Abstract
AML1-ETO and TEL-AML1 are chimeric proteins resulting from the t(8;21)(q22;q22) in acute myeloid leukemia, and the t(12;21)(p13;q22) in pre-B-cell leukemia, respectively. The Runt domain of AML1 in both proteins mediates DNA binding and heterodimerization with the core binding factor β (CBFβ) subunit. To determine whether CBFβ is required for AML1-ETO and TEL-AML1 activity, we introduced amino acid substitutions into the Runt domain that disrupt heterodimerization with CBFβ but not DNA binding. We show that CBFβ contributes to AML1-ETO's inhibition of granulocyte differentiation, is essential for its ability to enhance the clonogenic potential of primary mouse bone marrow cells, and is indispensable for its cooperativity with the activated receptor tyrosine kinase TEL-PDGFβR in generating acute myeloid leukemia in mice. Similarly, CBFβ is essential for TEL-AML1's ability to promote self-renewal of B cell precursors in vitro. These studies validate the Runt domain/CBFβ interaction as a therapeutic target in core binding factor leukemias.
Introduction
RUNX1 and CBFB are very frequent targets of mutations and gene rearrangements in leukemia.1,2 Among the most commonly identified rearrangements are the t(8;21)(q22;q22) in 12% of adult acute myeloid leukemia (AML) and the t(12;21)(p13;q22) in 25% of pediatric acute lymphocytic leukemia (ALL), both of which involve the RUNX1 gene.3 The molecular consequence of these translocations is the generation of fusion proteins, AML1-ETO and TEL-AML1, respectively.4-7
AML1-ETO contains the N-terminal 177 amino acids of the Runx1 DNA-binding subunit fused in frame with nearly all of ETO (821; encoded by RUNX1T1).4,5 Runx1 is required for normal hematopoiesis.8 The function of ETO itself is not particularly well understood. Its Drosophila homolog, Nervy, interacts directly with the transcription factor daughterless, and represses the activity of enhancers normally activated by the achaete-scute complex in the sensory organ precursor cell.9 Homozygous disruption of Runx1t1 in mice resulted in gastrointestinal defects, but no hematopoietic deficiencies.10
AML1-ETO has 5 conserved domains, one from Runx1 (the Runt domain) and 4 from ETO.11,12 The eTAFH (or NHR1) domain interacts with the nuclear hormone receptor corepressor (N-CoR),13 and also with the activation domain of E proteins (E2A and HEB).14 The HHR (NHR2) domain forms an α-helical tetramer that mediates oligomerization of AML1-ETO with itself and other ETO proteins and interacts with the corepressor Sin3, Gfi1, and histone deacetylases 1 and 3.12,15-20 Nervy (NHR3) is an α-helical domain that interacts with the regulatory subunit of type II cyclic AMP-dependent protein kinase.21,22 Myeloid-Nervy-DEAF-1 (MYND or NHR4) is a zinc-chelating domain structurally homologous to the PHD and RING finger domains and mediates interactions with N-CoR, the silencing mediator of retinoid and thyroid hormone receptor (SMRT), and the DNA binding protein SON.16,23-26 A foreshortened splice variant of AML1-ETO lacking both the Nervy and MYND domains (AML1-ETO9a), or full-length AML1-ETO with a mutation predicted to disrupt the structure of the MYND domain and shown to impair SON binding had increased leukemogenic potential.23,27,28
The Runt domain from Runx1 mediates both DNA binding and heterodimerization with CBFβ.29 Both of these properties are essential for normal Runx1 function in vivo.30 CBFβ enhances the affinity of the Runt domain for DNA by 7- to 10-fold through quenching conformational exchange in several dynamic regions31-36 and may additionally inhibit Runx1 degradation mediated by the ubiquitin proteasome pathway.37 An R174Q mutation in Runx1 found in AML of the M0 subtype and in familial platelet disorder with predisposition for AML (FPD/AML) that disrupts only DNA but not CBFβ binding generated a weakly dominant negative Runx1 allele.30,38-40 Single amino acid substitutions in Runx1 that impaired CBFβ but not DNA binding resulted in hypomorphic alleles that diminished but did not eliminate Runx1's in vivo activity.30
The most commonly accepted models of AML1-ETO–mediated leukemogenesis posit that it functions either as a dominant inhibitor of CBF function41-43 or as a constitutive repressor of CBF target gene expression.44 Inhibition or repression is presumably mediated by the recruitment of corepressor complexes interacting with ETO domains to genomic targets recognized by the Runt domain.11,12 However, increasing evidence in cell line models suggests that the real situation may be substantially more complex.14,45-48 For example, it was proposed that AML1-ETO may function by sequestering transcription factors (such as E-box proteins), SON, or other regulatory proteins into subnuclear compartments13,23 and that its DNA binding function may not be necessary.13 It has also been proposed that AML1-ETO functions by dysregulating the expression of E-box protein targets.14 The essential contribution of DNA binding by the Runt domain to overt leukemia was, however, recently and unequivocally established by demonstrating that an R174Q mutation that disrupts DNA but not CBFβ binding abolished AML1-ETO's activity.30,49 On the other hand, the role of CBFβ binding for AML1-ETO's leukemogenic activity has not been accessed.
TEL-AML1 contains the N-terminal, non-DNA binding region of TEL (translocation-ETS-leukemia; encoded by ETV6) fused to nearly all of Runx1.7,50 The components of TEL retained in the fusion protein include the Pointed domain, which forms a head-to-tail polymeric structure, as well as a corepressor binding domain, which together mediate interaction with N-CoR, SMRT, and mSin3a.6,51-55 The predominant model of TEL-AML1 function posits that it recruits corepressor complexes to CBF target genes in a Runt domain-dependent manner, resulting in inappropriate gene regulation.52,56,57 Mechanisms invoking off-DNA activities including sequestration of transcriptional complexes and disruption of wild-type TEL function have also been proposed.58-60 However, recent work using the equivalent of an AML1-ETO R174Q substitution in the Runt domain that specifically disrupted DNA binding resulted in a loss of TEL-AML1 activity in B-cell precursors, demonstrating the centrality of this function.61
These mechanistic uncertainties underscore the importance of characterizing the specific contributions of each domain in AML1-ETO and TEL-AML1 to their in vivo activities. Herein we analyzed the importance of CBFβ binding by the Runt domain for both AML1-ETO and TEL-AML1 function. The residues involved in DNA binding and heterodimerization are on distinct surfaces of the Runt domain, and extensive mutational analysis has been performed on both interfaces.30,34,36,62-65 Importantly, the majority of mutations in the Runt domain destabilize its structure, and negatively impact both DNA and CBFβ binding.30,63,65 We used the available biophysical information to introduce and carefully characterize amino acid substitutions that selectively target CBFβ binding. We incorporated these mutations into AML1-ETO and TEL-AML1 and obtained evidence that CBFβ binding is critical for their respective activities.
Methods
Cloning, expression, and protein purification
A retroviral vector expressing TEL-PDGFβR plus human CD4 (hCD4) was made by cloning a blunted EcoRI fragment from the TEL-PDGFβR cDNA into the HpaI site of the MSV2.2-IRES-hCD4 vector. The MigR1-AML1-ETO-IRES-EGFP, pMSCV-IRES-EGFP (MSCV-EGFP), and pMSCV-TEL-AML1-IRES-EGFP (MSCV-T/A) vectors were described previously.15,66
CBFβ (amino acids 1-141) and the wild-type Runt domain (residues 41-190) were cloned between the BamHI and EcoRI sites and the NcoI and XhoI sites of the pHis parallel vector,67 respectively. The proteins were expressed in Rosetta(DE3) cells (Novagen, San Diego, CA) by inducing with 0.8 mM isopropyl-β-D-thiogalactopyranoside (IPTG) at 25°C (for CBFβ) or 20°C (for Runt domain) when optical density (OD)600 nm reached 0.5 to approximately 0.7, and purified on a Ni-NTA column (QIAGEN, Valencia CA). The 6 × His tag was cleaved by AcTEV (Invitrogen, Carlsbad, CA), and proteins were further purified on an S-100 column (CBFβ) or SP-Sepharose column (Runt domain; GE Healthcare, Piscataway, NJ). All mutations were generated using the QuikChange XLII Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA).
Urea denaturation, electrophoretic mobility shift assays, and isothermal titration calorimetry measurements
Retroviral transduction
Bone marrow (BM) harvested from C57BL/6J mice treated 7 days previously with 150 mg/kg 5-fluorouracil (5-FU; Sigma-Aldrich, St Louis, MO) was suspended in 1 mL transplant media per mouse hind-leg [RPMI, 20% fetal calf serum (FCS), 100 IU/mL penicillin, 100 μg/mL streptomycin (Mediatech, Herndon, VA), 10 ng/mL interleukin-3 (IL-3), 20 ng/mL IL-6, 20 ng/mL stem cell factor (SCF; R&D Systems, Minneapolis, MN)] and incubated overnight at 37°C in ultra-low attachment plates (Costar, Corning, NY). One milliliter of each virus (MigR1-EGFP, AML1-ETO, and mutants thereof, TEL-PDGFβR, or MSCV-hCD4) was spun (1000g for 90 minutes at 31°C) onto an ultra-low attachment 6-well plate well, previously precoated with Retronectin (100 μg/well; Takara, Madison, WI), and the viral supernatant was subsequently removed. Cells were then resuspended in fresh transplant media with cytokines at 6 × 106 cells/3 mL and centrifuged (1 000g for 90 minutes) onto the virally preloaded plates with an additional 0.5 mL each virus. A second spinfection (150g for 10 minutes) was performed 12 hours later onto new virally precoated plates, and cells were incubated at 37°C for 3.5 hours.
Hematopoietic assays and flow cytometry
Granulocyte differentiation, BrDU staining, serial replating, and c-Kit+ B-cell progenitor colony forming assays were performed as described previously.15,25,61,66 Antibodies used were: allophycocyanin (APC), phycoerythrin (PE), or PerCPcy5.5 conjugated anti–Mac-1 (clone M1/70), APC or PerCP-Cy5.5 conjugated anti–Gr-1 (clone RB6-8C5), APC-conjugated anti-Sca1 (clone D7), PE-Cy5.5 conjugated anti-CD117 (clone 2B8), and PE-conjugated anti–human CD4 (clone L3T4; eBioscience, San Diego, CA).
Transplantation assays
Retrovirally transduced cells were washed and resuspended in phosphate-buffered saline (PBS). C57BL/6J males (5 to 6 weeks old) were lethally irradiated with a split dose of 5.5 Gy 3 to 4 hours apart and injected intravenously with 106 infected BM cells and 2 × 105 splenocytes from a C57BL6/J donor. Mice were monitored for development of leukemia through peripheral blood analysis for infected cells by fluorescence-activated cell sorting (FACS) and by observation of physical symptoms characterized by lethargy, anemia, and splenomegaly. Sick mice were euthanized, and all remaining mice were killed at 63 days. All animal procedures were approved by the Institutional Animal Care and Use Committee at Dartmouth College.
Western blot analysis
AML1-ETO and TEL-AML1 proteins were detected in retrovirally transduced NIH3T3 cells as described previously.15,25,61,66 Splenocyte extracts were prepared by lysing approximately 5 × 105 cells in 3× sodium dodecyl sulfate (SDS) sample buffer (New England Biolabs, Ipswich, MA) with 0.1 M dithiothreitol for 10 minutes on ice, boiling 5 minutes at 95°C, and the proteins were resolved through NuPAGE Novex 4%-12% Bis-Tris mini gels (Invitrogen) and transferred onto a polyvinylidene fluoride, (PVDF) membrane. Blots were probed with primary anti-ETO Ab-1 (2.5μg/mL, 2 hours; Calbiochem, San Diego, CA) and anti-actin (1:1 000, 1 hour; Sigma-Aldrich) antibodies, and secondary goat anti–rabbit IgG (H + L; 1:100 000, 45 minutes; Caltag Laboratories, Carlsbad, CA) horseradish peroxidase-conjugated antibody in PBS/0.2% Tween/5% nonfat milk. Band intensities were determined using ImageQuant 5.0 software.
Results
Generating Runt domain mutations that independently disrupt DNA and CBFβ binding
We compared the contribution of DNA versus CBFβ binding to AML1-ETO–mediated leukemogenesis by introducing amino acid substitutions at both interfaces. An R174Q substitution caused a greater than 40 000-fold reduction in DNA binding by the isolated Runt domain without perturbing heterodimerization with CBFβ, its structure, or its thermodynamic stability.30 A T161A mutation, on the other hand, caused a 40-fold reduction in CBFβ binding while having no effect on DNA binding.65 The T161A mutation did not perturb the Runt domain's structure as assessed in 15N-1H HSQC spectra,65 although it did moderately decrease its thermodynamic stability.30 Since the T161A mutation, when introduced into the Runx1 locus in mice, did not completely eliminate Runx1's in vivo function,30 we sought to disrupt CBFβ binding further by combining it with a second mutation. The majority (approximately two-thirds) of alanine substitutions in the Runt domain perturb its fold, particularly those within the β-barrel, one surface of which comprises the CBFβ interface.30,63,65 A Y113A mutation was one of only a few that decreased the affinity for CBFβ (by approximately 5-fold), while causing only minimal, local effects on the Runt domain fold as assessed in 15N-1H HSQC spectra.65 We showed that the Y113A/T161A dual mutations did not further decrease the Runt domain's thermodynamic stability compared with the T161A mutation alone (Figure 1B). The dissociation constant (K2; Figure 1C) of the Y113A/T161A mutant Runt domain for DNA was unaltered (Figure 1D). Since DNA binding is highly sensitive to mutations in the Runt domain that perturb its structure,30,63 we reasoned that the Y113A/T161A mutant Runt domain's fold is intact despite its modestly decreased thermodynamic stability.
Amino acid substitutions in the Runx1 Runt domain that specifically disrupt DNA and CBFβ binding. (A) Structures of the Runt domain and CBFβ are shown in gray and blue, respectively, and the DNA is purple.36 The R174 side chain in the Runt domain is green, and the T161 and Y113 side chains are orange. (B) Urea denaturation monitored by tryptophan fluorescence for the wild-type Runt domain and point mutants thereof. Plotted is the fraction of unfolded Runt domain in the presence of increasing concentrations of urea. Data from the wild-type Runt domain, R174Q, and T161A are from Matheny et al.30 (C) Diagram of the potential interactions between the Runt Domain, CBFβ, and DNA. (D) EMSA measuring the affinity of the Y113A/T161A Runt domain for DNA (K2). Shown is a representative example of 3 experiments. Triangles indicate decreasing concentrations of the Runt domain (2 × 10−6 to 4 × 10−15 M). Arrow indicates the lane in which the Runt domain concentration approximates K2. (E) Isothermal titration calorimetric measurements of CBFβ binding to the wild-type, T161A, and Y113A/T161A mutant Runt domains. Wild-type Runt domain (45 μM) was titrated with 440 μM CBFβ at 26°C, and 42 μM T161A or 38 μM Y113A/T161A were titrated with 396 μM CBFβ at 22°C. In each panel, the top portion is the raw data, and the bottom panel is a plot of the binding corrected for dilution enthalpy. Experimental data (squares) are fit to a one-site binding model (line). The equilibrium dissociation constants (K1) are indicated in the plots. The average values from 2 independent measurements (± standard deviation [SD]) are given.
Amino acid substitutions in the Runx1 Runt domain that specifically disrupt DNA and CBFβ binding. (A) Structures of the Runt domain and CBFβ are shown in gray and blue, respectively, and the DNA is purple.36 The R174 side chain in the Runt domain is green, and the T161 and Y113 side chains are orange. (B) Urea denaturation monitored by tryptophan fluorescence for the wild-type Runt domain and point mutants thereof. Plotted is the fraction of unfolded Runt domain in the presence of increasing concentrations of urea. Data from the wild-type Runt domain, R174Q, and T161A are from Matheny et al.30 (C) Diagram of the potential interactions between the Runt Domain, CBFβ, and DNA. (D) EMSA measuring the affinity of the Y113A/T161A Runt domain for DNA (K2). Shown is a representative example of 3 experiments. Triangles indicate decreasing concentrations of the Runt domain (2 × 10−6 to 4 × 10−15 M). Arrow indicates the lane in which the Runt domain concentration approximates K2. (E) Isothermal titration calorimetric measurements of CBFβ binding to the wild-type, T161A, and Y113A/T161A mutant Runt domains. Wild-type Runt domain (45 μM) was titrated with 440 μM CBFβ at 26°C, and 42 μM T161A or 38 μM Y113A/T161A were titrated with 396 μM CBFβ at 22°C. In each panel, the top portion is the raw data, and the bottom panel is a plot of the binding corrected for dilution enthalpy. Experimental data (squares) are fit to a one-site binding model (line). The equilibrium dissociation constants (K1) are indicated in the plots. The average values from 2 independent measurements (± standard deviation [SD]) are given.
We measured the affinity of the T161A and Y113A/T161A mutant Runt domains for CBFβ by isothermal titration calorimetry (Figure 1E). The dissociation constant (K1 in Figure 1C) for the T161A mutant Runt domain was increased by 63-fold, and for the Y113A/T161A mutant by 430-fold (Figure 1E). Whereas binding of the wild-type Runt domain to CBFβ is exothermic (release of heat upon binding), that of the mutant Runt domains is endothermic (absorption of heat upon binding). For this reason, the raw heat data in Figure 1E switches from being negative for the wild-type to positive for the T161A and Y113A/T161A mutant Runt domains. Apparently, the interactions mediated by T161 contribute strongly to the enthalpy of binding, and loss of these interactions results in a binding process that is no longer enthalpically favorable but remains entropically favorable, albeit of weaker affinity.
Disruption of DNA or CBFβ binding impairs AML1-ETO's ability to repress granulocyte differentiation.
We introduced the R174Q, T161A, and Y113A/T161A mutations into AML1-ETO (Figure 2A) and expressed AML1-ETO and its mutated derivates in lineage negative (CD5−, B220−, Mac-1−, Gr-1−, Ter119−) mouse BM cells (Lin− BM). The retroviruses also expressed the enhanced green fluorescent protein (EGFP) from an internal ribosomal entry site. We cultured cells following transduction in the presence of IL-3, IL-6, and SCF for 2 days, in the same cytokines plus granulocyte colony-stimulating factor (G-CSF) for an additional 7 days to induce granulocyte differentiation, and then analyzed EGFP+ cells for Mac-1 and Gr-1 expression (Figure 2B,C). Approximately 32% of cells expressing EGFP alone were Mac-1+Gr-1+ (Figure 2C) versus 10% of those expressing AML1-ETO. Disruption of DNA binding (R174Q) or impairment of CBFβ heterodimerization (Y113A/T161A) increased the percentage of Mac-1+Gr-1+ cells. A triple Y113A/T161A/R174Q mutation in AML1-ETO did not further increase the percentage of Mac-1+Gr-1+cells compared with the individually mutated AML1-ETO proteins, indicating that the residual inhibitory effect of AML1-ETO resides in more C-terminal ETO sequences, and is not mediated through CBF binding sites. The mutated AML1-ETO proteins accumulated to similar steady state levels in transduced NIH3T3 cells (Figure 2E).
AML1-ETO function is impaired by mutations that disrupt DNA or CBFβ binding. (A) Diagram of AML1-ETO and location of the mutations affecting DNA (R174Q) and CBFβ binding (T161A and Y113A/T161A). (B) Lin− BM cells infected with MigR1 retroviruses expressing AML1-ETO and mutated derivatives after 7 days of culture in the presence of IL-3, IL-6, SCF, and G-CSF. EGFP+ gated cells (not shown) were analyzed for Gr-1 and Mac-1 expression. (C) Summary of data illustrated in panel B compiled from 2 experiments, each with triplicate samples. **P ≤ .01 compared with AML1-ETO (#); ***P ≤ .001 (Dunnett test and analysis of variance [ANOVA]). (D) Serial replating analysis. Graphs represent the average number of colonies from each round of replating in the presence of IL-3, IL-6, and SCF. Week 1 represents colony numbers per 103 cells plated, weeks 2 and 3 are from 104 plated cells. Numbers are averaged from 2 experiments, each containing triplicate samples. (E) Western blot analysis probed with an antibody to the Runt domain, demonstrating expression of AML1-ETO and its mutated derivatives in MigR1-transduced NIH3T3 cells. (F) BrdU incorporation 48 hours after transduction of Lin− BM cells with AML1-ETO and its mutants. EGFP+ cells were analyzed after a 1 hour BrdU pulse for BrdU and 7-AAD incorporation. Shown is a representative of 3 experiments. (G) Average percentages of gated BrdU+ (S phase) cells from scatter plots in panel F (n = 3). Error bars indicate 95% confidence intervals. **P ≤ .01 compared with AML1-ETO (Dunnett test and ANOVA).
AML1-ETO function is impaired by mutations that disrupt DNA or CBFβ binding. (A) Diagram of AML1-ETO and location of the mutations affecting DNA (R174Q) and CBFβ binding (T161A and Y113A/T161A). (B) Lin− BM cells infected with MigR1 retroviruses expressing AML1-ETO and mutated derivatives after 7 days of culture in the presence of IL-3, IL-6, SCF, and G-CSF. EGFP+ gated cells (not shown) were analyzed for Gr-1 and Mac-1 expression. (C) Summary of data illustrated in panel B compiled from 2 experiments, each with triplicate samples. **P ≤ .01 compared with AML1-ETO (#); ***P ≤ .001 (Dunnett test and analysis of variance [ANOVA]). (D) Serial replating analysis. Graphs represent the average number of colonies from each round of replating in the presence of IL-3, IL-6, and SCF. Week 1 represents colony numbers per 103 cells plated, weeks 2 and 3 are from 104 plated cells. Numbers are averaged from 2 experiments, each containing triplicate samples. (E) Western blot analysis probed with an antibody to the Runt domain, demonstrating expression of AML1-ETO and its mutated derivatives in MigR1-transduced NIH3T3 cells. (F) BrdU incorporation 48 hours after transduction of Lin− BM cells with AML1-ETO and its mutants. EGFP+ cells were analyzed after a 1 hour BrdU pulse for BrdU and 7-AAD incorporation. Shown is a representative of 3 experiments. (G) Average percentages of gated BrdU+ (S phase) cells from scatter plots in panel F (n = 3). Error bars indicate 95% confidence intervals. **P ≤ .01 compared with AML1-ETO (Dunnett test and ANOVA).
Disruption of DNA binding or CBFβ binding abrogates AML1-ETO's ability to promote self-renewal and inhibit proliferation.
We assessed the importance of DNA binding and heterodimerization with CBFβ for AML1-ETO's ability to confer increased self-renewal capacity to hematopoietic progenitors in vitro.69-71 Cells expressing AML1-ETO could be propagated for at least 3 weeks in methylcellulose cultures (Figure 2D) and yielded primarily immature myeloid lineage cells and a smaller percentage of differentiated macrophages15,70 (not shown). Impaired DNA or CBFβ binding caused by the R174Q or Y113A/T161A mutations, respectively, completely abolished AML1-ETO's ability to sustain this clonogenic activity (Figure 2D). Interestingly, it appears that the threshold level above which AML1-ETO cannot tolerate disruption of CBFβ binding and maintain clonogenicity lies above 60-fold, as the T161A mutant retains activity for at least 3 weeks in this assay (Figure 2D).
CBFβ binding is required for AML1-ETO's leukemogenic activity.
Numerous murine models have demonstrated that full-length AML1-ETO alone is not capable of causing AML.69,72-76 Activating mutations in the platelet-derived growth factor family of receptor tyrosine kinases, including FLT3 and KIT, and in RAS are common in core binding factor leukemias,77-81 and correspondingly, AML1-ETO was shown to be capable of cooperating with activated forms of both platelet-derived growth factor receptor-b (TEL-PDGFβR) and FLT3 to generate AML in mice.82,83 Both aforementioned mouse studies reported that mutation of leucine 148 to aspartic acid (L148D) within the Runt domain of AML1-ETO, which disrupts DNA binding16 and presumably causes a severe disruption of domain stability,30,65 resulted in a loss of cooperation with either oncogene.82,83 These studies underscored the importance of the Runt domain for AML1-ETO's cooperative activity, but did not address whether disruption of DNA binding, CBFβ heterodimerization, or both were responsible for the observed effect.
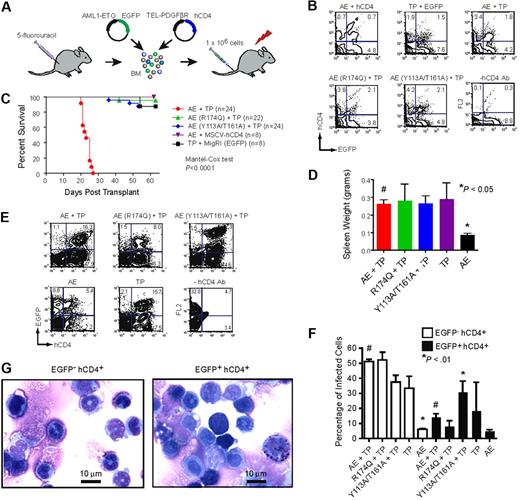
We retrovirally transduced AML1-ETO (and derivative mutants) and TEL-PDGFβR into BM cells from 5-FU–treated mice (Figure 3A). The retrovirus expressing AML1-ETO produced EGFP from an internal ribosome entry site, and the TEL-PDGFβR virus produced hCD4. The percentages of doubly transduced cells (EGFP+hCD4+) were similar for AML1-ETO and various mutants thereof at 2 days after spinfection (Figure 3B). We transplanted lethally irradiated primary recipient animals with 106 retrovirally transduced, unsorted cells and monitored the animals for disease. The AML that develops in mice transplanted with BM cells expressing both AML1-ETO and TEL-PDGFβR is marked clinically by splenomegaly, hepatomegaly, thrombocytopenia, and anemia with an accumulation of myeloid blasts in the BM and an infiltration thereof in the spleen and liver.82 TEL-PDGFβR will independently generate a chronic myeloproliferative disorder (CMPD) marked by splenomegaly, hepatomegaly, and neutrophilic leukocytosis.84 We predicted that if mutations in AML1-ETO impaired its leukemogenic activity, the disease would more closely resemble the CMPD caused by TEL-PDGFβR alone.
Mutations that disrupt DNA or CBFβ binding impair AML1-ETO's leukemogenic activity. (A) Schematic of transplantation scheme. BM mononuclear cells harvested from 5-FU–treated C57BL/6 mice were co-infected with MigR1 expressing AML1-ETO (and its mutated derivatives) and TEL-PDGFβR. Internal ribosome entry site (IRES)–mediated expression of EGFP marks AML1-ETO expressing cells, while hCD4 marks TEL-PDGFβR expressing cells. Retrovirally transduced cells (106) were transplanted along with 2 × 105 normal BM cells into lethally irradiated mice. (B) FACS plots of BM cells 2 days after transduction to show that all were equivalently infected. (C) Kaplan-Meier survival curve of mice after transplantation with retroviruses expressing AML1-ETO (AE) or its mutated derivatives and TEL-PDGFβR (TP). The study end point was 63 days. The number of mice in each group is indicated. (D) Spleen weights of mice upon sacrifice. Significant differences from AML1-ETO plus TEL-PDGFβR recipients (#) are indicated with * (Dunnett test and ANOVA). (E) Representative plots of BM cells in transplant recipients upon sacrifice demonstrating the presence of EGFP+ and hCD4+ cells. The relatively high EGFP fluorescence in the AML1-ETO (Y113A/T161A) plus TEL-PDGFβR BM cells was observed in 19 of 24 mice. (F) Average percentage of EGFP− hCD4+ and EGFP+ hCD4+ BM cells in transplant recipients upon sacrifice. Error bars indicate 95% confidence intervals. Differences relative to AML1-ETO plus TEL-PDGFβR (#) for each group were determined using Dunnett test and ANOVA. (G) Cytospin preparations of BM cells from an AML1-ETO plus TEL-PDGFβR leukemic mouse purified based on hCD4 and EGFP expression.
Mutations that disrupt DNA or CBFβ binding impair AML1-ETO's leukemogenic activity. (A) Schematic of transplantation scheme. BM mononuclear cells harvested from 5-FU–treated C57BL/6 mice were co-infected with MigR1 expressing AML1-ETO (and its mutated derivatives) and TEL-PDGFβR. Internal ribosome entry site (IRES)–mediated expression of EGFP marks AML1-ETO expressing cells, while hCD4 marks TEL-PDGFβR expressing cells. Retrovirally transduced cells (106) were transplanted along with 2 × 105 normal BM cells into lethally irradiated mice. (B) FACS plots of BM cells 2 days after transduction to show that all were equivalently infected. (C) Kaplan-Meier survival curve of mice after transplantation with retroviruses expressing AML1-ETO (AE) or its mutated derivatives and TEL-PDGFβR (TP). The study end point was 63 days. The number of mice in each group is indicated. (D) Spleen weights of mice upon sacrifice. Significant differences from AML1-ETO plus TEL-PDGFβR recipients (#) are indicated with * (Dunnett test and ANOVA). (E) Representative plots of BM cells in transplant recipients upon sacrifice demonstrating the presence of EGFP+ and hCD4+ cells. The relatively high EGFP fluorescence in the AML1-ETO (Y113A/T161A) plus TEL-PDGFβR BM cells was observed in 19 of 24 mice. (F) Average percentage of EGFP− hCD4+ and EGFP+ hCD4+ BM cells in transplant recipients upon sacrifice. Error bars indicate 95% confidence intervals. Differences relative to AML1-ETO plus TEL-PDGFβR (#) for each group were determined using Dunnett test and ANOVA. (G) Cytospin preparations of BM cells from an AML1-ETO plus TEL-PDGFβR leukemic mouse purified based on hCD4 and EGFP expression.
Mice transplanted with BM cells expressing both AML1-ETO and TEL-PDGFβR developed a completely penetrant lethal leukemia, as reported previously,82 and succumbed to their disease within 4 weeks (Figure 3C). In contrast, most control mice transplanted with cells expressing AML1-ETO alone (8/8), or TEL-PDGFβR alone (7/8) survived for 63 days, at which point they were killed for analysis. Diseased AML1-ETO plus TEL-PDGFβR transplanted mice presented with anemia, splenomegaly, and pale livers (not shown). Their BM contained blast cells characterized by a high nucleocytoplasmic ratio, heterochromatic nuclei, occasional cytoplasmic vacuoles, irregular nuclear contours, and prominent nucleoli (not shown). Blast cells were also present in peripheral blood (not shown). However the BM contained many more differentiated myeloid cells such as neutrophils with ring-shaped nuclei (characteristic of CMPD), presumably arising from the large population of EGFP−hCD4+ cells expressing TEL-PDGFβR alone (Figure 3E,F). As a result of the mixed diseases in these mice, BM differentials and histology were not useful diagnostic aids. We therefore isolated EGFP−hCD4+ and EGFP+hCD4+ cells from the BM of leukemic AML1-ETO plus TEL-PDGFβR mice and analyzed their morphology. The EGFP+hCD4+ population (expressing AML1-ETO plus TEL-PDGFβR) contained a markedly greater percentage of blast cells, consistent with AML, in comparison to the EGFP−hCD4+ population (Figure 3G). We were able to transplant AML1-ETO plus TEL-PDGFβR expressing cells into secondary recipients and reconstitute the disease, but only using large numbers (> 2 × 106) of freshly isolated splenocytes, and not with frozen cells (not shown), indicating that the percentage of leukemia initiating cells was low.
Most mice transplanted with BM expressing TEL-PDGFβR plus either the non-DNA binding or non-CBFβ binding AML1-ETO mutants (R174Q and Y113A/T161A, respectively) survived until the study end point of 63 days (Figure 3C). The majority of these mice had evidence of disease as manifested by enlarged spleens [15/22 for AML1-ETO (R174Q) and 22/24 for AML1-ETO (Y113A/T161A)]. Their average spleen sizes were similar to those of mice transplanted with BM expressing AML1-ETO plus TEL-PDGFβR or TEL-PDGFβR alone, and were significantly greater than those of mice transplanted with BM expressing AML1-ETO alone (Figure 3D), indicating that TEL-PDGFβR contributed significantly to the splenomegaly. A minority of the mice were anemic as evidenced by pale livers [2/22 for AML1-ETO (R174Q) and 6/24 for AML1-ETO (Y113A/T161A)], whereas anemia was more prevalent in the AML1-ETO plus TEL-PDGFβR mice (16/22). The BM of all groups contained EGFP−hCD4+ and EGFP+hCD4+ cells, but relatively few cells expressing EGFP (AML1-ETO) alone, suggesting a competitive advantage for cells that expressed TEL-PDGFβR (Figure 3E). Interestingly, most mice (19/24) transplanted with cells expressing the AML1-ETO (Y113A/T161A) mutant contained an EGFPhihCD4+ BM population, presumably resulting from a selection for cells expressing high AML1-ETO (Y113A/T161A) levels (Figure 3E). We speculate that the Y113A/T161A mutant has residual activity and that more highly expressing cells partially overcome the effects of the mutations and thus are positively selected. Despite having more EGFP+hCD4+ cells and higher AML1-ETO levels, the AML1-ETO (Y113A/T161A) plus TEL-PDGFβR group survived longer than the AML1-ETO plus TEL-PDGFβR group, thus AML1-ETO deficient for CBFβ binding has greatly attenuated activity. We were unable to detect AML1-ETO protein in spleen samples from any of the transplanted mice using antibodies that recognize either ETO or the Runt domain from Runx1, although we could detect the protein in Kasumi-1 cells which contain the t(8;21), and actin was clearly visible in all of the samples (not shown). We suspect our inability to detect AML1-ETO was due to the relatively low percentage of AML1-ETO expressing spleen cells (Figure 3F).
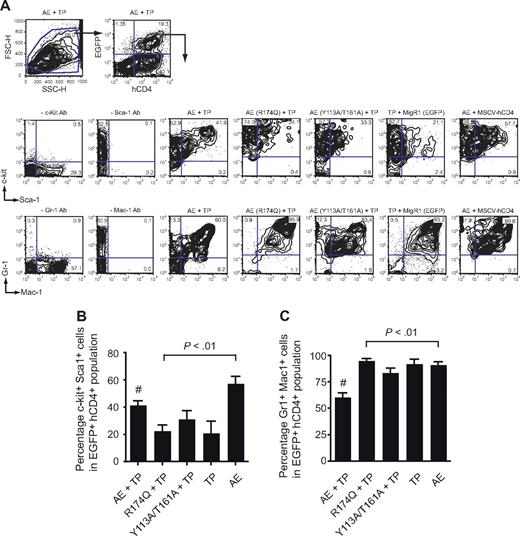
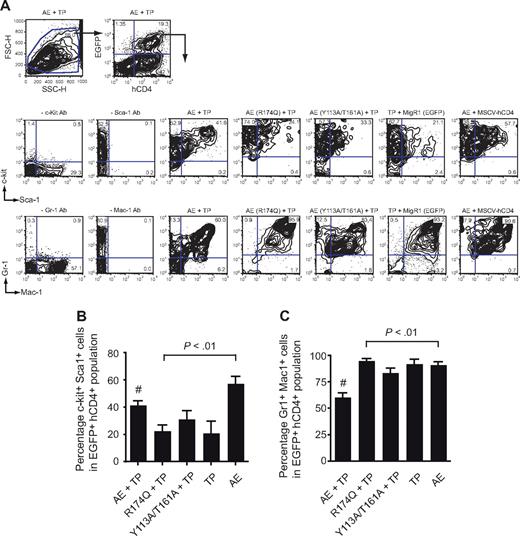
EGFP+hCD4+ BM cells (expressing AML1-ETO plus TEL-PDGFβR) from the AML1-ETO plus TEL-PDGFβR recipients contained significantly higher percentages of c-kit+ Sca-1+ cells than those expressing either of the mutated AML1-ETO proteins or TEL-PDGFβR alone (Figure 4A,B). The percentage of Gr1+Mac1+ cells in the EGFP+hCD4+ BM population was correspondingly lower in the AML1-ETO plus TEL-PDGFβR group than in any other, reflective of impaired granulocyte differentiation (Figure 4A,C). Interestingly, the FACS profiles of EGFP+hCD4+ BM cells from the AML1-ETO (Y113A/T161A) plus TEL-PDGFβR group were not identical to those of the AML1-ETO (R174Q) plus TEL-PDGFβR group and appeared to be intermediate between CMPD and AML. For instance, there were fewer Gr1hiMac1hi cells in the AML1-ETO (Y113A/T161A) plus TEL-PDGFβR group, suggesting that granulocyte differentiation was somewhat more impaired (Figure 4A).
Flow cytometric analysis of BM from primary transplant recipients. (A) Whole BM of diseased mice was gated based on forward and side scatter characteristics and subsequently analyzed for expression of hCD4 (TEL-PDGFβR) and EGFP (AML1-ETO). EGFP+hCD4+ cells were then analyzed for the expression of myeloid and progenitor markers. Plots are from a single animal that is representative of the experimental group. (B) Differences in the percentage of EGFP+hCD4+ BM cells that were positive for both c-kit and Sca-1. Error bars indicate 95% confidence intervals. AE + TP, n = 21 mice; R174Q + TP, n = 22; Y113A/T161A, n = 24; TP, n = 8; AE, n = 8. Differences relative to AML1-ETO plus TEL-PDGFβR (#) for each group were determined using Dunnett test and ANOVA. (C) Differences in the percentage of EGFP+hCD4+ BM cells that were positive for both Gr-1 and Mac-1. Error bars indicate 95% confidence intervals. Differences relative to AML1-ETO plus TEL-PDGFβR (#) for each group were determined using Dunnett test and ANOVA. The percentage of Gr-1+Mac-1+ cells in mice transplanted with AML1-ETO (Y113A/T161A) plus TEL-PDGFβR transduced cells was significantly lower (P < .001) than in mice transplanted with BM expressing AML1-ETO (R174Q) plus TEL-PDGFβR or TEL-PDGFβR alone.
Flow cytometric analysis of BM from primary transplant recipients. (A) Whole BM of diseased mice was gated based on forward and side scatter characteristics and subsequently analyzed for expression of hCD4 (TEL-PDGFβR) and EGFP (AML1-ETO). EGFP+hCD4+ cells were then analyzed for the expression of myeloid and progenitor markers. Plots are from a single animal that is representative of the experimental group. (B) Differences in the percentage of EGFP+hCD4+ BM cells that were positive for both c-kit and Sca-1. Error bars indicate 95% confidence intervals. AE + TP, n = 21 mice; R174Q + TP, n = 22; Y113A/T161A, n = 24; TP, n = 8; AE, n = 8. Differences relative to AML1-ETO plus TEL-PDGFβR (#) for each group were determined using Dunnett test and ANOVA. (C) Differences in the percentage of EGFP+hCD4+ BM cells that were positive for both Gr-1 and Mac-1. Error bars indicate 95% confidence intervals. Differences relative to AML1-ETO plus TEL-PDGFβR (#) for each group were determined using Dunnett test and ANOVA. The percentage of Gr-1+Mac-1+ cells in mice transplanted with AML1-ETO (Y113A/T161A) plus TEL-PDGFβR transduced cells was significantly lower (P < .001) than in mice transplanted with BM expressing AML1-ETO (R174Q) plus TEL-PDGFβR or TEL-PDGFβR alone.
In summary, cells expressing AML1-ETO plus TEL-PDGFβR gave rise to a rapid AML as reported previously.82 Mutations in AML1-ETO that impaired DNA binding significantly weakened its activity, resulting in an extended latency and disease specificity more closely resembling that caused by TEL-PDGFβR alone. Loss of CBFβ binding also weakened AML1-ETO's activity, resulting in an extended disease latency, selection of higher AML1-ETO expressing cells, and a cell surface phenotype intermediate between AML and CMPD. These results indicate that the mutated AML1-ETO proteins were unable to appropriately regulate a subset of genes required for the development of AML.
Disruption of CBFβ binding by the Runt domain of TEL-AML1 abrogates its ability to enhance self-renewal of B-cell progenitors.
TEL-AML1 is critically dependent upon DNA binding to enhance the self-renewal of B-cell progenitors in culture.61 To determine whether disrupting CBFβ binding above a specific threshold results in functional impairment, we introduced mutations analogous to T161A and Y113A/T161A (T188A and Y140A/T188A) into TEL-AML1. As the Runt domains of AML1-ETO and TEL-AML1 are identical, we would expect the biophysical characteristics of the mutated proteins to be comparable.
We transduced c-Kit+ fetal liver cells from embryonic day 12 C57BL/6 mice with retroviruses expressing TEL-AML1 or its mutated derivatives and EGFP,61,66 and serially replated them in methylcellulose under conditions that promote B-cell differentiation. Expression of similar steady-state levels of the TEL-AML1 mutant proteins in transduced NIH3T3 cells was confirmed by Western blot analysis, and retroviral transduction of c-kit+ cells was measured by EGFP fluorescence (Figure 5A,B). Cells expressing TEL-AML1 generated increased numbers of colonies and cells, which are B220 enriched, more than multiple rounds of replating relative to cells expressing EGFP alone (Figure 5C-E). A reduction in CBFβ heterodimerization by greater than 400-fold through introduction of the Y140A/T188A mutations severely impaired TEL-AML1's ability to enhance the clonogenic potential of these B-cell precursors, as the numbers of cells and colonies were very similar to those of EGFP-transduced cells. Cells expressing the T188A TEL-AML1 mutant, which is expected to have an approximately 60-fold reduction in CBFβ binding, produced numbers of colonies and cells similar to those of TEL-AML1–expressing cells during secondary replating, however, the numbers from the third round of replating were very significantly decreased and therefore somewhat intermediate between the TEL-AML1 and TEL-AML1 (Y140A/T188A) expressing cells. It appears that, as was the case for AML1-ETO, a decrease in CBFβ binding from 60- to 400-fold is accompanied by a progressive impairment in TEL-AML1's clonogenic activity. As the thermodynamic stability and DNA binding affinity of the T118A and Y140A/T188A mutant Runt domains are identical, the decreased activity can only be explained by more severely impaired CBFβ binding.
Disrupting heterodimerization with CBFβ substantially abrogates TEL-AML1's ability to enhance the clonogenic potential of c-kit+ B-cell precursors. (A) Detection of hemagglutinin (HA)–tagged TEL-AML1 and the T188A and Y140A/T188A mutants in NIH3T3 cells 48 hours after retroviral (murine stem cell virus [MSCV]) transduction. Vertical lines have been inserted to indicate a repositioned gel lane. (B) Flow cytometric analysis of EGFP expression in c-kit+ fetal liver cells 48 hours after infection with viral supernatants. (C) Transduced c-kit+ cells grown in methylcellulose serial replating assays in conditions promoting B-cell differentiation (IL-7, SCF, and FLT3 ligand). The number of colonies harvested from each culture, per 104 cells plated, is shown for the first (□), second ( ), and third (■) rounds of plating. Plots show means and SDs of duplicate cultures. The data shown are representative of 5 independent experiments. (D) Total number of cells, as in panel C. (E) Number of B220+ cells as in panel C.
), and third (■) rounds of plating. Plots show means and SDs of duplicate cultures. The data shown are representative of 5 independent experiments. (D) Total number of cells, as in panel C. (E) Number of B220+ cells as in panel C.
Disrupting heterodimerization with CBFβ substantially abrogates TEL-AML1's ability to enhance the clonogenic potential of c-kit+ B-cell precursors. (A) Detection of hemagglutinin (HA)–tagged TEL-AML1 and the T188A and Y140A/T188A mutants in NIH3T3 cells 48 hours after retroviral (murine stem cell virus [MSCV]) transduction. Vertical lines have been inserted to indicate a repositioned gel lane. (B) Flow cytometric analysis of EGFP expression in c-kit+ fetal liver cells 48 hours after infection with viral supernatants. (C) Transduced c-kit+ cells grown in methylcellulose serial replating assays in conditions promoting B-cell differentiation (IL-7, SCF, and FLT3 ligand). The number of colonies harvested from each culture, per 104 cells plated, is shown for the first (□), second ( ), and third (■) rounds of plating. Plots show means and SDs of duplicate cultures. The data shown are representative of 5 independent experiments. (D) Total number of cells, as in panel C. (E) Number of B220+ cells as in panel C.
), and third (■) rounds of plating. Plots show means and SDs of duplicate cultures. The data shown are representative of 5 independent experiments. (D) Total number of cells, as in panel C. (E) Number of B220+ cells as in panel C.
Discussion
Assessing which protein-protein interactions mediated by oncogenic transcription factors are essential for their leukemogenic activity, and determining the structures of the domains responsible for those interactions will greatly facilitate the development of specific inhibitors by structure-aided drug design. Here, we show that ablation of DNA binding by 40 000 fold30,49 or reduction of CBFβ heterodimerization by greater than 400-fold partially impaired AML1-ETO's ability to inhibit granulocyte differentiation, almost completely reversed its acute inhibition of proliferation, eliminated its ability to confer serial replating to myeloid progenitors, and severely compromised its cooperation with TEL-PDGFβR to cause AML, and thus impaired or delayed AML disease. Similarly, inhibition of both DNA61 and CBFβ binding by TEL-AML1 eliminated its ability to enhance the self-renewal of B-cell progenitors. We are optimistic that targeted disruption of either function may provide an effective therapeutic modality. The more promising of the 2 interactions for small molecule inhibitors is that between the Runt domain and CBFβ, since the Runt domain-DNA interface is highly charged and may be difficult to target with a small molecule. The Runt domain-CBFβ interface, on the other hand, contains a large hydrophobic area,36,62 which should be more conducive for binding small molecules with drug-like properties. Although small molecules targeting protein-protein interactions are relatively limited thus far, there has been an increase in activity and reported success in developing these inhibitors.85 For example, we recently developed a small molecule that binds CBFβ and allosterically inhibits CBFβ binding to the Runt domain, supporting the notion that disrupting this interaction with drug-like molecules is feasible.86 A potential drawback to this approach is that the inhibitors would also further impair the function of the normal core binding factors, in which case there would need to be a therapeutic window for them to be clinically useful. Alternatively, combined therapies using inhibitors targeting other functions specific for AML1-ETO (eg, oligomerization15,49,87 ) may provide specificity.
We find it encouraging that both AML1-ETO and TEL-AML1 rely on CBFβ for their activity, and therefore a single compound may prove efficacious in both disease settings. In fact, we predict that all the Runx1 fusion proteins will require CBFβ for their activity and that an inhibitor of the Runt domain-CBFβ interaction may be useful for targeting all of these proteins. Although CBFβ is clearly important for AML1-ETO and TEL-AML1 function, the nature of its activity is not completely understood. It has long been known that CBFβ increases the Runx proteins' affinity for DNA,88,89 and it was more recently shown that it protects them from ubiquitin-mediated proteolysis.37,90 However, genetic data in both flies and worms hint at other functions. For example, overexpression of a Drosophila Runt protein that could bind DNA but not CBFβ dominantly inhibited its wild-type counterpart, suggesting that it was occupying its DNA binding sites but forming nonfunctional complexes.91 More recently, it was shown that ectopic overexpression of CBFβ (BRO-1) could partially restore a Runx (RNT)–deficient defect in Caenorhabditis elegans.92 Thus we speculate that CBFβ participates in the formation of protein complexes that are necessary for AML1-ETO and TEL-AML1 function in vivo. If this is the case, and if the nature of those complexes differs from those formed by the wild-type Runx1-CBFβ heterodimer then other protein-protein interactions specific for the fusion protein complexes could lend themselves to targeting with small molecules.
It has been suggested that much of AML1-ETO's activity may be mediated in a manner that does not require binding to Runx-dependent promoters.13 Our data support the notion that some of AML1-ETO's activity indeed does not require either DNA or CBFβ binding, in that ectopic (retroviral) expression of a mutant defective in both functions could still significantly repress granulocyte differentiation of BM cells in vitro. Although repression of granulocyte differentiation is a consistent feature of AML1-ETO overexpression,15,25,70,73,75 it is not entirely clear how important it is to AML1-ETO's leukemogenic function. We found several AML1-ETO mutants that were able to partially repress granulocyte differentiation but had no leukemogenic activity, nor was a granulocyte differentiation defect observed in a conditional AML1-ETO knock-in model.69 What is clear, however, from our data and that of Yan et al,49 is that DNA binding by AML1-ETO is essential for its leukemogenic activity and that this observation must be incorporated into any mechanistic model for AML1-ETO function.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Gary Gilliland for the TEL-PDGFBR cDNA, Thomas Graf for the MSV2.2-IRES-hCD4 vector, Warren Pear for the MigR1 vector, Gary Ward for help with cell sorting, and Shah Kadri for help with the analysis of mice.
This study was supported by the Leukemia & Lymphoma Society (White Plains, NY; 7006-05, J.H.B. and N.A.S.) and the Leukemia Research Fund, London, United Kingdom (O.W.; T32GM08704, M.D.C.). Flow cytometry was supported in part by the Core Grant of the Norris Cotton Cancer Center (Hanover, NH; CA23108).
National Institutes of Health
Note added in proof:
Kwok et al (Proc Natl Acad Sci U S A. 2009 Feb 6. [Epub ahead of print] PMID: 19202074) confirmed our finding that a single amino acid substitution in the Runt domain at the CBFβ interface was insufficient to impair AML1-ETO activity.
Authorship
Contribution: M.D.C. performed the experiments described in Figures 1B,D and 2B-E; L.R. and E.M. performed the experiments described in Figures 3 and 4; W.C. performed the experiments described in Figure 2F,G; M.M. performed the experiments described in Figure 5; S.P. performed the experiments described in Figure 1E; C.L. performed Western blot analysis; P.K. analyzed the histology; O.W. participated in the design and interpretation of the experiments in Figure 5; and J.H.B. and N.A.S. participated in the design and interpretation of the experiments and writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nancy A. Speck, Abramson Family Cancer Research Institute and Department of Cell and Developmental Biology, University of Pennsylvania, 511 BRB 11/111, 421 Curie Blvd, Philadelphia, PA 19104; e-mail: nancyas@exchange.upenn.edu; or John H. Bushweller, Department of Chemistry, University of Virginia, 1300 Jefferson Park Ave, Charlottesville, VA 22908; e-mail: jhb4v@virginia.edu.
References
Author notes
*L.R. and M.D.C. contributed equally to this work.

![Figure 1. Amino acid substitutions in the Runx1 Runt domain that specifically disrupt DNA and CBFβ binding. (A) Structures of the Runt domain and CBFβ are shown in gray and blue, respectively, and the DNA is purple.36 The R174 side chain in the Runt domain is green, and the T161 and Y113 side chains are orange. (B) Urea denaturation monitored by tryptophan fluorescence for the wild-type Runt domain and point mutants thereof. Plotted is the fraction of unfolded Runt domain in the presence of increasing concentrations of urea. Data from the wild-type Runt domain, R174Q, and T161A are from Matheny et al.30 (C) Diagram of the potential interactions between the Runt Domain, CBFβ, and DNA. (D) EMSA measuring the affinity of the Y113A/T161A Runt domain for DNA (K2). Shown is a representative example of 3 experiments. Triangles indicate decreasing concentrations of the Runt domain (2 × 10−6 to 4 × 10−15 M). Arrow indicates the lane in which the Runt domain concentration approximates K2. (E) Isothermal titration calorimetric measurements of CBFβ binding to the wild-type, T161A, and Y113A/T161A mutant Runt domains. Wild-type Runt domain (45 μM) was titrated with 440 μM CBFβ at 26°C, and 42 μM T161A or 38 μM Y113A/T161A were titrated with 396 μM CBFβ at 22°C. In each panel, the top portion is the raw data, and the bottom panel is a plot of the binding corrected for dilution enthalpy. Experimental data (squares) are fit to a one-site binding model (line). The equilibrium dissociation constants (K1) are indicated in the plots. The average values from 2 independent measurements (± standard deviation [SD]) are given.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/13/10.1182_blood-2008-03-147207/4/m_zh80160933800001.jpeg?Expires=1769099500&Signature=VGEjowXJ62zyBozy8YpbMhYwnf8pTvW0kNyHv1NljL7B9kaNjxkqzH9chwU7EeggRzmhkqHChAln2r16s-AR9-dEsMOxxeImJCVbPJmF8HHvL8Rbbk9z1PGlx~JgeNoCwKUlw2zHYc7mwY~nvbUSMUrZihg1xnChCMx6KnFNNDmOIkfNUWwu51uW5MiZYFqe2TL3PKY3YO8uZppOSKymdF2WNM4kPznPw4c8yLrN~yhpgmuFWCHRhlFBe0D7h3WFXa2CBqlLUqob~q1fVbYg~y6aUkfgHg43BAODHjRXDEwEpeCyjFJcJeX1LJMmq0IiC4xzyVuAMqTWusjqfKCOXw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. AML1-ETO function is impaired by mutations that disrupt DNA or CBFβ binding. (A) Diagram of AML1-ETO and location of the mutations affecting DNA (R174Q) and CBFβ binding (T161A and Y113A/T161A). (B) Lin− BM cells infected with MigR1 retroviruses expressing AML1-ETO and mutated derivatives after 7 days of culture in the presence of IL-3, IL-6, SCF, and G-CSF. EGFP+ gated cells (not shown) were analyzed for Gr-1 and Mac-1 expression. (C) Summary of data illustrated in panel B compiled from 2 experiments, each with triplicate samples. **P ≤ .01 compared with AML1-ETO (#); ***P ≤ .001 (Dunnett test and analysis of variance [ANOVA]). (D) Serial replating analysis. Graphs represent the average number of colonies from each round of replating in the presence of IL-3, IL-6, and SCF. Week 1 represents colony numbers per 103 cells plated, weeks 2 and 3 are from 104 plated cells. Numbers are averaged from 2 experiments, each containing triplicate samples. (E) Western blot analysis probed with an antibody to the Runt domain, demonstrating expression of AML1-ETO and its mutated derivatives in MigR1-transduced NIH3T3 cells. (F) BrdU incorporation 48 hours after transduction of Lin− BM cells with AML1-ETO and its mutants. EGFP+ cells were analyzed after a 1 hour BrdU pulse for BrdU and 7-AAD incorporation. Shown is a representative of 3 experiments. (G) Average percentages of gated BrdU+ (S phase) cells from scatter plots in panel F (n = 3). Error bars indicate 95% confidence intervals. **P ≤ .01 compared with AML1-ETO (Dunnett test and ANOVA).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/13/10.1182_blood-2008-03-147207/4/m_zh80160933800002.jpeg?Expires=1769099500&Signature=xpj40MHoJdmA5BBccRxOTRkUwQxleKJFEusRx9E-VtaT3zELBaekeN68dZlHpYQM~V6VdVQA5467ut3~FoK4zz8D~HxskdhpuEe5RYa~t9eRZfEsW84WJ1wDV5ymChCNLXQxmo-V2ljbYkhfydovVP9qJLCZRcT6w-1LXZzrFjSUUgYZ3669qcTNil4s1M4F5KLtq8SW9OUDrXPraPCGqbbW5JeJp0Wb7P6kyEFPmxe5noXqA9bbEHogReF-rU8Jket6ezKR4I8j7K7BZ7jdYYm3gThxs6AAjLQWiA~lhlTle1tqmNuLxAXCiQQ0dC0zkRjqanm838~NHjT3z1K4Tg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Disrupting heterodimerization with CBFβ substantially abrogates TEL-AML1's ability to enhance the clonogenic potential of c-kit+ B-cell precursors. (A) Detection of hemagglutinin (HA)–tagged TEL-AML1 and the T188A and Y140A/T188A mutants in NIH3T3 cells 48 hours after retroviral (murine stem cell virus [MSCV]) transduction. Vertical lines have been inserted to indicate a repositioned gel lane. (B) Flow cytometric analysis of EGFP expression in c-kit+ fetal liver cells 48 hours after infection with viral supernatants. (C) Transduced c-kit+ cells grown in methylcellulose serial replating assays in conditions promoting B-cell differentiation (IL-7, SCF, and FLT3 ligand). The number of colonies harvested from each culture, per 104 cells plated, is shown for the first (□), second (), and third (■) rounds of plating. Plots show means and SDs of duplicate cultures. The data shown are representative of 5 independent experiments. (D) Total number of cells, as in panel C. (E) Number of B220+ cells as in panel C.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/13/10.1182_blood-2008-03-147207/4/m_zh80160933800005.jpeg?Expires=1769099500&Signature=nX9H-AhktPY9sqZjPoEx8wmJIlkBtrGFWmeDkirRgUUyMb8Ci9EuixopF64hEcEJ2viyL2RC643virdpjGfzdQQb1Lbnho-wrCcwTQp5zzZbY5ENDVIoGkV-er6~E9H3824fgBRjwx~CGaLTAhWdoQpKPAQjuDSbZ8Dq9xsZJLofSFHpxV--aTJKMEMbGS7t7HrGR4CHUcbf6Gi3yy~TJ-797zm8Ua2Il42SLe3luuGfZ03syglB-ISH3SYiJXlN0Hkf5obkoZiXVyMeqUJl68lZ-iS1ZzFpv-5-Wp7RQ7se8Q3f44jTVqeCXa4KhbwTdPuPFx~Ri34TYkiGdsJCOg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 1. Amino acid substitutions in the Runx1 Runt domain that specifically disrupt DNA and CBFβ binding. (A) Structures of the Runt domain and CBFβ are shown in gray and blue, respectively, and the DNA is purple.36 The R174 side chain in the Runt domain is green, and the T161 and Y113 side chains are orange. (B) Urea denaturation monitored by tryptophan fluorescence for the wild-type Runt domain and point mutants thereof. Plotted is the fraction of unfolded Runt domain in the presence of increasing concentrations of urea. Data from the wild-type Runt domain, R174Q, and T161A are from Matheny et al.30 (C) Diagram of the potential interactions between the Runt Domain, CBFβ, and DNA. (D) EMSA measuring the affinity of the Y113A/T161A Runt domain for DNA (K2). Shown is a representative example of 3 experiments. Triangles indicate decreasing concentrations of the Runt domain (2 × 10−6 to 4 × 10−15 M). Arrow indicates the lane in which the Runt domain concentration approximates K2. (E) Isothermal titration calorimetric measurements of CBFβ binding to the wild-type, T161A, and Y113A/T161A mutant Runt domains. Wild-type Runt domain (45 μM) was titrated with 440 μM CBFβ at 26°C, and 42 μM T161A or 38 μM Y113A/T161A were titrated with 396 μM CBFβ at 22°C. In each panel, the top portion is the raw data, and the bottom panel is a plot of the binding corrected for dilution enthalpy. Experimental data (squares) are fit to a one-site binding model (line). The equilibrium dissociation constants (K1) are indicated in the plots. The average values from 2 independent measurements (± standard deviation [SD]) are given.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/13/10.1182_blood-2008-03-147207/4/m_zh80160933800001.jpeg?Expires=1769099501&Signature=qRtIL~vFDowUIva2~ufOcrVTjBfGERziYRw7kz4hmuqZkdhmjHz1a3u8r9nb8hL79474OrnKyd5OdTEqIYHCIY-YOdiU7TwZzjFVZ0mVUwVO90eTfKKTRS3~KvDaZIu1CMm6dpvRrrlymigjtAwFQY-SN1qRw-We45DSmNk3eYmVIGPe61dCk1BXTF-q8t7hAiEEi2bv4C0tYzJPrr6WnS8an5rLCmwabueObxKZJv4C7eKCKbclFzfQ4~jFBBxCXxS4IzSIiDiQgh46Rvsyf1zT80f3JII7WGNoKX2J4NzibpNRXdCqCt3nodQFIVbusq7kOB3fIjdZXjoU6rARcg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. AML1-ETO function is impaired by mutations that disrupt DNA or CBFβ binding. (A) Diagram of AML1-ETO and location of the mutations affecting DNA (R174Q) and CBFβ binding (T161A and Y113A/T161A). (B) Lin− BM cells infected with MigR1 retroviruses expressing AML1-ETO and mutated derivatives after 7 days of culture in the presence of IL-3, IL-6, SCF, and G-CSF. EGFP+ gated cells (not shown) were analyzed for Gr-1 and Mac-1 expression. (C) Summary of data illustrated in panel B compiled from 2 experiments, each with triplicate samples. **P ≤ .01 compared with AML1-ETO (#); ***P ≤ .001 (Dunnett test and analysis of variance [ANOVA]). (D) Serial replating analysis. Graphs represent the average number of colonies from each round of replating in the presence of IL-3, IL-6, and SCF. Week 1 represents colony numbers per 103 cells plated, weeks 2 and 3 are from 104 plated cells. Numbers are averaged from 2 experiments, each containing triplicate samples. (E) Western blot analysis probed with an antibody to the Runt domain, demonstrating expression of AML1-ETO and its mutated derivatives in MigR1-transduced NIH3T3 cells. (F) BrdU incorporation 48 hours after transduction of Lin− BM cells with AML1-ETO and its mutants. EGFP+ cells were analyzed after a 1 hour BrdU pulse for BrdU and 7-AAD incorporation. Shown is a representative of 3 experiments. (G) Average percentages of gated BrdU+ (S phase) cells from scatter plots in panel F (n = 3). Error bars indicate 95% confidence intervals. **P ≤ .01 compared with AML1-ETO (Dunnett test and ANOVA).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/13/10.1182_blood-2008-03-147207/4/m_zh80160933800002.jpeg?Expires=1769099501&Signature=qR3Jxs6Y1o4h~JwP1MnRNdbW-e8WCNAHf1T4vp4upSMvp8FjxMlADN3q1Irzia3NIN7UUOkZLIJPPaspbnqaQ8vZ1OkSHT1of1AiNKDR406gxu6RctcY1pUilmIFqAFYF4j51s8mgsrG~i6CkwXB9bLAXBqpK1elQ5t20Uk4vYduwed2r9RMQiaSP3iW7Bi5KtdswEPN7EVmYQQq6e545TbzIPohACuMLsIIvpmGh556X6jxo4BjRCUfembGyNvdjCBaZ06NCfp4ajTi6PhEM7s39c8TLRaEwfDG5CRPcajTqNMoJbGIl3CXqMXVOuUYQ25atykgnVWfw2zo51BIPA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 5. Disrupting heterodimerization with CBFβ substantially abrogates TEL-AML1's ability to enhance the clonogenic potential of c-kit+ B-cell precursors. (A) Detection of hemagglutinin (HA)–tagged TEL-AML1 and the T188A and Y140A/T188A mutants in NIH3T3 cells 48 hours after retroviral (murine stem cell virus [MSCV]) transduction. Vertical lines have been inserted to indicate a repositioned gel lane. (B) Flow cytometric analysis of EGFP expression in c-kit+ fetal liver cells 48 hours after infection with viral supernatants. (C) Transduced c-kit+ cells grown in methylcellulose serial replating assays in conditions promoting B-cell differentiation (IL-7, SCF, and FLT3 ligand). The number of colonies harvested from each culture, per 104 cells plated, is shown for the first (□), second (), and third (■) rounds of plating. Plots show means and SDs of duplicate cultures. The data shown are representative of 5 independent experiments. (D) Total number of cells, as in panel C. (E) Number of B220+ cells as in panel C.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/13/10.1182_blood-2008-03-147207/4/m_zh80160933800005.jpeg?Expires=1769099501&Signature=eQUGxW~SEC8xsLSelVAuYex5M110yw9S7hFUJk-5sCxtDRr4h8X25iqCXY67zYQL~qr81L-n-AYBLhltIad2JdgRiNyzt2153vkO82r2aO0Jct7rM0lZjuze~CVSTXULplhtaSxNbHY2h6hFK9dIRSJbfPx7Uf9NC3hqNlB8vWv48nju5ohSkEn9LMJe8lM0JYjmQlake7m7Jhimdz7jRLFLi1bucA6iV-9MzwKDKpjUTTi2hAaZGUP9dJDR6obTfjpaKDi0tYx7wgPbisfGuqlZZPNDypYAo-ts-D4CgHNDcfyAubFCcGhYz9sVcMQQo7o3fcXgzzBP-gCwattIGw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
 ), and third (■) rounds of plating. Plots show means and SDs of duplicate cultures. The data shown are representative of 5 independent experiments. (D) Total number of cells, as in panel C. (E) Number of B220+ cells as in panel C.
), and third (■) rounds of plating. Plots show means and SDs of duplicate cultures. The data shown are representative of 5 independent experiments. (D) Total number of cells, as in panel C. (E) Number of B220+ cells as in panel C.