Abstract
We recently demonstrated that blockade of the platelet adhesion receptor glycoprotein (GP) Ibα protects mice from ischemic stroke. Although von Willebrand factor (VWF) is the major ligand for GPIbα, GPIbα can engage other counterreceptors on endothelial cells, platelets, and leukocytes (eg, Mac-1 or P-selectin) potentially involved in stroke outcome. To further analyze whether VWF is of particular relevance for stroke development, VWF−/− mice underwent 60 minutes of middle cerebral artery occlusion. After 24 hours, VWF−/− mice had significantly smaller infarctions (P < .05) and less severe neurologic deficits (P < .01) compared with controls. This effect was sustained after 1 week, and intracranial bleeding was absent in VWF−/− mice as revealed by serial magnetic resonance imaging. Hydrodynamic injection of a VWF-encoding plasmid restored the susceptibility for stroke in VWF−/− mice. This study indicates that VWF is critically involved in cerebral ischemia. Hence, targeted inhibition of the GPIbα-VWF pathway might become a promising therapeutic option.
Introduction
Ischemic stroke is mainly caused by thromboembolic occlusion of brain arteries. During the course of cerebral ischemia, platelet-derived thrombus formation at the site of the damaged endothelium occurs in defined steps comprising platelet adhesion, activation, and aggregation.1 Using novel antibodies against glycoproteins (GP) expressed on the surface of platelets,2 we recently demonstrated that inhibition of GPIbα protects mice from ischemic stroke without causing intracerebral hemorrhage.3 GPIbα can bind different counterreceptors on endothelial cells, platelets, and white blood cells such as von Willebrand factor (VWF), Mac-1 or P-selectin.4 The key question of which of these engagements is of particular relevance for stroke development awaits clarification. VWF is the principal ligand of GPIbα.4,5 Under conditions of high shear, present for instance in stenosed arteries prone to cause stroke, the interaction between GPIbα and VWF is indispensable for plug formation.6,7
We here show that VWF deficiency protects mice from ischemic stroke without causing intracerebral hemorrhage. Together with our previous findings,3 this study suggests that GPIbα-VWF interactions represent a central pathophysiologic event during cerebral ischemia. Inhibition of the GPIbα-VWF pathway might become a promising strategy to treat ischemic stroke in the future.
Methods
Induction of cerebral ischemia
Animal experiments were approved by the Institutional Review Board of the University of Wuerzburg, Wuerzburg, Germany, and conducted according to the recommendations for research in basic stroke studies.8 VWF−/− mice were described previously.9 C57BL/6 wild-type (WT) mice served as controls. Cerebral ischemia was induced in 6- to 8-week-old mice by 60 minutes of middle cerebral artery occlusion, as described (see Document S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).3,10 Laser-Doppler flowmetry was used to monitor cerebral blood flow, and cerebral vasculature was assessed by perfusion with black ink (see Document S1 and Figure S1).
Reconstitution of VWF plasma levels
Assessment of functional outcome
Determination of infarct size and histology
Edema-corrected infarct volumes were quantified by planimetry 24 hours after ischemic stroke as described.3,10 For morphologic assessment, paraffin-embedded brains were stained with hematoxylin and eosin (H&E) and examined under an Axioplan 2 microscope (Carl Zeiss, Jena, Germany) connected to a CCD camera (Spot Insight 4Meg FW Color Mosaic; Diagnostic Instruments, Sterling Heights, MI). For data acquisition, Metaview Software (Visitron Systems, Pucheim, Germany) was used.
Stroke assessment by magnetic resonance imaging
Magnetic resonance imaging (MRI) was performed repeatedly at 24 hours and 7 days after stroke on a 1.5-Tesla MR unit (Vision Siemens, Erlangen, Germany).3,10 For all measurements, a custom-made dual-channel surface coil designed for examination of mice was used (A063HACG; Rapid Biomedical, Wuerzburg, Germany). The image protocol comprised a coronal T2-w sequence (slice thickness 2 mm) and a coronal 3-dimensional T2-w gradient echo constructed interference in steady state (slice thickness 1 mm) sequence. MR images were assessed blinded to the experimental group with respect to infarct morphology and the occurrence of intracerebral bleeding.
Statistical analysis
Data are expressed as mean plus or minus SD. For statistical analysis, Prism Graph version 4.0 software (GraphPad Software, La Jolla, CA) was used. Infarct volumes and neurologic scores were tested for Gaussian distribution with the D‘Agostino and Pearson omnibus normality test and then analyzed using the unpaired 2-tailed Student t test. P values less than .05 were considered statistically significant.
Results and discussion
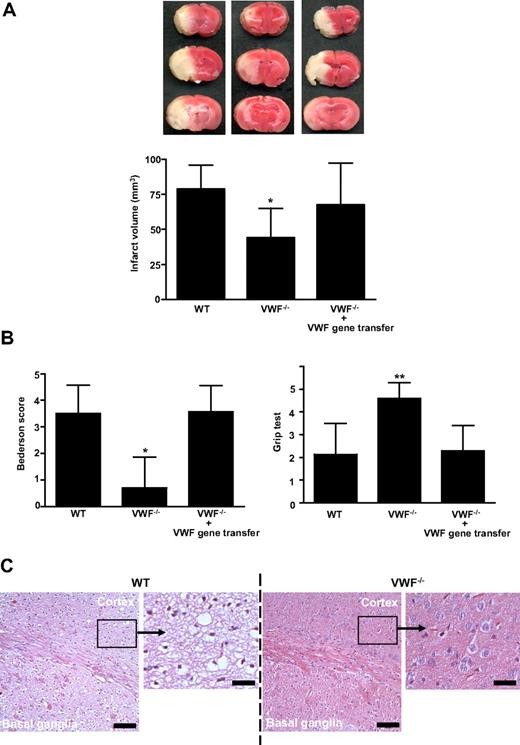
To further clarify whether GPIbα-VWF interactions are of particular relevance for stroke development,3 mice deficient in VWF9 were subjected to middle cerebral artery occlusion. The infarct volumes, 24 hours after reperfusion, in VWF−/− mice were reduced to approximately 60% of the infarct volumes in WT mice (44.0 ± 21,1 mm3 vs 78.8 ± 17.2 mm3; P < .05; Figure 1A). The reduction in infarct size was functionally relevant, as the Bederson score (0.70 ± 1.2 vs 3.5 ± 1.1; P < .05) assessing global neurologic function and the grip test (P < .01), which specifically measures motor function and coordination, were significantly better in VWF−/− mice (Figure 1B). Consistent with the triphenyltetrazolium chloride (TTC) stains, histology revealed large hemispheric infarctions in WT animals while tissue damage was restricted to the basal ganglia in VWF−/− mice (Figure 1C). Reconstitution of plasma VWF by hydrodynamic gene transfer11,12 (mean VWF plasma levels in reconstituted VWF−/− mice: 100% ± 49%, n = 8) restored the susceptibility of VWF−/− mice to ischemic stroke (Figure 1A,B). These rescue experiments provide strong evidence that the protection conferred by VWF deficiency is specifically caused by the absence of plasma VWF but not VWF derived from other compartments (eg, platelets or endothelial cells). Moreover, concomitant changes induced by the lack of VWF, such as absence of Weibel-Palade bodies, are obviously less important during ischemic brain damage.
Infarct volumes and functional outcomes 24 hours after transient middle cerebral artery occlusion in WT mice, VWF−/− mice, and VWF−/− mice after reconstitution with plasma VWF (gene transfer). (A top) Representative 2,3,5-TTC stains of 3 corresponding coronal brain sections of the 3 groups at day 1 after transient middle cerebral artery occlusion (tMCAO). (Bottom) Brain infarct volumes of the 3 groups as measured by planimetry at day 1 after tMCAO (n = 10 per group). (B) Neurologic Bederson score (left) and grip test score (right) of the 3 groups as assessed at day 1 after tMCAO (n = 10 per group). (C) Hematoxylin and eosin (H&E)–stained sections of corresponding territories in the ischemic hemispheres of wild-type (WT) and von Willebrand factor (VWF)−/− mice. Infarcts are restricted to the basal ganglia in VWF−/− mice but consistently include the neocortex in WT controls. Bar represents 100 μm or 20 μm (enlarged picture), **P < .01, *P < .05; unpaired 2-tailed Student t test compared with WT mice.
Infarct volumes and functional outcomes 24 hours after transient middle cerebral artery occlusion in WT mice, VWF−/− mice, and VWF−/− mice after reconstitution with plasma VWF (gene transfer). (A top) Representative 2,3,5-TTC stains of 3 corresponding coronal brain sections of the 3 groups at day 1 after transient middle cerebral artery occlusion (tMCAO). (Bottom) Brain infarct volumes of the 3 groups as measured by planimetry at day 1 after tMCAO (n = 10 per group). (B) Neurologic Bederson score (left) and grip test score (right) of the 3 groups as assessed at day 1 after tMCAO (n = 10 per group). (C) Hematoxylin and eosin (H&E)–stained sections of corresponding territories in the ischemic hemispheres of wild-type (WT) and von Willebrand factor (VWF)−/− mice. Infarcts are restricted to the basal ganglia in VWF−/− mice but consistently include the neocortex in WT controls. Bar represents 100 μm or 20 μm (enlarged picture), **P < .01, *P < .05; unpaired 2-tailed Student t test compared with WT mice.
In accordance with our observations, lack of VWF exhibited profound antithrombotic effects in other in vivo clotting models: Thrombus formation in mesenterial vessels after superfusion with ferric chloride was significantly reduced in VWF−/− mice.9,11 Moreover, antibodies against VWF reversed cyclic flow reductions after experimental femoral or coronary artery stenosis.15,16
Although our study using VWF−/− mice cannot definitely differentiate between the contribution of VWF-GPIbα and VWF-collagen interactions, the present results, in context with our previous complementary findings on the central role of GPIbα for platelet adhesion2 and stroke formation,3 emphasize the functional significance of the GPIbα-VWF pathway in the pathophysiology of ischemic stroke. In support of this notion, elevated serum levels of VWF are an independent stroke risk factor in humans,17,18 and polymorphisms of platelet GPIbα exist that are associated with an increased risk of stroke due to enhanced VWF-GPIbα engagement.19,20
Apart from GPIbα, VWF can also bind the platelet integrin αIIbβ3,4,5 providing an alternative explanation why VWF−/− mice are less sensitive to ischemic stroke. VWF-αIIbβ3 engagement is probably only functional, however, when platelets are already immobilized and activated, as αIIbβ3 requires inside-out activation for VWF to bind.4,5 At the high shear rates typically found during arterial stenosis or brain ischemia-reperfusion, platelet adhesion and even aggregation is entirely dependent on the GPIbα-VWF axis.6,7
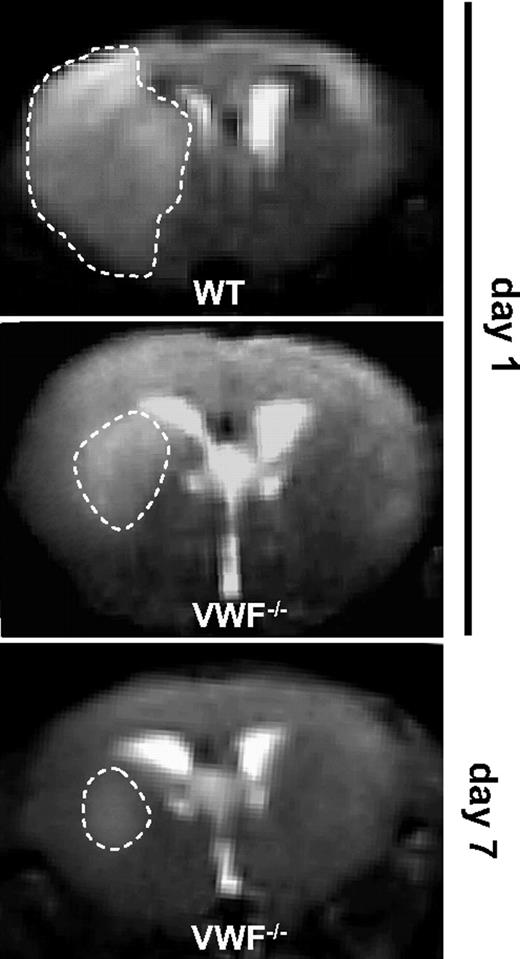
In contrast to GPIbα, VWF appears not to be essential for thrombus formation as platelet aggregation was strongly delayed but not absent in VWF−/− mice after vessel wall injury.21 Because deferred clotting in VWF−/− mice could influence stroke outcome at later time points, we analyzed the infarct course in individual animals over time by serial MRI. In line with the TTC stainings (Figure 1), infarctions at day 1 after stroke were smaller in VWF−/− mice than in WT mice (Figure 2). Importantly, infarctions in VWF−/− mice remained restricted to the basal ganglia at day 7, thus excluding delayed infarct growth (Figure 2). Infarctions in both VWF−/− mice and WT mice always appeared hyperintense on blood-sensitive gradient echo MRI (Figure 2). Hypointense areas, which would indicate intracerebral hemorrhage, were absent in all animals (WT and VWF−/−) after experimental stroke. These findings exclude an increased rate of intracerebral hemorrhage in VWF−/− mice and are in line with the observation that adult VWF−/− mice do not show spontaneous bleeding.9 Because mice treated with anti-GPIbα antibodies also did not suffer from intracerebral hemorrhage after middle cerebral artery occlusion,3 it additionally underlines that inhibition of GPIbα-VWF binding during stroke appears to be safe.
Serial magnetic resonance (MR) images of cerebral infarcts after tMCAO in WT and VWF−/− mice. Serial coronal T2-weighted gradient echo MR sequences show hyperintense (bright) ischemic lesions (white dashed lines) at day 1 after tMCAO in WT mice (top) and VWF−/− mice (middle). Infarcts at day 1 are smaller in VWF−/− mice than in WT mice and remain restricted to the basal ganglia at day 7 (bottom) excluding delayed infarct growth. Hypointense (dark) areas indicative of intracerebral hemorrhage were always absent during the infarct course in both VWF−/− mice (middle and bottom) and WT controls (top; n = 8 per group).
Serial magnetic resonance (MR) images of cerebral infarcts after tMCAO in WT and VWF−/− mice. Serial coronal T2-weighted gradient echo MR sequences show hyperintense (bright) ischemic lesions (white dashed lines) at day 1 after tMCAO in WT mice (top) and VWF−/− mice (middle). Infarcts at day 1 are smaller in VWF−/− mice than in WT mice and remain restricted to the basal ganglia at day 7 (bottom) excluding delayed infarct growth. Hypointense (dark) areas indicative of intracerebral hemorrhage were always absent during the infarct course in both VWF−/− mice (middle and bottom) and WT controls (top; n = 8 per group).
Our study demonstrates that deficiency of the main GPIbα ligand VWF, like blocking GPIbα itself,3 protects mice from brain ischemia without inducing excessive bleeding. These findings underline the important pathophysiologic role of VWF during ischemic stroke. Thus, inhibition of the GPIbα-VWF pathway might open new avenues for the safe treatment of stroke in the future.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Prof U. Walter for valuable comments and Melanie Glaser, Gabi Köllner, and Virgil Michels for excellent technical assistance.
This work was supported by the Deutsche Forschungsgemeinschaft (Bonn, Germany; SFB688 grants A1/B1 to B.N. and G.S.), the Interdisziplinäres Zentrum für Klinische Forschung, (University of Wuerzburg, Wuerzburg, Germany; IZKF grant E35 to C.K.), and by the Flemish Fonds voor Wetenschappelijk Onderzoek (FWO; Brussels, Belgium; grant G.0299.06 to H.D.). S.F.D.M. and K.V. are postdoctoral fellows of the FWO.
Authorship
Contribution: C.K. performed the stroke experiments, analyzed the data, designed the research, funded the project, and wrote the paper; S.F.D.M. designed the research, provided the VWF−/− mice, determined VWF plasma levels, analyzed the data, and corrected the manuscript; T.S. and K.V. performed VWF gene transfer and operated on the reconstituted VWF−/− mice; M.A. performed the stroke experiments, collected the functional scores, and analyzed the data; and B.N., H.D., and G.S. designed the research, funded the project, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christoph Kleinschnitz, MD, Department of Neurology, Julius-Maximilians-University of Wuerzburg, Josef-Schneider Strasse 11, D-97080 Wuerzburg, Germany; e-mail: christoph.kleinschnitz@mail.uni-wuerzburg.de.