Abstract
Second-generation tyrosine kinase inhibitors are effective in Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia (ALL) and chronic myeloid leukemia (CML). Occasionally, patients with Ph+ ALL, or accelerated phase (AP) or blast phase (BP) CML achieve a major cytogenetic response (MCyR) but not a complete hematologic response (CHR). We analyzed 126 patients with CML in AP or BP, or with Ph+ ALL treated with dasatinib or nilotinib after imatinib failure. Twenty patients received sequential treatment with both dasatinib and nilotinib for a total of 146 instances. CHR and MCyR rates were 54% and 37%, respectively in AP, 17% and 39% in BP, and 33% and 50% in Ph+ ALL. Failure to achieve a CHR at the time of achievement of a MCyR was associated with an inferior outcome, similar to that of patients without a MCyR (2-year survival rate, 37% and 35%, respectively). In contrast, patients with MCyR and concomitant CHR had a 77% 2-year survival rate. Twelve of 29 patients with MCyR without concomitant CHR later achieved a CHR; the 2-year survival rate for these patients was 55% compared with 22% for those who never achieved a CHR. These results suggest that achievement of a MCyR without concomitant CHR is associated with poor outcome.
Introduction
The second-generation tyrosine kinase inhibitors (TKIs) like nilotinib and dasatinib have demonstrated significant efficacy among patients with Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia (ALL) and chronic myeloid leukemia (CML) in all phases. Myelosuppression is a common adverse event for both agents; the incidence of grade 3 to 4 neutropenia is 30% in chronic phase CML with nilotinib and up to 50% with dasatinib after imatinib failure. The corresponding rates for grade 3 to 4 thrombocytopenia are 28% for nilotinib and 49% for dasatinib.1,2 These figures are higher in advanced phases of CML or with Ph+ ALL.3-12
In many instances, patients with Ph+ ALL or advanced phase CML treated with second generation TKIs achieve a cytogenetic response that does not meet the criteria for a complete hematologic response (CHR), often because of persistent cytopenias. For example, among patients treated with nilotinib in accelerated phase (AP) after imatinib failure, the reported rate of a major cytogenetic response (MCyR) is 31%, while the CHR rate was only 26%.11,12 In blast phase (BP), the MCyR rate was 38% to 48%, while the CHR rate was only 11% to 13%.10 Similarly, although calculation of response rates were slightly different, cytogenetic responses rates were 47% to 58% with dasatinib in BP after imatinib failure while the CHR rates were 26% to 29%.5,6 A similar trend was noted in Ph+ ALL with either agent.3,4,9 It has been suggested that this discordance may be due to the cytopenia induced by the TKI and that the achievement of a MCyR would be sufficient to favorably influence the long-term outcome of patients.
This analysis evaluated the significance of incomplete neutrophil or platelet recovery in the context of a MCyR achieved with a second generation TKI among patients with CML in advanced phases or with Ph+ ALL after imatinib failure.
Methods
Between April 2004 and April 2007, 126 patients with advanced phase Ph+ CML or Ph+ ALL who had failed imatinib started treatment with dasatinib or nilotinib, as part of multicenter trials, in Institutional Review Board–approved protocols. Informed consent was obtained in accordance with the Declaration of Helsinki. Twenty patients received sequential treatment with both dasatinib and nilotinib for a total of 146 instances of treatment with at least one second generation TKI (referred to as “cases” from here on). Sixteen of the 20 patients were initially treated with nilotinib and then with dasatinib; 4 patients were first treated with dasatinib and later with nilotinib. All patients changed treatment because of failure of the first second-generation TKI. The definitions of AP and BP were as previously described.13 Briefly, AP was defined by the presence of one or more of the following criteria: at least 15% but no more than 29% of blasts in blood or bone marrow, at least 30% blasts plus promyelocytes in peripheral blood or bone marrow (provided that < 30% of blasts were present in the peripheral blood and bone marrow), at least 20% basophils in peripheral blood or bone marrow, or platelets less than 100 × 109/L unrelated to therapy, and/or emergence during a prior treatment of clonal evolution. Clonal evolution was defined by the presence of additional chromosomal abnormalities in the Ph+ cells, excluding variant Ph translocations, a loss of chromosome Y, or constitutional abnormalities. BP was defined by the presence of at least 30% blasts in the blood or bone marrow and/or the presence of extramedullary disease. All patients had previously received at least 400 mg/day imatinib and had shown resistance or intolerance. Patients received variable starting doses of dasatinib or nilotinib, depending on the protocol in which they were enrolled. One hundred two patients (82%) received a standard daily dose, defined as 800 mg of nilotinib (n = 48), administered either as a single dose (n = 12) or as 400 mg twice daily (n = 36), or 140 mg of dasatinib (n = 54), either as a single dose (n = 10) or as 70 mg twice daily (n = 44). Twelve patients (8%; 2 dasatinib, 10 nilotinib) started with a daily dose higher than the standard, and 32 patients (22%; 13 dasatinib, 19 nilotinib) started with less than the standard dose. Dose interruptions and reductions were permitted for the management of toxicity, which was graded according to the National Cancer Institute (NCI) common terminology criteria version 3.0.14 Dose escalation was permitted in the absence of toxicity in case of inadequate response.
Patients were assessed with complete blood counts at least once weekly until a CHR, and with bone marrow and cytogenetic analysis every month for the first 3 months, then every 3 months for the first year, and then every 6 months.
Definitions of response
To be classified as achieving a CHR, a patient had to meet all of the following criteria15 : white blood cell count no more than the institutional upper limit of normal, absolute neutrophil count at least 109/L, platelet count at least 100 × 109/L and lower than the institutional upper limit of normal, differential without immature granulocytes with less than 2% basophils, and no extramedullary involvement (including no hepatomegaly or splenomegaly). A hematologic response had to be maintained for at least 4 weeks to be considered sustained. Cytogenetic responses were assessed by G banding with 20 metaphases analyzed, as previously described15 : complete (CCyR), 0% Ph+; partial (PCyR), 1% to 35%; minor 36% to 65%; and minimal 66% to 95%. A MCyR included CCyR and PCyR (ie, < 36% Ph+). Interphase fluorescence in situ hybridation (FISH) with a dual-fusion probe (Vysis LSI bcr/abl ES probe), performed on bone marrow, was used only when routine cytogenetic analysis yielded no evaluable metaphases (3 patients). For the purpose of this analysis, the cutoffs used for cytogenetic response definitions by FISH were the same as used for G banding.
Mutational analysis by direct sequencing was performed whenever possible before the start of therapy and was also performed later in patients who failed therapy.
Concomitant MCyR and CHR was defined as fulfilling all the criteria for a CHR by the time a MCyR was documented (ie, within 1 week). Patients who had a CHR only after a MCyR was achieved, or who had an early CHR that had been lost by the time a MCyR was documented, were not considered to have a concomitant CHR. Treatment failure was defined as loss of a MCyR, transformation to BP (for patients treated in AP), persistence of extramedullary disease, or a significant increase in the bone marrow blasts, even in the absence of a demonstration of loss of a MCyR, or treatment discontinuation because of toxicity or failure to respond.
Statistical considerations
Event-free survival (EFS) was measured from the start of a second generation TKI therapy until failure, as defined above, or death from any cause. Overall survival (OS) was defined from the start of a second generation TKI until the date of death or last follow-up. Time-to-event (treatment failure or death) analyses were performed using the Kaplan-Meier method, with statistical significance (P values) assessed using the log-rank test.
The Fisher exact test and Mann-Whitney U test were used for paired group comparisons. P values were calculated using the 2-tailed test.
Results
Table 1 summarizes the characteristics of the 126 patients. The median age was 56 years (range, 15-79 years) and they had received a median of 2 prior lines of treatment (range, 1-7) before receiving a second generation TKI. All patients had received imatinib for a median of 29 months (range, 2-78 months). Among the 146 study cases (Table 2), 83 were in AP, 57 in BP (myeloid 39, lymphoid 13) and 6 had Ph+ ALL at the time of treatment with a second generation TKI. Seventy-seven (53%) patients received nilotinib and 69 (47%) received dasatinib. At the start of therapy with a second generation TKI, 52 (49%) of 106 evaluable patients had 25 different mutations. The most common mutations were G250E (10), E255K (5), E355G (4), M351T (4), T315I (4), and F317L (3).
After a median follow-up of 17 months (range, 1-45 months), 72 (49%) patients achieved a CHR on at least one occasion, and 57 (39%) of them had a CHR sustained for at least 4 weeks. A MCyR was achieved in 55 (38%) patients, including 39 (27%) who obtained a CCyR. The median time to achieve a CHR was 27 days (range, 0-649 days) and to achieve a MCyR, 84 days (range, 12-560 days). The responses by disease category are summarized in Table 3. The 2-year survival rate for the total study group was 43% (61% in AP, 19% in BP, and 33% in Ph+ ALL). The 2-year survival rate for patients achieving a CHR was 63% compared with 22% for those who never achieved a CHR (P < .001). Similarly, the 2-year survival rate for those achieving a MCyR was 56% compared with 35% for those without a MCyR (P = .009). This difference was the same for patients in each disease category, regardless of the specific TKI used.
Twenty-six of the 55 (47%) patients who achieved a MCyR had a concomitant CHR at the time the MCyR was first documented, including 19/31 (61%) in AP, 6/21 (29%) in BP, and 1/3 Ph+ ALL. The main reasons for not meeting criteria for a CHR at the time of a MCyR were persistent thrombocytopenia in 24 patients (16 with grade 3-4), persistent neutropenia in 10 (all grade 3-4), persistent basophilia in 2, persistent thrombocytosis in one, and presence of blasts in the peripheral blood and/or in the bone marrow in 7 (6 of whom had > 5% blasts in the bone marrow; range, 6%-91%). Ten patients had more than one feature associated with the lack of a CHR. Of the 29 (53%) patients who had not achieved a CHR at the time they achieved a MCyR, 12 eventually achieved a CHR in at least one assessment; in 8 of them it was sustained.
Among the 20 patients who were treated with both TKIs, 10 patients failed both nilotinib and dasatinib: 5 were still alive at the last follow-up and 5 had died. Among patients who responded to at least one of the second-generation TKIs, 4 responded to both but eventually lost the response and died. Two more patients responded only to the first treatment (nilotinib in both): one survived and the other died after relapse and failure of the second TKI. The last 4 patients responded only to the second therapy: 2 were still alive at the last follow-up (both treated with dasatinib); 2 patients died (1 treated with nilotinib, 1 with dasatinib). Only 3 of these 20 patients achieved a MCyR with a concomitant CHR: one patient, treated with nilotinib and dasatinib, who finally died; one patient, treated with nilotinib as a first drug, who eventually lost the response and died after dasatinib; and the third patient, who achieved a concomitant CHR with dasatinib after nilotinib failure and was still alive 31 months after the start of dasatinib.
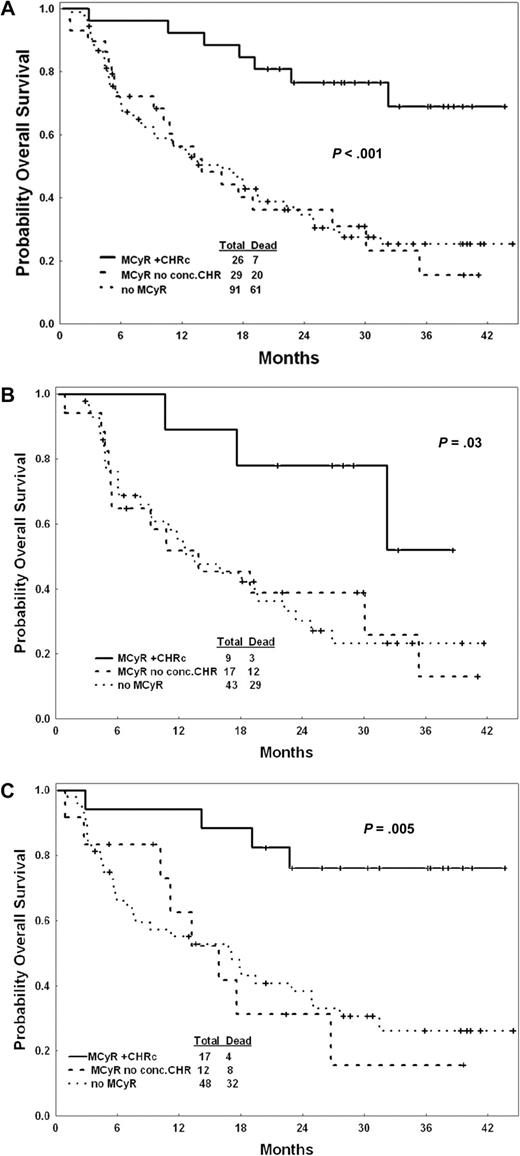
To investigate the prognostic significance of failure to meet the criteria for CHR at the time of a MCyR, we grouped patients into 3 categories: patients with a MCyR with a concomitant CHR (ie, the CHR criteria were met at the time the MCyR was documented; n = 26); those with a MCyR without a concomitant CHR (ie, those not fulfilling the CHR criteria at the time the MCyR was documented; n = 29); and patients without a MCyR (n = 91). Patients with a MCyR and a concomitant CHR had the best outcome, with a 2-year survival rate of 77%. In contrast, patients with a MCyR without a concomitant CHR and those without a MCyR had similarly poor outcomes (2-year survival rate, 37% and 35%, respectively; Figure 1A). We then analyzed separately patients treated with dasatinib or nilotinib and there was no difference between patients treated with either drug (Figure 1B,C). Similar differences were observed for EFS. Since 12 of the 29 (41%) patients who achieved a MCyR but had no concomitant CHR eventually achieved a CHR, we investigated whether achieving a CHR at an earlier (n = 2) or later (n = 10) time had any impact on prognosis. As shown in Figure 2, patients with a MCyR who achieved a CHR at other times but had no CHR at the time the MCyR was achieved had an improved survival compared with those who achieved a MCyR but never achieved a CHR, although still inferior to that of patients with a MCyR and a concomitant CHR. In addition, among patients without a MCyR, those who achieved a CHR had a significantly better outcome than those who did not achieve any response (2-year survival rate, 55% and 22%, respectively). Other factors associated with improved OS and EFS included: longer duration of imatinib treatment, higher hemoglobin, higher platelet count, lower percentage of peripheral and bone marrow blasts, and AP (vs BP or ALL; Table 4).
Overall survival by 3 groups. (A) Total population, (B) patients treated with dasatinib, and (C) patients treated with nilotinib. MCyR+CHRc indicates a MCyR plus a concomitant CHR; MCyR+no conc. CHR indicates a MCyR with no concomitant CHR. Censored cases are shown with vertical tick marks. P values were calculated by the log-rank test for heterogeneity.
Overall survival by 3 groups. (A) Total population, (B) patients treated with dasatinib, and (C) patients treated with nilotinib. MCyR+CHRc indicates a MCyR plus a concomitant CHR; MCyR+no conc. CHR indicates a MCyR with no concomitant CHR. Censored cases are shown with vertical tick marks. P values were calculated by the log-rank test for heterogeneity.
Overall survival by 5 groups. MCyR+CHRc indicates a MCyR plus a concomitant CHR; MCyR+CHR nc indicates a MCyR with no concomitant CHR. Censored cases are shown with vertical tick marks. P values were calculated by the log-rank test for heterogeneity.
Overall survival by 5 groups. MCyR+CHRc indicates a MCyR plus a concomitant CHR; MCyR+CHR nc indicates a MCyR with no concomitant CHR. Censored cases are shown with vertical tick marks. P values were calculated by the log-rank test for heterogeneity.
These trends were maintained when analyzing patients separately according to their disease stage, although this was not statistically significant, possibly because of the small numbers of patients per subset. For example, 10/19 (53%) patients in AP with a MCyR and a concomitant CHR eventually failed, compared with 9/12 (75%) of those without a CHR at the time of the MCyR. Three (50%) of the 6 patients in BP with a concomitant CHR eventually failed, compared with 14 of the 15 (93%) patients without a CHR at the time of the MCyR (Table 5).
The finding that patients with a MCyR but no CHR had an outcome similar to those with no response to a second generation TKI was unexpected. We thus analyzed the pattern of cytogenetic responses in these patients. Surprisingly, these patients achieved an MCyR significantly faster than those who achieved a MCyR and a CHR (whether concomitant or nonconcomitant), but these responses were short-lived (median duration, 2 months; range, 1-26 months).
Discussion
Achieving a cytogenetic response has been established as a surrogate marker for an improved probability of survival in CML. Among patients with CML in chronic phase treated with interferon alpha, those who achieved a CCyR had a 10-year survival rate of 78%.16 Although patients whose best response was a PCyR did not fare as favorably, they still had a significantly better outcome compared with patients who achieved a minor cytogenetic response or no cytogenetic response. Similarly, among patients treated with imatinib in chronic phase, those with a CCyR or PCyR at 12 months have the best probability of being alive and free from events at 5 years.17 A similar trend is seen for patients treated with second generation TKI after failure of imatinib.1,2
Favorable responses to second generation TKIs have been reported among patients in advanced stages of the disease (AP or BP, or Ph+ ALL) who have failed prior imatinib therapy. However, a significant proportion of such patients may achieve a MCyR but fail to meet criteria for a CHR.3-12 The reason for this is often persistent cytopenias, although residual blasts or basophils above the limits acceptable for the definitions of a CHR are seen in some patients. In our series, failure to achieve CHR criteria occurs in more than half of the patients who achieve a MCyR. It is frequently assumed that the achievement of a MCyR overrides the lack of a CHR; thus patients with a MCyR but no CHR are thought to have a prognosis as favorable as those who achieve a MCyR while also meeting the criteria for a CHR. The purpose of our analysis was to determine the significance of residual cytopenias, persistent basophilia, or increased blasts in the setting of a MCyR after second generation TKIs. Our results suggest that patients who do not achieve full recovery of normal hematopoiesis have a significantly inferior outcome whether they achieve a MCyR or not. These results would suggest that the lack of normal hematopoiesis is not an effect of the treatment itself but a manifestation of the biology of the disease.
Residual cytopenias in the context of a supposedly favorable response to therapy have also been reported and investigated in other settings. In acute myeloid leukemia, complete remission with incomplete marrow recovery (CRi) is a recognized response category,18 most frequently associated with incomplete platelet recovery (CRp). Patients with CRp may not have an outcome as favorable as that of patients with a complete remission (CR) and full recovery of hematopoiesis.19 In addition, in AML, it has been suggested that persistence of a few blasts in the peripheral blood may not adversely affect outcome if all other criteria for a CR are met,20 and such a finding in patients still considered to have a CR is indeed tolerated.17 The scenario is somewhat different in CML in that residual cytopenias may coexist with a level of response (cytogenetic) deemed to be of greater value than a morphologic CR (ie, CHR). Still, in our analysis it appears that residual cytopenias have an adverse impact on long-term prognosis. The achievement of a transient cytogenetic response despite the absence of a morphologic CHR may represent a transient recovery of Ph− hematopoiesis after therapy, in a way similar to what has been described after intensive chemotherapy.21 This Ph− recovery is short-lived and followed by a Ph+ recovery. In our series, these patients had the fastest time to achievement of a MCyR, but also the most rapid relapse. Interestingly, these patients frequently had residual blasts, particularly in the bone marrow. These patients had a particularly poor prognosis even when seen in the presence of a MCyR. It is possible that this increase in blasts in the setting of a vanishing Ph+ clone (represented by the decrease in Ph+ metaphases) may represent the appearance of a secondary Ph− leukemia. However, the Ph+ clone rapidly reemerged in all but one of these patients. More research would be required to fully understand this phenomenon.
In conclusion, our results demonstrate that many patients with Ph+ CML in advanced stages who achieve a MCyR on second generation TKI do so in the presence of residual cytopenias or increased blasts. Not achieving a CHR overrides the favorable prognosis of a MCyR and identifies a patient population with particularly poor outcome. Although the mechanism of this phenomenon is not understood, our results suggest that patients with Ph+ ALL or CML in advanced phase who do not achieve a CHR after treatment with second generation TKIs should be considered for alternative therapies, even if they do achieve a MCyR.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: C.F. and J.C. designed the research, wrote the paper, analyzed data, and reviewed the paper; J.S. performed statistical analysis, analyzed results, and made the figures; E.J. and M.B.R. analyzed data and approved the paper; and H.M.K., S.O., N.J., G.G.-M., F.R., S.V., and G.B. treated the patients and reviewed the paper.
Conflict-of-interest disclosure: J.C. and H.M.K. receive research support from Novartis AG and Bristol-Myers Squibb. E.J. serves on the speakers bureau for Novartis AG and Bristol-Myers Squibb. F.R. has received honorarium from Bristol-Myers Squibb and serves on the speakers' bureau for Novartis AG and Bristol-Myers Squibb. All other authors declare no competing financial interests.
Correspondence: Jorge Cortes, Department of Leukemia, Box 428, University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: jcortes@mdanderson.org.