Abstract
CX3CR1 is a chemokine receptor with a single ligand, the membrane-tethered chemokine CX3CL1 (fractalkine). All blood monocytes express CX3CR1, but its levels differ between the main 2 subsets, with human CD16+ and murine Gr1low monocytes being CX3CR1hi. Here, we report that absence of either CX3CR1 or CX3CL1 results in a significant reduction of Gr1low blood monocyte levels under both steady-state and inflammatory conditions. Introduction of a Bcl2 transgene restored the wild-type phenotype, suggesting that the CX3C axis provides an essential survival signal. Supporting this notion, we show that CX3CL1 specifically rescues cultured human monocytes from induced cell death. Human CX3CR1 gene polymorphisms are risk factors for atherosclerosis and mice deficient for the CX3C receptor or ligand are relatively protected from atherosclerosis development. However, the mechanistic role of CX3CR1 in atherogenesis remains unclear. Here, we show that enforced survival of monocytes and plaque-resident phagocytes, including foam cells, restored atherogenesis in CX3CR1-deficent mice. The fact that CX3CL1-CX3CR1 interactions confer an essential survival signal, whose absence leads to increased death of monocytes and/or foam cells, might provide a mechanistic explanation for the role of the CX3C chemokine family in atherogenesis.
Introduction
Chemokines are a family of chemotactic cytokines that activate specific G-protein-coupled 7-transmembrane receptors1 and have been categorized into C, CC, CXC, and CX3C families. The only known CX3C chemokine, CX3CL1, also known as fractalkine,1-3 is expressed by activated vascular endothelial cells,3 neurons,4 epithelial cells,5,6 smooth muscle cells,7 dendritic cells (DCs),8 and macrophages.9 The single known CX3CL1 receptor, CX3CR1,10 is expressed by T-cell and natural killer (NK) cell subsets,10,11 brain microglia,4,12,13 DC subsets13-15 as well as blood monocytes.10,13
Classical small-molecular-weight chemokines are secreted proteins considered to form gradients by binding to extracellular matrix proteoglycans. In contrast, CX3CL1 is synthesized as a transmembrane protein with its chemokine domain presented on an extended mucin-like stalk.2,3 In this form, CX3CL1 promotes tight, integrin-independent adhesion of CX3CR1-expressing leukocytes.7,16 In addition, constitutive and inducible cleavage by metalloproteases can result in release of a soluble CX3CL1 entity from the cell membrane.17-19 CX3CL1 thus potentially acts as an adhesion molecule and a chemoattractant; albeit the differential importance of these activities for the physiologic role of CX3CL1 remains unknown. In cell lines and cultured microglia, CX3CR1 engagement triggers the phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway resulting in cell survival and proliferation.20-23 However, the significance of this activity for the in vivo role of the CX3C chemokine system remains to be determined.
Atherosclerosis is characterized by the accumulation of lipids and fibrous elements in the large arteries and involves diverse factors, including components of the immune system.24 Human CX3CR1 gene polymorphisms were shown to be genetic risk factors for coronary artery diseases and atherosclerosis.25,26 Moreover, mice deficient for either CX3CR1 or CX3CL1 display a relative resistance to atherosclerosis development in the respective murine disease models.27-30 However, the mechanistic explanations for these phenotypes remain under debate.31
Monocytes are mononuclear phagocytes that are generated in the bone marrow (BM) and released to the bloodstream.32,33 From there they enter peripheral tissues, where they can give rise to macrophages and DCs.15,32-35 Undifferentiated monocytes represent a short-lived transitional state, with an estimated circulation half-life of approximately 71 hours in human36 and approximately 17 hours in mice.32,37 They are heterogeneous and can be divided into at least 2 main subpopulations,38 with human monocytes comprising CD14++CD16− and CD14+CD16+ cells.39 Murine monocytes have been divided into Gr1hi (Ly6Chi) and Gr1low (Ly6Clow) subsets,35,40 which are functionally distinct with respect to both their migration ability and differentiation potential.15,35,41,42 Although all monocytes express CX3CR1, the 2 subsets differ by distinct surface CX3CR1 levels, with human CD14+CD16+ and murine Gr1low monocyte subsets displaying significantly higher CX3CR1 amounts.35 The role of this prominent CX3CR1 expression for monocyte physiology remains unknown, although our own studies and reports by others suggest that CX3CR1-CX3CL1 interactions might control monocyte levels35,43 and be involved in monocyte migration.44,45
Here, we investigated the role of CX3CL1-CX3CR1 interaction during steady state and atherogenesis, using mutant mice as well as human blood monocyte isolates. We show that mice deficient for either CX3CR1 or CX3CL1 display a specific reduction in the frequency of circulating Gr1low blood monocytes, whereas Gr1hi monocytes remain unaffected. Using forced Bcl2 expression, we provide genetic evidence that this phenotype results from enhanced cell death. The protective CX3C signal is direct and evolutionary conserved, as addition of soluble CX3CL1 inhibits cell death of cultured human monocytes. Altered CX3CR1 function was reported to affect atherosclerosis progression in both humans and mice. Here, we show that the absence of the CX3C signal results in increased apoptosis of plaque-resident cells, including macrophages, whereas introduction of a Bcl2 transgene allows disease progression in the absence of CX3CR1. We suggest, therefore, that CX3CR1 plays a central role during monocyte homeostasis and atherogenesis by promoting essential cell survival.
Methods
Mice
This study involved the use of the following C57BL/6 mouse strains: Cx3cr1gfp mice13 ; cx3cl1−/− mice46 ; hMRP8bcl2 mice47 and apoe−/− mice (B6.129P2-Apoetm1Unc/J; The Jackson Laboratory, Bar Harbor, ME). Wild-type (wt) C57BL/6 mice were purchased from Harlan Laboratories (Rehovot, Israel). Mice used were 7 to 10 weeks of age. All mice were maintained under specific pathogen–free conditions and handled under protocols approved by the Weizmann Institute Animal Care Committee according to international guidelines.
BM chimera generation
For blood cell analysis, wt or cx3cl1−/− recipient mice were lethally irradiated with a 950-rad dose and 1 day later were intravenously (IV) injected with 5 × 106 BM cells isolated from donor femora and tibiae. BM recipients were allowed to rest for 8 weeks before analysis. For atherosclerosis studies, apoe−/− recipient mice were lethally irradiated 2 times with 650-rad doses, injected IV 1 day later with 5 × 106 donor BM cells, and allowed to rest for 4 weeks before being placed on high-fat diet.
Mouse model of atherosclerotic disease progression
Apoe−/− mice were fed an atherogenic diet containing 21% fat (0.15% cholesterol, 19.5% casein; Altromin, Lage, Germany), and after 12 weeks were fixed by in situ perfusion. The extent of atherosclerosis was quantified by Oil Red O staining of thoracoabdominal aortas prepared en face and in serial sections through the aortic root by computerized image analysis (MetaMorph version 6.0; Universal Imaging-Molecular Devices or Diskus software, Downingtown, PA), as previously described.48
Mouse cell isolation
Mice were bled from their tail veins, and blood was subjected to a Ficoll density gradient (Amersham Biosciences, Sunnyvale, CA) for erythrocyte and neutrophil removal. BM cells were isolated from mouse femora, followed by erythrocyte lysis using ACK buffer (0.15 M NH4Cl, 0.01 M KHCO3). Cells were suspended in phosphate-buffered saline (PBS) supplemented with 2 mM ethylenediaminetetraacetic acid (EDTA), 0.05% sodium azide, and 1% fetal calf serum (FCS).
Human blood cell studies
Human blood monocytes were isolated from white blood cell (WBC)–enriched blood (Israeli Blood Bank, Tel Hashomer, Israel). After erythrocyte and neutrophil removal through a Ficoll density gradient, monocyte subsets were magnetically separated using the Monocyte Isolation Kit II and CD16+ Monocyte Isolation Kit (both Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's protocols. For serum deprivation studies, the cells were washed with PBS to remove serum residues, and 5 × 104 cells per well were plated in RPMI supplemented with nonessential amino acids. RPMI was further supplemented with FCS (10%; Beit Haemek Industries, Israel) or recombinant mouse CX3CL1 (amino acids [aa] 1-337; R&D Systems, Minneapolis, MN). Cells were incubated for 4 hours before harvesting. For pertussis toxin (PTx) studies, cells were incubated 1 hour with PTx (Calbiochem, San Diego, CA) in serum supplemented medium, washed with PBS, and incubated as previously described. For the oxysterol studies 5 × 104 cells/well were incubated with serum-supplemented RPMI either further supplemented or nonsupplemented with rCX3CL1 for 6 hours, followed by incubation with 7-β-hydroxycholesterol (Sigma-Aldrich, St Louis, MO) for an additional 16 hours.
Flow cytometric analysis
The following monoclonal antibodies and staining reagents were used according to the manufacturers' protocols: human-specific biotin-conjugated anti-CD14 (Caltag Laboratories, Burlingame, CA), fluorescein isothiocyanate (FITC)–conjugated anti-CD16 (Caltag Laboratories), and phycoerythrin (PE)–conjugated anti-Bcl2 (Santa Cruz Biotechnology, Santa Cruz, CA) and mouse-specific PE-conjugated anti-CD115 (eBioscience, San Diego, CA) and allophycocyanin (APC)–conjugated anti-Gr1 (Ly6C/G; eBioscience). Propidium iodide (Sigma-Aldrich) was added to 5 minutes before sample acquisition. Cells were analyzed on a FACSCalibur cytometer (Becton Dickinson, Franklin Lakes, NJ) using CellQuest software (Becton Dickinson).
Immunohistochemistry
Plaque cellular composition was analyzed in transverse sections through the aortic root. Sections were stained with anti-MOMA-2 (AbD Serotec, Raleigh, NC) and anti–human Bcl2 (BD Biosciences, San Jose, CA) monoclonal antibodies, detected by alkaline phosphate substrate and the Vector Red Substrate Kit (Vector Laboratories, Burlingame, CA). Apoptotic nuclei were detected by terminal deoxynucleotidyl nick-end labeling (TUNEL) kit (Roche Applied Science, Indianapolis, IN), and nuclei were counterstained by 4′,6-diamidino-2-pheylindol (DAPI) when indicated. Images were recorded with a Leica DMLB fluorescence microscope and CCD camera (JVC).
Results
Absence of CX3CR1-CX3CL1 interactions results in a specific reduction of Gr1lowCX3CR1hi blood monocytes
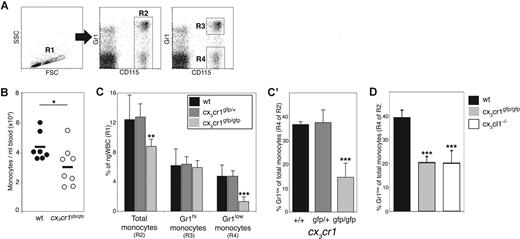
To study the physiologic role of CX3CR1, we took advantage of knockin mice that carry a targeted replacement of the cx3cr1 gene by a gene encoding green fluorescent protein (GFP).13 All circulating blood monocytes, as defined by expression of CD115 (also known as MCSF [CSF1] receptor, Figure 1A), express CX3CR1 and are, therefore, GFP-positive in cx3cr1gfp mice.13 The comparative analysis of leukocytes of wt and cx3cr1gfp/gfp mice revealed a slight, but consistent and significant, reduction of the absolute monocyte numbers in the absence of CX3CR1 (Figure 1B). This was also reflected in their reduced percentage among total nongranular WBCs (ngWBC; Figure 1C). Blood monocyte levels of heterozygote mutant mice were comparable with wt mice (Figure 1C).
Reduced numbers of Gr1low monocytes in CX3CR1-deficient mice under steady-state conditions. (A) Flow cytometric analysis of blood monocytes. Left dot plot shows total Ficoll-fractionated wt blood cells. Cells gated in region R1 are living, nongranular white blood cells (ngWBCs). Middle and right dot plots show CD115 and Gr1 staining of blood cells gated in R1. Region R2 gates monocytes, as indicated by their CD115 expression. Cells gated in R3 and R4 regions are Gr1hi and Gr1low monocytes, respectively. (B) Comparison of blood monocyte population size of wt (●) and cx3cr1gfp/gfp (○) mice. Diagram shows number of monocytes, identified as CD115+ cells (R2 gated cells), in 1 mL blood. Each circle represents a single mouse. (C,C′) Comparison between monocyte population size of wt (■), cx3cr1gfp/+ ( ) and cx3cr1gfp/gfp (
) and cx3cr1gfp/gfp ( ) mice. Bar diagram (C) shows for each group of mice the percentage of total blood monocytes (R2 gated cells), Gr1hi monocytes (R3 gated cells) and Gr1low monocytes (R4 gated cells) out of ngWBC gated in R1. (C′) Alternative presentation of the data showed in panel C. Bar diagram shows the percentage of Gr1low monocytes (R4 gated cells) among total monocytes (R2 gated cells) for each of the mouse strains; n = 5. (D) Comparison between blood monocytes of wt (■), cx3cr1gfp/gfp (
) mice. Bar diagram (C) shows for each group of mice the percentage of total blood monocytes (R2 gated cells), Gr1hi monocytes (R3 gated cells) and Gr1low monocytes (R4 gated cells) out of ngWBC gated in R1. (C′) Alternative presentation of the data showed in panel C. Bar diagram shows the percentage of Gr1low monocytes (R4 gated cells) among total monocytes (R2 gated cells) for each of the mouse strains; n = 5. (D) Comparison between blood monocytes of wt (■), cx3cr1gfp/gfp ( ) and cx3cl1−/− mice (□). Bar histogram shows for each group the percentage of Gr1low monocytes (R4 gated cells) of total monocytes (R2 gated cells) for each mouse strain; n = 5. *P < .05, **P < .005, **P < 5 × 10−5, all as compared with wt mice (Student t test).
) and cx3cl1−/− mice (□). Bar histogram shows for each group the percentage of Gr1low monocytes (R4 gated cells) of total monocytes (R2 gated cells) for each mouse strain; n = 5. *P < .05, **P < .005, **P < 5 × 10−5, all as compared with wt mice (Student t test).
Reduced numbers of Gr1low monocytes in CX3CR1-deficient mice under steady-state conditions. (A) Flow cytometric analysis of blood monocytes. Left dot plot shows total Ficoll-fractionated wt blood cells. Cells gated in region R1 are living, nongranular white blood cells (ngWBCs). Middle and right dot plots show CD115 and Gr1 staining of blood cells gated in R1. Region R2 gates monocytes, as indicated by their CD115 expression. Cells gated in R3 and R4 regions are Gr1hi and Gr1low monocytes, respectively. (B) Comparison of blood monocyte population size of wt (●) and cx3cr1gfp/gfp (○) mice. Diagram shows number of monocytes, identified as CD115+ cells (R2 gated cells), in 1 mL blood. Each circle represents a single mouse. (C,C′) Comparison between monocyte population size of wt (■), cx3cr1gfp/+ ( ) and cx3cr1gfp/gfp (
) and cx3cr1gfp/gfp ( ) mice. Bar diagram (C) shows for each group of mice the percentage of total blood monocytes (R2 gated cells), Gr1hi monocytes (R3 gated cells) and Gr1low monocytes (R4 gated cells) out of ngWBC gated in R1. (C′) Alternative presentation of the data showed in panel C. Bar diagram shows the percentage of Gr1low monocytes (R4 gated cells) among total monocytes (R2 gated cells) for each of the mouse strains; n = 5. (D) Comparison between blood monocytes of wt (■), cx3cr1gfp/gfp (
) mice. Bar diagram (C) shows for each group of mice the percentage of total blood monocytes (R2 gated cells), Gr1hi monocytes (R3 gated cells) and Gr1low monocytes (R4 gated cells) out of ngWBC gated in R1. (C′) Alternative presentation of the data showed in panel C. Bar diagram shows the percentage of Gr1low monocytes (R4 gated cells) among total monocytes (R2 gated cells) for each of the mouse strains; n = 5. (D) Comparison between blood monocytes of wt (■), cx3cr1gfp/gfp ( ) and cx3cl1−/− mice (□). Bar histogram shows for each group the percentage of Gr1low monocytes (R4 gated cells) of total monocytes (R2 gated cells) for each mouse strain; n = 5. *P < .05, **P < .005, **P < 5 × 10−5, all as compared with wt mice (Student t test).
) and cx3cl1−/− mice (□). Bar histogram shows for each group the percentage of Gr1low monocytes (R4 gated cells) of total monocytes (R2 gated cells) for each mouse strain; n = 5. *P < .05, **P < .005, **P < 5 × 10−5, all as compared with wt mice (Student t test).
Murine blood monocyte subsets differ in their level of CX3CR1 surface expression with Gr1hi monocytes expressing intermediate amounts of CX3CR1 and Gr1low monocytes being CX3CR1hi.35 Interestingly, Gr1hi monocyte levels remained unaffected by the CX3CR1 deficiency (Figure 1C). In contrast, Gr1low monocytes of CX3CR1 KO mice were found to be 3-fold reduced, as compared with wt and cx3cr1gfp/+ mice (Figure 1C). To avoid indirect effects on these quantifications due to a potential CX3CR1 requirement by other leukocytes,10,11,13 we plotted the fraction of Gr1low monocytes among the total monocyte population in individual mice (Gr1lowCD115+ cells out of total CD115+ cells). While in wt and cx3cr1gfp/+ naive mice approximately one-third of blood monocytes are Gr1low, in cx3cr1gfp/gfp mice the latter comprise only 15% of the total monocyte population (Figure 1C′). This way of presentation will be used throughout the remainder of this study.
Mice deficient for CX3CL1, the single known CX3CR1 ligand,3,10 have fewer F4/80+ blood monocytes than their wt littermates.46 We therefore next tested whether also the cx3cl1−/− phenotype is restricted to the Gr1lowCX3CR1hi monocytes. Indeed, cx3cr1gfp/gfp and cx3cl1−/− mice showed similar fractions of the Gr1low subset, which were significantly lower than in wt controls (Figure 1D).
The observed Gr1low monocyte reduction could result from a systemic defect in CX3CR1- and CX3CL1-deficient mice or be a direct outcome of the absence of the receptor on Gr1low monocytes. To test these options, we generated mixed BM chimeras by reconstituting lethally irradiated wt mice with a 1:1 mixture of wt and cx3cr1gfp/gfp BM cells. Eight weeks after transfer, the recipients' blood contained both GFP-positive (cx3cr1gfp/gfp) and GFP-negative (wt) monocytes (Figure 2A), allowing the comparison of cells of distinct genetic background in the same environment. As shown in Figure 2A, compared with their wt counterpart, CX3CR1-deficient Gr1low monocyte numbers were reduced 3-fold. Next, we transferred wt and cx3cr1gfp/gfp BM cells into irradiated cx3cl1−/− and wt recipients. In the absence of CX3CL1 expression by host cells, wt Gr1low monocyte levels were significantly lower compared with wt > wt chimeras and similar to those found in cx3cr1gfp/gfp > wt mice (Figure 2B). To conclude, CX3CR1- and CX3CL1-deficient mice display a specific reduction of their Gr1low monocytes, which is a direct result of CX3CR1 requirement by BM-derived cells.
Reduction of Gr1low monocytes in absence of CX3CR1 is due to impaired cell survival. (A) Comparison of wt and cx3cr1gfp/gfp monocytes in mixed BM chimera blood. BM cells were isolated from either wt or cx3cr1gfp/gfp donors, mixed to 1:1 ratio from each genotype and transferred into irradiated wt recipients. Eight weeks after transfer recipient mice were bled, and their blood content was analyzed. Dot plot shows discrimination between GFP-positive cx3cr1gfp/gfp monocytes (R5 gated cells) and GFP-negative wt monocytes (R6 gated cells). Bar diagram shows percentage of Gr1low monocytes (as gated in R4, Figure 1A) of either total wt (R6 gated cells, ■) or cx3cr1gfp/gfp (R5 gated cells,  ) monocytes; n = 8. (B) Comparison of monocyte levels in presence and absence of CX3CL1. Wt and cx3cl1−/− irradiated mice received 1:1 mix of BM cells from wt and cx3cr1gfp/gfp mice, and were bled 8 weeks later. Bar histogram shows percentage of Gr1low monocytes (R4 gated cells, Figure 1A) of total monocytes (R2 gated cells, Figure 1A) of wt (■) and cx3cl1−/− (□) recipients of either wt or cx3cr1gfp/gfp BM cells; n = 5. (C) Comparison of blood and BM monocytes of cx3cr1gfp/+ (
) monocytes; n = 8. (B) Comparison of monocyte levels in presence and absence of CX3CL1. Wt and cx3cl1−/− irradiated mice received 1:1 mix of BM cells from wt and cx3cr1gfp/gfp mice, and were bled 8 weeks later. Bar histogram shows percentage of Gr1low monocytes (R4 gated cells, Figure 1A) of total monocytes (R2 gated cells, Figure 1A) of wt (■) and cx3cl1−/− (□) recipients of either wt or cx3cr1gfp/gfp BM cells; n = 5. (C) Comparison of blood and BM monocytes of cx3cr1gfp/+ ( ) and cx3cr1gfp/gfp (
) and cx3cr1gfp/gfp ( ) littermates. Bar diagram shows the percentage of Gr1low cells (gated in R4, Figure 1A) out of total blood or BM monocytes (cells gated in R2, Figure 1A); n = 4. (D) Comparison of monocytes of hMRP8bcl2-transgenic cx3cr1gfp/gfp and cx3cr1gfp/+ mice. Bar histogram shows percentage of Gr1low monocytes (cells gated in R4, Figure 1A) of total monocytes (cells gated in R2, Figure 1A) of cx3cr1gfp/+ (
) littermates. Bar diagram shows the percentage of Gr1low cells (gated in R4, Figure 1A) out of total blood or BM monocytes (cells gated in R2, Figure 1A); n = 4. (D) Comparison of monocytes of hMRP8bcl2-transgenic cx3cr1gfp/gfp and cx3cr1gfp/+ mice. Bar histogram shows percentage of Gr1low monocytes (cells gated in R4, Figure 1A) of total monocytes (cells gated in R2, Figure 1A) of cx3cr1gfp/+ ( ) and cx3cr1gfp/gfp (
) and cx3cr1gfp/gfp ( ) mice, either hMRP8bcl2-transgenic (right bars) or nontransgenic (left bars); n = 3. *P < .0013 **P < .001, ***P < < .005 (Student t test).
) mice, either hMRP8bcl2-transgenic (right bars) or nontransgenic (left bars); n = 3. *P < .0013 **P < .001, ***P < < .005 (Student t test).
Reduction of Gr1low monocytes in absence of CX3CR1 is due to impaired cell survival. (A) Comparison of wt and cx3cr1gfp/gfp monocytes in mixed BM chimera blood. BM cells were isolated from either wt or cx3cr1gfp/gfp donors, mixed to 1:1 ratio from each genotype and transferred into irradiated wt recipients. Eight weeks after transfer recipient mice were bled, and their blood content was analyzed. Dot plot shows discrimination between GFP-positive cx3cr1gfp/gfp monocytes (R5 gated cells) and GFP-negative wt monocytes (R6 gated cells). Bar diagram shows percentage of Gr1low monocytes (as gated in R4, Figure 1A) of either total wt (R6 gated cells, ■) or cx3cr1gfp/gfp (R5 gated cells,  ) monocytes; n = 8. (B) Comparison of monocyte levels in presence and absence of CX3CL1. Wt and cx3cl1−/− irradiated mice received 1:1 mix of BM cells from wt and cx3cr1gfp/gfp mice, and were bled 8 weeks later. Bar histogram shows percentage of Gr1low monocytes (R4 gated cells, Figure 1A) of total monocytes (R2 gated cells, Figure 1A) of wt (■) and cx3cl1−/− (□) recipients of either wt or cx3cr1gfp/gfp BM cells; n = 5. (C) Comparison of blood and BM monocytes of cx3cr1gfp/+ (
) monocytes; n = 8. (B) Comparison of monocyte levels in presence and absence of CX3CL1. Wt and cx3cl1−/− irradiated mice received 1:1 mix of BM cells from wt and cx3cr1gfp/gfp mice, and were bled 8 weeks later. Bar histogram shows percentage of Gr1low monocytes (R4 gated cells, Figure 1A) of total monocytes (R2 gated cells, Figure 1A) of wt (■) and cx3cl1−/− (□) recipients of either wt or cx3cr1gfp/gfp BM cells; n = 5. (C) Comparison of blood and BM monocytes of cx3cr1gfp/+ ( ) and cx3cr1gfp/gfp (
) and cx3cr1gfp/gfp ( ) littermates. Bar diagram shows the percentage of Gr1low cells (gated in R4, Figure 1A) out of total blood or BM monocytes (cells gated in R2, Figure 1A); n = 4. (D) Comparison of monocytes of hMRP8bcl2-transgenic cx3cr1gfp/gfp and cx3cr1gfp/+ mice. Bar histogram shows percentage of Gr1low monocytes (cells gated in R4, Figure 1A) of total monocytes (cells gated in R2, Figure 1A) of cx3cr1gfp/+ (
) littermates. Bar diagram shows the percentage of Gr1low cells (gated in R4, Figure 1A) out of total blood or BM monocytes (cells gated in R2, Figure 1A); n = 4. (D) Comparison of monocytes of hMRP8bcl2-transgenic cx3cr1gfp/gfp and cx3cr1gfp/+ mice. Bar histogram shows percentage of Gr1low monocytes (cells gated in R4, Figure 1A) of total monocytes (cells gated in R2, Figure 1A) of cx3cr1gfp/+ ( ) and cx3cr1gfp/gfp (
) and cx3cr1gfp/gfp ( ) mice, either hMRP8bcl2-transgenic (right bars) or nontransgenic (left bars); n = 3. *P < .0013 **P < .001, ***P < < .005 (Student t test).
) mice, either hMRP8bcl2-transgenic (right bars) or nontransgenic (left bars); n = 3. *P < .0013 **P < .001, ***P < < .005 (Student t test).
Gr1low blood monocytes require CX3CR1 expression for their survival
Next, we investigated the cause for the decrease of Gr1low monocytes in the absence of CX3CL1-CX3CR1 interactions. Comparison of cx3cr1gfp/gfp and cx3cr1gfp/+ BM by us and others did not reveal any differences in Gr1low BM monocyte levels (Figure 2C).43 This indicates that, as opposed to other chemokine receptors such as CCR2,49 CX3CR1 is not required for BM egress.
The adoptive cotransfer of wt and CX3CR1-deficient Gr1low monocytes allows the comparison of monocyte fate, bypassing differences in monocyte production. We previously reported such cotransfers, 2 days after which we analyzed the recipients for the presence of grafted cells.35 We observed a 2.5-fold reduction in the level of retrieved grafted cx3cr1gfp/gfp monocytes as compared with cotransferred cx3cr1gfp/+ monocytes in recipient's blood and peripheral organs.35 The overall reduction in the level of grafted cx3cr1gfp/gfp monocytes suggested impaired survival.
To directly address increased monocyte apoptosis as the cause of the CX3CR1 knockout (KO) phenotype, we took advantage of mice transgenic for the human antiapoptotic factor Bcl2 under the control of the neutrophil- and monocyte-specific MRP8 promotor (hMRP8bcl2 mice47 ). As reported, enforced Bcl2 expression resulted in increased blood monocyte levels47 (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Interestingly, although intracellular staining for human Bcl2 revealed that both monocyte subsets expressed comparable transgene levels (Figure S1A), the observed increment was significantly more pronounced for the Gr1low subset (Figure 1B). This might indicate that Gr1low monocytes are more prone to die than Gr1hi monocytes, a notion also recently invoked by others.43
To test the influence of enforced Bcl2 expression on the CX3CR1 KO phenotype, we generated double transgenic cx3cr1gfp/gfp;hMRP6bcl2 and cx3cr1gfp/+;hMRP6bcl2 mice. Interestingly, analysis of the blood of these animals revealed that Bcl2 transgenic CX3CR1 knockout mice displayed Gr1low monocyte levels comparable with Bcl2-transgenic cx3cr1gfp/+ mice, levels which comprised approximately 70% of their respective total blood monocytes (Figure 2D). This was in contrast to the significant reduction of monocyte levels observed in nontransgenic cx3cr1gfp/gfp mice (Figures 1C′ and 2D). Hence, enforced monocyte survival restores Gr1low cell numbers of CX3CR1-deficient mice to the levels observed in CX3CR1-proficient mice, thus rescuing their phenotype.
To conclude, our results suggest that the specific reduction of Gr1low monocytes in cx3cr1gfp/gfp mice reflects impaired survival due to the absence of antiapoptotic CX3CR1-CX3CL1 interactions.
CX3CL1 has an antiapoptotic effect on cultured human blood monocytes
CX3CL1 has been shown to act as a survival factor for transformed cell lines,20,22 as well as cultured brain microglia.21 To directly study the role of CX3CR1-CX3CL1 interactions in monocyte survival, we resorted to an ex vivo model. In this system, we studied the ability of recombinant soluble CX3CL1 to rescue cultured isolated human blood monocytes from cell death induced by serum deprivation or exposure to oxysterol,50 a cytotoxic oxygenated cholesterol derivative known to accumulate in atherosclerosis lesions.51 Cultured monocytes underwent major apoptosis after 4 hours of serum deprivation (Figure 3B), which led us to choose this time point for our analysis. Independent repeats of these experiments resulted in distinct percentages of serum-deprived dying cells (compare Figure 3B,D, and G), presumably as a consequence of genetic variation among human donors and different blood bank storage periods. Addition of recombinant soluble CX3CL1 (full length without transmembrane anchor, aa 1-337) to monocytes either incubated with serum-free medium (Figure 3B) or with 7-β-hydroxycholesterol (Figure 3C) resulted in a significant reduction of the percentage of PI+ dying monocytes, as compared with nonsupplemented media. Decreased percentage of dead cells with the rising concentration of supplemented CX3CL1 (Figure 3D) indicated dose-dependency of its activity. Since CX3CR1 is a member of the 7-transmembrane G-protein coupled receptor (GPCR) family,10 its activity should be blocked in the presence of Pertussis toxin (PTx), a Gα-protein inhibitor. Indeed, addition of PTx to the cultured monocytes reversed the antiapoptotic effect of CX3CL1 (Figure 3E). This indicates that Gα-protein–dependent signaling by CX3CR1 is required for CX3CL1-mediated monocyte survival.
CX3CL1 rescues human blood monocytes from experimentally induced death. (A) Flow cytometric analysis of cultured human blood monocytes stained with propidium iodide (PI). Cells gated in region R7 (left dot plot) represent total analyzed population. Region R8 (right dot plot) gates PI+ cells. All bar diagrams shown in this figure present the portion of R8 gated PI+ dying cells out of R7 gated total cells. (B) Human blood monocytes (CD14++CD16−) were incubated in RPMI supplemented with 10% FCS, 10 nM soluble recombinant CX3CL1 (rCX3CL1), or nonsupplemented medium for 4 hours; n = 3. (C) Human blood monocytes (CD14++CD16−) were incubated in serum-supplemented RPMI either further supplemented or nonsupplemented with 10 nM soluble recombinant CX3CL1 (rCX3CL1). Six hours later, 30 μM 7-β-hydroxycholesterol (7β-OH-CH) was added to the cultures, and cells were analyzed after additional 16 hours; n = 3. (D) Human blood monocytes (CD14++CD16−) were incubated with RPMI supplemented with 1, 5, 10, or 100 nM CX3CL1. Diagram shows percentage of PI+ cells; n = 3. (E) Human blood monocytes (CD14+CD16+) were incubated in RPMI supplemented with 1 μg/mL pertussis toxin (PTx) for 1 hour followed by incubation with 10% FCS, 10 nM soluble recombinant CX3CL1 (rCX3CL1) or unsupplemented medium for additional 4 hours; n = 3. (F) Flow cytometric analysis of isolated CD14++CD16− (top dot plot) and CD14+CD16+ (bottom dot plot) human blood monocytes, before culture. Numbers indicate percentage of gated cells out of total population. (G) Isolated CD14++CD16− and CD14+CD16+ human blood monocytes incubated in RPMI (Medium; □) or RPMI supplemented with either 10% FCS (+FCS; ■) or 10 nM recombinant CX3CL1 (+CX3CL1;  ); n = 3. *P < .005, **P < .01, ***P < .05, NS = not significant (Student t test).
); n = 3. *P < .005, **P < .01, ***P < .05, NS = not significant (Student t test).
CX3CL1 rescues human blood monocytes from experimentally induced death. (A) Flow cytometric analysis of cultured human blood monocytes stained with propidium iodide (PI). Cells gated in region R7 (left dot plot) represent total analyzed population. Region R8 (right dot plot) gates PI+ cells. All bar diagrams shown in this figure present the portion of R8 gated PI+ dying cells out of R7 gated total cells. (B) Human blood monocytes (CD14++CD16−) were incubated in RPMI supplemented with 10% FCS, 10 nM soluble recombinant CX3CL1 (rCX3CL1), or nonsupplemented medium for 4 hours; n = 3. (C) Human blood monocytes (CD14++CD16−) were incubated in serum-supplemented RPMI either further supplemented or nonsupplemented with 10 nM soluble recombinant CX3CL1 (rCX3CL1). Six hours later, 30 μM 7-β-hydroxycholesterol (7β-OH-CH) was added to the cultures, and cells were analyzed after additional 16 hours; n = 3. (D) Human blood monocytes (CD14++CD16−) were incubated with RPMI supplemented with 1, 5, 10, or 100 nM CX3CL1. Diagram shows percentage of PI+ cells; n = 3. (E) Human blood monocytes (CD14+CD16+) were incubated in RPMI supplemented with 1 μg/mL pertussis toxin (PTx) for 1 hour followed by incubation with 10% FCS, 10 nM soluble recombinant CX3CL1 (rCX3CL1) or unsupplemented medium for additional 4 hours; n = 3. (F) Flow cytometric analysis of isolated CD14++CD16− (top dot plot) and CD14+CD16+ (bottom dot plot) human blood monocytes, before culture. Numbers indicate percentage of gated cells out of total population. (G) Isolated CD14++CD16− and CD14+CD16+ human blood monocytes incubated in RPMI (Medium; □) or RPMI supplemented with either 10% FCS (+FCS; ■) or 10 nM recombinant CX3CL1 (+CX3CL1;  ); n = 3. *P < .005, **P < .01, ***P < .05, NS = not significant (Student t test).
); n = 3. *P < .005, **P < .01, ***P < .05, NS = not significant (Student t test).
Similarly to those of mouse, human blood monocytes comprise 2 subsets that are distinct in their CX3CR1 expression levels.38 CD14++CD16− monocytes express intermediate levels of CX3CR1, and CD14+CD16+ monocytes express high levels of CX3CR1.35 In contrast to those of mouse, human CX3CR1high monocytes comprise only 5% of the total monocyte population in healthy individuals.38 We next compared the effect of CX3CL1 on the 2 human monocyte subsets (Figure 3F) upon serum depravation. Interestingly, both subsets responded similarly to 10 nM chemokine addition, as indicated by comparable levels of surviving cells (Figure 3G). In addition, no significant differences between monocyte subsets could be observed upon incubation with additional CX3CL1 concentrations (1, 5, or 100 nM, not shown). Therefore, in contrast to the murine in vivo data, the protective effect of CX3CL1 on cultured human monocytes is not restricted to CX3CR1hi cells.
To conclude, CX3CL1 specifically acts as a human blood monocyte survival factor. However, at least in vitro, it has similar effect on both monocyte subsets. Importantly, this activity does not require cell-cell interactions.
CX3CR1 deficiency causes increased apoptosis in atherosclerotic plaques
Mice deficient for apolipoprotein E (ApoE) develop atherosclerosis if subjected to a Western high-fat diet.52 However, when on a CX3CR1-deficient genetic background, apoe−/− mice display reduced atherosclerotic plaque formation.27,28 Moreover, CX3CL1-deficient mice also are relatively protected from atherosclerosis.29,30
To probe for a potential connection between the above-described prosurvival effect of CX3CL1 and the reported atherosclerosis phenotype of CX3CR1- and CX3CL1-deficient mice, we sublethally irradiated apoe−/− mice and reconstituted them with cx3cr1+/+, cx3cr1gfp/+, or cx3cr1gfp/gfp BM cells. Four weeks after BM transfer, the chimeras were exposed to a high-fat diet, and 3 months later their arteries were analyzed for the formation of atherosclerotic plaques. No differences in plaque areas between recipients of wt and cx3cr1gfp/+ BM were detected (Figure S2). Notably, the presence of ApoE-expressing donor cells in ApoE-deficient hosts alters disease progression in these mixed chimeras,53 potentially preventing the detection of subtle differences detected by others. However, importantly and in agreement with the published data,27,28,31 apoe−/− recipients of cx3cr1gfp/gfp BM cells developed significantly reduced atherosclerotic plaque area levels in both thoracoabdominal aorta and aortic roots, as compared with apoe−/− recipients of cx3cr1+/+ BM (Figure S2 and data not shown).
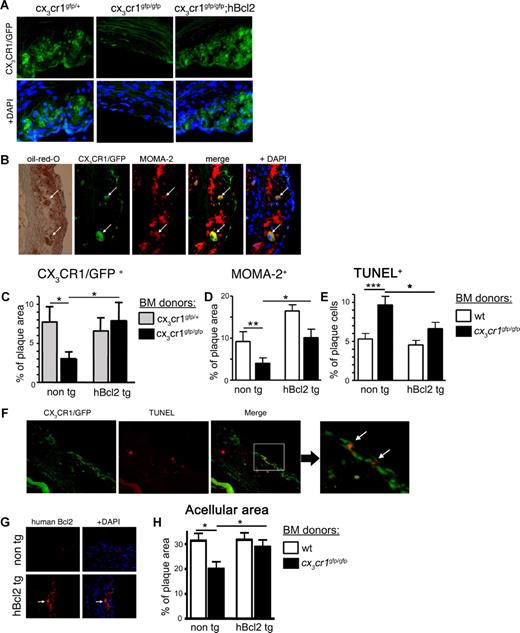
Atherosclerotic plaques display a complex composition of mononuclear phagocytes, including monocytes, DCs, and unique plaque-associated macrophages, the so-called foam cells.24,31,54 Immunohistochemical analysis of atheroma of the cx3cr1gfp/+ > apoe−/− BM chimeras revealed abundant CX3CR1/GFP expressing cells (Figure 4A); among them were cells displaying the marker MOMA-2, which is known to be expressed by monocytes and foam cells,55 as well as Oil Red O–positive fat deposits, a foam cell characteristic (Figure 4B). Interestingly, the number of both CX3CR1/GFP-positive and MOMA-2–positive cells was significantly reduced in apoe−/− recipients of cx3cr1gfp/gfp BM (Figures 4C,D non-tg bars).
CX3CR1-deficient atheromas show changes in cellular composition and increased apoptosis. Irradiated apoe−/− recipients of indicated BM cells were subjected to high-fat diet for 12 weeks, after which their plaque content was analyzed. (A) Histology of atherosclerotic plaque of representative recipient of cx3cr1gfp/+, cx3cr1gfp/gfp, and cx3cr1gfp/gfp;hMRP8bcl2 (cx3cr1gfp/gfp;hBcl2) BM cells. CX3CR1/GFP expression is shown in green; DAPI stain in blue. (B) Immunohistochemistry of atherosclerotic plaque of representative recipient of cx3cr1gfp/+ BM cells. CX3CR1/GFP expression is shown in green, anti–MOMA-2 staining in red, and DAPI in blue. Brightfield shows orange oil (Oil Red O) deposit. Arrows indicate large CX3CR1/GFP+ cells also positive for MOMA-2 expression and orange oil deposit. (C) Histology-based quantification of CX3CR1/GFP+ cells per total plaque areas. Bar diagram shows recipients of cx3cr1gfp/+ ( ) and cx3cr1gfp/gfp (■), both on either wt (non-tg) or hMRP8bcl2-transgenic background (hBcl2 tg); n = 12. (D) Immunohistochemistry-based quantification of MOMA-2 positive area out of total plaque area. Bar diagram shows recipient of wt (□) and cx3cr1gfp/gfp (■), both either on wt (non tg) or hMRP8bcl2 (hBcl2 tg)–transgenic background; n = 12. (E) Immunohistochemistry-based quantification of TUNEL+ cells out of total plaque cells (identified by DAPI staining). Bar diagram shows recipient of wt (□) and cx3cr1gfp/gfp (■), both on either wt (non-tg) or hMRP8bcl2-transgenic background (hBcl2 tg); n = 12. (F) Immunohistochemistry of atherosclerotic plaque of cx3cr1gfp/gfp BM cell recipient. CX3CR1/GFP expression is shown in green, TUNEL staining is shown in red. Right panel shows higher magnification of area marked by white rectangular. Arrows indicate cells double positive for CX3CR1/GFP and TUNEL. (G) Immunohistochemistry of atherosclerotic plaque of wt (non-tg) or hMRP8bcl2-transgenic (hBcl2 tg) BM were stained with an antibody specific for human Bcl2 (red). DAPI is shown in blue. Arrow indicates human Bcl2-positive cells. (H) Histology-based quantification of acellular plaque area. Bar diagram shows recipient of wt (□) and cx3cr1gfp/gfp (■), both on either wt (non-tg) or hMRP8bcl2-transgenic background (hBcl2 tg); n = 12. *P < .05, **P < .01, ***P < .005 (Student t test).
) and cx3cr1gfp/gfp (■), both on either wt (non-tg) or hMRP8bcl2-transgenic background (hBcl2 tg); n = 12. (D) Immunohistochemistry-based quantification of MOMA-2 positive area out of total plaque area. Bar diagram shows recipient of wt (□) and cx3cr1gfp/gfp (■), both either on wt (non tg) or hMRP8bcl2 (hBcl2 tg)–transgenic background; n = 12. (E) Immunohistochemistry-based quantification of TUNEL+ cells out of total plaque cells (identified by DAPI staining). Bar diagram shows recipient of wt (□) and cx3cr1gfp/gfp (■), both on either wt (non-tg) or hMRP8bcl2-transgenic background (hBcl2 tg); n = 12. (F) Immunohistochemistry of atherosclerotic plaque of cx3cr1gfp/gfp BM cell recipient. CX3CR1/GFP expression is shown in green, TUNEL staining is shown in red. Right panel shows higher magnification of area marked by white rectangular. Arrows indicate cells double positive for CX3CR1/GFP and TUNEL. (G) Immunohistochemistry of atherosclerotic plaque of wt (non-tg) or hMRP8bcl2-transgenic (hBcl2 tg) BM were stained with an antibody specific for human Bcl2 (red). DAPI is shown in blue. Arrow indicates human Bcl2-positive cells. (H) Histology-based quantification of acellular plaque area. Bar diagram shows recipient of wt (□) and cx3cr1gfp/gfp (■), both on either wt (non-tg) or hMRP8bcl2-transgenic background (hBcl2 tg); n = 12. *P < .05, **P < .01, ***P < .005 (Student t test).
CX3CR1-deficient atheromas show changes in cellular composition and increased apoptosis. Irradiated apoe−/− recipients of indicated BM cells were subjected to high-fat diet for 12 weeks, after which their plaque content was analyzed. (A) Histology of atherosclerotic plaque of representative recipient of cx3cr1gfp/+, cx3cr1gfp/gfp, and cx3cr1gfp/gfp;hMRP8bcl2 (cx3cr1gfp/gfp;hBcl2) BM cells. CX3CR1/GFP expression is shown in green; DAPI stain in blue. (B) Immunohistochemistry of atherosclerotic plaque of representative recipient of cx3cr1gfp/+ BM cells. CX3CR1/GFP expression is shown in green, anti–MOMA-2 staining in red, and DAPI in blue. Brightfield shows orange oil (Oil Red O) deposit. Arrows indicate large CX3CR1/GFP+ cells also positive for MOMA-2 expression and orange oil deposit. (C) Histology-based quantification of CX3CR1/GFP+ cells per total plaque areas. Bar diagram shows recipients of cx3cr1gfp/+ ( ) and cx3cr1gfp/gfp (■), both on either wt (non-tg) or hMRP8bcl2-transgenic background (hBcl2 tg); n = 12. (D) Immunohistochemistry-based quantification of MOMA-2 positive area out of total plaque area. Bar diagram shows recipient of wt (□) and cx3cr1gfp/gfp (■), both either on wt (non tg) or hMRP8bcl2 (hBcl2 tg)–transgenic background; n = 12. (E) Immunohistochemistry-based quantification of TUNEL+ cells out of total plaque cells (identified by DAPI staining). Bar diagram shows recipient of wt (□) and cx3cr1gfp/gfp (■), both on either wt (non-tg) or hMRP8bcl2-transgenic background (hBcl2 tg); n = 12. (F) Immunohistochemistry of atherosclerotic plaque of cx3cr1gfp/gfp BM cell recipient. CX3CR1/GFP expression is shown in green, TUNEL staining is shown in red. Right panel shows higher magnification of area marked by white rectangular. Arrows indicate cells double positive for CX3CR1/GFP and TUNEL. (G) Immunohistochemistry of atherosclerotic plaque of wt (non-tg) or hMRP8bcl2-transgenic (hBcl2 tg) BM were stained with an antibody specific for human Bcl2 (red). DAPI is shown in blue. Arrow indicates human Bcl2-positive cells. (H) Histology-based quantification of acellular plaque area. Bar diagram shows recipient of wt (□) and cx3cr1gfp/gfp (■), both on either wt (non-tg) or hMRP8bcl2-transgenic background (hBcl2 tg); n = 12. *P < .05, **P < .01, ***P < .005 (Student t test).
) and cx3cr1gfp/gfp (■), both on either wt (non-tg) or hMRP8bcl2-transgenic background (hBcl2 tg); n = 12. (D) Immunohistochemistry-based quantification of MOMA-2 positive area out of total plaque area. Bar diagram shows recipient of wt (□) and cx3cr1gfp/gfp (■), both either on wt (non tg) or hMRP8bcl2 (hBcl2 tg)–transgenic background; n = 12. (E) Immunohistochemistry-based quantification of TUNEL+ cells out of total plaque cells (identified by DAPI staining). Bar diagram shows recipient of wt (□) and cx3cr1gfp/gfp (■), both on either wt (non-tg) or hMRP8bcl2-transgenic background (hBcl2 tg); n = 12. (F) Immunohistochemistry of atherosclerotic plaque of cx3cr1gfp/gfp BM cell recipient. CX3CR1/GFP expression is shown in green, TUNEL staining is shown in red. Right panel shows higher magnification of area marked by white rectangular. Arrows indicate cells double positive for CX3CR1/GFP and TUNEL. (G) Immunohistochemistry of atherosclerotic plaque of wt (non-tg) or hMRP8bcl2-transgenic (hBcl2 tg) BM were stained with an antibody specific for human Bcl2 (red). DAPI is shown in blue. Arrow indicates human Bcl2-positive cells. (H) Histology-based quantification of acellular plaque area. Bar diagram shows recipient of wt (□) and cx3cr1gfp/gfp (■), both on either wt (non-tg) or hMRP8bcl2-transgenic background (hBcl2 tg); n = 12. *P < .05, **P < .01, ***P < .005 (Student t test).
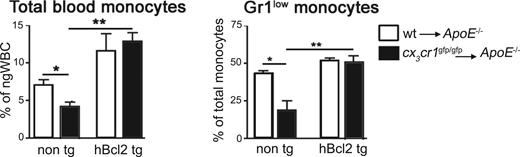
Similar to steady-state conditions (Figure 1), analysis of apoe−/− recipients of wt or cx3cr1gfp/gfp BM cells subjected to high-fat diet revealed that the CX3CR1 deficiency caused a significant reduction of blood monocytes levels also under systemic inflammatory conditions (Figure 5A non-tg bars). Furthermore, this reduction was again more pronounced for the Gr1low monocyte population (Figure 5B non-tg bars).
Reduced numbers of Gr1low monocytes in CX3CR1-deficient mice under atherosclerotic condition and rescue by Bcl2 expression. Blood monocyte levels of irradiated apoe−/− recipients of wt (□) and cx3cr1gfp/gfp (■) BM cells, either transgenic (hBcl2 tg) or nontransgenic (non-tg) for hMRP8bcl2. (A) Summary of total monocytes, identify as CD115+ cells (R2 gated cells, Figure 1A), as a percentage of ngWBC (R1 gated cells, Figure 1A); n = 12. (B) Summary of Gr1low monocytes levels (R4 gated cells, Figure 1A) as a percentage of total CD115+ monocyte levels (R2 gated cells, Figure 1A); n = 12. *P < .01, **P < .05 (Student t test).
Reduced numbers of Gr1low monocytes in CX3CR1-deficient mice under atherosclerotic condition and rescue by Bcl2 expression. Blood monocyte levels of irradiated apoe−/− recipients of wt (□) and cx3cr1gfp/gfp (■) BM cells, either transgenic (hBcl2 tg) or nontransgenic (non-tg) for hMRP8bcl2. (A) Summary of total monocytes, identify as CD115+ cells (R2 gated cells, Figure 1A), as a percentage of ngWBC (R1 gated cells, Figure 1A); n = 12. (B) Summary of Gr1low monocytes levels (R4 gated cells, Figure 1A) as a percentage of total CD115+ monocyte levels (R2 gated cells, Figure 1A); n = 12. *P < .01, **P < .05 (Student t test).
The observed decrease of CX3CR1/GFP-positive cells in atherosclerotic plaques could thus result from impaired monocyte recruitment. Alternatively, but not mutually exclusively, CX3CL1-CX3CR1 interactions might be required locally for cell survival within the atheroma. The latter is an inherently hostile environment due to the abundant presence of oxidized cholesterols.56 Of note, both activated endothelium and intimal smooth muscle cells express CX3CL13,7,57,58 and, therefore, could serve as a source of survival signals for plaque-resident CX3CR1-expressing cells.
To probe for this local scenario, we analyzed plaque apoptotic cell content using a TUNEL assay on tissue sections of the various apoe−/− BM chimeras. As seen in Figure 4E, atheromas of (cx3cr1gfp/gfp > apoe−/−) BM chimeras displayed a significantly increased frequency of TUNEL+ apoptotic cells, as compared with atheromas of (wt > apoe−/−) BM chimeras. Notably, we could detect apoptotic cells, which were also CX3CR1/GFP+ in (cx3cr1gfp/gfp > apoe−/−) chimera, further indicating a CX3CR1 requirement during cell survival (Figure 4F).
To investigate the involvement of cell death in the reduced plaque cell levels in the absence of CX3CR1, we took advantage of the hMRP8bcl2-transgenic mice, which express human Bcl2 tg under monocyte- and macrophage-specific promotors.59 To this end, we sublethally irradiated apoe−/− mice and reconstituted them with hMRP8bcl2-transgenic cx3cr1+/+, cx3cr1gfp/+, or cx3cr1gfp/gfp BM. After recovery the mice were subjected to high-fat diet as described earlier. As in steady state (Figure 2D), introduction of the hBcl2 transgene increased the frequency of total monocytes (Figure 5A) and restored the ratio of the monocyte subsets in CX3CR1 BM-deficient atherosclerotic mice (Figure 5B). Immunostaining for hBcl2 protein indicated that in addition to the blood, the transgene is also expressed in the atheroma (Figure 4G). Furthermore, hBcl2+ plaque cells were large in size, reminiscent of foam cells.
Next, we assessed the influence of enforced cell survival on plaque cellular composition. Immunohistochemical analysis revealed that the level of TUNEL+ apoptotic cells in apoe−/− recipient of cx3cr1gfp/gfp;hMRP8bcl2-transgenic BM was restored to that found in CX3CR1-proficient mice (Figure 4E). Importantly, the frequency of plaque-resident CX3CR1/GFP (Figure 4A,C) and MOMA-2 (Figure 4D) expressing cells was increased to wt levels, rescuing the cx3cr1gfp/gfp phenotype.
Taken together, our data indicate that atherosclerotic plaques contain CX3CR1-expressing cells, among them foam cells, that depend on CX3CL1-CX3CR1 interaction for their survival.
Enforced survival of plaque resident cells causes enhanced atherosclerosis in CX3CR1-deficient mice
We have shown requirement of CX3CR1 for the survival of blood and atheroma cells, including monocytes and foam cells. To test whether the absence of CX3C-mediated cell survival signals could provide the mechanistic explanation for the reduced atherosclerosis susceptibility of CX3CR1-deficient mice, we asked whether enforced cell survival would affect their protection from disease progression. To this end, we sublethally irradiated apoe−/− mice and reconstituted them with hMRP8bcl2-transgenic BM, either cx3cr1+/+ or cx3cr1gfp/gfp. Plaques of CX3CR1-deficient mice showed a decreased acellular area, also known as necrotic core (Figure 4H non-tg bar), indicating less progressed disease,56 a phenotype that was restored upon hBcl2 expression (Figure 4H). As seen in Figure 6, Bcl2 transgenesis raised total plaque area levels in both the aorta and the aortic roots of apoe−/− recipients even in the presence of CX3CR1-expressing BM cells. Most importantly, recipients of both cx3cr1+/+;hMRP8bcl2 and cx3cr1gfp/gfp;hMRP8bcl2 BM cells showed similar disease scores (Figure 6A′,B′). Thus, expression of the Bcl2 transgene, leading to enforced monocyte and foam cell survival (Figure 4), overrode the protection imposed by the CX3CR1 deficiency in cx3cr1gfp/gfp BM recipients.
Enforced cell survival promotes atherogenesis in the absence of CX3CR1. Quantitative analysis of atherosclerotic lesion area in apoe−/− recipients of wt (○) and cx3cr1gfp/gfp (●) BM cells, either from hMRP8bcl2-transgenic (hBcl2 tg) or nontransgenic (non tg) donors. Mice were subjected to high-fat diet for 12 weeks, after which atherosclerotic plaques were analyzed in aortic roots (A,A') and en face prepared aortas (B,B′) by Oil Red O staining. Panels A′ and B′ show a summary of Oil Red O+ plaque area for each group, a representative of which is shown in panels A and B, respectively; n = 12, representative of 2 independent experiments. *P < .05, **P < .01 (Student t test).
Enforced cell survival promotes atherogenesis in the absence of CX3CR1. Quantitative analysis of atherosclerotic lesion area in apoe−/− recipients of wt (○) and cx3cr1gfp/gfp (●) BM cells, either from hMRP8bcl2-transgenic (hBcl2 tg) or nontransgenic (non tg) donors. Mice were subjected to high-fat diet for 12 weeks, after which atherosclerotic plaques were analyzed in aortic roots (A,A') and en face prepared aortas (B,B′) by Oil Red O staining. Panels A′ and B′ show a summary of Oil Red O+ plaque area for each group, a representative of which is shown in panels A and B, respectively; n = 12, representative of 2 independent experiments. *P < .05, **P < .01 (Student t test).
To conclude, our results suggest that increased cell death in absence of CX3CR1-CX3CL1 interactions is the cause for the reduced atherogenesis observed in mice deficient for this chemokine receptor/ligand pair. Our results thus provide a mechanistic explanation for the well-established requirement for CX3CR1 during atherogenesis, in both mice and humans.
Discussion
The physiologic function of the CX3C chemokine family, as represented by the membrane-tethered ligand CX3CL1 (fractalkine) and its sole receptor, CX3CR1, remains poorly understood. Here, we establish a novel role for CX3CR1-CX3CL1 interactions in mononuclear phagocyte survival. First, we show that in the blood of both CX3CL1- and CX3CR1-deficient mice, Gr1low monocyte levels are specifically reduced. Enforced monocytic expression of the antiapoptotic factor Bcl2 reverted the phenotype, providing genetic evidence that this reduction results from impaired monocyte survival. In support of the in vivo data, recombinant CX3CL1 specifically rescued cultured human monocytes from serum deprivation and oxysterol-induced death. This indicates a direct and evolutionary conserved role for CX3CR1-CX3CL1 interaction in cell survival. Finally, we demonstrate that CX3C survival signals can provide the mechanistic explanation for one of the few known phenotypes of CX3CR1- and CX3CL1-deficient mice (ie, their relative protection from diet-induced atherosclerosis).27-30 Thus, enforced survival of monocytes and foam cells in CX3CR1 knockout mice restored atherogenesis scores to those found in wt mice, suggesting that CX3CL1-CX3CR1–mediated survival signals are critical for atheroma formation and progression.
CX3CR1-proficient mice expressing the human Bcl2 transgene show higher blood monocyte levels as well as increased atherosclerosis compared with nontransgenic controls (Figures 2,5). The Bcl2 phenotype could, thus, mask the CX3CR1 KO phenotype rather than rescuing it. However, if those 2 phenotypes are not linked, CX3CR1-deficient mice would be expected to have, at least to some extent, lower monocyte levels and decreased atherosclerosis even on a Bcl2-transgenic background. Yet, in both cases, despite dramatic discrepancies between CX3CR1-proficient and -deficient mice, no difference could be observed between groups of Bcl2-transgenic mice, supporting an antiapoptotic role for CX3CR1. Moreover, the direct involvement of CX3CR1 in cell survival control is also implied by the increased level of apoptotic cells in the plaques of atherosclerotic CX3CR1-deficient mice (Figure 4E).
Although chemokines were originally defined as chemoattractants, a growing body of evidence indicates their additional involvement in the control of cell survival.60 Interestingly, addition of the Gα-protein inhibitor PTx in our study increased apoptosis of serum-cultured monocytes (Figure 3E), which is compatible with the notion that serum contains chemokines that trigger survival promoting GPCRs. CX3CL1 itself was reported to promote the survival of cancer cell lines,20,22 as well as cultured microglia.21 Other chemokines, such as CXCL12/SDF1 and CXCL9/MIG protect CXCR4- and CXCR3-expressing cultured tumor cells from death.61,62 Furthermore, CCL5/RANTES was shown to contribute to the in vivo survival of pulmonary macrophages during viral infection.63 Interestingly, our recent finding of impaired foam cell levels and atherosclerotic lesion formation in ccr5−/− mice48,64 might suggest a potential role of CCR5-mediated survival also under these conditions.
We show that CX3CL1 can protect both human monocyte subsets from cell death in vitro. In contrast, in vivo survival of the murine Gr1hi monocytes subset did not depend on CX3CR1 engagement (Figure 1). Of note, Gr1hi monocytes, as well as their human CD14++CD16− counterpart, have an extensive chemokine receptor repertoire, as compared with Gr1low and CD14+CD16+ cells.35,38 Therefore, their survival in vivo could be ensured by other factors, including chemokines, to which Gr1low and CD14+CD16+ monocytes might not respond. The differential sensitivity to CX3CR1 deficiency of the monocyte subsets might also result from their distinct propensity to die, a notion supported by their distinct response to Bcl2 overexpression in this study as well as in other reports.43
Monocytes are critical for the development, maintenance, and resolution of atherosclerosis.65,66 In the plaque, they are believed to give rise to foam cells, a special macrophage subset found in those structures.54 Foam cells were shown to play a key role in the development of early atherosclerotic plaques and their maintenance.65 Accordingly, foam cell death during early disease stages has been shown to correlate with decreased atherogenesis, whereas their death during late stages leads to thrombosis and disease progression.56 CX3CL1 is prominently expressed by multiple nonhematopoetic cell types in the lesions, including muscle and endothelial cells.5,7 Here, we suggest that in absence of its sole receptor, CX3CR1, monocytes and foam cells undergo increased cell death, which together with potential effects on recruitment leads to a reduced foam cell load and the inhibition of plaque progression.
Our results suggest that inhibition of CX3CR1-CX3CL1 interactions during early atherogenesis stages might be beneficial in controlling this disease. However, since CX3C signals are likely to be required for foam cell survival also during later atheroma stages, such inhibition could result in disease exacerbation. The development of therapeutic protocols based on interference with the CX3CR1-CX3CL1 axis will, therefore, require an in-depth understanding of the system.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank members of the Jung laboratory for critical reading of the manuscript and are grateful to Y. Chermesh and O. Amram for animal husbandry.
This work was supported by the MINERVA Foundation (Munich, Germany; S.J.) and the Israeli Science Foundation (Jerusalem, Israel; S.J.) as well as the Deutsche Forschungsgemeinschaft (FOR809; Bonn, Germany; A.Z. and C.W.). S.J. is the incumbent of the Pauline Recanati Career Development Chair.
Authorship
Contribution: L.L., L.B.-O., and A.Z. designed and performed research; K.K., R.K., and E.S. provided critical technical assistance; S.A.L. and I.L.W. contributed vital reagents; and S.J., L.L., and C.W. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Steffen Jung, PhD, Department of Immunology, The Weizmann Institute of Science, Rehovot 76100, Israel; e-mail: s.jung@weizmann.ac.il.
References
Author notes
*L.L., L.B.-O., and A.Z. contributed equally to this work.