Abstract
The fact that the Xid mutation of Btk impairs the ability of pleckstrin homo-logy domain of Btk to bind phosphatidylinositol-(3,4,5)-trisphosphate, a product of class IA phosphoinositide-3 kinases (PI3Ks), has been considered strong evidence for the hypothesis that Btk functions downstream of PI3Ks. We demonstrate here that the Xid mutation renders the Btk protein unstable. Furthermore, class IA PI3K- and Btk-deficient mice show different phenotypes in B-cell development, collectively indicating that PI3Ks and Btk differentially function in BCR signal transduction. Nevertheless, both PI3K and Btk are required for the activation of NF-κB, a critical transcription factor family for B-cell development and function. We demonstrate that PI3Ks maintain the expression of NF-κB proteins, whereas Btk is known to be essential for IκB degradation and the translocation of NF-κB to the nucleus. The loss of PI3K activity results in marked reduction of c-Rel and to a lesser extent RelA expression. The lentivirus-mediated introduction of c-Rel corrects both developmental and proliferative defects in response to BCR stimulation in class IA PI3K-deficient B cells. These results show that the PI3K-mediated control of c-Rel expression is essential for B-cell functions.
Introduction
The nuclear factor κB (NF-κB) family of transcription factors plays an essential role in the expression of genes required for proliferation, survival, and development in many cell types. NF-κB functions as a homodimer or heterodimer consisting of a variety of combinations of 5 different Rel proteins: RelA (p65), c-Rel, RelB, NF-κB1 (p50), and NF-κB2 (p52). The latter 2 are generated through proteolytic processing from their precursor molecules p105 and p100.1 In most resting cells, NF-κB dimers are sequestered in the cytoplasm through binding to the ankyrin repeats of molecules termed inhibitor of NF-κBs (IκBs).2 The precursors of NF-κB1 and NF-κB2 also contain ankyrin repeats and therefore act as inhibitors for Rel proteins. The activation of NF-κB signaling pathway ultimately leads to IκB kinase (IKK)–mediated phosphorylation and subsequent degradation of IκBs, releasing NF-κBs to translocate to the nucleus where they induce NF-κB–dependent gene expression.3,4
NF-κB family proteins play pivotal roles in the diverse functions of B cells such as proliferation, isotype switching, and cytokine production.5,6 Furthermore, the NF-κB family is indispensable for the development of B-cell lineage, as demonstrated by the analyses of genetically manipulated mice deficient for more than one of the NF-κB subunits. In p50/p52 double-knockout mice, B-cell development is blocked at the immature transitional stage, shortly after B cells exit from the bone marrow (BM).7 Adoptive transfer experiments using mixed fetal liver cells showed that p50−/−RelA−/− B cells cannot develop into marginal zone (MZ) B cells,8 whereas RelA−/−c-Rel−/− fetal liver cells fail to give rise to mature follicular (FO) B cells.9 In addition, a recent report revealed that the survival of mature B cells depends on the IKK-mediated activation of NF-κB.10
Another set of molecules important in B-cell development and function are the class IA phosphoinositide 3-kinases (PI3Ks), which are a family of heterodimeric lipid kinases consisting of a catalytic (p110α, p110β, and p110δ) and a regulatory (p85α, p55α, p50α, p85β, and p55γ) subunit, and generate phospholipid second messengers that signal downstream of tyrosine kinases in the immune system.11,12 These PI3K products (phosphatidylinositol-(3,4)-bisphosphate PIP2 or phosphatidylinositol-(3,4,5)-trisphosphate [PIP3]) are bound by various proteins containing pleckstrin homology (PH) domains, such as Akt and phosphoinositide-dependent kinase 1 (PDK1). Thus, these PH-containing proteins function downstream of PI3K.12
Mice deficient for the gene encoding p85α, the most abundantly and ubiquitously expressed regulatory subunit of class IA PI3K, show the loss of B1 cells, reduced numbers of mature B cells, and failure to proliferate following BCR cross-linking.13 This phenotype is similar to that of Btk−/− mice or mice with the Xid mutation (a natural mutation in the PH domain of Btk in which an arginine residue critical for binding PIP3 is replaced with cysteine).14 Btk, an essential tyrosine kinase in BCR signaling, facilitates the activation of PLCγ2, which triggers NF-κB signaling through IKK activation.15,16 The BCR-induced activation of NF-κB is indeed blocked in Btk−/− B cells.17,18 p85α−/− B cells also show a defect in the BCR-induced NF-κB activation.19 In addition, LY294002, a potent PI3K inhibitor, blocks NF-κB activation induced by simultaneous stimulation with BCR and LPS.20 These observations are consistent with the dogma that PI3K functions upstream of Btk by recruiting Btk to the plasma membrane through the binding of the PH domain of Btk to PIP3 generated by PI3K.21 However, we have demonstrated that Btk activation upon BCR stimulation is unaffected by the inhibition of PI3K or the lack of p85α, raising the possibility that PI3K regulates the NF-κB signaling pathway independent of Btk.19 Here we show that Xid mutation renders the Btk protein unstable and Xid B cells express greatly reduced amounts of Btk protein that can still be activated upon BCR stimulation, likely resulting in the impaired B-cell signaling. We also show that PI3K activity is essential for maintaining the expression of NF-κB proteins such as c-Rel and RelA in B cells, whereas previous studies have shown that Btk is essential for IκB degradation.17,18 Forced expression of c-Rel restored the development and proliferative responses of p85α−/− B cells. Our results indicate that PI3K is involved in the B-cell homeostasis through the expression of NF-κB proteins.
Methods
Mice
p85α−/− mice13 were backcrossed to C57BL/6 or BALB/c mice for 12 generations before intercrossing heterozygous mice. Rag-2−/− mice on a BALB/c background (stock no. 000601) and B6.SJL, a C57BL/6 congenic strain expressing the CD45.1 allele (stock no. 004007), were obtained from Taconic (Germantown, NY). Btk−/−(y) mice on a (C57BL/6 × 129/Sv) mixed background (stock no. 002536) and Xid mice on a CBA background (stock no. 001011) were obtained from The Jackson Laboratory (Bar Harbor, ME). Although Btk is encoded on the X chromosome and Btk-deficient female and male mice have the Btk−/− and Btk−/y genotypes, respectively, we designate here Btk-deficient mice as Btk−/− mice. All mice were maintained at Taconic or in our animal facility under specific pathogen-free conditions. All experiments were approved by the animal care and use committee at Keio University and were performed in accordance with institutional guidelines.
Reagents
Antibodies to c-Rel (sc-70), RelA (sc-109), ERK2 (sc-154), Btk (sc-1107), and IκBα (sc-371) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-GFP (JL-8) was obtained from Clontech (Mountain View, CA). Anti–phospho-ERK (E10) was purchased from Cell Signaling Technology (Beverly, MA). Anti-Btk mAb, 43-3B,22 was a generous gift from Dr S. Tsukada (Osaka University, Osaka, Japan). Antiphosphotyrosine mAb (4G10)23 was a generous gift from Dr T. Roberts (DFCI, Boston, MA). Wortmannin was purchased from Calbiochem (La Jolla, CA).
Cell stimulation and immunoblotting
B cells were purified from total splenocytes using anti-B220–coated magnetic beads and AutoMACS (Miltenyi Biotec, Sunnyvale, CA), where the purity was more than 95%. Purified B cells were stimulated with or without the F(ab′)2 fragment of a goat polyclonal antibody against mouse IgM (20 μg/mL anti-IgM F(ab′)2; Jackson ImmunoResearch, West Grove, PA) at 37°C for the indicated times. The cell lysates were prepared and subjected to either immunoblot analysis or immunoprecipitation as described previously.19
Flow cytometric analysis
Fluorescein isothiocyanate (FITC)–conjugated anti–mouse IgM, FITC-conjugated anti-CD21, FITC-conjugated anti–mouse IgD, phycoerythrin (PE)–conjugated anti-CD23, PE-conjugated anti-IgM, PE-conjugated anti-CD21, biotinylated anti-CD45.2, biotinylated anti-CD23, and allophycocyanin-cyanin 7 (APC-Cy7)–conjugated anti-B220 were purchased from BD Biosciences (San Jose, CA). PE-conjugated anti-AA4.1 was purchased from eBioscience (San Diego, CA). The binding of biotinylated mAbs was detected with streptavidin-APC (BD Biosciences). One to 2 million cells were stained with the designated antibodies in HBSS with 2% fetal calf serum (FCS) and subjected to analysis on a FACSAria using FACSDiva software (BD Biosciences). Dead cells were eliminated from the analysis by staining with 7-amino-actinomycin D.
Generation of recombinant lentiviral vectors
A lentiviral vector system24 consisting of pCAG-HIVgp, pCMV-VSVG-RSV-Rev, and CSII-EF1α-IRES2-Venus was kindly provided by Dr H. Miyoshi (RIKEN, Tsukuba, Japan). Mouse c-Rel cDNA was amplified by polymerase chain reaction (PCR) using pMSCVIRES-c-Rel (kindly provided by Dr T. Kurosaki, RIKEN RCAI, Yokohama, Japan) as a template, whereas human p85α cDNA was amplified by reverse-transcription (RT)–PCR using total RNA from Jurkat T lymphocytes as a mRNA source, followed by cloning into the BamHI site of CSII-EF1α-IRES2-Venus. Resultant vectors along with pCAG-HIVgp and pCMV-VSVG-RSV-Rev were transiently transfected into 293T cells to generate HIV-1–based lentivirus vectors pseudotyped with the vesicular stomatitis virus G glycoprotein. BM cells were collected from the femurs of p85α+/− or p85α−/− mice 2 days after the intraperitoneal injection of 5-fluorouracil (Sigma-Aldrich, St Louis, MO) at a dose of 3 mg per mouse, and were cultured in RPMI-1640 medium containing 10% FCS, 50 μM 2-mercaptoethanol, 100 U/mL each of penicillin and streptomycin, nonessential amino acids (Invitrogen, Carlsbad, CA), 10 mM Hepes, and 1 mM sodium pyruvate supplemented with 10 ng/mL mouse IL-3, 5 ng/mL mouse IL-6, and 10 ng/mL stem cell factor (all from Peprotech, London, United Kingdom). After 48-hour incubation, nonadherent BM cells were collected and seeded on 24-well plates at 5 × 106 cells/mL in the medium supplemented with IL-3, IL-6, and stem cell factor. Lentivirus vectors were then infected by centrifugation (700g) for 2 hours in the presence of 5 μg/mL polybrene (Sigma-Aldrich). At 48 hours after infection, BM cells were injected into sublethally (3 Gy) irradiated Rag-2−/− mice.
Btk expression
A mammalian expression vector, pcDNA3.1(+) (Invitrogen) was modified by the introduction of the IRES-EGFP fragment, derived from pIRES-EGFP (Clontech), resulting in a vector named pcDNA3.1-IRESEGFP. A cDNA clone for human Btk25 was generously provided by Dr S. Tsukada (Osaka University). Three different cDNAs (WT, wild type; R28P, a mutant where arginine 28 was substituted with proline; and R28C, a mutant where arginine 28 was substituted with cysteine) tagged with a Myc-epitope (EQKLISEEDL) at their C-termini were generated and cloned into the pcDNA3.1-IRESEGFP vector. The expression vector for Btk was in vitro transcribed and translated using TNT T7 system (Promega, Madison, WI) in the presence of Transcend tRNA (Promega) to generate biotinylated Btk protein.
Cell-cycle analysis
Purified splenic B cells (5 × 106 cells/mL in 24-well plates) were stimulated with or without 20 μg/mL anti-IgM F(ab′)2. Lentivirus vectors were then infected by centrifugation (700g) for 2 hours in the presence of 5 μg/mL polybrene. At 48 hours after infection, the cells were incubated with 10 μg/mL Hoechst33342 (Calbiochem), followed by the evaluation of cell-cycle progression on a FACSAria according to the manufacturer's instruction.
Statistical analysis
Statistical analysis was performed using the Mann-Whitney U test with Microsoft Excel software (Redmond, WA), with a P value less than .05 considered significant.
Results
Reduced expression of Btk in Xid B cells
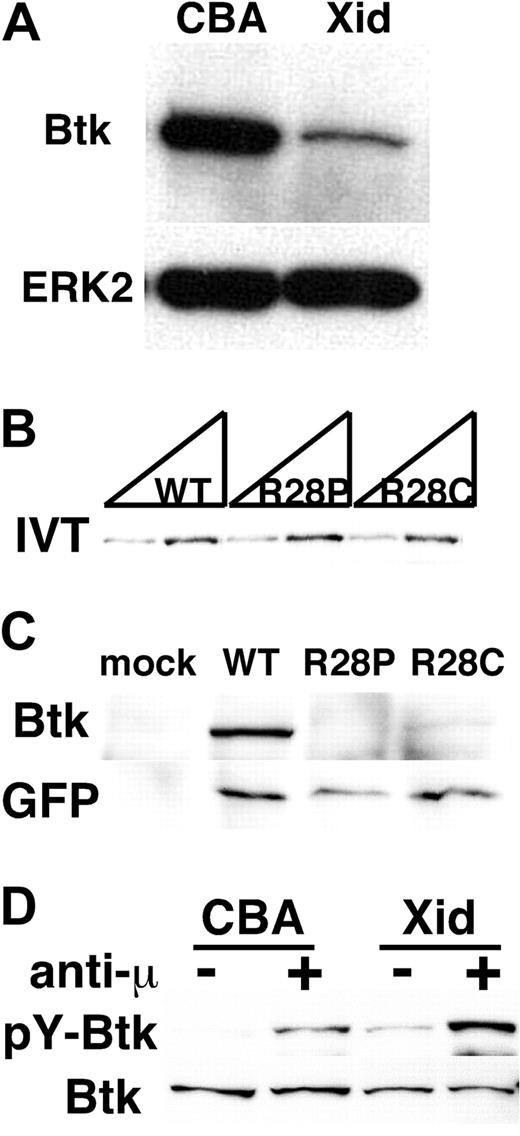
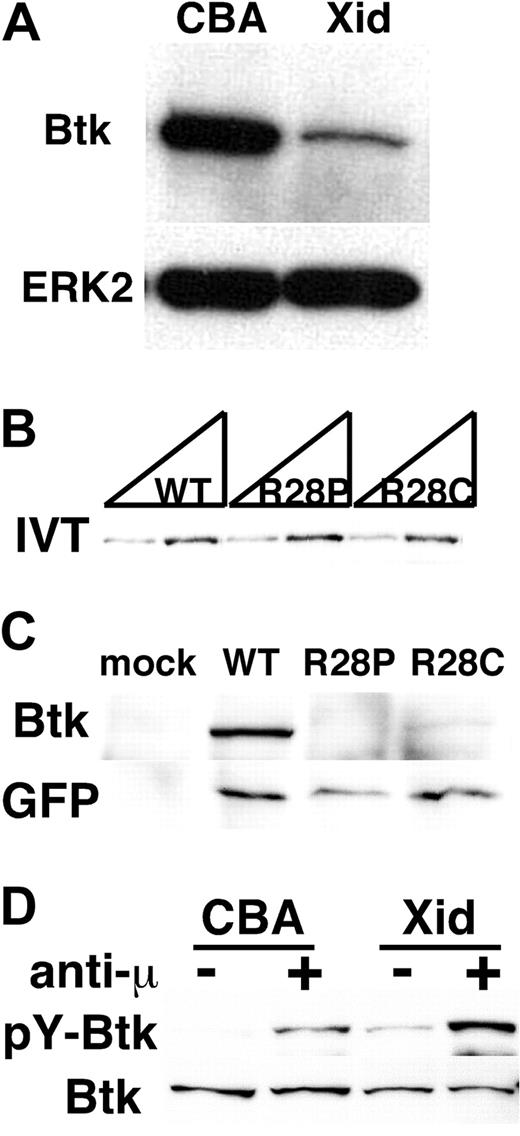
The presence of Xid mutation in both mice14 and humans25 has been considered strong evidence for the hypothesis that Btk is activated downstream of PI3K through interaction between the PH domain of Btk and PIP3, a PI3K product (reviewed in Satterthwaite et al21 ). However, our previous study demonstrated that the interaction between PIP3 and the PH domain of Btk is dispensable for the Btk function,19 raising a fundamental question: how does the Xid mutation in the PH domain perturb Btk function? Since a point mutation often renders the protein unstable within cells,26,27 we considered the possibility that the Xid mutation affects the expression of Btk. This was indeed the case with splenic B cells as B cells from Xid mice contained reduced amounts of Btk protein compared with control CBA mice (Figure 1A). We next examined the expression of human Btk proteins containing a point mutation found in XLA patients25 at the same arginine residue critical for PIP3 binding in the PH domain. Mutant and wild-type Btk proteins were expressed similarly when translated in an in vitro translation system (Figure 1B). However, mutant Btk constructs showed reduced expression of mutant proteins compared with wild-type Btk protein when transfected into Jurkat T lymphocytes (Figure 1C top panel). On the other hand, the amounts of EGFP protein translated from the same mRNA via the internal ribosomal entry site (IRES) were comparable (Figure 1C bottom panel), indicating that similar amounts of mRNA were transcribed from all 3 constructs. These results suggest that the mutations in the PH domain decrease the stability of Btk proteins within a cell. Despite its lower expression, the Xid mutant Btk was tyrosine-phosphorylated upon BCR-stimulation as wild-type Btk (Figure 1D). These results taken together suggest that the defect of BCR signaling in Xid mice is likely due to the reduced amounts of Btk protein but not a lack of Btk activation.
Xid mutation affects the stability of Btk protein. (A) Expression levels of Btk protein in Xid splenic B cells. The whole-cell lysates of splenic resting B lymphocytes obtained from control CBA/J (CBA) or Xid (Xid) mice were subjected to immunoblot analysis with an anti-Btk mAb (43-3B). The membrane was reblotted with anti-ERK2. Data are representative of 3 independent experiments with similar results. (B) The expression vector (0.5 μg and 1.0 μg per reaction, respectively) for either human wild-type Btk (WT) or XLA-derived mutants (R28P and R28C) was in vitro transcribed and translated in the presence of Transcend tRNA (Promega). The amounts of biotinylated Btk proteins were evaluated with streptavidin-HRP. (C) Jurkat T lymphocytes were transiently transfected with the expression vector for either human wild-type Btk (WT) or XLA-derived mutants (R28P and R28C), which contains EGFP downstream of an IRES. Whole-cell lysates were subjected to immunoblot with an anti-Btk antibody (top panel), followed by reblot with an anti-GFP antibody (bottom panel). (D) In vivo activation of Btk protein with Xid mutation. Splenic B cells obtained from 2 control CBA or 20 Xid mice were stimulated with or without 20 μg/mL anti-IgM F(ab′)2 (anti-μ) at 37°C for 3 minutes. Btk was then immunoprecipitated with an anti-Btk antibody, followed by immunoblot analysis with 4G10 (pY-Btk). The membrane was reblotted with 43-3B (Btk). Data in panels B through D are representative of 2 independent experiments with similar results.
Xid mutation affects the stability of Btk protein. (A) Expression levels of Btk protein in Xid splenic B cells. The whole-cell lysates of splenic resting B lymphocytes obtained from control CBA/J (CBA) or Xid (Xid) mice were subjected to immunoblot analysis with an anti-Btk mAb (43-3B). The membrane was reblotted with anti-ERK2. Data are representative of 3 independent experiments with similar results. (B) The expression vector (0.5 μg and 1.0 μg per reaction, respectively) for either human wild-type Btk (WT) or XLA-derived mutants (R28P and R28C) was in vitro transcribed and translated in the presence of Transcend tRNA (Promega). The amounts of biotinylated Btk proteins were evaluated with streptavidin-HRP. (C) Jurkat T lymphocytes were transiently transfected with the expression vector for either human wild-type Btk (WT) or XLA-derived mutants (R28P and R28C), which contains EGFP downstream of an IRES. Whole-cell lysates were subjected to immunoblot with an anti-Btk antibody (top panel), followed by reblot with an anti-GFP antibody (bottom panel). (D) In vivo activation of Btk protein with Xid mutation. Splenic B cells obtained from 2 control CBA or 20 Xid mice were stimulated with or without 20 μg/mL anti-IgM F(ab′)2 (anti-μ) at 37°C for 3 minutes. Btk was then immunoprecipitated with an anti-Btk antibody, followed by immunoblot analysis with 4G10 (pY-Btk). The membrane was reblotted with 43-3B (Btk). Data in panels B through D are representative of 2 independent experiments with similar results.
Differential defects in B-cell development between p85α−/− and Btk−/− mice
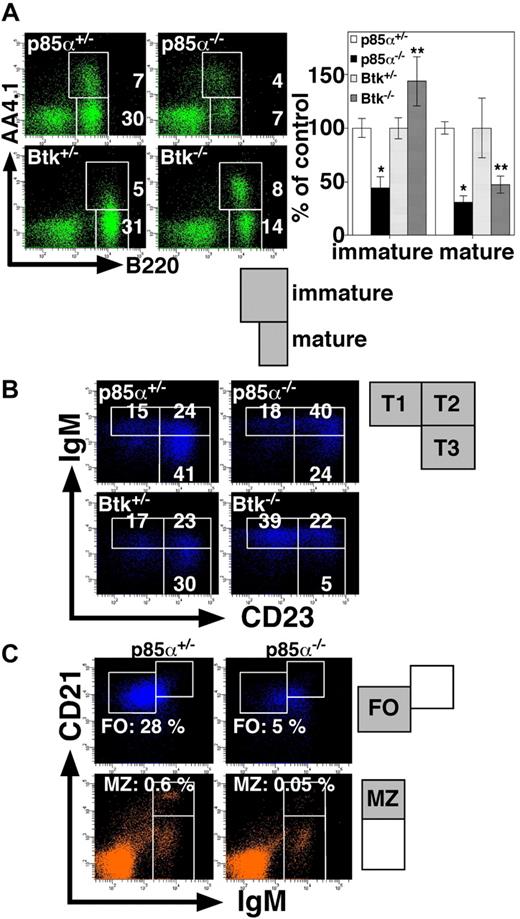
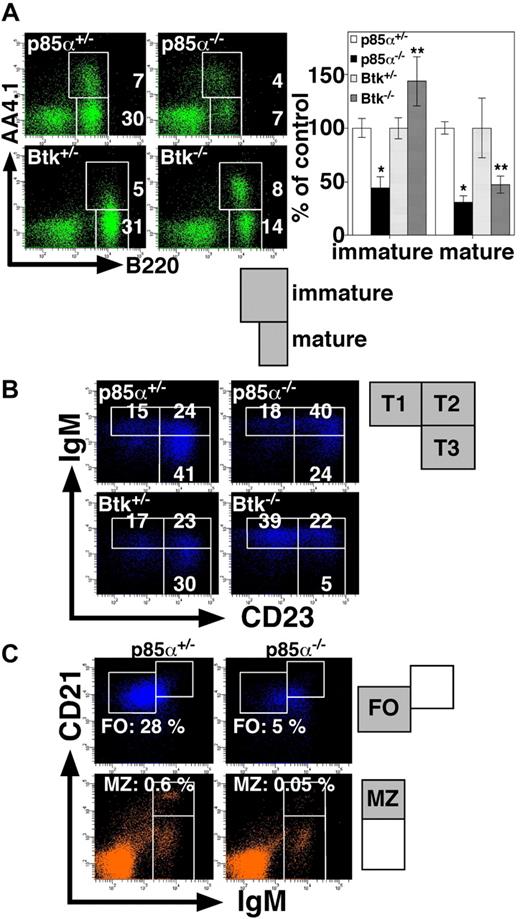
We next examined the phenotypes of p85α−/− and Btk−/− mice by comparing splenic B-cell subsets (Figure 2; Table 1). Note that p85α−/− and control mice were on a BALB/c background, whereas Btk−/− and control mice were on a (C57BL/6 × 129/Sv) mixed background. We stained splenocytes with antibodies to B220 and AA4.1, the latter of which is a complement C1q receptor and serves as an immature B-cell marker.28 Consistent with the previous reports demonstrating that PI3K deficiency leads to a decrease in the number of mature B cells in the periphery,11-13 the percentage of B220+AA4.1− cells corresponding to mature B cells was greatly decreased in p85α−/− mice (Figure 2A). In the p85α−/− spleen, the percentage of B220+AA4.1+ immature B cells was also decreased. In marked contrast, Btk−/− mice had an increased proportion of B220+AA4.1+ immature B cells compared with wild-type mice, whereas the percentage of B220+AA4.1− mature B cells was decreased (Figure 2A). Both p85α−/− and Btk−/− mice had significantly reduced number of splenic B cells compared with wild-type mice (Suzuki et al19 and data not shown). The present study further demonstrates that p85α−/− mice have reduced numbers of both mature and immature B cells, whereas Btk−/− mice have defects in only mature B cells. These results are consistent with the previous report showing that splenic B-cell development in mice with the Xid mutation is arrested at the immature stage.29
Phenotypes of p85α−/− and Btk−/− mice. (A) Splenocytes from the indicated mice were stained with anti-B220 and anti-AA4.1, and analyzed on a FACSAria. The percentages of immature B cells (B220+AA4.1+) and mature B cells (B220+AA4.1−) are indicated (left). Results are presented as mean plus or minus SD (percentages of heterozygotes) (right). Significant differences between p85α+/− and p85α−/− mice or Btk+/− and Btk−/− mice are indicated. *P < .01; **P < .05. (B) Analysis of B220+AA4.1+ immature B cells for surface expression of IgM and CD23: T1 represents IgM+CD23lo; T2, IgM+CD23+; and T3, IgMloCD23+. Among B220+AA4.1+ cells, the percentages of the T1, T2, and T3 subsets are indicated. (C) Splenocytes from the indicated mice were stained with anti-CD23, anti-CD21, and anti-IgM. Among CD23+ (top panels) and CD23− (bottom panels) lymphocyte populations, the percentages of CD23+CD21intIgMlo FO B cells identified as the cells in the square region (top panels) or CD23−CD21hiIgMhi MZ B cells identified as the cells in the square region (bottom panels) are indicated. Data are representative of 6 mice each for p85α+/− and p85α−/− mice on a BALB/c background or 4 mice each for Btk+/− and Btk−/− mice on a (C57BL/6 × 129/Sv) mixed background.
Phenotypes of p85α−/− and Btk−/− mice. (A) Splenocytes from the indicated mice were stained with anti-B220 and anti-AA4.1, and analyzed on a FACSAria. The percentages of immature B cells (B220+AA4.1+) and mature B cells (B220+AA4.1−) are indicated (left). Results are presented as mean plus or minus SD (percentages of heterozygotes) (right). Significant differences between p85α+/− and p85α−/− mice or Btk+/− and Btk−/− mice are indicated. *P < .01; **P < .05. (B) Analysis of B220+AA4.1+ immature B cells for surface expression of IgM and CD23: T1 represents IgM+CD23lo; T2, IgM+CD23+; and T3, IgMloCD23+. Among B220+AA4.1+ cells, the percentages of the T1, T2, and T3 subsets are indicated. (C) Splenocytes from the indicated mice were stained with anti-CD23, anti-CD21, and anti-IgM. Among CD23+ (top panels) and CD23− (bottom panels) lymphocyte populations, the percentages of CD23+CD21intIgMlo FO B cells identified as the cells in the square region (top panels) or CD23−CD21hiIgMhi MZ B cells identified as the cells in the square region (bottom panels) are indicated. Data are representative of 6 mice each for p85α+/− and p85α−/− mice on a BALB/c background or 4 mice each for Btk+/− and Btk−/− mice on a (C57BL/6 × 129/Sv) mixed background.
B220+AA4.1+ immature B cells can be subdivided into 3 transitional subsets, T1 (IgM+CD23lo), T2 (IgM+CD23+), and T3 (IgMloCD23+), according to the surface expression of IgM and CD23.28 p85α−/− mice had a reduction in the size of all 3 populations (Table 1). In addition, the proportion of T2 B cells in the p85α−/− immature B-cell compartment was increased, whereas the proportion of T3 B cells was decreased (Figure 2B). On the other hand, the proportion of T1 B cells, but not that of T2 B cells, was increased in the splenic immature B-cell compartment of Btk−/− mice, whereas the proportion of T3 B cells was markedly decreased (Figure 2B). The reduction in cell size of T3 population in Btk−/− mice was significant (Table 1). Considering the possibility that some of the phenotypic differences we observed in p85α−/− mice might be strain specific, we examined B-cell development in the spleen of p85α−/− mice on a C57BL/6 background and obtained essentially the same results (Figure S1 [available on the Blood website; see the Supplemental Materials link at the top of the online article]; Figure 3). We thus conclude that the defects in the development of splenic B-cell subsets are different between p85α−/− and Btk−/− mice.
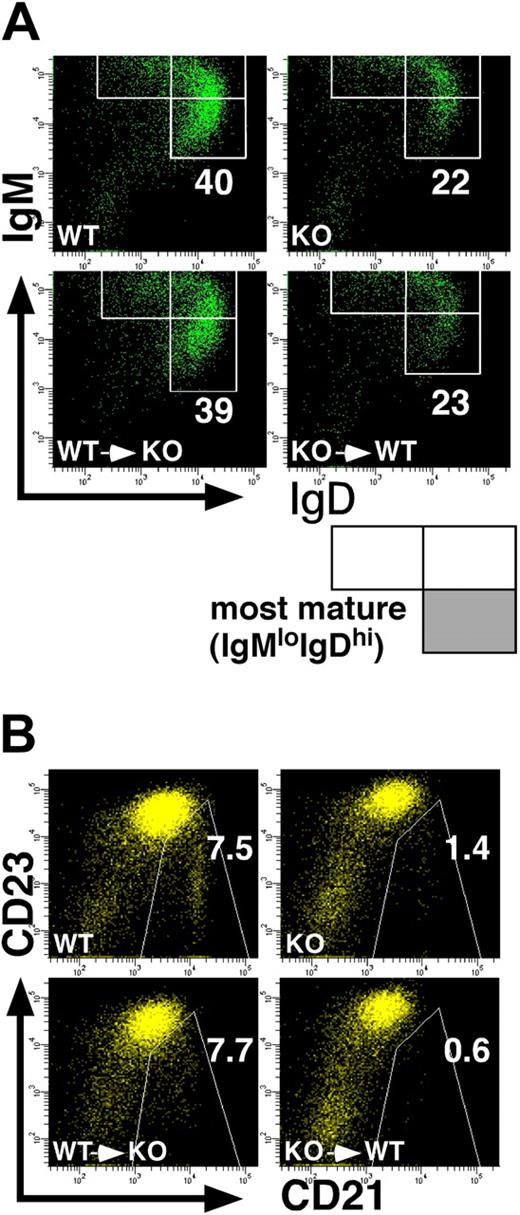
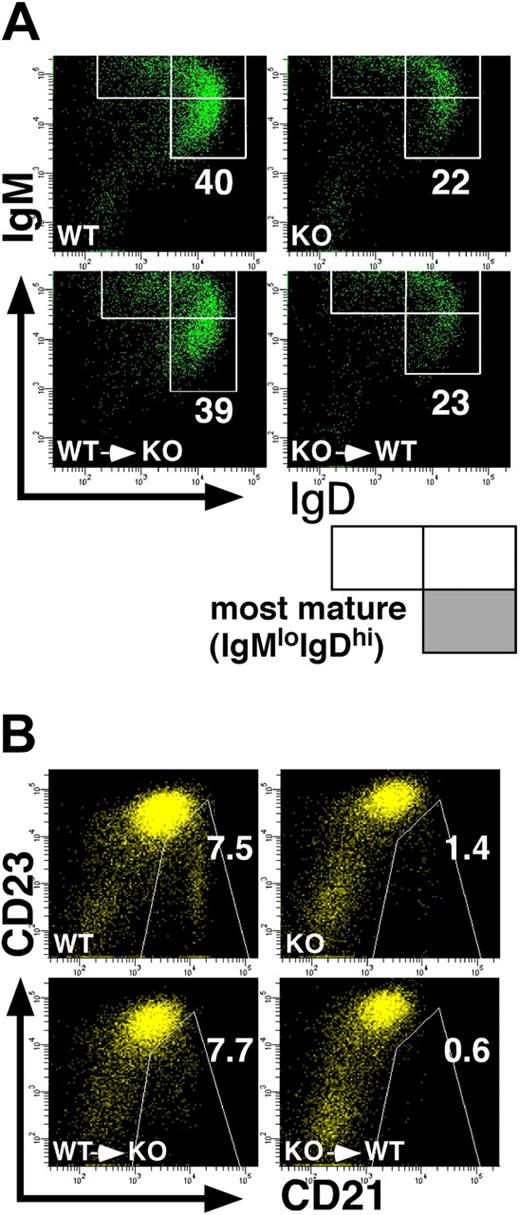
Impaired development of splenic B cells in p85α−/− mice is B-cell intrinsic. BM cells from the wild-type (p85α+/+CD45.1+) and p85α-deficient (p85α−/−CD45.2+) mice on a C57BL/6 background were transplanted into sublethally (3 Gy) irradiated PI3K-deficient (CD45.2+) and wild-type (CD45.1+) mice, respectively (bottom panels). Eight weeks after transplantation, splenocytes from recipient mice were stained with a combination of antibodies against B220, IgM, IgD, and CD45.2 (A) or B220, CD21, CD23, and CD45.2 (B), and analyzed on a FACSAria. Wild-type (WT) and p85α−/− (KO) mice were also analyzed (top panels). Among B220+CD45.2+ cells (for p85α−/− mouse–derived cells) or B220+CD45.2− cells (for the wild-type mouse-derived cells), the percentages of the most mature IgMloIgDhi subset (A) and CD21hiCD23lo MZ B cells (B) are indicated. Data are representative of 3 mice in each type of transplantation.
Impaired development of splenic B cells in p85α−/− mice is B-cell intrinsic. BM cells from the wild-type (p85α+/+CD45.1+) and p85α-deficient (p85α−/−CD45.2+) mice on a C57BL/6 background were transplanted into sublethally (3 Gy) irradiated PI3K-deficient (CD45.2+) and wild-type (CD45.1+) mice, respectively (bottom panels). Eight weeks after transplantation, splenocytes from recipient mice were stained with a combination of antibodies against B220, IgM, IgD, and CD45.2 (A) or B220, CD21, CD23, and CD45.2 (B), and analyzed on a FACSAria. Wild-type (WT) and p85α−/− (KO) mice were also analyzed (top panels). Among B220+CD45.2+ cells (for p85α−/− mouse–derived cells) or B220+CD45.2− cells (for the wild-type mouse-derived cells), the percentages of the most mature IgMloIgDhi subset (A) and CD21hiCD23lo MZ B cells (B) are indicated. Data are representative of 3 mice in each type of transplantation.
To further analyze mature B-cell populations in p85α−/− mice, splenocytes were stained with antibodies to IgM, CD21, and CD23, and then separated into CD23+ (Figure 2C top panels) and CD23− (Figure 2C bottom panels) populations. Among the CD23+ cells, p85α−/− mice had a markedly decreased proportion of FO B cells (CD23+CD21intIgMlo) compared with wild-type mice (Figure 2C top panels). In the CD23− cell subset, p85α−/− mice had a reduced percentage of MZ B cells (CD23−CD21hiIgMhi) compared with wild-type mice (Figure 2C bottom panels). The reduction of FO and MZ B cells in p85α−/− mice was significant (Table 1). These results strongly indicate that the development of the most mature FO and MZ B cells is severely impaired in p85α−/− mice. In contrast to the defects in B-cell development, p85α−/− mice had no apparent defect in T-cell development in either CD8/CD4 expression profile in the thymus13 or CD44/CD62L expression profile in splenic CD4+ T cells (Figure S2), the latter of which reflects the spontaneous activation of T cells and the generation of memory-type cells during aging, indicating that p85α deficiency impairs mostly B-cell development without apparent defects in T-cell development.
B cell–intrinsic developmental defect in p85α−/− mice
Immature B cells that enter the spleen express high levels of IgM and very little IgD (IgMhiIgDlo), and undergo maturational progression through an IgMhiIgDhi stage to the most mature IgMloIgDhi stage that is reportedly equivalent to the FO B-cell subset.30 In accordance with the data shown in Figure 2C, the percentage of the most mature IgMloIgDhi cells was severely decreased in p85α−/− mice on a C57BL/6 background (Figure 3A top panels). In addition, expression profile of CD21 and CD23 in the B220+ cell population, another criteria for MZ B cells, revealed that p85α−/− mice had a reduced percentage of MZ B cells compared with wild-type mice (Figure 3B top panels). It is of interest to note that the amount of CD23 was higher in p85α−/− B cells compared with wild-type B cells (Figure 3B), although the molecular mechanism for this increase remains to be determined.
Since PI3K is involved in various signal transduction pathways,11,12 PI3K deficiency may affect a variety of cells including stroma cells that can influence B-cell development. To determine whether the defect of B-cell development in p85α−/− mice is due to an intrinsic abnormality of the B cells, an abnormality of the splenic microenvironment, or a combination, we transplanted BM cells from wild-type (CD45.1+ on a C57BL/6 background) mice into sublethally irradiated p85α−/− (CD45.2+ on a C57BL/6 background) mice and vice versa. We analyzed B-cell development 8 weeks after transplantation. p85α−/− recipients that received wild-type CD45.1+ BM cells showed normal B-cell development, whereas wild-type CD45.1+ recipients that received p85α−/− BM cells had reduced percentages of MZ B cells and mature IgMloIgDhi B cells as observed with PI3K−/− mice (Figure 3A,B bottom panels). These data demonstrate that the splenic microenvironment in p85α−/− mice is able to support the development of p85α-sufficient B cells, and that the defects in B-cell development observed in p85α−/− mice are B-cell autonomous.
Requirement for PI3K in the expression of NF-κB proteins
Previous studies have shown that BCR-induced NF-κB activation is impaired in both p85α−/− and Btk−/− B cells.17-19 Since the differences in phenotypes between p85α−/− and Btk−/− mice as well as our previous study19 show that PI3K and Btk function independently, PI3K and Btk likely have distinct roles in the NF-κB activation. Btk is essential for PLCγ2 activation and sustained elevation in intracellular Ca2+ following BCR engagement.15 PLCγ2 activation then triggers PKCβ activation,16 followed by the activation of signaling cascade consisting of CARMA1,31,32 Bcl10,33 and paracaspase (also termed MALT1)34 leading to the degradation of IκBs and the release of active NF-κB. Indeed, IκB degradation is impaired in Btk−/− B cells.17,18 Since the molecular mechanism(s) underlying the impairment of BCR-induced NF-κB activation in p85α−/− B cells remains obscure, we examined whether PI3K shares this signaling pathway with Btk. Consistent with the previous report,35 BCR-induced Ca2+ mobilization in p85α−/− B cells was nearly intact, albeit the peak intensity was slightly decreased (data not shown). ERK phosphorylation upon BCR stimulation, which is regulated downstream of PLCγ2 through the activation of the RasGRP3/Ras pathway,36 was also induced in p85α−/− B cells to a level comparable to wild-type B cells (Figure 4A). In addition, PI3K deficiency had little effect on the BCR-induced tyrosine phosphorylation of PLCγ2 and PKCβ-mediated Btk phosphorylation at Ser18037 (Figure S3). Since PKCβ is another downstream effector of PLCγ2, these results taken together indicate that PLCγ2 activation in response to BCR stimulation is unaffected in p85α−/− B cells. Considering that PKCβ plays a critical role in BCR-induced IκBα degradation,16 we next examined whether PI3K deficiency affects this process. As shown in Figure 4B, BCR stimulation triggered the degradation of IκBα in p85α−/− B cells in a time course similar to wild-type B cells.
PI3K activity is essential for maintenance of c-Rel expression. (A) PI3K-independent activation of ERK. Splenic B cells obtained from p85α−/− (p85α−/−) or control (p85α+/−) mice on a BALB/c background were stimulated with or without 20 μg/mL anti-IgM F(ab′)2 (anti-μ) at 37°C for the indicated times. BCR-induced activation was evaluated by immunoblotting with a specific antibody against phospho-ERK (p-ERK). The membrane was reblotted with an anti-ERK2 antibody (ERK2). (B) PI3K-independent degradation of IκBα. Splenic B cells were stimulated as in panel A. The degradation of IκBα was evaluated by immunoblotting with an anti-IκBα antibody. The membrane was reblotted with an anti-ERK2 antibody. IκBα levels were normalized by ERK2 levels and indicated as percentage relative to that of the unstimulated lysate (ratio). (C) The whole-cell lysates of splenic B cells obtained from p85α−/− (−/−) or control (+/−) mice on a BALB/c background were subjected to immunoblot analysis with antibodies against c-Rel, RelA, and ERK2. (D) PI3K-dependent maintenance of c-Rel expression. Splenic B cells of C57BL/6 mice were cultured at 5 × 106 cells/mL in the presence or absence of either 100 nM wortmannin (WN) or 10 μg/mL rapamycin (Rap) for the indicated times, and evaluated for the expression level of c-Rel by immunoblotting with an anti–c-Rel antibody. Rapamycin, a potent inhibitor for mTOR, was chosen as a control since wortmannin is known to suppress mTOR activity besides PI3K. The membrane was reblotted with an anti-ERK2 antibody. Data in panels A-D are representative of 3 independent experiments with similar results.
PI3K activity is essential for maintenance of c-Rel expression. (A) PI3K-independent activation of ERK. Splenic B cells obtained from p85α−/− (p85α−/−) or control (p85α+/−) mice on a BALB/c background were stimulated with or without 20 μg/mL anti-IgM F(ab′)2 (anti-μ) at 37°C for the indicated times. BCR-induced activation was evaluated by immunoblotting with a specific antibody against phospho-ERK (p-ERK). The membrane was reblotted with an anti-ERK2 antibody (ERK2). (B) PI3K-independent degradation of IκBα. Splenic B cells were stimulated as in panel A. The degradation of IκBα was evaluated by immunoblotting with an anti-IκBα antibody. The membrane was reblotted with an anti-ERK2 antibody. IκBα levels were normalized by ERK2 levels and indicated as percentage relative to that of the unstimulated lysate (ratio). (C) The whole-cell lysates of splenic B cells obtained from p85α−/− (−/−) or control (+/−) mice on a BALB/c background were subjected to immunoblot analysis with antibodies against c-Rel, RelA, and ERK2. (D) PI3K-dependent maintenance of c-Rel expression. Splenic B cells of C57BL/6 mice were cultured at 5 × 106 cells/mL in the presence or absence of either 100 nM wortmannin (WN) or 10 μg/mL rapamycin (Rap) for the indicated times, and evaluated for the expression level of c-Rel by immunoblotting with an anti–c-Rel antibody. Rapamycin, a potent inhibitor for mTOR, was chosen as a control since wortmannin is known to suppress mTOR activity besides PI3K. The membrane was reblotted with an anti-ERK2 antibody. Data in panels A-D are representative of 3 independent experiments with similar results.
The fact that PI3K deficiency has little effect on the signaling pathway leading to IκBα degradation, which is impaired in Btk−/− B cells,17,18 raises the possibility that PI3K regulates BCR-induced NF-κB activation in a different way from Btk. Immunoblot analysis indeed revealed that c-Rel expression was markedly decreased in p85α−/− B cells compared with wild-type B cells (Figure 4C top panel). In addition, a slight reduction of RelA expression was observed in PI3K−/− B cells as well (Figure 4C middle panel). To exclude the possibility that the decrease of c-Rel reflects the developmental defects of p85α−/− B cells, we further compared c-Rel expression levels between p85α+/− and p85α−/− mature B cells (B220+AA4.1−) and found that c-Rel protein was severely reduced in PI3K-deficient mature B cells (Figure S4A). On the other hand, Btk deficiency had little effect on the expression of these NF-κB components (Figure S4B). As shown in Figure 4D, treatment of wild-type B cells with wortmannin, a potent inhibitor of PI3K, also results in the reduction of c-Rel expression, whereas the amounts of ERK2 were unaffected. Although wortmannin also inhibits mTOR downstream of PI3K, rapamycin, an inhibitor for mTOR, showed no effect on the expression of c-Rel (Figure 4D). These results indicate that the PI3K activity is essential for the maintenance of c-Rel expression in splenic B cells.
Restoration of B-cell defects in p85α−/− mice by c-Rel expression
Considering that the decreased amounts of c-Rel may underlie the defects in B-cell development and the blockade of cell cycle progression upon BCR stimulation in p85α−/− B cells, we examined whether the forced expression of c-Rel could overcome the defects due to the loss of PI3K. We used a lentivirus-mediated approach, in which we injected sublethally irradiated Rag-2−/− mice with p85α−/− BM cells transfected with c-Rel or p85α as a positive control. We cloned genes of interest into a lentivirus vector24 that contains Venus, a derivative of EGFP downstream of IRES, thus ensuring that Venus-positive cells also expressed lentivirally produced c-Rel or p85α. We analyzed splenocytes of reconstituted mice 10 weeks after transplantation. As shown in Figure 5A, p85α−/− cells reconstituted with p85α had MZ B cells (B220+CD21hiCD23lo) to the level comparable with mock-infected wild-type cells, whereas control vector failed to restore the phenotype of p85α−/− cells, demonstrating the feasibility of this system. The introduction of c-Rel into p85α−/− BM cells also resulted in the restoration of MZ B cells to normal percentages, indicating that the developmental defect of p85α−/− MZ B cells is due to the reduced amount of c-Rel expression. On the other hand, c-Rel expression failed to fully restore p85α−/− B-cell maturation to IgMloIgDhi cells (Figure 5B). It is likely that the overexpression of c-Rel alone is insufficient to fully compensate the lack of PI3K signaling pathway during B-cell development.
Forced expression of c-Rel partially rescues B-cell defects in p85α−/− mice. Recipient Rag-2−/− mice on a BALB/c background were reconstituted with BM cells from p85α−/− (−/−) or control (+/−) mice on a BALB/c background expressing either c-Rel (c-Rel), p85α (p85), or Venus alone (mock). Spleen cells were analyzed 10 weeks after transplantation. Among Venus+B220+ cells, the percentages of CD21hiCD23lo MZ B cells are shown (A). Among Venus+ cells, the percentages of IgMloIgDhi most mature B cells identified as the cells in the lower right quadrant are indicated (B). (C) Splenic B cells from p85α−/− (p85α−/−) or control (p85α+/−) mice on a BALB/c background were unstimulated or stimulated with anti-IgM F(ab′)2 (+ αIgM) and infected with lentivirus vector encoding c-Rel (c-Rel) or Venus alone (mock). Among Venus+ cells, BCR-induced cell-cycle progression was evaluated on a FACSAria using Hoechst 33342 as an indicator for DNA content. Shown are percentages of cells in S + G2/M. Mice are all on a BALB/c background. Data are representative of 3 (A,B) or 5 (C) independent experiments with similar results.
Forced expression of c-Rel partially rescues B-cell defects in p85α−/− mice. Recipient Rag-2−/− mice on a BALB/c background were reconstituted with BM cells from p85α−/− (−/−) or control (+/−) mice on a BALB/c background expressing either c-Rel (c-Rel), p85α (p85), or Venus alone (mock). Spleen cells were analyzed 10 weeks after transplantation. Among Venus+B220+ cells, the percentages of CD21hiCD23lo MZ B cells are shown (A). Among Venus+ cells, the percentages of IgMloIgDhi most mature B cells identified as the cells in the lower right quadrant are indicated (B). (C) Splenic B cells from p85α−/− (p85α−/−) or control (p85α+/−) mice on a BALB/c background were unstimulated or stimulated with anti-IgM F(ab′)2 (+ αIgM) and infected with lentivirus vector encoding c-Rel (c-Rel) or Venus alone (mock). Among Venus+ cells, BCR-induced cell-cycle progression was evaluated on a FACSAria using Hoechst 33342 as an indicator for DNA content. Shown are percentages of cells in S + G2/M. Mice are all on a BALB/c background. Data are representative of 3 (A,B) or 5 (C) independent experiments with similar results.
Since c-Rel is involved in cell-cycle progression,38 it is possible that the defect in proliferation upon BCR stimulation in p85α−/− B cells is also attributed to the reduced amount of c-Rel expression. We thus investigated whether the forced expression of c-Rel can overcome the proliferative defect in p85α−/− B cells. It was technically difficult to sort out lentivirally transfected B cells with high purity on the basis of Venus expression because of the limited transfection efficiency into primary B cells. Therefore, we estimated the BCR-induced cell-cycle progression through flow cytometric analysis using Hoechst 33342. As indicated by the percentage of cells in S and G2/M phases of the cell cycle, mock virus–infected p85α−/− B cells failed to proliferate in response to BCR stimulation compared with the mock-infected wild-type B cells (Figure 5C). In contrast, the percentage of p85α−/− B cells undergoing cell cycling was markedly increased by the forced expression of c-Rel, although it was still lower than that of wild-type cells. We noted that uninfected cells (Venus− cells) failed to enter the S and G2/M phases (data not shown). It is well established that the BCR stimulation of mature B cells leads to activation and proliferation, whereas the same signals on developing B cells result in an arrest in an unresponsive state or cell death.39,40 Therefore, the partial blockade of BCR-induced proliferation in p85α−/− B cells with lentivirally transfected c-Rel may reflect the reduced percentage of the most mature IgMloIgDhi population (Figure 5B).
Discussion
Present observations that NF-κB activation is differentially regulated by PI3K and Btk and that p85α−/− mice and Btk−/− mice show different developmental defects support the conclusion of our previous study that PI3K and Btk independently function in BCR signal transduction.19 The presence of Xid mutation has long been considered strong evidence for the hypothesis that Btk functions downstream of PI3K.14,25 On the other hand, as shown here, both mouse and human Btk proteins with Xid mutation are unstable and degraded. As a result, BCR cross-linking is unable to transmit sufficient signals in Xid B cells. It is thus likely that the interaction of PIP3 with the PH domain of Btk is dispensable for the BCR-induced activation of Btk, although PH domain does interact with PIP3 as shown previously by fusion proteins between GFP and the PH domain of Btk.41
We have demonstrated here that p85α−/− mice exhibit impaired development of 2 major populations of mature splenic B cells: FO and MZ B cells in a cell-autonomous manner. We also found that PI3K deficiency leads to significant decreases in immature B-cell subsets in agreement with the observation by Donahue et al.35 On the other hand, as previously reported, Btk-deficient mice had increased percentages of immature B-cell populations.30 Consistent with these differences between p85α−/− and Btk−/− mice, PI3K deficiency affects B-cell activation in a way distinct from Btk deficiency: the former perturbs the expression of NF-κB components such as c-Rel, whereas the latter causes the impairment of BCR-induced IKK activation. Since the inhibition of PI3K by wortmannin also resulted in the loss of c-Rel expression in wild-type B cells, the PI3K-Akt signaling pathway at its basal activity seems essential for the maintenance of c-Rel expression. It is possible that the BCR in p85α−/− B cells fails to transmit a basal signal without apparent ligand binding, so-called tonic signaling, which is required for maintaining NF-κB expression.42
Our previous observation that p85α−/− B cells fail to induce the target genes of the NF-κB pathway, such as cyclin D2 and Bcl-xL, upon BCR stimulation19,43 can be explained by the loss of c-Rel expression in p85α−/− B cells. However, the molecular mechanism underlying the reduction of c-Rel and/or RelA expression is yet to be determined. It has been shown that a lack of BCAP, an adaptor protein functioning downstream of the BCR, leads to a reduction of c-Rel expression at the transcriptional level,44 prompting us to examine c-Rel gene expression in p85α−/− B cells. Our preliminary experiments, however, suggest that the reduced amounts of c-Rel in p85α−/− B cells are likely due to perturbations in posttranscriptional regulation (data not shown). Consistent with this, there are several differences between p85α−/− B cells and BCAP−/− B cells. First, BCAP-deficient mice show a defect in B-cell development of the most mature IgMloIgDhi subset but not of MZ B cells. Second, the forced expression of c-Rel in BCAP−/− BM cells corrects the developmental defect nearly completely, unlike in p85α−/− BM cells. BCR-induced Ca2+ flux is impaired in BCAP−/− cells, whereas p85α−/− B cells show only slight decrease in Ca2+ flux. Finally, BCAP−/− B cells exhibit BCR-induced PI3K activation as strong as wild-type B cells.
Reduced numbers of FO and MZ B cells in p85α−/− mice is likely due to the impairment of the NF-κB pathway. Accordingly, forced expression of c-Rel significantly, but not completely, restored the defect in B-cell development as well as the proliferative defect upon BCR stimulation in p85α−/− mice. One can argue that defective c-Rel protein stability in p85α−/− B cells may attenuate the effect of lentiviral c-Rel overexpression. Since PI3K deficiency also results in the reduction of RelA expression, incomplete restoration by c-Rel overexpression may also reflect the remaining defect caused by the reduced amounts of RelA. Alternatively, developmental defects in p85α−/− B cells could be ascribed, at least in part, to signaling defects other than the NF-κB pathway. For instance, the PI3K-Akt pathway is involved in the regulation of forkhead family transcription factors such as FOXO145 as well as cell motility in response to chemokines,46 which play a critical role during B-cell development.
Class IA PI3K regulatory subunits function as a molecular chaperone to bind and stabilize their cognate catalytic subunits.11,12 We have previously shown that the expression of p110δ, the most abundantly expressed catalytic subunit in lymphocytes, is nearly completely lost in p85α−/− B cells.19 It has been shown, however, that a lack of p110δ activity results in reduced numbers of MZ B cells, but not of FO B cells.12 Moreover, p110δ deficiency has, if any, only a marginal effect on LPS-induced B-cell proliferation, whereas p85α−/− B cells fail to respond to LPS.11-13 It seems reasonable to assume that the lack of p85α affects the expression level of other catalytic subunits such as p110α, which could be more important for cell proliferation than p110δ. Consistently, in our preliminary experiment, the expression level of p110α in PI3K-deficient B cells was estimated as approximately 50% of that in wild-type B cells using an anti-p110α mAb (kindly provided by Dr L. T. Williams; data not shown). Interestingly, it has been reported by microinjecting neutralizing antibodies specific for p110α, p110β, and p110δ that in macrophages p110α, but not p110β or p110δ, is critical for cell proliferation and survival, whereas p110β and p110δ are important for cell migration.47 The contribution of p110α to B-cell function, especially in LPS-induced proliferation, will be clarified in a future study using a conditional knockout strategy.
Given that the NF-κB signaling pathway plays a critical role in B-cell development, survival, and activation, a comprehensive understanding of the PI3K signaling essential for maintenance of c-Rel expression in B cells will provide the molecular basis for explaining immunologic disorders such as XLA-like common-variable immunodeficiency and B-cell lymphomas.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs N. Watanabe and M. Handa for help in some experiments; Drs T. Miyawaki and H. Kanegane for discussion on XLA patients; Dr D. Rawlings for a specific antibody against Btk phosphorylated at Ser180 (pS180) and valuable discussion; Drs H. Miyoshi and A. Miyawaki for vector constructs; M. Motouchi and N. Yumoto for animal care; and Dr L. K. Clayton for critical reading of the paper and valuable suggestions.
This work was supported by a grant from the Mitsubishi Foundation (Tokyo, Japan; S.K.), a grant from the Kato Memorial Bioscience Foundation (Tokyo, Japan; S.M.), a grant from Nagao Memorial Fund (Tokyo, Japan; S.M.), a Keio University Special Grant-in-Aid for Innovative Collaborative Research Project (Tokyo, Japan), a Grant-in-Aid for Scientific Research for Young Scientist (16790293 [S.M.]) and a Grant-in-Aid for Scientific Research (B) (16390146 [S.K.]) from the Japan Society for the Promotion of Science (Tokyo, Japan), a Grant-in-Aid for Scientific Research on Priority Areas (16043248 [S.M.]), a National Grant-in-Aid for the Establishment of a High-Tech Research Center in a private university, and a Scientific Frontier Research Grant from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (Tokyo, Japan).
Authorship
Contribution: S.M. designed and performed research and wrote the paper; Y.M., M.O., M.F., Y.H., and A.M. performed research; Y.T. and T.K. contributed vital new reagents or analytical tools; and S.K. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shigeo Koyasu, Department of Microbiology and Immunology, Keio University School of Medicine, 35 Shinanomachi, Shinjuku-ku, Tokyo 160-8582, Japan; e-mail: koyasu@sc.itc.keio.ac.jp.