific chymotryptic activity by means of a specific fluorogenic peptide, as in Figure 3. (A) Spontaneous accumulation of polyubiquitinated proteins in primary MMCs. While in the CD138− fraction polyubiquitinated proteins accumulate only upon treatment with PIs (16 hours with 100 nM bortezomib [Btz] in the bottom left panel), CD138+ MMCs (hallmarked by Ig-L expression) show Fk2+ fluorescence in basal conditions (2 representative cases shown). Images were deconvoluted with DeltaVision and SoftWorx (see “Methods”); single z sections are shown. Size bars equal 5 μm. (B) Polyubiquitinated proteins are highly specific of Ig-L+ MMCs. Automated quantification of fluorescence in Ig-L+ and Ig-L− nucleated cells was performed using the IN Cell Investigator software. The box plot shows the average fluorescence intensity (FI), with 2.5 top and bottom percentiles; *P < .001. (C) Intrapatient positive correlation between polyubiquitinated accumulation and Ig-L content. Each dot corresponds to a single MM cell, and each plot corresponds to 1 patient. Scatters from 3 representative patients are shown. r > 0.6; P < .001. (D) Positive correlation of polyubiquitinated protein accumulation and Ig-L content among different patients. MFI indicates mean fluorescence intensity. r = 0.95; P < .05. (E) Primary MM endowed with high proteasome capacity is intrinsically less responsive to Btz. In 4 primary samples we measured overall proteasome activity in cell lysates by means of a fluorogenic peptide specifically probing the 26S chymotryptic activity (LLVY-amc).13,14 Proteasome-specific chymotryptic activity is expressed as nanomoles probe cleaved per minute per milligram of protein. Dose-response curves were generated by 24-hour treatments with Btz. To determine the intrinsic sensitivity of each MM clone, EC50 values were calculated using nonlinear regression with Prism version 5.0 software (GraphPad Software). (F) A model of proteasome-related determinants of apoptotic sensitivity to PI. Relatively PI-resistant MMCs are equipped with high proteasome pools, both in absolute terms, and relative to degradative demands. As a result, few proteins await degradation, and high levels of free ubiquitin are available to target new proteasome substrates. In contrast, PI-sensitive MMCs are equipped with poor proteasome levels, despite high metabolic demands. As a result, huge amounts of polyubiquitinated proteins accumulate upstream of overloaded proteasomes, and little ubiquitin is available for proteins to be degraded. Although cells are healthy, stresses that further challenge the ubiquitin-proteasome system will unveil a lower apoptotic threshold.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/13/10.1182_blood-2008-08-172734/4/m_zh80150933240006.jpeg?Expires=1763875608&Signature=47qEzRApCkcX2Zy8paVPpmcBYq2M1jfjwr2s6OVQ76fE5S8~172zmnqoOXazim7OeSvPF-Wuv9bu6rxurgB-~6LRPVIXja~QlQmDeeuWVw0v7R4ECDmiDHl~GNgSqGbmrzdUX~xNcakD4I6LmA0S8~nL2CxEBCbkLP4UgcpU51nTJkCuMhoWEhu2Q2ZJrJ8IZ1wGXd7mACkLMq4s~1EuT4wPWRWFrVH6S9JxhHk1ZPSXwyfhf~WvGB~CPeZy-bLW4iOZe5HZAMaJeLw9uuJ8ohnersibfakWIUKUF6-RqseMptkufn8dnB66rFtyhYSE2BS7BDVa9Q2H6AWsYMXAyA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 6. Proteasome stress predicts sensitivity of primary MMCs to bortezomib. Primary, patient-derived MMCs were selected by immunomagnetic purification from bone marrow biopsies and divided in 3 pools. The first pool was seeded onto polylysinated slides and assayed for accumulation of polyubiquitinated proteins and Ig light chain (Ig-L) expression by immunofluorescence. The second pool was plated in multiwell plates, challenged with increasing doses of bortezomib for 24 hours, and apoptotic responses assessed by highly sensitive FACS analysis (LSRII) on labeling with conjugated annexin V and propidium iodide. The third pool was assayed in vitro for proteasome-specific chymotryptic activity by means of a specific fluorogenic peptide, as in Figure 3. (A) Spontaneous accumulation of polyubiquitinated proteins in primary MMCs. While in the CD138− fraction polyubiquitinated proteins accumulate only upon treatment with PIs (16 hours with 100 nM bortezomib [Btz] in the bottom left panel), CD138+ MMCs (hallmarked by Ig-L expression) show Fk2+ fluorescence in basal conditions (2 representative cases shown). Images were deconvoluted with DeltaVision and SoftWorx (see “Methods”); single z sections are shown. Size bars equal 5 μm. (B) Polyubiquitinated proteins are highly specific of Ig-L+ MMCs. Automated quantification of fluorescence in Ig-L+ and Ig-L− nucleated cells was performed using the IN Cell Investigator software. The box plot shows the average fluorescence intensity (FI), with 2.5 top and bottom percentiles; *P < .001. (C) Intrapatient positive correlation between polyubiquitinated accumulation and Ig-L content. Each dot corresponds to a single MM cell, and each plot corresponds to 1 patient. Scatters from 3 representative patients are shown. r > 0.6; P < .001. (D) Positive correlation of polyubiquitinated protein accumulation and Ig-L content among different patients. MFI indicates mean fluorescence intensity. r = 0.95; P < .05. (E) Primary MM endowed with high proteasome capacity is intrinsically less responsive to Btz. In 4 primary samples we measured overall proteasome activity in cell lysates by means of a fluorogenic peptide specifically probing the 26S chymotryptic activity (LLVY-amc).13,14 Proteasome-specific chymotryptic activity is expressed as nanomoles probe cleaved per minute per milligram of protein. Dose-response curves were generated by 24-hour treatments with Btz. To determine the intrinsic sensitivity of each MM clone, EC50 values were calculated using nonlinear regression with Prism version 5.0 software (GraphPad Software). (F) A model of proteasome-related determinants of apoptotic sensitivity to PI. Relatively PI-resistant MMCs are equipped with high proteasome pools, both in absolute terms, and relative to degradative demands. As a result, few proteins await degradation, and high levels of free ubiquitin are available to target new proteasome substrates. In contrast, PI-sensitive MMCs are equipped with poor proteasome levels, despite high metabolic demands. As a result, huge amounts of polyubiquitinated proteins accumulate upstream of overloaded proteasomes, and little ubiquitin is available for proteins to be degraded. Although cells are healthy, stresses that further challenge the ubiquitin-proteasome system will unveil a lower apoptotic threshold.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/13/10.1182_blood-2008-08-172734/4/m_zh80150933240006.jpeg?Expires=1763875609&Signature=knDMfXgfByue77aeReZS1J2PUVgKIkHM4B7~bHLlS3QPbIhrGZqNTbg8DmePG~TXZAepib~zJTnrmK47EO8PPzHhRP-NJHVCrgz46cV0J~V5Shs3FGNPfJ5A5Hh9UPE82J2Pqq5tqviUOSdfVqMR8cTrEt34tlfbtHZ-oT5TyaUM4QFqImjnCq8fu48vbm8HPQQgAltDPAALdRL~Ihyqcn98LmmD8f1m8sGqBEoiPiDLUTNqiAARx7la4vPMZnGJ~bwCRtE-zgLzfTxqBYXn0keeWzz3iMqdRaoXNLO0LzdFyY72k5FAXM4LqZvZwsiosbnRraWr6Yoq0vLAY1zbuQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)