Abstract
IFN-λ1 (IL-29) plays a novel, emerging role in the inhibition of human Th2 responses. Here, we demonstrate that both naive and memory human CD4+ T cells express mRNA for the IFN-λ1–specific receptor, IL-28Rα, and are responsive to IFN-λ1. Expression of Th2 cytokines (IL-4 and IL-13) was suppressed in naive and memory CD4+ T cells by IFN-λ1, without affecting their proliferation. Further, acquisition of IL-4Rα expression after stimulation was inhibited by IFN-λ1, as was GATA3 expression. Finally, IFN-λ1 diminished the change in cell-surface phenotype that accompanies differentiation of “central memory” T cells into “effector memory” T cells. Taken together, our data describe unique immunomodulatory effects of IFN-λ1 and identify novel mechanisms for the reduction of existing Th2 responses and the regulation of new ones, in circulating naive and memory CD4+ T cells.
Introduction
The polarization of CD4+ T cells toward a Th1 or Th2 phenotype underlies the generation of protective and pathologic immune responses.1,2 An imbalance in this regulation is a hallmark component of asthma. Although asthma development is multifactorial, Th2 cytokines are highly associated with the disease, especially IL-4, IL-5, and IL-13, which are secreted after antigen challenge in allergic asthma.3-5 Understanding how to control the initiation of such responses may have significant healthcare potential.
Naive CD4+ T cells do not express mRNA for the Th1 or Th2 cytokines.6 The differentiation of naive CD4+ T cells into Th1 or Th2 effectors involves specific cytokines, interactions of cell-signaling proteins with transcription factors, and coordinated chromatin remodeling.7,8 Once committed, T helper 1 (Th1) cells secrete IL-2, IFN-γ, and lymphotoxin (LT), and direct cell-mediated immunity against intracellular pathogens. T helper 2 (Th2) cells produce IL-4, IL-5, IL-6, IL-9, and IL-13, driving the development of humoral immunity and allergic reactions. The properties of established Th2 memory cells determine the nature of recall immune responses, driven by a rapid production of polarized cytokines9 and Th2 expansion after restimulation.10,11 In this way, Th2 memory CD4+ T cells can play a major role in the flare-up of allergic diseases.
In light of the potential implications of long-term Th2 commitment, it is important to gain greater understanding of the mechanisms involved in polarization of naive CD4+ T cells. The mechanisms by which dendritic cells (DCs) are stimulated to drive Th1 responses are well described (eg, Kapsenberg12 ). DCs are activated through Toll-like receptors (TLRs), providing a potent negative signal for Th2 polarization13 independently of positive “Th1 signals” from IL-12 and IFN-γ. Although the underlying mechanisms are still not clear, it has been shown that TLR-activated DCs inhibit the early IL-4 production of CD4+ T cells by down-regulation of GATA3 expression.14 Several other studies have shown that the transcription factor T-bet is central to Th1 differentiation, inducing both transcriptional competence of the IFN-γ locus and selective responsiveness to IL-12.15,16
Less is known about how Th2 responses are driven and controlled. Th2 responses are characterized by activation of eosinophils, mast cells, and mucosal epithelial cells, and production of IgE; they can be induced by infection with organisms such as helminths.17 In addition, Th2 responses can be induced by simple protein allergens,18 which typically have little in common with infectious parasites. These precipitating events, exact cell types, and cellular mechanisms that drive Th2 differentiation have not yet been properly defined; yet, it is their regulation that is subverted in asthma.3-5
However, it is well established that IL-4 is essential for Th2 differentiation. IL-4 not only acts in a positive manner to activate IL-4 receptors on naive T cells and to initiate the Th2 differentiation process, but it also acts in a negative manner to extinguish IL-12 receptor expression, committing the cells to the Th2 pathway.19-21 During Th2 differentiation, stimulation through the IL-4R leads to phosphorylation of STAT6 and, consequently, up-regulation of GATA3 expression. Either type-I IL-4 receptor (IL-4Rα and the common γ chain) or type-II IL-4R (IL-4Rα and IL-13Rα1) is required for the intracellular signal responses in naive T cells. It is not clear whether IL-4 is essential for promoting memory Th2 responses.
IFN-γ, the only known type-II IFN, is the signature Th1 cytokine and activates T-bet expression by STAT1. This leads to T-bet transcription, which itself up-regulates IFN-γ expression. Recent studies have shown that IFN-γ fails to suppress Th2 cytokine production in polarized Th2 cells.22
The IFN-λ family comprises 3 ligands (IFN-λ1/2/3) in humans.23 In mice, IFN-λ1 is disrupted.24 IFN-λ1/2/3 have been named “type-III” interferons and are also known as IL-29, IL-28A, and IL-28B, respectively. This family of interferons is distantly related to both the IL-10 family of ligands and the type-I interferon family. The receptor “alpha chain” (IL-28Rα) used by members of this new family is not used by any other known ligand, although the “beta chain” (IL-10Rβ) chain is also part of the IL-10, IL-22, and IL-26 receptors.25 This receptor heterodimer is distinct from the type-I interferon receptor. Many investigators have studied the antiviral actions of the IFN-λ family (eg, Meager et al26 ), and it has been shown recently that IFN-λ1 has a lesser protective action compared with IFN-α.27 Thus, IFN-λ1/2/3 appear to act in a more specific fashion than the broadly active type-I interferons.
The chronic elevation of Th2 cytokines seen in patients with asthma has been associated with a marked deficiency in IFN-λ1 production.28 These findings complemented our investigations of the effect of IFN-λ1 on Th1/Th2 polarization. We have previously shown IFN-λ1 dampens Th2 responses in Con A–stimulated peripheral blood mononuclear cells (PBMCs) by inhibiting the transcription and production of the signature Th2 cytokines IL-4, IL-5, and IL-13, without affecting Th1 responses.29,30 Defining the mechanisms involved in the regulation of Th2 responses by IFN-λ1 will provide greater insight into the initiation and control of T-cell polarization.
Methods
Subjects
Human peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats purchased from the Newark Blood Bank (Blood Center of New Jersey, East Orange, NJ). These buffy coats were completely anonymous, and it was not possible to identify the donors. More than 30 buffy coats were used in this study.
Cell preparation
PBMCs were harvested by centrifugation over Ficoll Paque (Sigma-Aldrich, St Louis, MO). Cells were collected and washed twice in RPMI 1640 medium (Invitrogen, Carlsbad, CA). The cells were resuspended at a final density of 106/mL in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum (FCS; Invitrogen).
Isolation of naive and memory CD4+ T cells
“Untouched” naive (CD3+CD4+CD45RA+CD45RO−) and memory (CD3+CD4+CD45RO+) CD4+ T cells were prepared from PBMCs with the use of negative magnetic separation kits (StemCell Technologies; Vancouver, BC), according to the manufacturer's instructions. In brief, memory CD4+ T cells were negatively isolated with a cocktail of antibodies against CD8, CD14, CD16, CD19, CD20, CD36, CD56, CD123, TCRγ/δ, glycophorin A, and dextran-coated magnetic nanoparticle microbeads. For naive CD4+ T-cell isolation, PBMCs were preincubated with biotinylated anti-CD45RO antibody before adding the cocktail of antibodies. The purities of these 2 populations were each greater than 95%.
Cell culture conditions
After washing, cells were adjusted to a density of 106 cells/mL per well in 24-well flat-bottom plates. Cells were stimulated with microbeads coated with anti-CD2/CD3/CD28 (“beads”; 5 × 105/mL; bead-to-cell ratio, 1:2; Miltenyi Biotec, Auburn, CA) in the presence or absence of 100 ng/mL IFN-λ1 (PeproTech, Rocky Hill, NJ), in 1-mL cultures. For Th2 cell polarization, naive CD4+ T cells were cultured in “Th2 conditions” with (beads + 10 ng/mL IL-4; PeproTech). Supernatants were harvested at different time points as indicated, whereas cells were stored at −80°C in lysis buffer (Stratagene, La Jolla, CA) for RNA extraction. RPMI 1640 culture medium supplemented with 10% (vol/vol) heat-inactivated fetal calf serum was used throughout (complete medium).
Immunophenotype analysis by flow cytometry
For immunophenotype analysis, PBMCs and purified naive or memory CD4+ T cells were quadruple stained with fluorescent-conjugated mAbs specific for cell-surface markers and analyzed by flow cytometry using a FACSCalibur (Becton Dickinson, San Jose, CA) instrument. FITC-labeled anti-CD3, PE-labeled anti-CD45RA, PE-labeled anti-CCR7, PECy5.5-labeled anti-CD4 were purchased from eBioscience (San Diego, CA). PE-labeled CD124 (IL-4Rα), PECy5-labeled anti-CD62L, APC-labeled anti-CD45RO were purchased from BD PharMingen (San Diego, CA). For surface staining, cells were incubated with the respective mAbs at 4°C in the dark for 30 minutes. The cells were washed twice and fixed in 0.5% paraformaldehyde before acquisition.
Quantitation of secreted cytokines by enzyme-linked immunoabsorbent assay
Accumulated IFN-γ and IL-13 levels were determined by enzyme-linked immunoabsorbent assay (ELISA) from 24-hour and 3-day cultures. Antibody pairs for IFN-γ were purchased from eBioscience and for IL-13 from R&D Systems (Minneapolis, MN). Manufacturers' protocols were followed, and all washes were performed with phosphate-buffered saline (PBS) containing 0.05% (vol/vol) Tween-20 (Sigma-Aldrich). Briefly, flat-bottom, 96-well plates were coated with the appropriate capture antibody and incubated at 4°C overnight in the dark. After washing, plates were blocked with 1% (wt/vol) bovine serum albumin (BSA; Sigma-Aldrich), then standards and culture supernatants were plated in triplicate. After incubation at 37°C for 2 hours, plates were washed and exposed first to the relevant biotinylated antibodies, then to streptavidin-conjugated horseradish peroxidase, and finally to the chromogen, TMB. After 20 minutes, the reaction was halted by the addition of 2 M sulfuric acid and the optical density at 450 nm was determined. Cytokine concentrations were calculated from the standard curve present on each plate.
Real-time quantitative reverse transcription-polymerase chain reaction analysis
Total RNA was extracted from cells (Stratagene), and cDNA was prepared and subsequently assayed using a 2-step procedure (AffinityScript; Stratagene). Quantitative (real-time) reverse transcription–polymerase chain reaction (qRT-PCR) was carried out using a SYBR Green method in a Stratagene MX-3000 instrument. cDNA samples were amplified as follows: 10 minutes at 95°C then 40 cycles of 95°C for 30 seconds, 60°C for 60 seconds, and 72°C for 30 seconds. A melting curve analysis was carried out to verify that the Ct values were based on a single PCR product. All primer concentrations were at 300 nM, except those for EF-1α (150 nM). Primer pairs for cytokine analysis were IL-28RαF, 5′-CCA.GCC.AGT.CCA.GAT.CAC.TCT-3′; IL-28RαR, 5′-ACA.GCA.GTA.TCA.GAA.GCG.ATG.G-3′; T-betF, 5′-ACC.ACC.TGT.TGT.GGT.C-3′; T-betR, 5′-CCT.TTC.CAC.ACT.GCA.C-3′; GATA3F, 5′-TCA. AGG.CAA.CCA.CGT.C-3′; and GATA3R, 5′-GAT.GGA.CGT.CTT.GGA.G-3′.
Relative levels of these cDNAs and the effects of IFN-λ1 were established using the ΔΔCt method against the housekeeping gene EF-1a, as follows: EF-1aF, 5′-CTG.AAC.CAT.CCA.GGC.CAA.AT-3′; and EF-1aR, 5′-GCC.GTG.TGG.CAA.TCC.AAT-3′.
Western blotting analysis of GATA3
After stimulation of naive CD4+ T cells with beads in the presence or absence of IL-4 or IFN-λ1 (or both) as described, cells were pelleted and washed in ice-cold PBS, pH 7.4. Cell pellets were resuspended in 100 μL lysis buffer (Fermentas, Glen Burnie MD). Protein (80 μg) was run on 4% to 12% gradient bisacrylamide gels (Bio-Rad, Hercules CA). The protein was transferred to PVDF membrane, and the blots were blocked with 5% nonfat dry milk in PBS-Tween for 1 hour at room temperature. The blots were then incubated overnight with either anti-GATA3, 1:1000 (BD PharMingen) or anti–β-actin, 1:1000 (Sigma-Aldrich). After washing, HRP-conjugated secondary antibodies were applied at 1:4000 dilution for 1 hour at room temperature. The blots were developed using the enhanced chemiluminescence (ECL) procedure (Thermo Electron, Waltham, MA).
Proliferation of CFSE-labeled naive and memory CD4+ T cells
Purified naive or memory CD4+ T cells were resuspended in complete RPMI 1640 medium at 107 cells/mL. Carboxyfluorescein diacetate succinimidyl ester (CFSE; Invitrogen) was added at a final concentration of 5 μM, and the cells were incubated for 10 minutes at 37°C in 5% CO2. The stain was quenched using a 5× volume of ice-cold complete medium for 5 minutes. The cells were then washed 3 times and resuspended in complete medium before stimulating them with CD2/CD3/CD28 beads. After 6 days of culture, cells were acquired with the FACSCalibur and analyzed using FlowJo software (TreeStar, Ashland, OR).
Results
IFN-λ1 primarily inhibits IL-13 production and gives rise to IFN-γ production in PBMCs
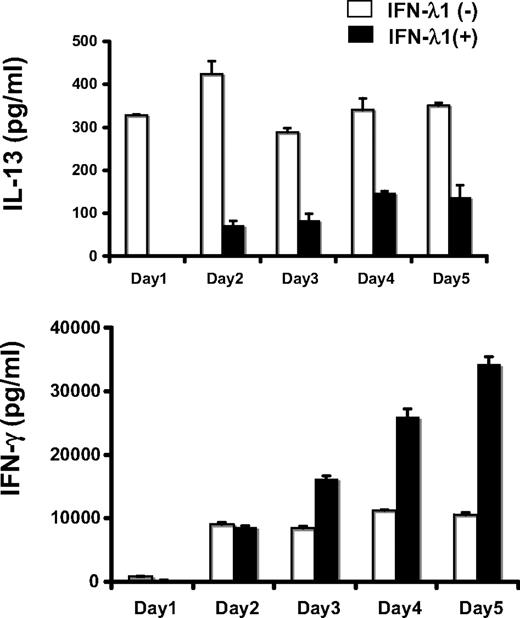
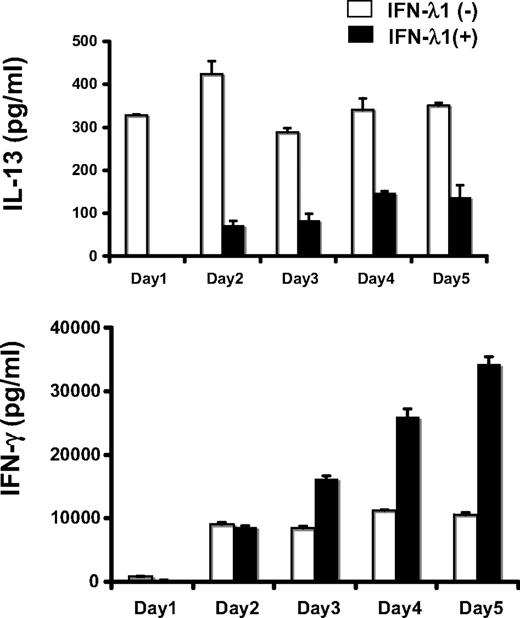
We have previously shown that IFN-λ1 modulates IL-13 (representative of Th2 responses) with little effect on IFN-γ production (representative of Th1 responses) after mitogen stimulation of PBMCs. Here, we extended those findings to a more physiologically relevant stimulation using anti–CD2/3/28-coated beads to stimulate T cells directly through their T-cell receptor complex, with appropriate costimulation. PBMCs were stimulated with beads for 3 days in the presence or absence of IFN-λ1. IFN-λ1 inhibited IL-13 production and elevated IFN-γ levels.
To determine whether IL-13 production was inhibited by IFN-λ1 directly (produced early, in response to the bead stimulation), a daily time course for this experiment was undertaken. IFN-λ1 blocked IL-13 production completely at day 1, with consistent reductions from day 2 to day 5 (56.8%-82.9% inhibition; Figure 1). In contrast, we did not find any differences in IFN-γ production by IFN-λ1 treatment at day 1 and day 2. Thus, IFN-λ1 acted primarily to inhibit the production of IL-13, as we had previously observed.29,30 The subsequent rise in IFN-γ was therefore most likely a consequence of the reduction of Th2 cytokines. Given that the stimulation used was specific for T cells, we hypothesized that IFN-λ1 was acting directly on T cells to inhibit IL-13 production.
Time course studies on the effect of IFN-λ1 on Th2 (IL-13) and Th1 (IFN-γ) production by bead-stimulated PBMCs. PBMCs were stimulated for 1 to 5 days with beads bound to anti-CD2, CD3, and CD28 antibodies, in the presence (■) or absence (□) of IFN-λ1 (100 ng/mL). The secretion of IL-13 (top panel) and IFN-γ (bottom) at each day was determined by ELISA. Result s are shown as means ± SDs.
Time course studies on the effect of IFN-λ1 on Th2 (IL-13) and Th1 (IFN-γ) production by bead-stimulated PBMCs. PBMCs were stimulated for 1 to 5 days with beads bound to anti-CD2, CD3, and CD28 antibodies, in the presence (■) or absence (□) of IFN-λ1 (100 ng/mL). The secretion of IL-13 (top panel) and IFN-γ (bottom) at each day was determined by ELISA. Result s are shown as means ± SDs.
IFN-λ1 receptor IL-28Rα is expressed on both naive and memory CD4+ T cells
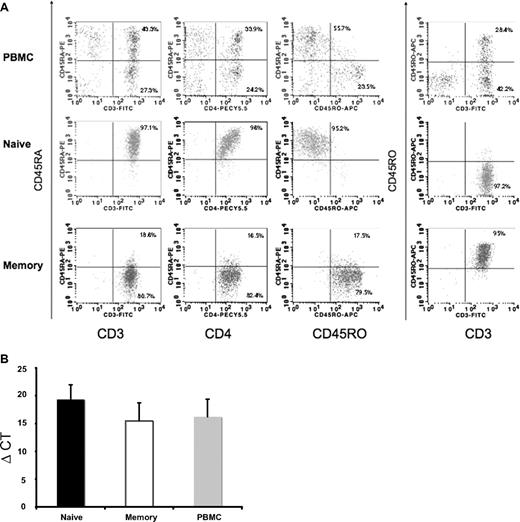
To identify IFN-λ1–responsive cell types in human PBMCs, we assayed various cell populations for the presence of the IFN-λ1 receptor (IL-28Rα) mRNA by qRT-PCR. Although others have reported IL-28Rα expression on fresh blood dendritic cells,31 the results from Figure 1 prompted investigation of human naive and memory CD4+ T cells. “Untouched” populations of naive and memory CD4+ T cells were purified using negative isolation (Figure 2A). Greater than 95% of the naive T cells were CD3+CD4+CD45RA+CD45RO− cells. Greater than 95% of the memory T cells were CD3+CD4+CD45RO+ cells, with a small proportion (18.6%) expressing both CD45RO and CD45RA. Figure 2B shows the ΔCT of IL-28Rα mRNA expression in PBMCs and naive and memory T cells. With the use of this approach, higher ΔCT values signify lower mRNA expression levels. These data show that, within the total PMBC population, both naive and memory CD4+ T cells express IL-28Rα mRNA to broadly the same extent as the PBMC population overall. These data lend strong support to the hypothesis that IFN-λ1 can act directly on multiple CD4+ T-cell populations.
Expression of IFN-λ1 receptor, IL-28Rα, by human naive and memory T cells. (A) Phenotype of purified human naive and memory T cells. Populations of PBMCs (top), purified naive (middle) or memory (bottom) T cells were stained with the indicated antibodies and analyzed by flow cytometry to assess purity. Numbers indicate percentage of naive or memory T cells which coexpress CD45RA, CD3, CD4, and CD45RO. The purity of naive and memory T cells was at least 95%. Results are from a representative routine experiment. (B) Detection of IL-28 Rα by real-time PCR. Total RNA was harvested from naive and memory T cells and from PBMCs. The levels of IL-28Rα mRNA expression were determined by real-time PCR. PBMCs were used as positive controls. Negative controls included both non-RT and nontemplate control (data not shown). Results (shown as means ± SDs) from 3 individual donors are presented as ΔCT, with lower values representing higher levels of mRNA.
Expression of IFN-λ1 receptor, IL-28Rα, by human naive and memory T cells. (A) Phenotype of purified human naive and memory T cells. Populations of PBMCs (top), purified naive (middle) or memory (bottom) T cells were stained with the indicated antibodies and analyzed by flow cytometry to assess purity. Numbers indicate percentage of naive or memory T cells which coexpress CD45RA, CD3, CD4, and CD45RO. The purity of naive and memory T cells was at least 95%. Results are from a representative routine experiment. (B) Detection of IL-28 Rα by real-time PCR. Total RNA was harvested from naive and memory T cells and from PBMCs. The levels of IL-28Rα mRNA expression were determined by real-time PCR. PBMCs were used as positive controls. Negative controls included both non-RT and nontemplate control (data not shown). Results (shown as means ± SDs) from 3 individual donors are presented as ΔCT, with lower values representing higher levels of mRNA.
Differential effects of IL-4 on IL-13 production in naive and memory CD4+ T cells
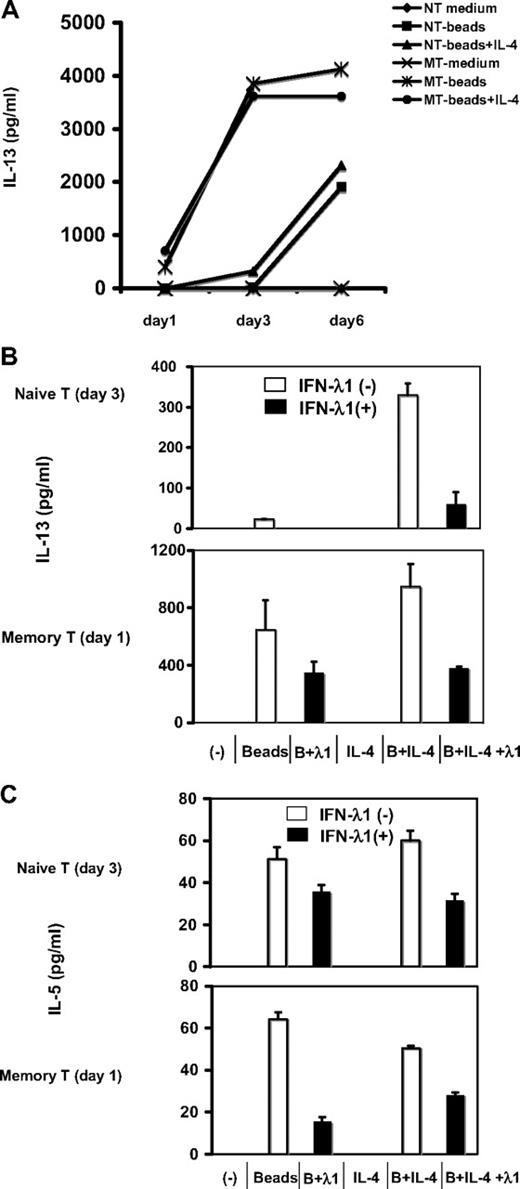
To determine the capacity of naive and memory CD4+ T cells to produce the Th2 cytokine IL-13 in the presence of IL-4 (ie, under Th2 conditions), highly purified naive and memory T cells from the same donor were stimulated with anti-CD2/3/28 beads under neutral (beads only) or Th2 conditions (beads + IL-4). At different time points (days 1, 3, and 6), the supernatant was collected, and IL-13 production was examined by ELISA. As shown in Figure 3A, naive T cells did not produce IL-13 immediately at day 1 and produced less at day 3 than memory cells were producing on day 1; as expected, memory T cells produced a large amount of IL-13 from day 1, reaching peak levels at day 3. Interestingly, IL-4 specifically increased IL-13 production by naive T cells but did not affect IL-13 production by memory T cells at day 1 and day 3 and actually slightly inhibited their IL-13 production at day 6.
IFN-λ1 directly inhibits Th2 cytokine production by activated memory T cells and Th2 polarization of naive T cells. (A) IL-13 production by naive and memory CD4+ T cells. Purified populations of each were stimulated under neutral (anti-CD2/3/28 beads only) or Th2 conditions (anti-CD2/3/28 beads + 10 ng/mL IL-4) in 24-well plates, at a concentration of 106 cells/mL for 1, 3, and 6 days. Supernatants (SNs) were harvested and analyzed for the presence of IL-13 by ELISA. The effect of IFN-λ1 on production of Th2 cytokines was determined by culturing purified naive (top) or memory CD4+ T cells (bottom) under neutral or Th2 conditions for 1 or 3 days. Where indicated, cells were cultured in the presence or absence of 100 ng/mL IFN-λ1. SNs were harvested from memory T cells at day 1 and from naive T cells at day 3, and tested for IL-13 (B) and IL-5 (C) by ELISA. The results from 5 individual donors are presented as means ± SDs.
IFN-λ1 directly inhibits Th2 cytokine production by activated memory T cells and Th2 polarization of naive T cells. (A) IL-13 production by naive and memory CD4+ T cells. Purified populations of each were stimulated under neutral (anti-CD2/3/28 beads only) or Th2 conditions (anti-CD2/3/28 beads + 10 ng/mL IL-4) in 24-well plates, at a concentration of 106 cells/mL for 1, 3, and 6 days. Supernatants (SNs) were harvested and analyzed for the presence of IL-13 by ELISA. The effect of IFN-λ1 on production of Th2 cytokines was determined by culturing purified naive (top) or memory CD4+ T cells (bottom) under neutral or Th2 conditions for 1 or 3 days. Where indicated, cells were cultured in the presence or absence of 100 ng/mL IFN-λ1. SNs were harvested from memory T cells at day 1 and from naive T cells at day 3, and tested for IL-13 (B) and IL-5 (C) by ELISA. The results from 5 individual donors are presented as means ± SDs.
IFN-λ1 directly inhibits Th2 cytokine production by either naive or memory T cells
Given that both naive and memory cells expressed IL-28Rα (Figure 2), we next characterized the direct effect of IFN-λ1 on Th2 cytokine production. In the absence of other cell types (including dendritic cells), purified naive or memory CD4+ T cells were stimulated under neutral (beads alone) or Th2 conditions (beads + IL-4), in the presence or absence of IFN-λ1. Supernatants were assayed by ELISA for the Th2 cytokines IL-13 and IL-5. As shown in Figure 3B and C, naive CD4+ T cells did not produce any Th2 cytokines at day 1 (data not shown); only after the cells were driven toward a Th2 phenotype did they produce IL-13 and IL-5 (day 3 or day 6). Likewise, IL-4 significantly enhanced IL-13 production by naive T cells. Notably, this increased IL-13 production was greatly reduced by IFN-λ1, suggesting it may act specifically to antagonize the activity of IL-4. In contrast, under the same Th2-polarizing conditions, IL-13 and IL-5 production were induced in memory T cells immediately after overnight stimulation. Interestingly, IFN-λ1 immediately inhibited their IL-13 and IL-5 production under both neutral and Th2 conditions. Similar inhibitions were seen at day 3, and less of an effect was observed at day 6 (data not shown). In sum, IL-13 and IL-5 secretions were inhibited by IFN-λ1 under neutral and Th2 conditions, in naive and memory CD4+ T cells. This occurred independently of a rise in Th1-associated cytokine, and was also observed in the presence of neutralizing antibody to IFN-γ (not shown).
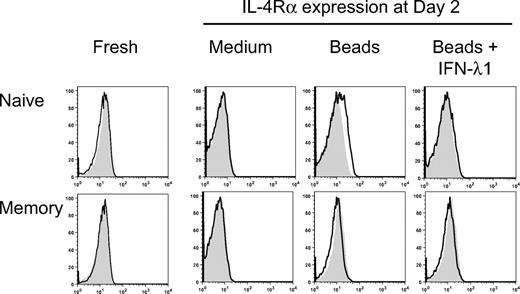
Differential expression of IL-4Rα on naive and memory T cells and the effect of IFN-λ1 on IL-4Rα expression
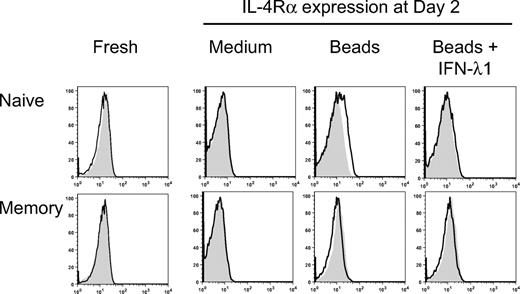
The results described in Figure 3 above show a selective effect of IL-4 on naive T cells. To determine whether IFN-λ1 specifically antagonized the activity of IL-4 on naive T cells through the regulation of the IL-4 signaling pathway, IL-4Rα expression on naive and memory T cells was measured by flow cytometry, with or without stimulation, in the presence or absence of IFN-λ1 at different time points. As shown in Figure 4, freshly isolated naive and memory CD4+ T cells did not express IL-4Rα on their surface. However, IL-4Rα expression was detected after 48-hour stimulation with anti-CD2/3/28 beads, but only on naive T cells. Remarkably, naive T cells that were stimulated in the presence of IFN-λ1 did not express detectable cell-surface expression levels of IL-4Rα, suggesting that IFN-λ1 down-regulated, or prevented, the expression of IL-4Rα. In contrast, IL-4Rα expression was not detectable on memory T cells with or without stimulation, in any culture conditions through the whole culture period (6 days). This is consistent with our observation that IL-4 was not required for IL-13 production by memory CD4+ T cells.
Modulation of IL-4Rα expression on naive and memory T cells by IFN-λ1. Naive and memory T cells were stimulated with anti-CD2/3/28 beads, in the presence or absence of IFN-λ1. At different time points, the cells were surface stained with anti–IL-4Rα (black line). The filled histograms show staining with isotype-matched controls. Data for day 2 are shown, along with expression in freshly isolated cells.
Modulation of IL-4Rα expression on naive and memory T cells by IFN-λ1. Naive and memory T cells were stimulated with anti-CD2/3/28 beads, in the presence or absence of IFN-λ1. At different time points, the cells were surface stained with anti–IL-4Rα (black line). The filled histograms show staining with isotype-matched controls. Data for day 2 are shown, along with expression in freshly isolated cells.
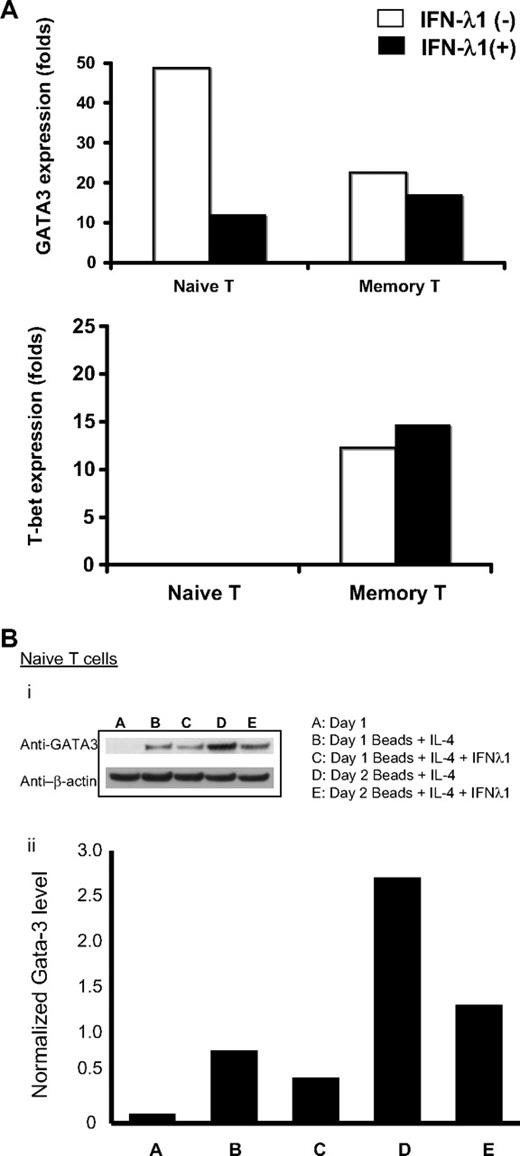
IFN-λ1 regulates expression of GATA3 and T-bet
Our data indicated that IFN-λ1 specifically antagonizes the IL-4 activity by down-regulating its receptor (IL-4Rα) expression. We next addressed whether downstream signaling can be modulated by IFN-λ1. As widely recognized, the polarized cytokine profiles of Th1 and Th2 cells are primarily dictated by the mutually exclusive expression of the “master” Th1 and Th2 transcription factors, T-bet and GATA3.15,32 IL-4 stimulation through the IL-4R leads to phosphorylation of STAT6 and up-regulation of GATA3 expression, whereas IL-12 and IFN-γ perform the complementary function of up-regulating T-bet. Given the effect of IFN-λ1 on the expression of Th2 cytokines and IL-4Rα, we hypothesized that the effect of IFN-λ1 on Th2 cytokines may be mediated through the regulation of GATA3 or T-bet or both.
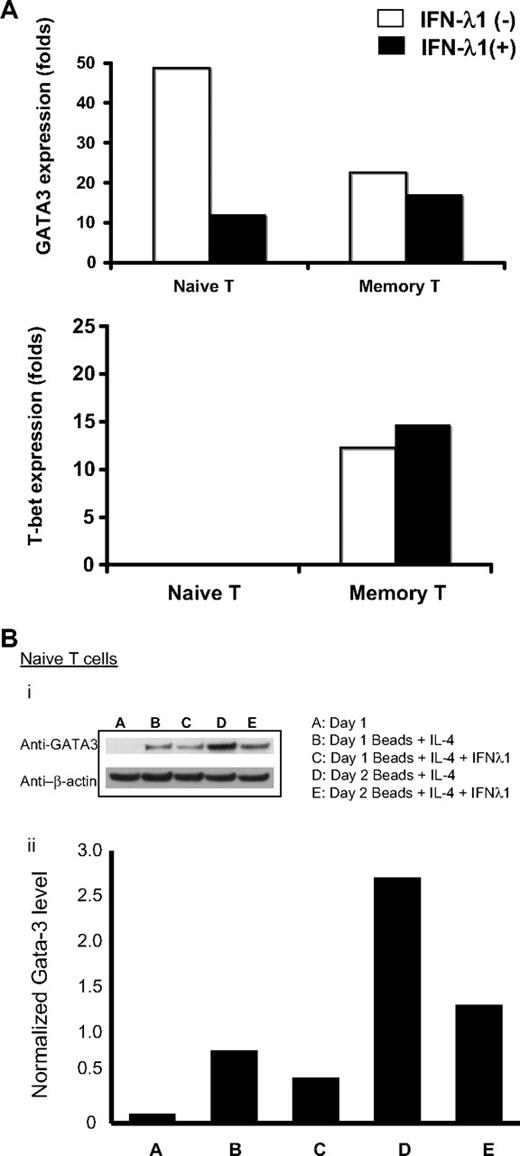
In these experiments, purified naive or memory CD4+ T cells were stimulated under Th2-polarizing conditions (beads + IL-4) for 18 hours, in the presence or absence of IFN-λ1. RNA was harvested for qRT-PCR quantitation of T-bet and GATA3 expression. As shown in Figure 5A, expression of GATA3 mRNA by naive T cells was decreased 4-fold (3- to 6-fold, depending on the donor) on treatment with IFN-λ1. When we examined the degree of expression of GATA3 protein, we found concordance with mRNA expression. As shown in Figure 5B, freshly isolated naive CD4+ T cells did not contain GATA3 protein; this was present after stimulation with beads + IL-4 on day 1 and day 2, and in both instances, the levels were markedly reduced by the presence of IFN-λ1. Memory T cells, however, exhibited only small changes in either GATA3 or T-bet expression (protein data not shown). As expected, T-bet mRNA expression was barely detectable after stimulation of naive CD4+ T cells under Th2 conditions.
IFN-λ1 regulates the expression of GATA3 and T-bet. (A) Purified naive CD4+ T cells and memory T cells were cultured under Th2 (anti-CD2/3/28 beads + 10 ng/mL IL-4) or control conditions (10 ng/mL IL-4), in the presence (■) or absence (□) of IFN-λ1. Total RNA was harvested after 18 hours, and expressions of GATA3 and T-bet were measured by real-time PCR. Graphs show expression levels (fold change) of cells stimulated under Th2 conditions, normalized to unstimulated cells under control conditions. (B) Purified naive CD4+ T cells were harvested, and protein was extracted and probed for the presence of GATA3, which was normalized by densitometry against levels of β-actin. One representative experiment of 3 is shown.
IFN-λ1 regulates the expression of GATA3 and T-bet. (A) Purified naive CD4+ T cells and memory T cells were cultured under Th2 (anti-CD2/3/28 beads + 10 ng/mL IL-4) or control conditions (10 ng/mL IL-4), in the presence (■) or absence (□) of IFN-λ1. Total RNA was harvested after 18 hours, and expressions of GATA3 and T-bet were measured by real-time PCR. Graphs show expression levels (fold change) of cells stimulated under Th2 conditions, normalized to unstimulated cells under control conditions. (B) Purified naive CD4+ T cells were harvested, and protein was extracted and probed for the presence of GATA3, which was normalized by densitometry against levels of β-actin. One representative experiment of 3 is shown.
Thus, our data support the direct inhibition of Th2 polarization by IFN-λ1, through the regulation of the Th2-restricted transcription factor GATA3, and that this effect is most pronounced in naive CD4+ T cells.
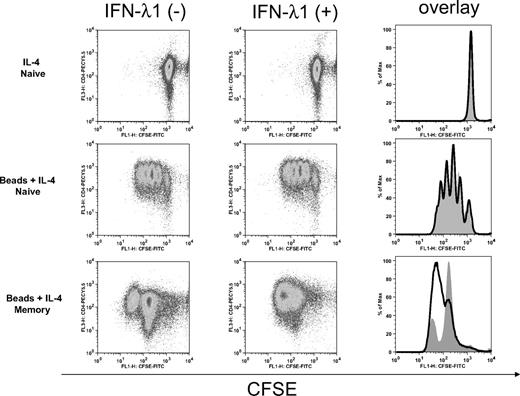
IFN-λ1 does not modulate the proliferation of naive CD4+ T cells
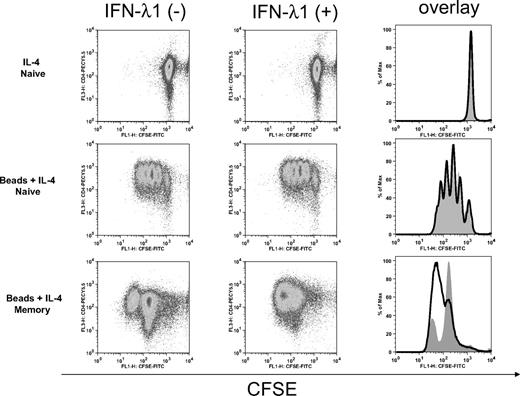
To characterize the potential effects of IFN-λ1 on T-cell function and to determine whether the decrease in cytokine production was due to inhibition of T-cell proliferation, we assessed its effect on T-cell proliferation. With the use of the CFSE dilution method, we defined the effect of IFN-λ1 on CD4+ T-cell proliferation in naive and memory cells. In these experiments, IL-4 alone did not induce proliferation of naive or memory T cells (data not shown). As shown in Figure 6, naive T cells proliferated more extensively than memory T cells under Th2 conditions. IFN-λ1 did not affect proliferation of naive CD4+ T cells, which undertook 4 rounds of division over the 6-day culture period. A minor enhancement of proliferation was noted in memory cells. No difference in the morphology of expanded naive T cells or memory T cells was observed in the presence or absence of IFN-λ1 (data not shown).
IFN-λ1 does not affect T-cell proliferation. Naive and memory CD4+ T cells were labeled with CFSE and cultured under Th2 (anti-CD2/3/28 beads + 10 ng/mL IL-4) or control (10 ng/mL IL-4) conditions for 6 days in the absence (left) or presence (middle) of IFN-λ1. The cells were stained with PECy5.5-labeled anti-CD4 antibody and analyzed by flow cytometry to assess cell division. Histogram overlays (right) compare CFSE staining in IFN-λ1–treated (bold line) to untreated (gray filled) cells. One representative experiment of 3 is shown.
IFN-λ1 does not affect T-cell proliferation. Naive and memory CD4+ T cells were labeled with CFSE and cultured under Th2 (anti-CD2/3/28 beads + 10 ng/mL IL-4) or control (10 ng/mL IL-4) conditions for 6 days in the absence (left) or presence (middle) of IFN-λ1. The cells were stained with PECy5.5-labeled anti-CD4 antibody and analyzed by flow cytometry to assess cell division. Histogram overlays (right) compare CFSE staining in IFN-λ1–treated (bold line) to untreated (gray filled) cells. One representative experiment of 3 is shown.
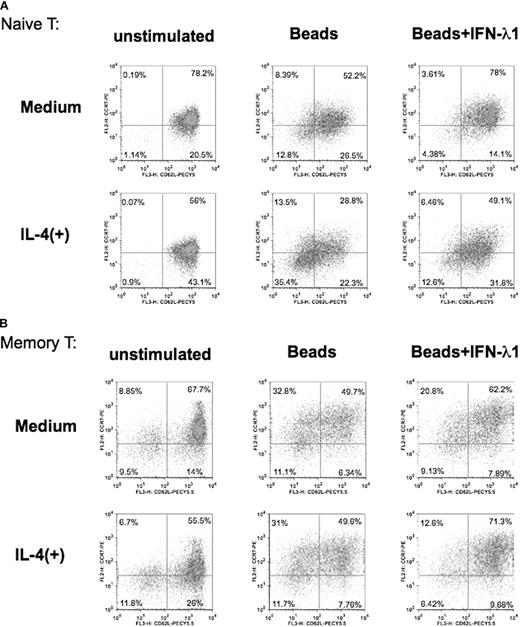
IFN-λ1 inhibits T-cell differentiation
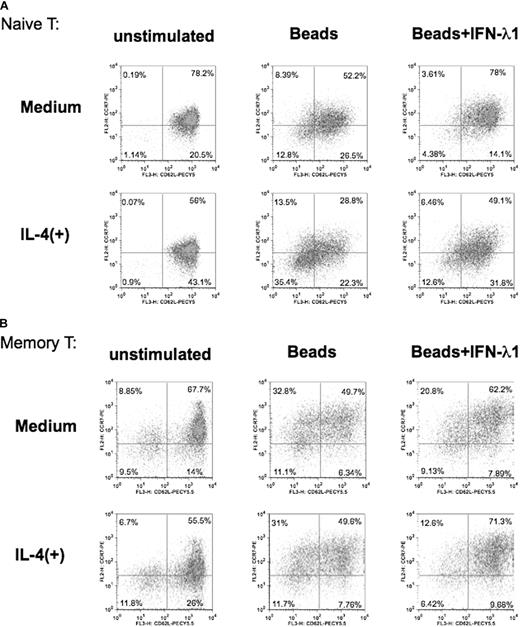
Finally, we investigated whether IFN-λ1 affects the phenotype or differentiation of naive and memory T cells on activation. In memory T cells, coexpression of CD62L and CCR7 (CD62L+CCR7+) leads to preferential retention in lymphoid tissue, whereas loss of these markers from the surface liberates these cells to the periphery and nonlymphoid sites.33-36 Their role on naive T cells is less well defined, but here our freshly isolated naive CD4+ T cells were also CD62L+CCR7+ (data not shown). We examined the expression of these 2 homing receptors on naive and memory CD4+ T cells after stimulation under either neutral or Th2 conditions, in the presence or absence of IFN-λ1.
In naive cells (Figure 7A), activation with beads decreased the expression of both CD62L and CCR7, with resultant reduction of the number of double-positive cells by greater than 25% and the corresponding elevation of both of the single-positive and the double-negative populations (8.39% CD62L−CCR7+, 26.5% CD62L+CCR7−, 12.8% CD62L−CCR7−). This effect was almost completely inhibited by the inclusion of IFN-λ1, showing that, although it was not acting on cell proliferation in naive cells (Figure 6), IFN-λ1 was preventing this aspect of naive CD4+ T-cell activation/differentiation. When the bead stimulation was carried out in the presence of IL-4, the loss of double-positive cells was further increased over beads alone (13.5% CD62L−CCR7+, 22.5% CD62L+CCR7−, 35.4% CD62L−CCR7−). Once again, the presence of IFN-λ1 worked against this loss of the double-positive population but did not cause a complete reversal, as was the case in the absence of IL-4.
Altered T-cell differentiation in the presence of IFN-λ1. Purified naive (A) or memory (B) CD4+ T cells were stimulated under neutral (only anti-CD2/3/28 beads) or Th2 conditions (anti-CD2/3/28 beads + 10 ng/mL IL-4). Each cell type was cultured in the presence or absence of IFN-λ1 for 3 days, harvested, and stained with PE-labeled anti-CCR7 and PECy5.5-labeled anti-CD62. Percentages shown denote expression of CD62L and CCR7 as analyzed by flow cytometry. One representative experiment of 4 is shown.
Altered T-cell differentiation in the presence of IFN-λ1. Purified naive (A) or memory (B) CD4+ T cells were stimulated under neutral (only anti-CD2/3/28 beads) or Th2 conditions (anti-CD2/3/28 beads + 10 ng/mL IL-4). Each cell type was cultured in the presence or absence of IFN-λ1 for 3 days, harvested, and stained with PE-labeled anti-CCR7 and PECy5.5-labeled anti-CD62. Percentages shown denote expression of CD62L and CCR7 as analyzed by flow cytometry. One representative experiment of 4 is shown.
In memory CD4+ T-cells, activation with beads (with and without IL-4) led to broadly similar loss of the double-positive population and increased numbers of the other 3 phenotypes. This was expected, because the memory CD4+ T cells lacked evidence of IL-4Rα chain or its induction on activation (Figure 4). Once again, IFN-λ1 inhibited the loss of the double-positive population under either activation protocol. Thus, IFN-λ1 prevented the activation-induced differentiation of memory T cells and may represent a mechanism by which effector cells are sequestered in the lymph node, rendered incapable of entry into the periphery by modulation of their homing receptor expression.
Discussion
We have recently reported on the IFN-λ1–mediated inhibition of Th2-associated cytokine production in PBMCs.29,30 Several immediate questions, however, remained unanswered, including the identification of the IFN-λ1 responsive cells and the mechanisms involved in driving this effect. To address this issue, we used purified naive and memory CD4+ T cells to conduct a detailed analysis of TCR/CD28/CD2-induced differentiation and activation in vitro, and we examined the capacity of IFN-λ1 to inhibit Th2 polarization and naive and memory Th2 cell responses. Our findings provide insight into the role of IFN-λ1 as a regulator of Th2 cytokine production by showing several points. First, IFN-λ1 directly inhibits IL-13 production by both naive and memory CD4+ T cells. This effect was facilitated through the expression of the IFN-λ1 receptor, IL-28Rα, on CD4+ T cells. Second, the down-regulation of IL-4Rα and GATA3 expression by IFN-λ1 was correlated with its inhibition of Th2 polarization. Third, the inhibitory effects of IFN-λ1 on Th2 responses in memory T cells were not only manifest through changes in cytokine production, but also by changes in differentiation-associated cell-surface phenotype. We observed that the differentiation of central memory T cells into effector memory T cells was clearly inhibited by the presence of IFN-λ1. Fourth, the increased production of the Th1 cytokine, IFN-γ, came later than the reduction of Th2 cytokines, indicating that IFN-λ1 acted first to inhibit Th2 responses. Finally, our results indicated clearly that T-cell proliferation was not affected by IFN-λ1.
Priming naive CD4+ T cells in the presence of IL-12 or IL-4 results in the generation of Th1 or Th2 effector cells, respectively. Several lines of evidence indicate that when naive T cells are primed in vivo in IL-4–deficient or IL-4R–deficient animals, or during blocking with αIL-4 antibodies, the development of Th2 cells and the cytokines they produce is diminished.37-39 However, natural silencing of the IL-4 gene plays a crucial role in the development and maintenance of Th1 cells.40,41 Therefore, both IL-4 and IL-4R are critical for Th2 polarization. Consistent with these studies, we found that IL-4 significantly promoted IL-13 production by naive CD4+ T cells and enhanced IL-4R expression. IFN-λ1 antagonized both of these events and prevented activation-induced cell-surface changes in naive T cells. Moreover, we further showed that GATA3 expression was significantly reduced by IFN-λ1 in naive T cells. GATA3 is sufficient to induce a Th2 phenotype and acts not only through the induction of IL-4, IL-5, and IL-13 but also through selective growth of Th2 cells.42 Therefore, the inhibition of IL-13 and IL-5 production by IFN-λ1 was most likely mediated by its regulation of GATA3 expression. Our data showed that only small changes of GATA3 expression were found in memory T cells. Given the heterogeneous nature of memory T cells, it is difficult to gauge what percentage are Th2 polarized and potentially IFN-λ1 responsive. Future experiments will focus on the effects of IFN-λ1 on separate groups of committed cells.
Memory T cells are heterogeneous and are composed of both uncommitted central memory and committed Th1 and Th2 effector memory T cells.43 After stimulation, central memory T cells have been shown only to produce IL-2; in contrast, effector memory T cells produced high levels if IL-4, IL-5, and IFN-γ.33 Therefore, it seems very likely that IFN-λ1 suppressed the IL-13 production of memory T cells by interfering with the differentiation of central memory T cells into effector cells. In addition, preventing the differentiation of central memory T cells into effector T cells may well effectively hold activated T cells in secondary lymphoid organs and interfere with their recruitment to peripheral tissues. By acting in this manner, IFN-λ1 disables activated effector T cells and prevents them from functioning in the periphery. Artificially inhibiting T-cell differentiation with IFN-λ1 could prevent recruitment of Th2 memory T cells to the airway in patients with asthma or other clinically relevant sites in additional diseases.
It is well established that naive and memory T cells mediate quantitatively and qualitatively distinct types of immune responses, manifest as differences in cytokine secretion, adhesive and trafficking properties, and activation requirements.44 Differences in proximal signal transduction through TCR/CD3 have also been identified between naive and memory T cells.45 Our parallel studies of naive and memory T cells show that differential responsiveness to IL-4 is correlated with the differential expression of IL-4R on both populations. This is consistent with previous studies that have found a marked up-regulation of IL-4Rα chain on naive T cells, directly induced by IL-4; in contrast, IL-4Rα expression was not detected on memory T cells.46
Recently, the Notch pathway has been identified as an IL-4–independent pathway to initiate Th2 polarization47,48 It was also shown that the Notch pathway is required for TCR-mediated activation of peripheral T cells49 and stimulates GATA3 expression directly, independently of IL-4.50 Our data show that IL-4 barely induced memory CD4+ T cells to produce IL-13 or IL-5. However, IFN-λ1 was still able to exert its inhibitory effect on these 2 cytokines, suggesting that the IL-4–independent pathway might play a preferred role in memory T cells. Nonetheless, the idea that IFN-λ1 may act on both IL-4–dependent and –independent pathways is intriguing. Studies to define the effect of IFN-λ1 on Notch signaling are currently underway.
Recent studies have shown deficient induction of IFN-λ1 by rhinovirus virus in primary bronchial epithelial cells and alveolar macrophages28 of patients with asthma. Consistent with this study, we have found that IFN-λ1 was able to alter cytokine-mediated Th biasing when naive T cells were exposed to allogeneic mDCs.29 It has been shown that only dendritic cells and epithelial cells respond to IFN-λ in the mouse.25 However, in this study, we have found the IL-28Rα mRNA expression in both human naive and memory CD4+ T-cell subsets, which strongly suggests a broader and direct effect of IFN-λ1 on human CD4+ T cells. Mennechet and Uze31 have shown that human dendritic cells matured in the presence of IFN-λ1 direct the generation of T regulatory (Treg) cells from naive CD4+ T cells. The resultant cells were FOXP3+, known to influence the balance of Th1 and Th2 responses through a separate and distinct mechanism from that described here. In our studies, IFN-λ1 exerted direct effects on CD4+ T cells, in the absence of other cell types and independently of a rise in Th1 cytokine production or change in proliferation. The results therefore identify a novel pathway by which IFN-λ1 acts to regulate T-cell polarization, through direct activity on these cells, independently of DC-mediated responses.
In summary, we report here that IFN-λ1 acts directly on CD4+ T cells to inhibit activation-induced differentiation and Th2 polarization through the regulation of IL-4R and GATA3 expression. These findings are likely to facilitate further investigation to elucidate the differential effects of IFN-λ1 on Th2 memory T cells.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported intramurally by HUMIGEN.
Authorship
Contribution: J.D. designed and performed research, analyzed data, and wrote the manuscript; N.J.M. designed and performed research, revised the manuscript, analyzed data, and contributed to preparing the resubmission; G.E.G. performed research and analyzed data; R.Y.L.Y. designed and performed research and analyzed data; and G.G. designed research, analyzed data, wrote and edited the manuscript, and wrote the resubmission.
Conflict-of-interest disclosure: All authors are employees of HUMIGEN.
Correspondence: Grant Gallagher, HUMIGEN, The Institute for Genetic Immunology, 2439 Kuser Rd, Hamilton, NJ 08690; e-mail: g.gallagher@humigen.org.