Abstract
Drug-induced immune thrombocytopenia (DITP) is caused by drug-dependent antibodies (DDAbs) that are nonreactive in themselves but bind tightly to specific platelet membrane glycoproteins (GP) when soluble drug is present at pharmacologic concentrations. This reaction takes place without covalent linkage of drug to the target, indicating that drug does not function as a classical hapten to promote antibody binding. Studies to define other mechanism(s) responsible for this interaction have been frustrated by the polyclonal nature of human DDAbs and limited quantities of antibody usually available. We produced 2 monoclonal antibodies (mAbs), 314.1 and 314.3, from a mouse immunized with purified human GPIIb/IIIa and quinine that recognize the N terminus of the GPIIb β propeller domain only when soluble quinine is present. Both monoclonals closely mimic the behavior of antibodies from patients with quinine-induced immune thrombo-cytopenia in their reactions at various concentrations of quinine and quinine congeners. Sequencing studies showed that the 2 mAbs are closely related structurally and that mAb 314.3 probably evolved from mAb 314.1 in the course of the immune response. These monoclonal reagents are the first of their kind and should facilitate studies to define the molecular basis for drug-dependent antibody binding and platelet destruction in DITP.
Introduction
Drug-induced immune thrombocytopenia (DITP) is a recognized side effect of treatment with numerous medications.1-3 In many cases of DITP, platelet destruction is caused by a remarkable type of immunoglobulin (Ig; drug-dependent antibody [DDAb]) that is nonreactive in the absence of drug, but binds avidly to a specific epitope(s) on a platelet membrane glycoprotein (GP; usually GPIIb/IIIa and/or GPIb/V/IX) when soluble drug is present at pharmacologic concentrations.3-6 Because there is no requirement for covalent linkage of the drug to the target GP for this reaction to take place,3-5 the drug does not act as a classical hapten7-9 to facilitate antibody binding. Various hypotheses have been offered,3-5,10 but the mechanism(s) responsible for induction of DDAbs and for their drug-dependent reactions with specific targets are not yet understood. Murine monoclonal antibodies (mAbs) that mimic the behavior of human DDAbs could provide valuable tools with which to study molecular mechanisms of DDAb binding and, possibly, the immune response leading to their production. Here, we describe the properties of 2 unique murine mAbs closely resembling the DDAbs that cause platelet destruction in patients sensitive to quinine, a common cause of DITP
Methods
Reagents
Unless otherwise stated, reagents were purchased from Sigma-Aldrich (St Louis, MO). Protein G sepharose was from GE Healthcare (Piscataway, NJ); fetal bovine serum (FBS) from Hyclone (Logan, UT); F12K media, phosphate-buffered saline (PBS), G418, and gentamycin from Mediatech (Herndon, VA); zeocin, Trizol, and Superscript II from Invitrogen (Carlsbad, CA); Fugene 6 from Roche (Indianapolis, IN); biotin sulfo-NHS from Pierce Biotechnology (Rockford, IL); and fluorescein isothiocyanate (FITC) goat (Fab′)2 anti–mouse IgG (H + L), FITC goat (Fab′)2 anti–human IgG (H + L), and phycoerythrin (PE)–labeled streptavidin from Jackson ImmunoResearch Laboratories (West Grove, PA). Human GPIIb/IIIa was purified from platelets by the method of Fitzgerald et al11 or purchased from Enzyme Research (South Bend, IN). mAbs AP3 (anti-GPIIIa), 312.6 and 312.8 (anti-GPIIb), AP2 (anti-GPIIb/IIIa), and AP1 and 142.11 (anti-GPIbα) were from the monoclonal laboratory of the Blood Research Institute (Milwaukee, WI). Monoclonal 10E5 (anti-GPIIb) was a gift from Dr Barry Coller, Rockefeller University (New York, NY).
Immunization of mice and preparation of hybridomas
Mice were immunized, and stable hybridoma lines were produced by standard methods.12,13 Platelet-derived human GPIIb/IIIa was covalently linked to quinine though free amino groups as previously described.10,14 BALB/c mice were immunized biweekly for 6 weeks with a mixture of 7.5 μg quinine-linked GPIIb/IIIa, 7.5 μg unlinked GPIIb/IIIa, and 90 μg soluble quinine injected intraperitoneally in RIBI adjuvant (Sigma-Aldrich). An intravenous injection of the same material without adjuvant was given at 8 weeks. Mice were humanely killed 3 days later. Splenocytes were isolated, fused with NP-3 cells in a 1:5 ratio, and cultured in hypoxanthine aminopterin thymidine (HAT) medium.12 Fused cells were transferred to multiple 96-well plates, and supernatants were screened after 10 days in culture.
Identification of candidate clones
Culture supernatants (0.025 mL) were added to 5 × 106 washed human platelets isolated from citrated blood and 0.4 mM quinine in a total volume of 50 μL. After 60 minutes, the platelets were washed twice in PBS, containing 0.4 mM quinine, and platelet-bound mouse IgG was detected by flow cytometry10 using FITC-labeled goat F(ab′)2 specific for mouse Ig (H + L chain). Supernatants giving positive reactions were rescreened against human platelets in the presence and absence of quinine, and those that reacted more strongly in the presence than in the absence of drug were selected for subculturing and further study.
Expression of GPIIb/IIIa constructs in Chinese hamster ovary cells
Stably transfected Chinese hamster ovary (CHO) cell lines expressing mixed GPIIb/IIIa integrins of rat and human origin were as previously described15 or produced in our laboratory using standard methods.15,16 All constructs were validated by sequencing of cDNA incorporated into the expression vectors. CHO cells were cotransfected with chimeric GPIIb in the expression vector pcDNA3 with neomycin selection (Invitrogen) and nonmutated human GPIIIa in the expression vector pcDNA3.1 with zeocin selection with Fugene 6, using the manufacturer's suggested protocol. Stably transfected cell lines were selected with 0.2 mg/mL zeocin and 0.6 mg/mL neomycin in F12K media. Cells expressing GPIIb/IIIa were sorted with the GPIIb/IIIa-specific mAb AP2 (Blood Research Instititute) on a FACSAria cell sorter (Becton Dickinson, Franklin Lakes, NJ).
Sequencing and analysis of mouse Ig genes
Mouse Ig genes expressed in selected hybridomas were sequenced as described by Wang et al.17 Hybridoma cells from a confluent t-75 flask were lifted and washed with PBS. The cells were lysed with Trizol according to the manufacturer's instructions. Isolated RNA was reverse-transcribed with Superscript II and poly T (Invitrogen). The resulting cDNA was used as the template for the polymerase chain reaction (PCR), and resulting PCR products were cloned and sequenced using standard methods.18 Mouse Ig sequences were analyzed and compared using VBASE219 and the international ImMunoGeneTics (IMGT) information system (http://imgt.cines.fr).20-22
Research approvals
Human studies were approved by the Institutional Review Board of the BloodCenter of Wisconsin. Written informed consent was obtained from all human participants in accordance with the Declaration of Helsinki. Murine studies were approved by the Institutional Animal Care and Use Committee of the Medical College of Wisconsin.
Results
Two unique clones were identified that produced quinine-dependent, platelet-reactive antibodies
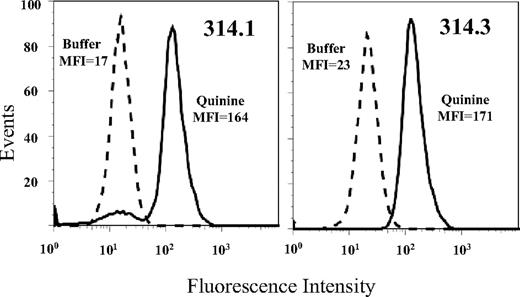
Approximately 5000 hybrid clones derived from immunization of 9 mice were screened for quinine-dependent antibodies using flow cytometry. Although many clones secreting platelet-reactive mAbs were identified, no quinine-dependent mAbs were detected until the seventh immunization, when 3 of 530 clones (314.1, 314.2, and 314.3) were found to have the desired properties (Figure 1). Each clone possessed an IgG1 heavy chain and κ light chain. Sequencing of genes encoding complementarity-determining regions (CDR) showed that clones 314.1 and 314.2 were identical. Subsequent studies were done with clones 314.1 and 314.3.
Monoclonals 314.1 and 314.3 reacted with normal human platelets. Histograms show reaction in the presence (solid histograms) but not the absence (dashed histograms) of soluble quinine. Mean fluorescent intensity (MFI) values for each histogram are shown.
Monoclonals 314.1 and 314.3 reacted with normal human platelets. Histograms show reaction in the presence (solid histograms) but not the absence (dashed histograms) of soluble quinine. Mean fluorescent intensity (MFI) values for each histogram are shown.
Quinine-dependent mAbs mimicked the behavior of human DDAbs in their drug-dependent reactions with platelets
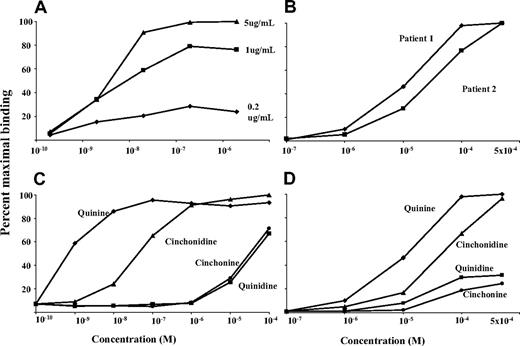
As shown in Figure 2A, mAb 314.1, used at concentrations ranging from 5.0 to 0.2 μg/mL, reacted strongly with intact human platelets in the presence of quinine at concentrations as low as 5.0 × 10−8 M. A weaker reaction was obtained with quinine at 5 × 10−9 M. Reactions of 2 antibodies from typical patients with quinine-induced immune thrombocytopenia were qualitatively similar to those of mAbs 314.1 and 314.3, but drug concentrations more than 1000-fold higher were needed to achieve antibody binding (Figure 2B). Cinchondine (desmethoxy-quinine) also supported binding of mAbs 314.1 and 314.3, but approximately 100 times more drug was required relative to quinine (Figure 2C). With quinidine, the diastereoisomer of quinine, concentrations approximately 100 000-fold greater were necessary. Cinchonine (desmethoxy-quinidine) behaved like quinidine. Reactions of mAb 314.3 with these compounds were essentially identical to those of mAb 314.1 (not shown). Drug-dependent reactions of a typical quinine-dependent human antibody with the quinine congeners were qualitatively similar to those of the 2 monoclonals (Figure 2D).
Drug-dependent reactions of monoclonal 314.1 with intact platelets were similar to those of 2 human quinine-dependent antibodies. All studies were done with flow cytometry. (A) Reactions of mAb 314.1 (0.2, 1, and 5 μg/mL) at various concentrations of quinine. (B) Reactions of 2 human quinine-dependent antibodies at various concentrations of quinine. (C,D) Reactions of mAb 314.1 (1.0 μg/mL; C) and 2 human quinine-dependent antibodies (D) at various concentrations of quinine and quinine congeners. Drug concentrations are shown on the abscissa. Reactions of mAb 314.3 (not shown) were essentially identical to those of mAb 314.1.
Drug-dependent reactions of monoclonal 314.1 with intact platelets were similar to those of 2 human quinine-dependent antibodies. All studies were done with flow cytometry. (A) Reactions of mAb 314.1 (0.2, 1, and 5 μg/mL) at various concentrations of quinine. (B) Reactions of 2 human quinine-dependent antibodies at various concentrations of quinine. (C,D) Reactions of mAb 314.1 (1.0 μg/mL; C) and 2 human quinine-dependent antibodies (D) at various concentrations of quinine and quinine congeners. Drug concentrations are shown on the abscissa. Reactions of mAb 314.3 (not shown) were essentially identical to those of mAb 314.1.
mAbs 314.1 and 314.3 recognized the GPIIb component of the intact GPIIb/IIIa complex
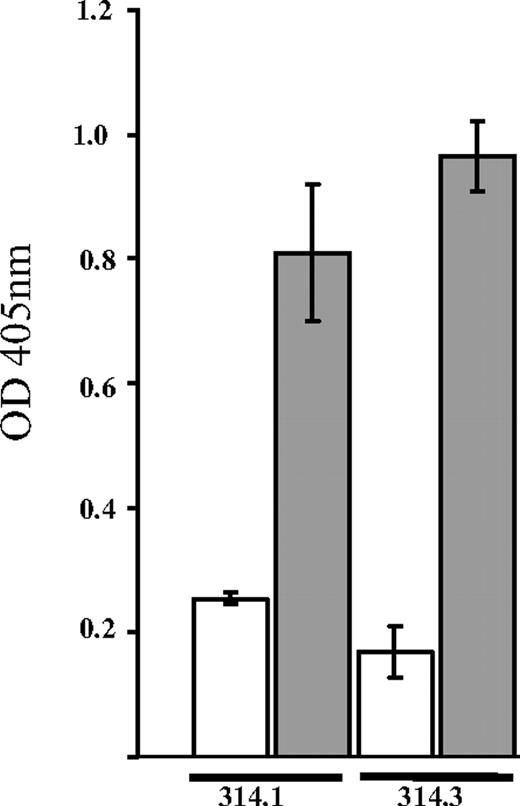
As shown in Figure 3, mAbs 314.1 and 314.3 reacted with GPIIb/IIIa in the presence of 0.4 mM quinine, but not in the absence of drug. In a similar assay using the GPIb-specific monoclonal AP1 for antigen capture, neither antibody reacted demonstrably with the GPIb/IX complex, also a common target for human quinine-induced DDAbs6,23 (data not shown). After treatment of platelets with EDTA at 37°C for 30 minutes to dissociate the integrin into its 2 components,24,25 reactions of the 2 quinine-dependent mAbs and the complex-specific mAb AP2 were lost, but binding of the GPIIIa-specific mAb AP3 was unaffected (not shown).
Monoclonals 314.1 and 314.3 reacted with isolated GPIIb/IIIa in the presence, but not in the absence of quinine. Platelet GPIIb/IIIa was captured from a platelet lysate by monoclonal AP2 (anti-GPIIb/IIIa complex) immobilized in microtiter wells. Biotinylated mAbs 314.1 and 314.3 were incubated with this target in the presence (■) and absence (□) of 0.4 mM quinine. Bound mAb was detected with alkaline-phosphatase-labeled streptavidin. Brackets show the mean plus or minus 1 standard deviation of triplicate determinations.
Monoclonals 314.1 and 314.3 reacted with isolated GPIIb/IIIa in the presence, but not in the absence of quinine. Platelet GPIIb/IIIa was captured from a platelet lysate by monoclonal AP2 (anti-GPIIb/IIIa complex) immobilized in microtiter wells. Biotinylated mAbs 314.1 and 314.3 were incubated with this target in the presence (■) and absence (□) of 0.4 mM quinine. Bound mAb was detected with alkaline-phosphatase-labeled streptavidin. Brackets show the mean plus or minus 1 standard deviation of triplicate determinations.
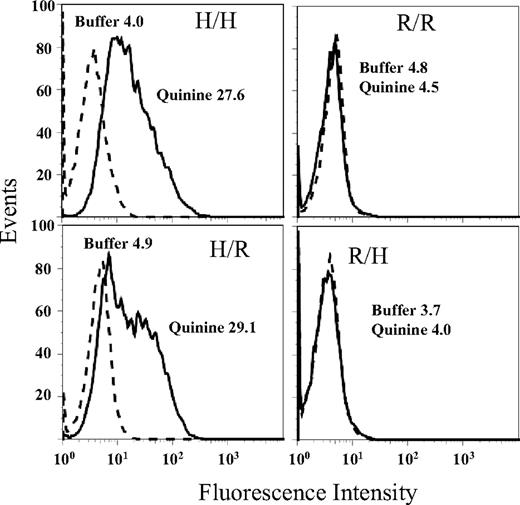
Reactions of mAb 314.1 with CHO cells expressing all-human GPIIb/IIIa (H/H), all-rat GPIIb/IIIa (R/R), or human/rat mixed GPIIb/IIIa integrins (H/R, R/H) are shown in Figure 4. Quinine-dependent mAb binding occurred with constructs H/H and H/R, but not with R/R or R/H. The same pattern of reactions was obtained with mAb 314.3 (not shown).
Reactions of mAb 314.1 with CHO cells expressing mixed (human/rat) GPIIb/IIIa integrins. In the presence of quinine (solid histograms) mAb 314.1 reacted with cells expressing human GPIIb paired with either human (H/H) or rat (H/R) GPIIIa but failed to react with cells expressing rat GPIIb paired with either human (R/H) or rat (R/R) GPIIIa. No reactions were obtained in the absence of quinine (dashed histograms). Essentially identical reactions were obtained with mAb 314.3 (not shown).
Reactions of mAb 314.1 with CHO cells expressing mixed (human/rat) GPIIb/IIIa integrins. In the presence of quinine (solid histograms) mAb 314.1 reacted with cells expressing human GPIIb paired with either human (H/H) or rat (H/R) GPIIIa but failed to react with cells expressing rat GPIIb paired with either human (R/H) or rat (R/R) GPIIIa. No reactions were obtained in the absence of quinine (dashed histograms). Essentially identical reactions were obtained with mAb 314.3 (not shown).
Together, the findings indicate that mAbs 314.1 and 314.3 recognize an epitope on human GPIIb. Failure of the mAbs to react with GPIIb/IIIa dissociated by EDTA treatment suggests that GPIIb must be stabilized by human GPIIIa or GPIIIa from another species (in this case rat) for the target epitope(s) to be in a configuration appropriate for antibody binding.
The binding sites for mAbs 314.1 and 314.3 were localized to an epitope near the N terminus of GPIIb in the β propeller domain
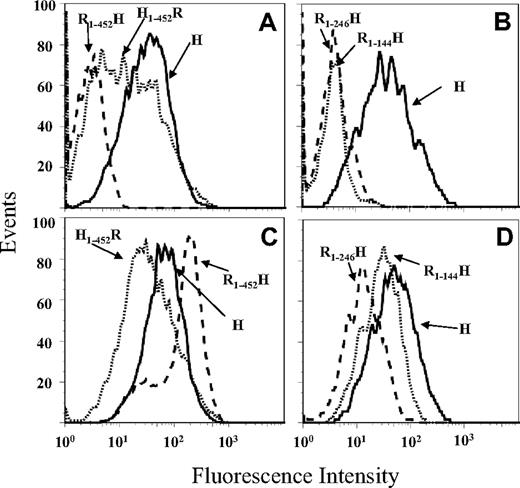
Binding sites on GPIIb for mAbs 314.1 and 314.3 were further characterized by examining reactions of the monoclonals with transfected CHO cells expressing GPIIb, in which rat sequences were substituted for the corresponding human sequences in selected domains and subdomains. Crystallographic studies of the human integrin αv, closely homologous to GPIIb,26 and of the N-terminal domains of human GPIIb in a complex with a fragment of GPIIIa18 have shown that the GPIIb heavy chain is organized into 4 major structural domains designated (from the N terminus) β propeller, thigh, calf-1, and calf-2. As shown for mAb 314.1 in Figure 5A, the 2 quinine-dependent mAbs recognized GPIIb containing human sequence in the β propeller domain and rat sequence in the remaining extracellular domains (H1-452R), but failed to react with a reciprocal construct containing β propeller derived from rat and human sequence in the remainder of the molecule (R1-452H). The β propeller domain contains 7 repeats making up its “blades” and a cap subdomain made up of amino acid sequences contributed by repeats 1, 2, and 3.18 As shown in Figure 5B, mAb 314.1 failed to react with chimeric GPIIb containing rat sequence in the first 2 (R1-144H) or 3 (R1-246H) N-terminal repeats and human sequence in the remainder of the molecule. Reactions of mAb 314.3 with these constructs were essentially the same as those of mAb 314.1 (not shown). Reactions of mAb AP2 (anti-GPIIb/IIIa) with these cell lines showed that all constructs were satisfactorily expressed (Figure 5C,D). Together, the findings suggest that epitopes recognized by the quinine-dependent mAbs are located in the first 2 N-terminal repeats of GPIIb or in the adjacent cap subdomain.
Quinine-dependent reactions of mAb 314.1 with CHO cells expressing human/rat chimeric or wild-type human GPIIb paired with human GPIIIa. (A) mAb 314.1 reacted with wild-type GPIIb (H, solid histogram) and with a GPIIb containing human sequence in the N-terminal β propeller domain (H1-452R, dotted histogram) but not with the inverse construct containing rat sequence in the β propeller domain (R1-452H, dashed histogram). (B) mAb 314.1 binding was lost when the first 2 (R1-144H, dotted histogram) or 3 (R1-246H, dashed histogram) N-terminal repeats (blades) of the human β propeller domain were replaced by a rat sequence. All reactions contained quinine 0.4 mM. There were no significant reactions in the absence of quinine (not shown). (C,D) Reactions of mAb AP2 (specific for the GPIIb/IIIa complex) with the same CHO cell lines showed that all constructs were satisfactorily expressed.
Quinine-dependent reactions of mAb 314.1 with CHO cells expressing human/rat chimeric or wild-type human GPIIb paired with human GPIIIa. (A) mAb 314.1 reacted with wild-type GPIIb (H, solid histogram) and with a GPIIb containing human sequence in the N-terminal β propeller domain (H1-452R, dotted histogram) but not with the inverse construct containing rat sequence in the β propeller domain (R1-452H, dashed histogram). (B) mAb 314.1 binding was lost when the first 2 (R1-144H, dotted histogram) or 3 (R1-246H, dashed histogram) N-terminal repeats (blades) of the human β propeller domain were replaced by a rat sequence. All reactions contained quinine 0.4 mM. There were no significant reactions in the absence of quinine (not shown). (C,D) Reactions of mAb AP2 (specific for the GPIIb/IIIa complex) with the same CHO cell lines showed that all constructs were satisfactorily expressed.
Independent support for localization of the 314.1 and 314.3 binding site(s) at the N terminus of GPIIb was obtained by determining the ability of these mAbs to interfere with the binding of 3 other mAbs (10E5, 312.6, and 312.8) specific for the GPIIb β propeller domain. Reaction patterns of these 3 mAbs and the 2 quinine-dependent mAbs with wild-type GPIIb and with GPIIb R1-144H and R1-246H coexpressed with human GPIIIa in CHO cells are summarized in Table 1, where it can be seen that mAbs 314.1 and 314.3 recognized only wild-type GPIIb, mAbs 10E5, and 312.8 recognized wild-type GPIIb and GPIIb R1-144H but not GPIIb R1-246H, and mAb 312.6 reacted with all 3 constructs. Thus, binding of mAbs 314.1, 314.3, 10E5, and 312.8, but not that of 312.6, was adversely affected by one or both of the substitutions (rat for human) at the N terminus of the β propeller domain. Monoclonal 10E5 has been shown in crystallographic studies to be specific for the cap subdomain of GPIIb, where it contacts residues contributed by the first, second, and third repeats.18 Therefore, its reaction with R1-144H (in which the first 2 “blades” of the propeller are replaced by rat sequence) was unexpected. This seeming discrepancy is probably explained by the fact that in the crystal structure deduced by Xiao et al, major residues from the first 2 “blades” that contribute to the interface between 10E5 and the cap subdomain (Arg77 and Gln83) are identical in human and rat.18
Each of the 5 mAbs was labeled with biotin, a modification shown in preliminary studies not to interfere with binding to GPIIb. The ability of each unlabeled antibody (preincubated with target platelets at high concentration) to block binding of each biotin-labeled probe was then determined using PE-labeled streptavidin and flow cytometry to detect platelet-bound biotin. Results are summarized in Table 2. As expected, each unlabeled mAb completely blocked the binding of its biotin-labeled counterpart. Inhibitory effects of the 5 mAbs on each other can be summarized as follows: (1) the quinine-dependent mAbs 314.1 and 314.3 and mAb 10E5 were mutually inhibitory, suggesting that their binding sites overlap; (2) mAb 312.6 inhibited mAbs 314.1 and 314.3 but not 10E5, indicating that its binding site overlaps that of the quinine-dependent mAbs, but not that of 10E5; and (3) mAb 312.8 failed to significantly inhibit any of the other 4 mAbs except for a minor effect on mAb 10E5.
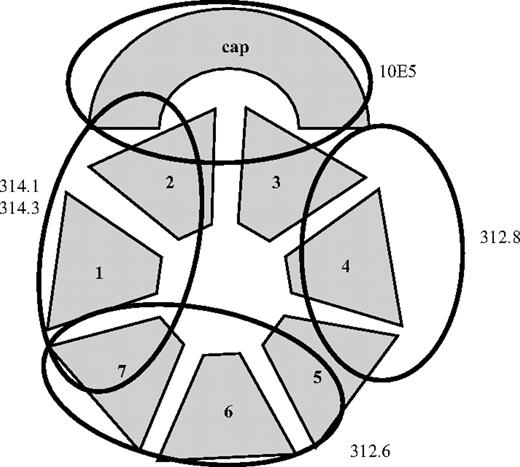
Together, the findings suggest that mAbs 314.1 and 314.3 recognize an epitope in or near the cap subdomain of the GPIIb propeller located at least partially in the “footprint” of 10E5. The direct reactions and inhibitory effects of mAbs 312.6 and 312.8 are consistent with the recognition sites shown in Figure 6. Other interpretations are possible if mAb binding produces significant long-range changes in β propeller structure.
Probable binding sites of monoclonals 314.1, 314.3, 10E5, 312.6, and 312.8 in the GPIIb β propeller domain. N-terminal repeats (“blades”) of the propeller 1 to 7 and the CAP subdomain26 are shown schematically. The binding site shown for 10E5 is based on the crystallographic data of Xiao et al.18 Proposed binding sites for the other 4 mAbs are based on their reactions with chimeric GPIIb/IIIa (Table 1) and the results of competitive binding studies (Table 2).
Probable binding sites of monoclonals 314.1, 314.3, 10E5, 312.6, and 312.8 in the GPIIb β propeller domain. N-terminal repeats (“blades”) of the propeller 1 to 7 and the CAP subdomain26 are shown schematically. The binding site shown for 10E5 is based on the crystallographic data of Xiao et al.18 Proposed binding sites for the other 4 mAbs are based on their reactions with chimeric GPIIb/IIIa (Table 1) and the results of competitive binding studies (Table 2).
mAbs 314.1 and 314.3 were closely related in structure
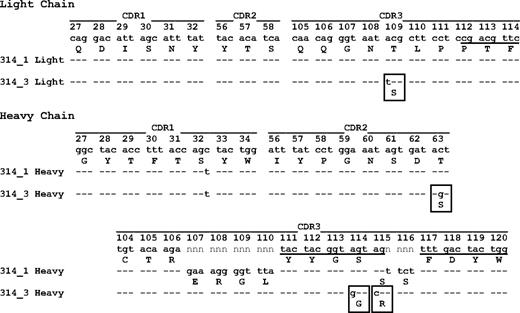
How drug-dependent antibodies are induced is unknown. It was therefore of interest to determine V gene sequences of mAbs 314.1 and 314.3. Sequencing studies showed that mAb 314.1 uses the IGHV671, D1-1*01, JH2*02, and the IGKV137 and JK1*02 gene segments for synthesis of heavy and light chains, respectively. The cDNAs encoding CDRs 1, 2, and 3 of both the light and heavy chains of mAb 314.1 were identical in sequence to the corresponding germline sequences except for a silent (AGC to AGT) mutation encoding 32S in the heavy chain CDR1 (Figure 7). Six additional amino acids were inserted in the heavy chain CDR3 as a result of “n” nucleotide addition during gene recombination. The mAb 314.3 sequence was identical to that of mAb 314.1 except for 4 additional mutations leading to 1 amino acid switch in the light chain CDR3, 1 in the heavy chain CDR2, and 2 in the heavy chain CDR3 (Figure 7). The findings suggest that clone 314.3 evolved by somatic hypermutation from clone 314.1 during the immune response. We sequenced 8 “conventional” (non–drug-dependent) mAbs specific for GPIIb or GPIIIa, and found that, in contrast to mAb 314.1, cDNA encoding the light and heavy chain CDRs contained an average of 6 mutations (range, 2-12) leading to an average of 4 amino acid substitutions (range, 2-8).
Light and heavy chain CDR sequences of monoclonals 314.1 and 314.3. Underlining indicates sequences of J gene IgKJ1*02 (light chain), D gene IgHD1*01 (heavy chain), and J gene IgHJ2*02 (heavy chain); n indicates nucleotides inserted during gene recombination. Amino acids deviating from those predicted by germline sequence are boxed. Amino acid residues are numbered according to the IMGT information system.20
Light and heavy chain CDR sequences of monoclonals 314.1 and 314.3. Underlining indicates sequences of J gene IgKJ1*02 (light chain), D gene IgHD1*01 (heavy chain), and J gene IgHJ2*02 (heavy chain); n indicates nucleotides inserted during gene recombination. Amino acids deviating from those predicted by germline sequence are boxed. Amino acid residues are numbered according to the IMGT information system.20
Discussion
A remarkable feature of antibodies that cause thrombocytopenia in patients sensitized to drugs such as quinine, nonsteroidal anti-inflammatory agents, and antibiotics is that they are nonreactive in the absence of the sensitizing drug but bind tightly to platelet membrane GPs (usually GPIIb/IIIa and/or Ib/IX) when soluble drug is present at pharmacologic concentrations.3,4,27 In general, antibodies from patients with quinine-induced immune thrombocytopenia react best in the presence of quinine and less well when tested with quinine congeners in the order: quinine > cinchonidine > quinidine > cinchonine.28 Human drug-dependent antibodies exhibit dose-dependent binding to their targets up to saturation and are not inhibited by excess drug at concentrations more than 100 000 times greater than the number of antibody binding sites,4,10,29,30 a property that distinguishes them from classic hapten-specific antibodies.7,8 Figures 1 and 2 show that monoclonals 314.1 and 314.3 mimicked the behavior of quinine-dependent human antibodies in each of these respects.
At the 1.0-μg/mL (intermediate) dose of mAb used in the study shown in Figure 2A, the concentration of mAb 314.1 in the reaction mixture was 0.67 × 10−8 M and the concentration of target GPIIb/IIIa molecules was approximately 1.3 × 10−8 M (assuming approximately 80 000 GPIIb/IIIa complexes on the surface of each platelet).31 At these concentrations of mAb and target, quinine promoted strong antibody binding at a concentration as low as 2 × 10−8 M. Thus, binding occurred at concentrations of the 3 reactants approximating 10−8 M, indicating that the effective kD for drug-dependent, antibody binding is on the order of 10−8 M. It is of interest that more than 40 years ago, N. R. Shulman estimated the kD for binding of a single, high-titer quinidine-dependent human antibody to platelets to be approximately 2.5 × 10−8 M.29 However, this calculation was based on tenuous assumptions made necessary by lack of knowledge about the nature of the target molecules for which the antibody was specific and by limitations of available analytical tools.
As expected, mAbs 314.1 and 314.3 recognized the GPIIb/IIIa complex used for immunization in the presence, but not in the absence of quinine (Figure 3). Neither antibody recognized GPIb/IX immobilized with mAb AP1 (not shown). Studies using CHO cells expressing human/rat chimeric GPIIb/IIIa localized the antibody binding sites to GPIIb (Figure 4). Failure of the mAbs to recognize GPIIb dissociated from GPIIIa by treatment with EDTA suggests that stabilization by rat or human GPIIIa is necessary to maintain GPIIb in a configuration suitable for antibody recognition. Further studies (Figure 5 and Table 1) provided evidence that mAbs 314.1 and 314.3 each recognize an epitope or epitopes located in one or both of the first 2 N-terminal repeats of the β propeller or the adjacent cap subdomain (Figure 6). Reciprocal competition between mAbs 314.1 and 314.3 for binding (Table 2) and their similar CDR sequences (Figure 7) are consistent with the possibility that their target epitopes are almost identical. Studies to define the recognition sites more precisely by determining the effect of selected amino acid substitutions on antibody binding are in progress.
The finding that both heavy and light chains of mAb 314.1 contained CDR 1, 2, and 3 sequences identical to germline (Figure 7) was unexpected and was in distinct contrast to the CDR sequences of 8 non–drug-dependent mAbs specific for GPIIb/IIIa, which contained 2 to 12 mutations, leading to an average of 4 (range, 2-8) amino acid substitutions. The findings suggest that mAb 314.1 originated in a naive B cell that was expanded upon exposure to GPIIb and quinine and produced antibody initially with no hypermutation of the CDRs, although class switching to IgG1 had occurred. Clone 314.3 shares junctional sequences with clone 314.1 and represents a hypermutated variety of the latter. The B1 subset of B lymphocytes, present mainly in the peritoneum and at mucosal surfaces, is the source of most “naturally occurring” immunoglobulins, tends to have nonmutated B cell receptors, and is capable of an adaptive immune response,32,33 but produces antibodies largely restricted to the IgM and IgA classes.32 Studies in rats provided evidence that B cells of the marginal zone (MZ) subset have receptors that are mainly germline in sequence,34 but clearly MZ B cells in rodents can receive help from both T cells35 and NKT cells,36 and human MZ B cells appear to be hypermutated.37,38 We are now attempting to produce additional drug-dependent, platelet-reactive mAbs by immunizing mice with platelet GPs and drugs other than quinine. It is unclear whether naive splenic B cells that are activated by quinine and platelet GPs are of follicular or MZ B lymphoid origin, but it appears that the immunogen used is capable of inducing a T-dependent immune response against the drug of interest as indicated by class switching to IgG and somatic hypermutation noted in mAb 314.3.
How a drug can promote binding of a drug-dependent antibody to its target without acting as a classical hapten (ie, without linking covalently to the target) is poorly understood.4,5,10 It is now widely accepted that B cells, especially those of the B1 and MZ subsets, are positively selected in development for their ability to interact weakly with self-antigens.32,37,39,40 We have proposed that drugs capable of causing DITP have structural elements that enable them to react with both a weakly autoreactive Ig and its target epitope (in this case on a platelet GP) to improve the fit between the 2 macromolecules and increase the effective KA sufficiently for antibody binding to occur at concentrations of drug, antibody, and GP achieved in the circulation after treatment with the sensitizing drug.3,10 The polyclonal and polyspecific nature of drug-dependent antibodies from patients with DITP and the limited amounts of antibody usually available have frustrated attempts to validate this model. Availability of monoclonals 314.1 and 314.3 in unlimited quantities and localization of their approximate binding site(s) will facilitate characterization of the molecular interactions involved in drug-dependent antibody binding and may enable the molecular character of the presumptive trimolecular complex produced when this reaction takes place to be defined.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to Dr Shiv Pillai of the Massachusetts General Hospital and Harvard Medical School (Boston, MA) for review of the paper and helpful comments and to Scott Ahl for outstanding technical assistance.
This work was supported by grant HL-13629 from the National Heart, Lung, and Blood Institute (Bethesda, MD) and the Blood Research Foundation of the BloodCenter of Wisconsin (Milwaukee, WI).
National Institutes of Health
Authorship
Contribution: D.W.B. and R.H.A. designed the study, provided oversight of laboratory work, and prepared the manuscript; M.P. provided CHO cells stably transfected with GPIIb/IIIa and reviewed and commented on the paper; and J.B. and M.R. performed laboratory studies.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Daniel W. Bougie, Blood Research Institute, 8727 Watertown Plank Road, Milwaukee, WI 53226; e-mail: dan.bougie@bcw.edu.