Abstract
Allograft vasculopathy is the leading cause of death in patients with heart transplantation. Accelerated endothelial regeneration mediated by enhanced endothelial progenitor cell (EPC) incorporation may attenuate the development of allograft vasculopathy. We investigated the hypothesis that modulation of EPC biology and attenuation of allograft vasculopathy by increased high-density lipoprotein cholesterol after human apo A-I (AdA-I) transfer requires scavenger receptor (SR)–BI expression in bone marrow–derived EPCs. After AdA-I transfer, the number of circulating EPCs increased 2.0-fold (P < .001) at different time points in C57BL/6 mice transplanted with SR-BI+/+ bone marrow but remained unaltered in mice with SR-BI−/− bone marrow. The effect of high-density lipoprotein on EPC migration in vitro requires signaling via SR-BI and extracellular signal-regulated kinases and is dependent on increased nitric oxide (NO) production in EPCs. Human apo A-I transfer 2 weeks before paratopic artery transplantation reduced intimal area at day 21 3.7-fold (P < .001) in mice with SR-BI+/+ bone marrow but had no effect in mice with SR-BI−/− bone marrow. AdA-I transfer potently stimulated EPC incorporation and accelerated endothelial regeneration in chimeric SR-BI+/+ mice but not in chimeric SR-BI−/− mice. In conclusion, human apo A-I transfer accelerates endothelial regeneration mediated via SR-BI expressing bone marrow–derived EPCs, thereby preventing allograft vasculopathy.
Introduction
Cardiac allograft vasculopathy is the leading cause of late heart failure and death after heart transplantation.1,2 Transplantation vasculopathy is caused by recognition of major histocompatibility complex class I and class II antigens on the endothelium of the allograft by recipient T lymphocytes. This initiates an inflammatory cascade leading to macrophage activation and proliferation of smooth muscle cells.3 Denuding endothelial injury in allografts occurs as a consequence of peritransplantation ischemia/reperfusion injury and of cellular immunity. Because the endothelium regulates vascular tone, inflammation, smooth muscle cell proliferation, and thrombosis,4 restoration of endothelial integrity and function is pivotal to attenuate the development of allograft vasculopathy.5
Disruption of the endothelial lining resulting from endothelial cell apoptosis can be restored by proliferation of adjacent endothelial cells but also by incorporation of circulating bone marrow–derived endothelial progenitor cells (EPCs).6-9 Hu et al10 have demonstrated that EPCs contribute to endothelial regeneration and to microvessel formation in allografts. Recently, we have shown that increased high-density lipoprotein (HDL) cholesterol levels after hepatocyte-directed human apo A-I transfer in C57BL/6 apo E−/− mice increase circulating EPC number, enhance the incorporation of bone marrow–derived EPCs into the injured endothelium, and reduce neointima formation in a murine model of transplantation arteriosclerosis.11 Evaluation of EPC function in vitro showed that addition of HDL improved EPC adhesion, migration, and invasion.11 This prior study suggests that attenuation of allograft vasculopathy after human apo A-I transfer is mediated by enhanced EPC incorporation into allografts. However, because HDL has pleiotropic effects on lipoprotein metabolism and vascular biology, the mechanistic link between the effects of HDL on EPC biology and on allograft vasculopathy remains uncertain. A more stringent experimental approach that dissociates effects of HDL on EPCs from other actions of HDL is required to investigate the role of enhanced EPC number and function in the attenuation of allograft vasculopathy after human apo A-I transfer.
Scavenger receptor (SR)–BI is an HDL receptor that plays a critical role in reverse cholesterol transport by mediating selective lipid uptake from HDL particles.12,13 SR-BI is expressed in hepatocytes,12 steroidogenic tissues,12 Kupffer cells,14 and macrophages,15 but also in endothelial cells.16 HDL stimulates endothelial cell migration in vitro via SR-BI.16 In addition, the effects of HDL on endothelium-dependent vasoreactivity appear to depend on the binding of HDL to SR-BI and subsequent stimulation of NO formation.17 Whether SR-BI plays a role in EPC biology has not been evaluated before. In the current study, we investigated the hypothesis that modulation of EPC biology by increased HDL cholesterol after hepatocyte-directed human apo A-I transfer requires SR-BI expression on bone marrow–derived EPCs. If this hypothesis is correct, bone marrow transplantation of SR-BI–deficient bone marrow may abrogate the effect of HDL on bone marrow–derived EPCs and at the same time leave other effects of HDL intact. To investigate whether the inhibition of allograft vasculopathy by human apo A-I transfer requires SR-BI expression in bone marrow cells, transplantation of bone marrow obtained from SR-BI+/+ or SR-BI−/− donor mice was performed in C57BL/6 mice before gene transfer or saline injection and artery transplantation. We show for the first time that enhanced EPC number, function and incorporation after human apo A-I transfer is strictly dependent on SR-BI expression in bone marrow–derived EPCs. Human apo A-I transfer accelerates EPC-mediated endothelial regeneration in allografts and thereby prevents the development of transplantation arteriosclerosis in chimeric SR-BI+/+ mice but not in chimeric SR-BI−/− mice. The effect of HDL on EPC biology requires signaling via SR-BI and extracellular signal-regulated kinases (ERK) and is dependent on enhanced NO production. Taken together, the results of the current study indicate that increased EPC number, function, and incorporation induced by therapeutic HDL levels after human apo A-I transfer is probably the main effector of the beneficial effects of increased HDL cholesterol on allograft vasculopathy.
Methods
Animals
All experimental procedures were approved by the ethical committee for animal experimentation of the Katholieke Universiteit Leuven. The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (Publication No. 85-23, revised 1996). C57BL/6 (H2b) and BALB/c (H2d) mice were obtained from the specific pathogen-free facility of the Center for Molecular and Vascular Biology. SR-BI−/− mice, originally developed by Rigotti et al,18 were backcrossed to the C57BL/6 background at the University of Leiden for 5 generations. Bone marrow cells and spleens obtained from these SR-BI−/− mice were kindly provided by M.v.E. Mice were fed normal chow ad libitum.
Gene transfer and artery transplantations
Gene transfer was performed with 5 × 1010 particles of AdA-I or Adnull at the age of 13 weeks. AdA-I is an E1E3E4-deleted adenoviral vector containing the human α1-antitrypsin promoter upstream of the genomic human apo A-I sequence and 4 copies of the human hepatic control region-1.19,20 The E1E3E4-deleted control vector Adnull does not contain an expression cassette.20 Two weeks after gene transfer or saline injection, a common carotid artery of a female BALB/c donor mouse was transplanted paratopically into the recipient male C57BL/6 mice as described previously.11,21 In selected experiments, to determine incorporation of bone marrow–derived EPCs, recipient C57BL/6 mice were female.
Bone marrow transplantations
C57BL/6 mice were lethally irradiated with 9.5 Gy at the age of 9 weeks. Transplantation of 3 × 106 bone marrow cells obtained from male SR-BI+/+ or male SR-BI−/− mice was performed 24 hours after irradiation via tail vein injection. Gene transfer and artery transplantation were subsequently performed in recipient mice at the age of 13 and 15 weeks, respectively.
Human apo A-I ELISA
Human apo A-I levels were determined by sandwich enzyme-linked immunosorbent assay (ELISA) as described previously.22
Separation of lipoproteins by gel filtration
Mouse plasma lipoproteins were fractioned by fast performance liquid chromatography gel filtration of 100 μL plasma as described previously.22 Cholesterol levels in non-HDL and HDL fractions were determined by Amplex Red Kit (Invitrogen, Carlsbad, CA).
HDL isolation by density gradient ultracentrifugation
FACS
The number of circulating EPCs or the number of EPCs in bone marrow was determined by quantification of the number of Flk-1 Sca-1 double-positive cells by fluorescence-activated cell sorter (FACS) as described before.11
Evaluation of SR-BI expression by immunocytochemistry
Murine spleen EPC culture and human EPC culture were performed as described previously.11 To study SR-BI expression in EPCs, circulating human mononuclear cells and murine spleen cells were cultured for 4 and 7 days, respectively, and incubated with DiI-acLDL (6.6 μg/mL; Invitrogen) for 4 hours before fixation in 4% paraformaldehyde. After overnight incubation with a 1:1000 dilution of rabbit anti–mouse SR-BI primary antibody (Abcam, Cambridge, United Kingdom) and subsequent incubation for 1 hour with a horseradish peroxidase–conjugated goat anti–rabbit antibody (Dako Denmark, Glostrup, Denmark) in a 1:100 dilution, a positive signal was detected using the NEL 704 Kit (PerkinElmer Life and Analytical Sciences, Waltham, MA) according to the instructions of the manufacturer. Images were acquired using Zeiss Axiovision 4.6 software on a Zeiss AxioVert 100M microscope equipped with a Zeiss AxioCam MRc5 digital camera at a 20×/0.4 magnification (Carl Zeiss, Jena, Germany). Post-hoc image analysis was performed using KS300 software (Carl Zeiss). In parallel, cultured murine EPCs from SR-BI−/− mice were used as a negative control.
Murine bone marrow EPC culture and quantification
Bone marrow mononuclear cells were isolated from bone marrow–transplanted C57BL/6 mice 21 days after artery transplantation by density gradient centrifugation using Histopaque-1077 (Sigma Chemie, Deisenhofen, Germany) as described.24 Immediately after isolation, cells were plated onto fibronectin (40 μg/mL)–coated 24-well plates at a density of 4 × 106 cells/well and cultured in EGM-2MV Bullet Kit medium (Cambrex, East Rutherford, NJ). After 7 days of culture, medium was removed and adhered cells were stained for DiI-acLDL (6.6 μg/mL; Invitrogen) for 4 hours and then fluorescein isothiocyanate (FITC)–labeled isolectin (10 μg/mL; Invitrogen) for 1 hour. The number of EPCs, identified as DiI-acLDL isolectin double-positive cells, per microscopy field was quantified using Zeiss Axiovision 4.6 acquisition software on a Zeiss AxioPlan 2 microscope equipped with a Zeiss AxioCam MRc5 digital camera at a 20×/0.4 magnification (Carl Zeiss).
EPC migration assay
EPC migration assay was performed as described before.11 Migration was performed in the presence of HDL (100 μg/mL) or an equivalent amount of bovine serum albumin (Roche Diagnostics, Mannheim, Germany). To investigate whether the effect of HDL on EPC migration requires signaling via ERKs and depends on NO, the effect of HDL on migration of SR-BI+/+ EPCs was studied in the presence of the mitogen-activated protein kinase kinase 1 (MEK1) and MEK2 inhibitor U0126 (10 μM; Cell Signaling Technology, Danvers, MA) and the NO synthase inhibitor NG-monomethyl-L-arginine (LNMA, 2 mM; Sigma Chemie), respectively.
Methods corresponding to EPC experiments are described in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Evaluation of p-ERK expression by Western blot
After 7 days of culture of bone marrow mononuclear cells obtained from SR-BI+/+ or SR-BI−/− mice, HDL (100 μg/mL) or an equivalent amount of bovine serum albumin was added for 2.5 minutes. Cells were subsequently harvested in lysis buffer (20 mM Tris, pH 7.5, 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid, 1 mM ethylene glycol-bis-N,N,N′,N′-tetraacetic acid, 1% Triton, 1 mM sodium vanadate, 1× PhosSTOP (Roche Diagnostics), and complete proteinase inhibitor; Roche Diagnostics). After separation of the lysate (50 μg per lane) in a 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) system, proteins were transferred to a nitrocellulose membrane (GE Healthcare, Little Chalfont, United Kingdom). After overnight incubation with a 1:1000 dilution of rabbit antimouse p42 (ERK-2)/p44 (ERK-1) ERK or p-p42/p-p44 ERK antibodies (Cell Signaling Technology) and subsequent incubation for 1 hour with horseradish peroxidase–labeled goat antirabbit antibodies (Dako Denmark) in a 1:1000 dilution, the membrane was developed using enhanced chemiluminescence detection reagent (GE Healthcare). Films were scanned on an Epson Perfection V700 Photo scanner (Seiko Epson, Nagano, Japan), and p-ERK and ERK levels were quantified using Image J software (Wayne Rasband, National Institutes of Health, http://www.rsb.info.nih.gov/ij/).
Quantification of NO production
Nitrite, nitrate, and S-nitrosothiol levels (NOx) were quantified as stable oxidative products of NO in cell supernatant or bone marrow plasma by the Griess method using a Sievers chemiluminescent NO analyzer as previously described.25 Negative control values obtained from unconditioned medium were subtracted from the sample values. NOx values were normalized to protein concentration in medium or bone marrow plasma.
Histologic analysis
C57BL/6 artery transplantation recipients were killed for histologic analysis of the allografts 21 days after artery transplantation. Perfusion fixation, tissue processing, and morphometric analysis were performed as described before.11
Immunohistochemistry
Paraffin sections were stained with rat antimouse CD45 (1:100; BD Biosciences, San Jose, CA), rat antimouse Mac-3 (1:50; BD Biosciences), rabbit antihuman CD3 (1:50; Dako), and rat antimouse CD31 (1:500; BD Biosciences) to detect leukocytes, macrophages, T lymphocytes, and endothelial cells, respectively. Images were acquired on a Zeiss AxioPlan 2 microscope at a 20×/0.4 magnification (Carl Zeiss). Inflammatory cells were quantified in a blinded fashion by computer assisted image analysis using KS300 software (Carl Zeiss). The percentage of leukocytes, macrophages, and T lymphocytes was determined as the ratio of CD45, Mac-3, and CD3+ cells, respectively, in the neointima divided by the total number of cells in the neointima and multiplied by 100. The absolute number of endothelial cells per section was determined by CD31 immunohistochemistry and quantification via computer assisted image analysis using KS300 software (Carl Zeiss).
In situ hybridization for the murine Y-chromosome–bone marrow transplantations with bone marrow of male SR-BI+/+ or male SR-BI−/− bone marrow were performed at the age of 9 weeks in female C57BL/6 recipients. Artery transplantation using common carotid arteries obtained from female BALB/c donors was performed at the age of 15 weeks, 2 weeks after gene transfer or saline injection. To quantify incorporation of EPCs in the allografts, in situ hybridization for the murine Y-chromosome was performed in the laboratory of M.E. essentially as described previously.26,27 Briefly, paraffin sections were dewaxed in xylol and permeabilized in 2 mg/mL pepsin (Sigma Chemie) in 0.1 M HCl at 37°C for 20 minutes. Subsequently, slides were fixed in 4% formaldehyde in phosphate-buffered saline (PBS) for 10 minutes at room temperature and dehydrated in graded ethanol series. The PY353B probe,28 which hybridizes specifically to a series of repetitive sequences on the murine Y-chromosome, was nick-translated in the presence of digoxigenin-11-dUTP using a commercial kit (DIG-Nick Translation Mix; Roche Diagnostics). Next, the digoxigenin-labeled Y-probe was denatured in 50% formamide/10% dextran sulfate/2× sodium saline citrate buffer (0.2 M NaCl, 30 mM sodium citrate, pH 7.0) at 75°C for 5 minutes in the presence of salmon sperm DNA, and hybridized with sections at 37°C overnight (30 ng/slide). After washing with Tris-buffered saline (TBS; 50 mM Tris, 0.05% Tween 20, pH 7.0), signals were detected using antidigoxigenin Fab conjugated with alkaline phosphatase followed by a nitroblue tetrazolium/5-bromo-4-chloro-indolyl-phosphatase substrate conversion (Rembrand RISH and AP Detection Kit; KREATECH Diagnostics, Amsterdam, The Netherlands). Finally, sections were counterstained for nuclear fast red. Positive hybridization of the Y-chromosome bearing cells was identified by the presence of a small, discrete area of blue precipitate in the nucleus.
To quantify the incorporation of EPCs in the allograft, successive slides containing adjacent sections were stained for CD31 as described earlier. Images were obtained on a Zeiss AxioPla 2 microscope at a 20×/0.4 magnification (Carl Zeiss). The percentage of Y-chromosome and CD31 double-positive cells was calculated manually by superposing pictures of both stainings on adjacent slides using KS300 software (Carl Zeiss).
Statistical analysis
Data are expressed as mean plus or minus SEM. Cholesterol values or the number of Flk-1 Sca-1 double-positive cells at different time points after gene transfer were compared with baseline values by one-way analysis of variance (ANOVA) followed by the Dunnett multiple comparisons test using Instat3 (GraphPad Software, San Diego, CA). Data of EPC functional assays and histologic parameters were compared by ANOVA followed by Tukey multiple comparisons test. When indicated, a logarithmic or square root transformation or nonparametric test was performed. A 2-way ANOVA to test interaction between the factor gene transfer and the factor bone marrow transplantation on intimal area in allografts was performed using Statistica (Statsoft Benelux, Groningen, The Netherlands). A 2-sided P value of less than .05 was considered statistically significant.
Results
SR-BI is expressed on EPCs
To investigate whether effects of HDL on EPC biology may be mediated via scavenger receptor-BI, we first evaluated whether SR-BI is expressed on EPCs. Immunocytochemistry demonstrated SR-BI expression on both murine (data not shown) and human EPCs (Figure S1). No signal was detected in EPCs obtained from SR-BI−/− mice. FACS analysis showed SR-BI expression in 41% plus or minus 1.9% (n = 4) of circulating Flk-1 Sca-1–positive cells obtained from wild-type mice.
Human apo A-I gene transfer induces a persistent elevation of HDL cholesterol in chimeric C57BL/6 mice with SR-BI+/+ or SR-BI−/− bone marrow
Persistent human apo A-I plasma levels (Figure 1A) resulted in a 2.0-fold (P < .001) and 1.8-fold (P < .001) increase of HDL cholesterol at day 14 and day 35, respectively, after transfer with 5 × 1010 particles of AdA-I in C57BL/6 mice transplanted with SR-BI+/+ or SR-BI−/− bone marrow (Table 1). After human apo A-I transfer, human apo A-I containing HDL was polydisperse, and murine apo A-I levels decreased to less than 25% of baseline levels (data not shown). Gene transfer with the control vector Adnull, containing no expression cassette, did not result in a significant alteration of lipoprotein levels (Table 1). Lipoprotein profiles after transfer with AdA-I and Adnull are shown in Figure S2.
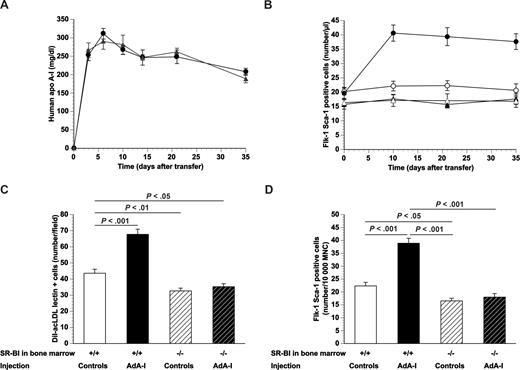
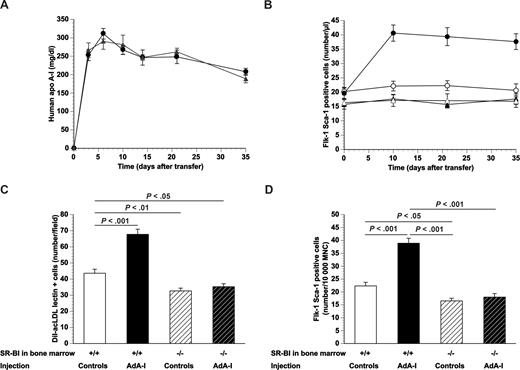
Human apo A-I gene transfer and EPC number in chimeric C57BL/6 SR-BI+/+ mice and chimeric C57BL/6 SR-BI−/− mice. (A) Human apo A-I expression levels after adenoviral gene transfer with 5 × 1010 particles of AdA-I in male C57BL/6 mice transplanted with SR-BI+/+ (●; n = 10) or SR-BI−/− (▲; n = 10) bone marrow 4 weeks before gene transfer. (B) Time course of the number of Flk-1 Sca-1 double-positive cells in AdA-I (●, ▲)– or Adnull (○, △)–treated C57BL/6 mice with SR-BI+/+ (●, ○) or SR-BI−/− (▲, △) bone marrow (n = 10 for each group). (C) Bar graph showing the number of DiI-acLDL FITC-isolectin double-positive cells after 7 days of ex vivo culture of bone marrow mononuclear cells isolated at day 35 after Adnull transfer or AdA-I transfer in C57BL/6 mice with SR-BI+/+ or SR-BI−/− bone marrow (n = 10 for each group). (D) Bar graph showing the number of Flk-1 Sca-1 double-positive cells in the bone marrow of C57BL/6 mice with SR-BI+/+ or SR-BI−/− bone marrow at day 35 after transfer with Adnull or AdA-I. Data are expressed as number/10 000 mononuclear cells (MNC) (n = 10 for each group). Data are mean plus or minus SEM.
Human apo A-I gene transfer and EPC number in chimeric C57BL/6 SR-BI+/+ mice and chimeric C57BL/6 SR-BI−/− mice. (A) Human apo A-I expression levels after adenoviral gene transfer with 5 × 1010 particles of AdA-I in male C57BL/6 mice transplanted with SR-BI+/+ (●; n = 10) or SR-BI−/− (▲; n = 10) bone marrow 4 weeks before gene transfer. (B) Time course of the number of Flk-1 Sca-1 double-positive cells in AdA-I (●, ▲)– or Adnull (○, △)–treated C57BL/6 mice with SR-BI+/+ (●, ○) or SR-BI−/− (▲, △) bone marrow (n = 10 for each group). (C) Bar graph showing the number of DiI-acLDL FITC-isolectin double-positive cells after 7 days of ex vivo culture of bone marrow mononuclear cells isolated at day 35 after Adnull transfer or AdA-I transfer in C57BL/6 mice with SR-BI+/+ or SR-BI−/− bone marrow (n = 10 for each group). (D) Bar graph showing the number of Flk-1 Sca-1 double-positive cells in the bone marrow of C57BL/6 mice with SR-BI+/+ or SR-BI−/− bone marrow at day 35 after transfer with Adnull or AdA-I. Data are expressed as number/10 000 mononuclear cells (MNC) (n = 10 for each group). Data are mean plus or minus SEM.
SR-BI deficiency in bone marrow lowers baseline EPC count and abrogates the increase of circulating EPCs induced by human apo A-I transfer in C57BL/6 mice
The number of Flk-1 Sca-1 double-positive cells in the peripheral blood at different time points after AdA-I transfer or Adnull transfer in C57BL/6 mice transplanted with SR-BI+/+ or SR-BI−/− bone marrow is shown in Figure 1B. The baseline number of circulating Flk-1 Sca-1 double-positive cells was 25% (P < .01) lower in C57BL/6 mice transplanted with SR-BI−/− bone marrow compared with mice transplanted with SR-BI+/+ bone marrow. Adnull gene transfer had no effect on the number of circulating Flk-1 Sca-1 double-positive cells at any time point. After AdA-I transfer, the number of circulating Flk-1 Sca-1+ cells increased 2.0-fold (P < .001) at different time points after transfer in mice transplanted with SR-BI+/+ bone marrow, whereas no change occurred in mice transplanted with SR-BI−/− bone marrow. The number of DiI-acLDL FITC-isolectin double-positive cells after 7 days of ex vivo culture of bone marrow mononuclear cells isolated at day 35 after Adnull transfer (Figure 1C) was 1.3-fold (P < .01) lower in mice transplanted with SR-BI−/− bone marrow than in mice transplanted with SR-BI+/+ bone marrow. A phenotypic characterization of cultured EPCs is described in Document S1. The EPC number in the bone marrow after AdA-I transfer was 1.6-fold (P < .001) higher than after Adnull transfer in mice transplanted with wild-type bone marrow, whereas no significant difference was observed in mice transplanted with SR-BI−/− bone marrow. These data were confirmed by quantification of the number of Flk-1 Sca-1+ cells in the bone marrow (Figure 1D).
The effect of HDL on EPC function requires signaling via SR-BI and extracellular signal-regulated kinases and is dependent on NO
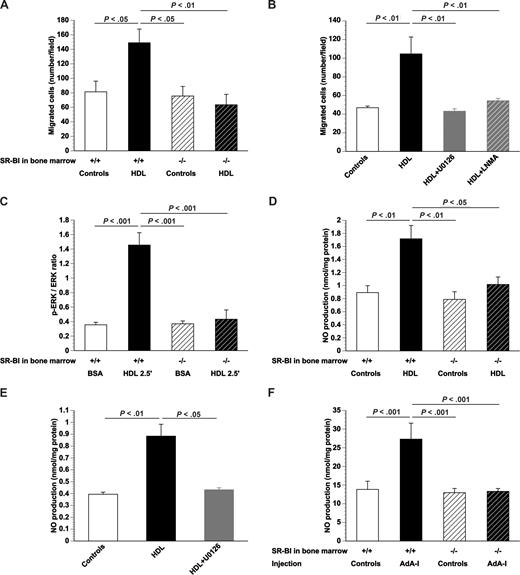
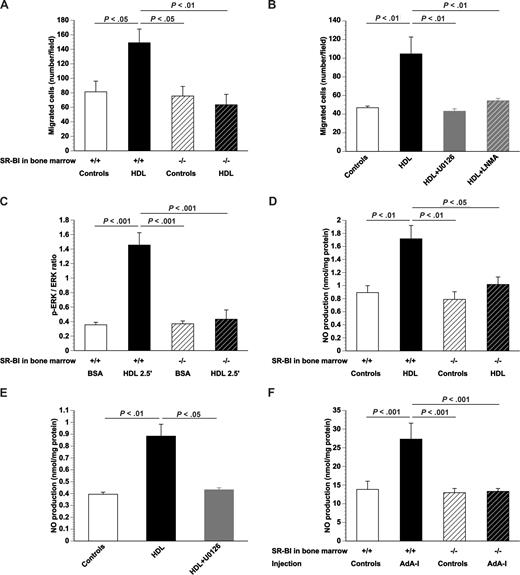
To evaluate whether SR-BI deficiency not only affects EPC number in vivo but also EPC function, migration was quantified in vitro for cultured SR-BI+/+ and SR-BI−/− bone marrow EPCs. Migration in the presence of 100 μg/mL HDL was 1.8-fold (P < .05) higher for SR-BI+/+ EPCs, whereas no effect was observed for SR-BI−/− EPCs (Figure 2A). These results were confirmed using cultured EPCs derived from Sca-1+ isolated cells (Document S1; Figure S3). To investigate whether the effect of HDL on EPC migration requires signaling via ERKs and depends on NO, the effect of HDL on migration of SR-BI+/+ EPCs was studied in the presence of the MEK1 and MEK2 inhibitor U0126 or the NO synthase inhibitor LNMA. U0126 as well as LNMA abrogated the stimulation of EPC migration by HDL (Figure 2B). In agreement, Western blot analysis indicated that the p-ERK/ERK ratio increased 4.1-fold (P < .001), 2.5 minutes after the addition of 100 μg/mL HDL to SR-BI+/+ EPCs (Figure 2C). In contrast, the p-ERK/ERK ratio was not significantly altered by addition of HDL to EPCs obtained from SR-BI−/− mice (Figure 2C). Figure 2D shows that NO production in cultured SR-BI+/+ EPCs was increased 1.8-fold (P < .001) by addition of HDL. HDL did not result in increased NO production in cultured SR-BI−/− EPCs (Figure 2D). Addition of HDL to cultured SR-BI+/+ EPCs did not increase NO production in the presence of U0126 (Figure 2E). Taken together, these data indicate that the effect of HDL on EPC migration is mediated via NO and that enhanced NO production requires signaling via SR-BI and ERK. Increased NO production was also observed after human apo A-I transfer in vivo. AdA-I transfer enhanced NO production 2.0-fold (P < .05) in the bone marrow of C57BL/6 mice transplanted with SR-BI+/+ bone marrow but not in mice with SR-BI−/− bone marrow (Figure 2F). The increased NO production in the bone marrow of chimeric SR-BI+/+ mice may stimulate EPC mobilization.
SR-BI and signal transduction induced by HDL in EPCs. (A) Bar graph showing the number of migrated EPCs in modified Boyden chambers. After 7 days of culture, bone marrow EPCs isolated from control mice or AdA-I–treated C57BL/6 mice with SR-BI+/+ or SR-BI−/− bone marrow were seeded in the upper chamber. The lower chamber was supplemented with either HDL (100 μg/mL) or an equivalent amount of bovine serum albumin and the number of migrated cells per microscopy field was quantified after 5 hours (n = 4 for each group). (B) Bar graph showing the number of migrated EPCs in modified Boyden chambers. After 7 days of culture, EPCs isolated from chimeric SR-BI+/+ C57BL/6 mice (n = 6) were seeded in the upper chamber. The lower chamber was supplemented with either bovine serum albumin (100 μg/mL) or HDL (100 μg/mL). To inhibit ERK signaling and NO synthase activity, experiments were performed in the presence of U0126 (10 μM) and LNMA (2 mM), respectively. The number of migrated cells per microscopy field was quantified after 5 hours (n = 6 for each group). (C) p-ERK/ERK ratio determined by Western blot. After 7 days of culture, bone marrow EPCs isolated from SR-BI+/+ or SR-BI−/− mice were exposed to either bovine serum albumin (100 μg/mL) or HDL (100 μg/mL) for 2.5 minutes (n = 4 for each group). (D) NO production in cultured bone marrow EPCs. After 7 days of culture, bone marrow EPCs isolated from SR-BI+/+ or SR-BI−/− mice were exposed to either bovine serum albumin (100 μg/mL) or HDL (100 μg/mL) for 24 hours, and NO production (nanomoles per milligram of protein) was measured (n = 5 for each group). (E) NO production in cultured EPCs. After 7 days of culture, EPCs isolated from chimeric SR-BI+/+ C57BL/6 mice (n = 4) were exposed to either bovine serum albumin (100 μg/mL) or HDL (100 μg/mL). To inhibit ERK signaling, experiments were performed in the presence of U0126 (10 μM). NO production (nanomoles per milligram of protein) was determined (n = 4 for each group). (F) NO production in the bone marrow at day 35 after transfer with Adnull or saline (Controls) or AdA-I in C57BL/6 mice with SR-BI+/+ (n = 8 for each group) or SR-BI−/− bone marrow (n = 18 for each group). Data are mean plus or minus SEM.
SR-BI and signal transduction induced by HDL in EPCs. (A) Bar graph showing the number of migrated EPCs in modified Boyden chambers. After 7 days of culture, bone marrow EPCs isolated from control mice or AdA-I–treated C57BL/6 mice with SR-BI+/+ or SR-BI−/− bone marrow were seeded in the upper chamber. The lower chamber was supplemented with either HDL (100 μg/mL) or an equivalent amount of bovine serum albumin and the number of migrated cells per microscopy field was quantified after 5 hours (n = 4 for each group). (B) Bar graph showing the number of migrated EPCs in modified Boyden chambers. After 7 days of culture, EPCs isolated from chimeric SR-BI+/+ C57BL/6 mice (n = 6) were seeded in the upper chamber. The lower chamber was supplemented with either bovine serum albumin (100 μg/mL) or HDL (100 μg/mL). To inhibit ERK signaling and NO synthase activity, experiments were performed in the presence of U0126 (10 μM) and LNMA (2 mM), respectively. The number of migrated cells per microscopy field was quantified after 5 hours (n = 6 for each group). (C) p-ERK/ERK ratio determined by Western blot. After 7 days of culture, bone marrow EPCs isolated from SR-BI+/+ or SR-BI−/− mice were exposed to either bovine serum albumin (100 μg/mL) or HDL (100 μg/mL) for 2.5 minutes (n = 4 for each group). (D) NO production in cultured bone marrow EPCs. After 7 days of culture, bone marrow EPCs isolated from SR-BI+/+ or SR-BI−/− mice were exposed to either bovine serum albumin (100 μg/mL) or HDL (100 μg/mL) for 24 hours, and NO production (nanomoles per milligram of protein) was measured (n = 5 for each group). (E) NO production in cultured EPCs. After 7 days of culture, EPCs isolated from chimeric SR-BI+/+ C57BL/6 mice (n = 4) were exposed to either bovine serum albumin (100 μg/mL) or HDL (100 μg/mL). To inhibit ERK signaling, experiments were performed in the presence of U0126 (10 μM). NO production (nanomoles per milligram of protein) was determined (n = 4 for each group). (F) NO production in the bone marrow at day 35 after transfer with Adnull or saline (Controls) or AdA-I in C57BL/6 mice with SR-BI+/+ (n = 8 for each group) or SR-BI−/− bone marrow (n = 18 for each group). Data are mean plus or minus SEM.
SR-BI deficiency in bone marrow abrogates the inhibitory effect of human apo A-I transfer on allograft vasculopathy in C57BL/6 mice
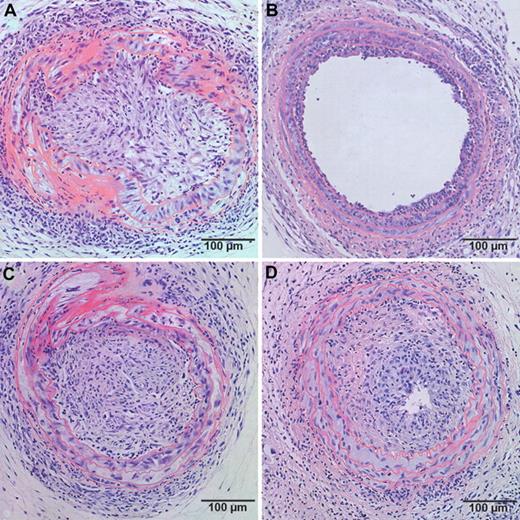
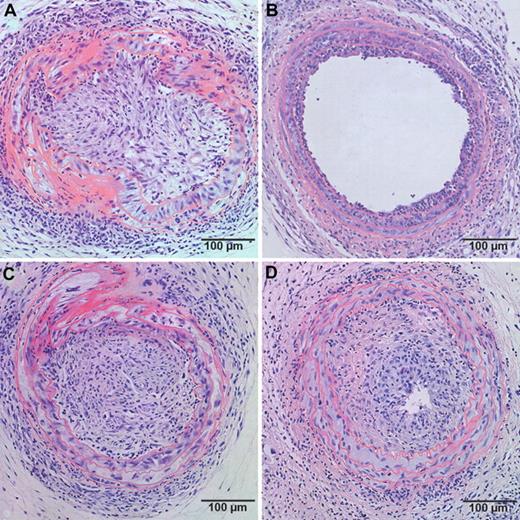
Based on the observation that the effect of human apo A-I transfer on EPC number and function requires the expression of SR-BI in bone marrow cells, we evaluated a potential interaction between human apo A-I gene transfer and SR-BI expression in bone marrow cells in a murine model of transplantation arteriosclerosis. A 2-way ANOVA to test interaction between the factor gene transfer and the factor bone marrow transplantation on intimal area indicated that the interaction term is significant (P < .002). Human apo A-I transfer 2 weeks before artery transplantation reduced intimal area at day 21 3.7-fold (P < .001) in mice reconstituted with SR-BI+/+ bone marrow but had no effect in mice with SR-BI−/− bone marrow (Table 2). No significant difference in intimal area was observed between control mice with SR-BI+/+ bone marrow and control mice with SR-BI−/− bone marrow. Representative sections of neointima formation at day 21 after transfer in control and AdA-I–treated mice with SR-BI+/+ or SR-BI−/− bone marrow are shown in Figure 3.
Effect of human apo A-I transfer on allograft vasculopathy in chimeric C57BL/6 SR-BI+/+ mice and chimeric C57BL/6 SR-BI−/− mice. Representative sections of allograft vasculopathy at day 21 after transplantation in control (A,C) and AdA-I (B,D)–treated C57BL/6 mice with SR-BI+/+ (A,B) or SR-BI−/− (C,D) bone marrow.
Effect of human apo A-I transfer on allograft vasculopathy in chimeric C57BL/6 SR-BI+/+ mice and chimeric C57BL/6 SR-BI−/− mice. Representative sections of allograft vasculopathy at day 21 after transplantation in control (A,C) and AdA-I (B,D)–treated C57BL/6 mice with SR-BI+/+ (A,B) or SR-BI−/− (C,D) bone marrow.
SR-BI deficiency in bone marrow abrogates potentiation of endothelial regeneration and EPC incorporation induced by human apo A-I transfer in allografts
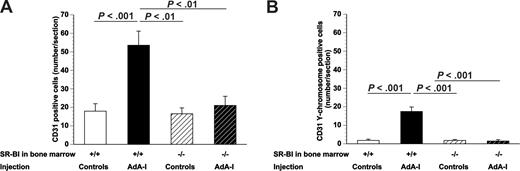
Endothelial regeneration was enhanced after AdA-I transfer in mice with SR-BI+/+ bone marrow as evidenced by a 3.0-fold (P < .001) higher number of CD31+ endothelial cells lining the lumen compared with control mice with SR-BI+/+ bone marrow (Figure 4A). In contrast, AdA-I transfer had no effect on endothelial regeneration in mice with SR-BI−/− bone marrow (Figure 4A). To evaluate whether SR-BI expression in bone marrow–derived cells affects EPC incorporation after human apo A-I transfer, bone marrow transplantations were performed with male SR-BI+/+ or SR-BI−/− bone marrow into female C57BL/6 mice, and artery transplantation was performed with arteries from female BALB/c donors (n = 8 for each experimental group). In situ hybridization was performed for the murine Y-chromosome, and the number of CD31 Y-chromosome–positive cells was quantified 21 days after transplantation. This number was increased 9.7-fold (P < .001) in AdA-I–treated chimeric SR-BI+/+ mice, whereas no effect was observed after AdA-I transfer in chimeric SR-BI−/− mice (Figure 4B), indicating that EPC incorporation requires SR-BI expression in bone marrow and bone marrow–derived cells. The percentage of CD31 Y-chromosome–positive cells increased 4.7-fold (P < .001) after AdA-I transfer in chimeric SR-BI+/+ mice (35% ± 3.0% vs 7.4% ± 2.2%), whereas no change was observed in chimeric SR-BI−/− mice (4.9% ± 2.4% vs 5.0% ± 1.4%).
Effect of human apo A-I transfer on endothelial regeneration and EPC incorporation in chimeric C57BL/6 SR-BI+/+ mice and chimeric C57BL/6 SR-BI−/− mice. The effect of AdA-I transfer on endothelial cell regeneration in allografts is dependent on SR-BI expression in bone marrow–derived cells. (A) Bar graph showing the number of CD31+ endothelial cells at day 21 after artery transplantation in control and AdA-I–treated C57BL/6 mice with SR-BI+/+ or SR-BI−/− bone marrow. (B) Bar graph showing the number of CD31 Y-chromosome–positive endothelial cells at day 21 after artery transplantation in control and AdA-I–treated C57BL/6 mice with SR-BI+/+ or SR-BI−/− bone marrow. Data are mean plus or minus SEM.
Effect of human apo A-I transfer on endothelial regeneration and EPC incorporation in chimeric C57BL/6 SR-BI+/+ mice and chimeric C57BL/6 SR-BI−/− mice. The effect of AdA-I transfer on endothelial cell regeneration in allografts is dependent on SR-BI expression in bone marrow–derived cells. (A) Bar graph showing the number of CD31+ endothelial cells at day 21 after artery transplantation in control and AdA-I–treated C57BL/6 mice with SR-BI+/+ or SR-BI−/− bone marrow. (B) Bar graph showing the number of CD31 Y-chromosome–positive endothelial cells at day 21 after artery transplantation in control and AdA-I–treated C57BL/6 mice with SR-BI+/+ or SR-BI−/− bone marrow. Data are mean plus or minus SEM.
Increased HDL cholesterol after human apo A-I transfer attenuates inflammation in allografts in chimeric SR-BI+/+ mice but not in chimeric SR-BI−/− mice
HDL exerts anti-inflammatory effects on the endothelium.29 We evaluated whether enhanced endothelial regeneration in chimeric SR-BI+/+ mice after human apo A-I transfer was associated with reduced allograft inflammation. Table 3 shows that AdA-I gene transfer significantly reduced leukocyte and macrophage infiltration (Figure S4) at day 21 after artery transplantation in mice with SR-BI+/+ bone marrow but not in mice with SR-BI−/− bone marrow.
Discussion
The main findings of this study are that (1) the effect of increased HDL cholesterol after human apo A-I transfer on EPC number and function requires SR-BI expression in bone marrow and bone marrow–derived cells; (2) accelerated endothelial regeneration, increased EPC incorporation, and attenuation of allograft vasculopathy induced by hepatocyte-directed human apo A-I transfer in a murine model of transplantation arteriosclerosis are strictly dependent on SR-BI expression in bone marrow and bone marrow–derived cells; and (3) the effect of HDL on EPC migration in vitro is mediated via NO, and enhanced NO production requires signaling via SR-BI and ERK. Taken together, the current study shows the crucial role of SR-BI–expressing bone marrow–derived EPCs in the protective effect of increased HDL cholesterol induced by overexpression of human apo A-I on allograft vasculopathy in mice.
Bone marrow transplantations with SR-BI−/− or SR-BI+/+ bone marrow were a cornerstone in the design of experiments to unravel the mechanistic link between the effect of human apo A-I transfer on EPC biology and on allograft vasculopathy. We cannot exclude that part of the effects of increased HDL cholesterol on bone marrow–derived EPCs are mediated indirectly via cargo molecules of SR-BI–bound HDL, like sphingosine-1-phosphate (S1P).30,31 S1P receptor 1 (S1P1) and S1P receptor 3 (S1P3) are expressed on EPCs.32 The S1P3 receptor has been shown to mediate the stimulation of the functional capacity of progenitor cells by S1P.32 Regardless of the potential involvement of S1P receptors, our data conclusively demonstrate that SR-BI is strictly required for the effects of human apo A-I transfer on EPC biology. Reconstituted HDL, containing no S1P, has been shown to promote the differentiation of human peripheral mononuclear cells to EPCs.33 In light of this observation, our results suggest that SR-BI may be sufficient for HDL-mediated effects on EPCs.
EPC mobilization from the bone marrow requires increased bone marrow NO levels.34 In the current study, we show that human apo A-I transfer resulted in increased NO production in the bone marrow of C57BL/6 mice transplanted with SR-BI+/+ bone marrow but not in mice transplanted with SR-BI−/− bone marrow. Increased EPC mobilization is probably a major factor in the increase of EPC number in the peripheral blood after human apo A-I transfer. In addition, EPC numbers are increased after AdA-I transfer in the bone marrow of chimeric SR-BI+/+ but not in chimeric SR-BI−/− mice. Our data suggest that a direct action of HDL in the bone marrow is an essential effector in the HDL-induced attenuation of allograft vasculopathy.
EPC incorporation experiments demonstrate that SR-BI expression in EPCs is required to observe enhanced incorporation after human apo A-I transfer. These in vivo experimental results are in line with our in vitro data showing that enhanced EPC migration requires SR-BI and ERK signaling and is dependent on NO. In CHO cells, it has been demonstrated that HDL signaling via SR-BI involves protein kinase C–independent activation of Ras, which is completely blocked by pertussis toxin, thus implicating heterotrimeric G proteins.35 Subsequent phosphorylation of Raf-1 and MEK1 results in phosphorylation and activation of ERK.36-38 ERK 1/2 directly phosphorylate endothelial NO synthase (eNOS) in endothelial cells.39 Phosphorylation and activation of eNOS and production of NO in turn contribute to ERK activation and increased NO production through a positive feed-forward mechanism.39 The role of NO in the effects of HDL is in line with its role in the beneficial effects of statin therapy, erythropoietin, and physical exercise on EPC biology.6,40,41
EPC number in the bone marrow and in the peripheral circulation was slightly reduced in chimeric SR-BI−/− mice compared with chimeric SR-BI+/+ mice. No significant differences of EPC incorporation, endothelial regeneration, and allograft vasculopathy were observed between control chimeric SR-BI+/+ mice and control chimeric SR-BI−/− mice. These results suggest that a threshold exists to observe augmented EPC incorporation and accelerated endothelial regeneration by HDL. This threshold is not reached at endogenous murine apo A-I–containing HDL concentrations, and supraphysiologic levels of human apo A-I-containing HDL are required to overcome this threshold. In contrast, carotid artery reendothelialization after perivascular electric injury has been shown to be blunted in SR-BI−/− mice compared with wild-type mice, suggesting an effect of endogenous HDL levels on endothelial regeneration in this model.16 We speculate that the relative contribution of EPCs to endothelial regeneration in the paratopic transplantation model is significantly higher than in the model of perivascular injury. This may be the result of continuing immunologic insult against donor-derived endothelial cells and the isogeneic nature of recipient EPC-derived endothelial cells in allografts. Endogenous HDL may be sufficient for endothelial regeneration mediated by SR-BI–expressing adjacent endothelial cells in the model of perivascular injury. In contrast, supraphysiologic levels of human apo A-I–containing HDL appear to be required for accelerated EPC-mediated endothelial regeneration in the transplantation model. This is in line with reports showing that administration of exogenous reconstituted HDL increases the recruitment of EPCs into the aortic endothelium after endothelial damage induced by lipopolysaccharide in C57BL/6 mice42 and enhances the contribution of bone marrow–derived cells to neovascularization in a mouse hindlimb ischemia model.33 Because an HDL concentration gradient exists between blood plasma and bone marrow plasma, SR-BI–mediated effects on endothelial cells may occur at lower HDL levels than SR-BI–mediated effects in the bone marrow. Because our data show that human apo A-I–containing HDL increases EPC number in the bone marrow and may enhance EPC mobilization from the bone marrow, this action may require higher circulating HDL levels. EPCs may not only directly contribute to accelerated endothelial regeneration by incorporation in the endothelium but also indirectly by paracrine effects on endothelial cells.43 Because incorporating EPCs only partially account for the increase in endothelial cell number after AdA-I transfer in chimeric SR-BI+/+ mice, this strongly suggests that the paracrine action of bone marrow–derived cells on endothelial regeneration is also enhanced by HDL in an SR-BI-dependent pathway.
Allograft inflammation was reduced after human apo A-I transfer in mice transplanted with wild-type bone marrow but not in mice transplanted with SR-BI−/− bone marrow. This reduced allograft inflammation is probably the result of improved endothelial regeneration and endothelial function.29 However, we cannot exclude that HDL is having direct effects on inflammatory cells via SR-BI that may have contributed to the attenuation of allograft vasculopathy.
In conclusion, the current study shows that the protective effect of hepatocyte-directed human apo A-I transfer on allograft vasculopathy requires SR-BI expression in bone marrow and bone marrow–derived cells. HDL modulates EPC biology via SR-BI. The action of HDL on bone marrow–derived EPCs appears to be a critical effector of a therapeutic HDL raising intervention in a murine model of allograft vasculopathy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank J. Hendrix and Z. Zhang for excellent technical assistance.
This work was supported by the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen (grant G.0564.05). The Center for Molecular and Vascular Biology is supported by Excellentiefinanciering Katholieke Universiteit (KU) Leuven (EF/05/013). M.v.E. is an Established Investigator of the Netherlands Heart Foundation (grant 2007T056). E.V.C. is a Research Assistant of the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen. F.J. is a Research Assistant of the Instituut voor de Aanmoediging van Innovatie door Wetenschap en Technologie in Vlaanderen. M.T. is a Fellow of the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen and is supported by a German Leibniz-Award (DFG 600/8-1).
Authorship
Contribution: Y.F., E.V.C., F.J., and V.C. acquired, analyzed, and interpreted the data; M.v.E. provided an essential animal model for the current study; M.E. acquired data and supervised research on in situ hybridization for the Y chromosome; and Y.F., S.V.L., M.T., and B.D.G. conceived and designed research. All authors made critical revision of the manuscript for important intellectual content.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bart De Geest, Center for Molecular and Vascular Biology, University of Leuven, Campus Gasthuisberg, Herestraat 49, 3000 Leuven, Belgium; e-mail: bart.degeest@med.kuleuven.be.