Abstract
Clinical phenotype in systemic mastocytosis (SM) is markedly variable, which complicates prognostication and decision making regarding the choice and timing of therapy. In a retrospective study of 342 consecutive adult patients with SM seen at the Mayo Clinic between 1976 and 2007, disease subdesignation according to the World Health Organization (WHO) proposal was indolent (ISM) in 159 (46%), with associated clonal hematologic non–mast cell lineage disease (SM-AHNMD) in 138 (40%), aggressive (ASM) in 41 (12%), and mast cell leukemia in 4 (1%). KITD816V was detected in bone marrow–derived DNA by allele-specific polymerase chain reaction (PCR) in 68% of 165 patients evaluated (ISM, 78%; ASM, 82%; SM-AHNMD, 60%; P = .03); JAK2V617F was detected in 4%, all in SM-AHNMD. Compared with those with nonindolent SM, life expectancy in ISM was superior and not significantly different from that of the age- and sex-matched US population. In addition, multivariable analysis identified advanced age, weight loss, anemia, thrombocytopenia, hypoalbuminemia, and excess bone marrow blasts as independent adverse prognostic factors for survival. The current study validates the prognostic relevance of the WHO subclassification of SM and provides additional information of value in terms of both risk stratification and interpretation of clinical presentation and laboratory results.
Introduction
Mastocytosis is a heterogeneous disorder characterized by the abnormal growth and accumulation of morphologically and immunophenotypically abnormal mast cells (MCs) in one or more organs. The clinical presentation of mastocytosis is diverse, and many patients do not fit the classical description—namely, a variably long history of urticaria pigmentosa (UP), followed by the insidious onset of flushing, cramping abdominal pain, diarrhea, bone pain, and hepatosplenomegaly.1-4 Unlike pediatric cases, most adults with UP-like skin lesions have systemic disease (ie, systemic mastocytosis [SM]) at presentation, a condition generally confirmed by a bone marrow (BM) biopsy.5 MCs are derived from CD34+/KIT+ pluripotent hematopoietic cells in the bone marrow6 ; its neoplastic counterparts are morphologically atypical (spindled shape, hypogranular cytoplasm, nuclear atypia),7,8 and express abnormal cell surface markers (CD25 and/or CD2).9,10 Most, if not all, adult mastocytosis patients carry gain-of-function KIT receptor mutations, most commonly D816V in the tyrosine kinase domain.11,12
The natural history of SM, ranging from indolent forms spanning years to more aggressive subtypes that rapidly progress to leukemia, complicates decision making regarding the choice of therapeutic modalities and timing of intervention. In 1988, Mayo Clinic investigators proposed a classification wherein SM patients were grouped into the following subtypes: (1) indolent SM (ISM); (2) SM with associated hematologic disorders (SM-AHD); (3) aggressive SM (ASM); and (4) mast cell leukemia (MCL), based on distinct clinicopathologic features.2 In 2001, the World Health Organization (WHO) formalized this classification and further refined it by incorporating recent advances in SM, including identification of KITD816V, aberrant expression of cell surface markers on neoplastic MCs, and elevated tryptase level in serum.13 Although the WHO proposal represents a major advance in identifying clinically distinct SM subgroups based on defined criteria and, consequently, has been widely adopted in clinical practice, it has hitherto not been validated in a large cohort of SM patients.
The aim of the current study was (1) to describe the clinical and laboratory features at presentation in a large cohort of SM patients; (2) to describe the risk of leukemic transformation and estimate mortality of SM patients within the context of the current WHO classification; (3) to estimate life expectancy relative to age- and sex-matched controls as a basis for clinical decision making; (4) to evaluate prognostic relevance of the WHO subclassification of SM, and to identify additional clinical, laboratory, and/or bone marrow histologic features that have prognostic value; and (5) to evaluate the prevalence and prognostic relevance of KITD816V and JAK2V617F mutations in this cohort.
Methods
Patients
The current study was approved by the Mayo Clinic Institutional Review Board. All patients provided informed authorization for use of their medical records for research purposes, and research was carried out in accordance with the principles of the Declaration of Helsinki. Prospective SM patients 18 years of age or older were identified by querying institutional electronic databases from January 1976 to October 2007. The flagged medical charts were thoroughly reviewed; study inclusion patients were required to have BM pathology reviewed at our institution and presence of SM confirmed. Clinical and laboratory features at diagnosis, survival data, and progression to leukemia were documented, and SM was subclassified according to the 2001 WHO proposal.14
Bone marrow studies
BM aspirates and trephine sections were reviewed by one of us (C.-Y.L.). All patients satisfied the 2001 WHO criteria for the diagnosis of SM. Special immunohistochemical stains were used to identify MC in biopsy material, including tryptase (n = 228; 67%) and KIT (n = 123; 36%). The other stains used were Giemsa, toluidine blue, chloroacetate esterase, aminocaproate esterase, and acridine orange. Pathognomonic MC infiltrates were confirmed and overall BM cellularity and MC burden were recorded for each case. The percentage BM involvement by MC was based on review of trephine specimens. The pattern of MC infiltration, percentage of BM blasts in aspirate smears, and degree of eosinophilic infiltration were also recorded. CD2 and/or CD25 expression on BM MCs was studied by immunohistochemistry and/or flow cytometry in a subset of cases (n = 84; 25%), as previously described.10 Cytogenetic analysis was performed at diagnosis and the karyotype was classified using the International System for Cytogenetic Nomenclature Criteria.
Molecular studies
Mutation analysis was performed in DNA derived from archived cytogenetic pellets obtained at the time of BM biopsy. KITD816V was evaluated using an allele-specific oligonucleotide polymerase chain reaction assay with fragment analysis performed on an ABI 3130xl genetic analyzer (Applied Bioscience, Foster City, CA). Briefly, polymerase chain reaction (PCR) was used to amplify a fragment containing the mutation site in 2 separate tubes: one containing a reverse primer complementary to the unmutated sequence and the other containing a reverse primer complementary to the mutated sequence. Each reverse primer was labeled with a fluorescent tag and both tubes contained an identical, nonlabeled forward primer. Both primer sets amplified a 200-bp fragment that differed only at the mutation site. Samples negative for mutation lacked an amplified fragment in the mutated reaction tube, whereas positive samples showed amplified fragments in both the unmutated and mutated tubes. The test is sensitive to 0.01% as determined by dilution of DNA from a positive cell line (HMC-1, containing homozygous mutation) in DNA from a negative cell line (HL-60). The primer sequences were as follows: forward primer: 5′-AATATAAGCAACACTATAGT-3′; reverse primer unmutated: 5′-FAM-ATTAGAATCATTCTTGATGA-3′; reverse primer mutated: 5′-HEX-ATTAGAATCATTCTTGATGT-3′. PCRs were performed in a total volume of 25 μL using approximately 50 ng DNA, 20 pmol each primer, 5 nmol dNTPs, 1.75 units AmpliTaq-Gold, and standard PCR buffer (1.5 mM MgCl2, 10 mM Tris-HCl, pH 8.3, 50 mM KCl). JAK2V617F analysis was performed as previously described.15
Statistical analyses
Actuarial probability of survival and leukemia-free survival were estimated using the Kaplan-Meier product limit method. Overall survival was defined as the time between diagnosis and death (as a result of all causes) or end of follow-up (censored observations). Leukemia-free survival was calculated from diagnosis to progression to acute leukemia/MCL or end of follow-up. Comparison between Kaplan-Meier curves was carried out by the log-rank test. Univariate and multivariate analyses were performed by Cox proportional hazards regression model. Expected survival curves were calculated using Hakulinen cohort method and were matched by age and sex to the US population for the appropriate time period based on diagnosis date.16-18 The Fisher exact test or χ2 test for 2 × 2 contingency tables and Wilcoxon rank-sum test or Kruskal-Wallis test were used to test for differences in proportions of nominal variables and medians of continuous variables, respectively. Correlation between 2 continuous variables was determined with the nonparametric Spearman rank-order correlation coefficient. Statistical analyses of the data were carried out using the StatView software package (SAS version 9.1; SAS Institute, Cary, NC).
Results
Clinical and laboratory features
The demographic, clinical, and laboratory features of the 342 study patients are summarized in Tables 1 and 2 and compared between WHO SM subgroups in Table 3. One hundred fifty-nine patients (46%) had ISM; 138 (40%), SM-AHNMD; 41 (12%), ASM; and 4 (1%), MCL. One patient each with SM-AHNMD and MCL presented with a histologically confirmed focal mast cell sarcoma-like lesion (iliac bone and chest wall, respectively). A greaterproportion of SM-AHNMD patients were male compared with ISM and ASM (P < .001). ISM patients were younger (P < .001) and exhibited a longer duration of symptoms before diagnosis (P < .001) compared with the other WHO subgroups. ISM patients also exhibited a higher prevalence of UP-like skin lesions (P < .001), cutaneous symptoms (P < .001), MC mediator-related symptoms (P < .001), anaphylactoid reactions (P < .001), and gastrointestinal symptoms (P = .05). In contrast, patients with ASM and SM-AHNMD exhibited a greater frequency of constitutional symptoms (P < .001), hepatosplenomegaly (P < .001), and lymphadenopathy (P = .005).
Approximately one-third and one-quarter of SM-AHNMD and ASM patients, respectively, had significant anemia (Hgb < 100 g/L [10 g/dL]) and thrombocytopenia (platelets < 100 × 109/L), and 51% of SM-AHNMD patients exhibited leukocytosis (P < .001). Fifty-six (16%) patients exhibited prominent eosinophilia (absolute eosinophil count > 1.5 × 109/L [1500/μL]): 31% and 22% with SM-AHNMD and ASM, respectively, versus 3% with ISM (P < .001). Relatively few ISM patients exhibited increased levels of serum uric acid (3%), lactate dehydrogenase (4%), bilirubin (11%), or ferritin (18%); or hypoalbuminemia (9%)—in contrast, a 2- to 3-fold greater proportion of SM-AHNMD and ASM patients displayed these laboratory abnormalities (P ≤ .004). Serum tryptase (normal < 11.5 ng/mL) was measured in 160 patients (47%), and virtually all (96%) had an elevated level (median 64 ng/mL; range 4 to 2000 ng/mL). A greater proportion of ASM and SM-AHNMD patients exhibited a serum tryptase level 200 ng/mL or higher compared with ISM patients (P = .009).
Bone marrow histology
Bone marrow histopathologic findings are described in Table 4. The median BM cellularity for the ISM, ASM, SM-AHNMD, and MCL subgroups was 50% (range 20%-100%), 75%, (30%-100%) 90%, (30%-100%), and 88%, (80%-95%), respectively (P < .001). The median BM MC percentage for the whole group of patients was 10% (range 1%-90%) and was significantly (P = .003) higher in MCL (median, 75%; range, 60%-90%) compared with ASM (median, 13%; range, 1%-70%), SM-AHNMD (median, 11%; range, 1%-70%), or ISM (median, 10%; range, 1%-90%). All 27 patients with BM blasts 5% or more were diagnosed with SM-AHNMD (9 had refractory anemia with excess blasts-2 [RAEB-2]; 7, chronic myelomonocytic leukemia-1 [CMML-1]; 4, myeloproliferative neoplasm (MPN)-unclassifiable; 2, RAEB-1; 2, AML-M2; 1, CMML-2; 1, chronic eosinophilic leukemia; and 1, essential thrombocythemia).
Cytogenetic and molecular studies
Cytogenetic data were available for 186 (54%) patients, 37 of whom (20%) had chromosomal aberrations (excluding 5 patients with sole deletion of chromosome Y). Recurrent chromosomal abnormalities included trisomy 8, monosomy 7, del(13q), del(5q), trisomy 10, del(20q), trisomy 6, trisomy 19, and trisomy X. Abnormal karyotype was more frequently detected in SM-AHNMD (31%) and ASM (20%), as opposed to ISM (5%; P < .001). All 7 patients with more than one chromosomal abnormality had SM-AHNMD. Fifty-six patients displayed prominent eosinophilia; 52% of 23 patients screened for FIP1L1-PDGFRA and 62% of 34 patients screened for KITD816V carried the mutation. Eight patients with eosinophilia were screened for FIP1L1-PDGFRA and KITD816V: there were no instances of concomitant presence of these mutations. Archived bone marrow was available in 165 (48%) patients for KITD816V and JAK2V617F analysis. By allele-specific PCR, KITD816V mutation was detected in 113 patients (68%): ISM, 78%; ASM, 82%; and SM-AHNMD, 60% (P = .03). The sole MCL leukemia patient who was tested did not harbor this mutation. JAK2V617F mutation was detected (mutant allele burden > 1%) in 6 patients (4%); all had SM-AHNMD, including 4 with non-MC lineage myeloproliferative neoplasm. Five (83%) of the 6 patients harbored KITD816V and JAK2V617F mutations concomitantly.
Survival studies and leukemic transformation
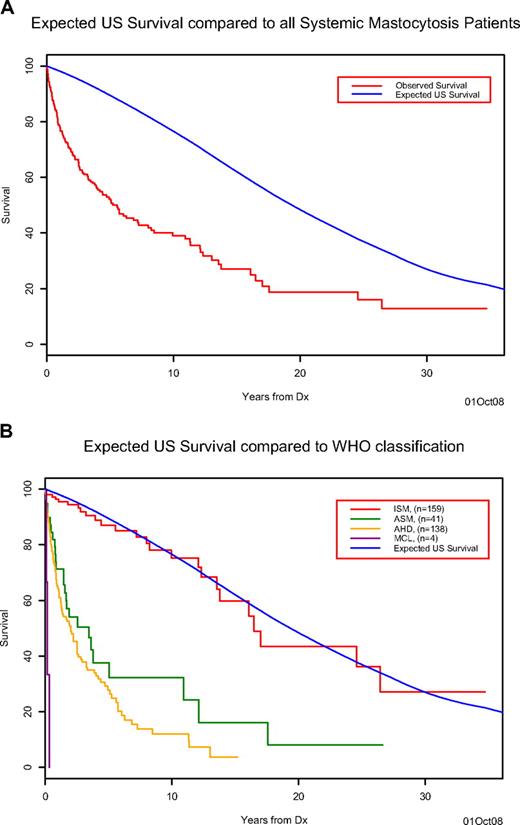
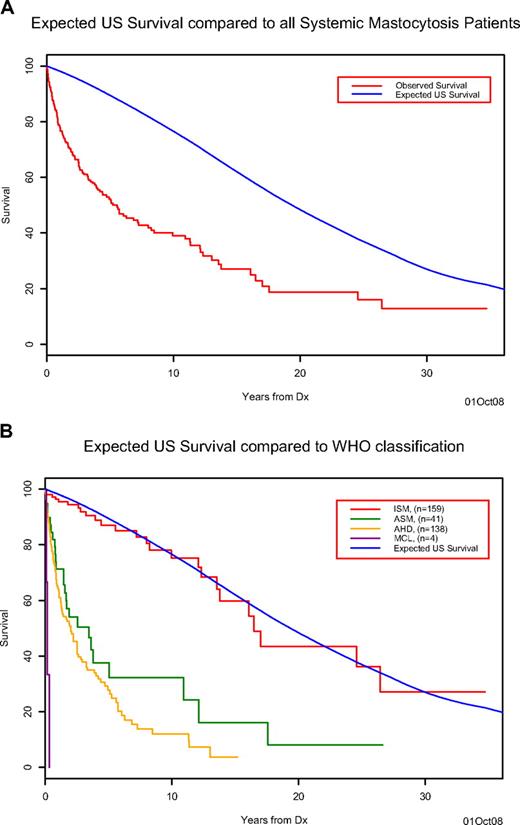
After a median follow-up of 20.7 months (range = 0-417 months), 153 (45%) deaths were recorded (26 ISM, 25 ASM, 99 SM-AHNMD, 3 MCL). The median survival of the cohort was 63 months, which is inferior compared with the age- and sex-matched US population (Figure 1A). Survival in patients with ISM (median, 198 months) was superior (P < .001) to that of patients with ASM (median, 41 months), SM-AHNMD (median, 24 months), or MCL (median, 2 months) and not significantly different from that of the control population (Figure 1B). Information regarding the cause of death was available for 13 (50%) of the 26 ISM patients: disease progression to ASM (n = 3), development of solid tumors (n = 3), comorbid cardiovascular conditions (n = 3), complications from massive MC mediator release (n = 2), acute myeloid leukemia (n = 1), and infection (n = 1). Among 21 (6%) cases of leukemic transformation in the overall cohort (18 with AML and 3 MCL), 18 (86%) occurred in the setting of SM-AHNMD; 2, ASM; and 1, ISM (P < .001; Table 3).
Survival of systemic mastocytosis patients. (A) The observed Kaplan-Meier survival for systemic mastocytosis patients (red) compared with the expected age- and sex-matched US population's survival (blue). (B) The observed Kaplan-Meier survival for systemic mastocytosis patients classified by disease type ISM (red), ASM (green), AHNMD (yellow), and MCL (purple) compared with the expected age- and sex-matched US population's survival (blue) for the entire cohort.
Survival of systemic mastocytosis patients. (A) The observed Kaplan-Meier survival for systemic mastocytosis patients (red) compared with the expected age- and sex-matched US population's survival (blue). (B) The observed Kaplan-Meier survival for systemic mastocytosis patients classified by disease type ISM (red), ASM (green), AHNMD (yellow), and MCL (purple) compared with the expected age- and sex-matched US population's survival (blue) for the entire cohort.
Prognostic factors
In addition to specific diagnosis based on the WHO proposal, other demographic, clinical, and laboratory variables were examined for their prognostic relevance (Table 5). In this regard, the pitfalls of considering multiple variables must be kept in mind and certain variables (eg, splenomegaly, hypoalbuminemia; identified by § in Table 5) represent B- or C-findings and are therefore already accounted for in the WHO classification. Multivariable analysis showed a significant and independent association between inferior survival and WHO subtype (P < .001), advanced age (P < .001), history of weight loss (P = .01), anemia (P = .007), thrombocytopenia (P < .001), hypoalbuminemia (P < .001), and excess BM blasts (> 5%; P = .004).
Discussion
To our knowledge, the current study is the largest SM series reported in the literature. The clinical findings pertain to an adult SM population with bone marrow histology–confirmed disease that was seen at a tertiary care center. Consequently, extrapolation to an SM population identified primarily on the basis of UP-like skin lesions, or one that includes pediatric patients, may not be valid.
Consistent with previous studies, the current cohort had a slight male preponderance; the relatively long duration between onset of symptoms and diagnosis is consistent with absence of UP-like skin lesions in a majority of patients, and also to the referral nature of our practice. The life expectancy of SM patients was shorter relative to age- and sex-matched controls. As initially observed by Travis et al,2 survival decreased rapidly after diagnosis: to 60% at 3 years, with a subsequent slower decline to 50% at 5 years. Beyond 5 years, the slope of the survival curve was similar to that of the control population. This observation confirms that the excess deaths in SM patients occur within the first 3 (and up to 5) years after diagnosis.
The current study validates the prognostic relevance of the WHO classification for SM. Patients with ISM have a significantly better prognosis in terms of overall survival and leukemia-free survival compared with ASM and SM-AHNMD patients. Furthermore, the life expectancy of ISM patients was not significantly different from the age- and sex-matched US population for the appropriate time period based on the diagnosis date. Furthermore, leukemic transformation rarely occurs in ISM patients. In contrast, the median survival of SM-AHNMD and ASM patients was 2 and 3.5 years, respectively; MCL patients, not surprisingly, had the poorest prognosis with median survival of 2 months. These data are clinically relevant and serve as a basis for guiding therapeutic decisions in SM patients. It supports the view that, in general, therapy for ISM ought to be symptom directed and potentially immunosuppressive and leukemogenic therapies are best avoided in these patients given the relatively good prognosis and normal life expectancy.19,20 For patients with ASM or MCL, or the rare ISM patient with recurrent severe anaphylaxis or syncope not responding to conventional approaches, the goal of therapy is to reduce the systemic MC burden. Published data suggest that interferon-alpha and 2-chlorodeoxyadenosine (2-CdA) have significant inhibitory activity against the malignant MC clone, however both agents are plagued by problems, namely, poor tolerability and uncertainty regarding optimal dose/duration of therapy (IFN-α), or potential mutagenic effects, myelosuppression, and immunosuppression (2-CdA).21,22 Imatinib is relatively ineffective in KITD816V-positive SM,23 however may have activity in the rare SM case with other KIT mutations (eg, KITF522C),24 or in patients with eosinophilia and MC proliferation who harbor the FIP1L1-PDGFRA mutation.25-27 Preliminary data suggest that dasatinib has limited therapeutic activity in SM28,29 ; however, its role in combination with chemotherapeutic agents is under active investigation.30,31
In the WHO proposal, “B-” and “C-”findings serve as surrogate markers for a high MC burden, and impaired organ function resulting therefrom, respectively, and are used to classify SM patients. Given that B- and C-findings have been defined somewhat arbitrarily (for instance, is Hgb < 100 g/L [10 g/dL] as prognostically relevant as Hgb < 80 g/L [8 g/dL], or Hgb < normal? Similarly, is presence of pathologic fracture as prognostically relevant as hypoalbuminemia?), we conducted a multivariate analysis to identify those variables that may have prognostic relevance within the context of the WHO subgroups. Among the demographic variables, advanced age (≥ 65 years) was independently associated with shorter survival. Anemia and thrombocytopenia were also independently associated with shorter survival; notably, the threshold for identifying poor risk patients was lower than the corresponding C-findings (defined as hemoglobin < 100 g/L [10 g/dL] and platelet count < 100 × 109/L). Weight loss and hypoalbuminemia were also independently associated with shorter survival regardless of whether this resulted from malabsorption. Among the histologic variables, only excess BM blasts (> 5%) was independently associated with shorter survival. It has been reported that the percentage of MCs in BM smears may distinguish between clinical variants of SM32 ; this aspect was not examined in our study because MCs were quantified only in trephine biopsy specimens.
In the current analysis, prevalence of KITD816V using a sensitive (0.01%) assay was broadly consistent with published data,11 considering our use of DNA from unfractionated BM cells. Detection of KITD816V was not significant in the survival analysis; however, the mutation was significantly associated with presence of C-findings (83% vs 64%; P = .03) and higher BM MC burden (median, 13% vs 10%; P = .01). JAK2V617F was infrequently detected in SM patients and appeared to be restricted to SM-AHNMD, where it frequently coexisted with KITD816V. In contrast, none of the FIP1L1-PDGFRA–positive patients in our cohort harbored KITD816V, an observation that is consistent with the complete clinical and histologic responses seen with imatinib therapy in these patients.
In summary, we have described the clinical, laboratory, and BM histologic features at presentation in a large cohort of adult SM patients. Survival of this cohort is shorter relative to an age- and sex-matched control population. When patients are classified into WHO subgroups, there are significant differences in disease characteristics. ISM patients have a normal life expectancy and leukemic transformation is rarely seen in these patients; in contrast, the other SM subgroups have a significantly poorer prognosis. We have validated the prognostic relevance of the WHO proposal for SM, and have identified additional risk factors (advanced age, weight loss, anemia, thrombocytopenia, hypoalbuminemia, and excess bone marrow blasts) that are significantly associated with shorter survival.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We acknowledge the contribution of Diane Grill in the Biostatistics Division, Mayo Clinic, for generating the survival curves.
Authorship
Contribution: K.-H.L. collected and analyzed the data and wrote the paper; A.T. designed the study, analyzed the data, and wrote the paper; T.L.L. and C.F. performed the molecular analysis; M.P. and J.H.B. collected and analyzed the data; R.F.M. performed the KIT mutation analysis; C.-Y.L. performed the bone marrow histology analysis; and A.P. designed the study, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: A. Pardanani, Division of Hematology, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: pardanani.animesh@mayo.edu.