Abstract
Graft-versus-host disease (GVHD) is a significant complication of allogeneic stem cell transplantation (alloSCT). The proteasome inhibitor bortezomib has immunomodulatory properties of potential benefit for GVHD control. We undertook a phase 1 trial of bortezomib, tacrolimus, and methotrexate for GVHD prophylaxis after reduced-intensity conditioning alloSCT using human leukocyte antigen–mismatched unrelated donors. Twenty-three patients were enrolled. Bortezomib dose levels of 1, 1.3, and 1.5 mg/m2 were evaluated with 5, 3, and 5 patients, respectively. Ten additional patients were accrued at the 1.3 mg/m2 bortezomib dose level. Bortezomib-related toxicity was minimal. With a 12-month median follow-up, grade II-IV acute GVHD occurred in 3 patients, a 180-day cumulative incidence of 13%. Chronic GVHD occurred in 9 patients, a 1-year cumulative incidence of 41%. At 1-year, the nonrelapse mortality was zero, cumulative incidence of relapse/progression was 29%, and overall, progression-free, and event-free survival were 75%, 64%, and 59%, respectively. Bortezomib is a promising novel immunomodulatory agent in allogeneic transplantation. This study was registered at http://www.clinicaltrials.gov as #NCT00369226.
Introduction
Acute graft-versus-host disease (GVHD) remains a significant barrier to allogeneic stem cell transplantation (alloSCT), especially for those lacking human leukocyte antigen (HLA)–matched donors. Donor T-cell depletion (eg, antithymocyte globulin) can control GVHD but is associated with impaired immune reconstitution, increased graft rejection, infections, posttransplantation lymphoproliferative disease, and malignant disease relapse.1 In the non–T-depleted context, we documented a 46% grade II-IV acute GVHD incidence in 52 patients mismatched at one or more HLA locus (-A, -B, -C, -DRB1, -DQB1) after reduced-intensity conditioning (RIC) alloSCT with calcineurin-inhibitor plus prednisone or calcineurin-inhibitor plus mini-methotrexate–based GVHD prophylaxis.2
Bortezomib is a proteasome inhibitor effective against multiple myeloma via inhibition of nuclear factor–κB, attenuation of interleukin-6–mediated cell growth, a direct apoptotic effect, and possibly antiangiogenic and other mechanisms.3 Nuclear factor–κB plays an important role in cytokine signaling and T-cell activation, proliferation, and apoptosis.4-7 Bortezomib inhibits antigen-presenting cells by attenuating Toll-like-receptor 4–mediated activation, with reduced cytokine production and immunostimulatory activity.8 In the preclinical setting, bortezomib preferentially and specifically depletes alloreactive T lymphocytes.9 In mouse models of HLA-mismatched alloSCT, early administration of bortezomib protects against acute GVHD without impairing engraftment.10-12 It also preserves graft-versus-tumor responses.10 However, delayed bortezomib administration can enhance colonic toxicity.11
We hypothesized that bortezomib administered early after alloSCT may improve GVHD control. We report on phase 1 of a phase 1/2 trial of HLA-mismatched RIC unrelated donor (URD) alloSCT using bortezomib, tacrolimus, and methotrexate for GVHD prophylaxis.
Methods
This study was approved by the Institutional Review Board of the Dana-Farber Cancer Institute/Harvard Cancer Center. Informed consent was obtained before patient enrollment in accordance with the Declaration of Helsinki. Hematologic malignancy patients enrolled had URD grafts with 1 or 2 antigen or allele mismatches at HLA class I (HLA-A, -B, -C), or 1 antigen or allele mismatch at HLA class II (HLA-DRB1). Excluded were patients with HIV infection, active hepatitis B or C disease, abnormal renal (serum creatinine > 2 mg/dL) or liver function tests (serum total bilirubin > 2 mg/dL, serum alanine aminotransferase > 90 U/L), Eastern Cooperative Oncology Group performance status more than 2, or peripheral neuropathy grade 2 or higher within 21 days before transplantation.
RIC consisted of fludarabine (30 mg/m2 intravenously) and busulfan (0.8 mg/kg intravenously) on days −5, −4, −3, and −2. The donor peripheral blood stem cell dose was more than or equal to 5 × 106 CD34+ cells/kg. GVHD prophylaxis comprised (1) tacrolimus at 0.05 mg/kg orally twice a day (target serum level of 5-10 ng/mL), starting on day −3 and finishing by day +180, with a suggested one-third taper at weeks 9, 17, and 26; (2) low-dose methotrexate at 5 mg/m2 intravenously on days +1, +3, +6, and +11; and (3) bortezomib at 1 of 3 dose levels. All patients received filgrastim at 5 μg/kg subcutaneously daily, from day +1 until an absolute neutrophil count (ANC) more than 1000 cells/μL; and 12 months of Pneumocystis jiroveci and herpes simplex virus/varicella-zoster virus prophylaxis.
Three bortezomib dose levels were evaluated: 1, 1.3, and 1.5 mg/m2 intravenously on days +1, +4, and +7. We enrolled 3 to 5 patients per dosing cohort, and cohort dose escalation was permitted if dose-limiting toxicity (DLT) occurred in no more than 0 of 3 or 1 of 5 patients.
DLT was defined as (1) Common Toxicity Criteria grade 3 or higher nonhematologic toxicity directly related to bortezomib (eg, neuropathy); (2) failure to achieve an ANC more than 500 cells/μL; and (3) less than 50% total donor chimerism (by day +45). Neutrophil and platelet engraftment were assessed by the number of days to ANC more than or equal to 500/μL and platelets more than or equal to 20=000/μL, respectively, in the absence of transfusions. Acute GVHD was assessed per consensus grading.13
Event-free survival was measured from the date of transplantation to the first event (graft failure, relapse/progression, death) or last contact. Progression-free survival was measured from the date of transplantation to disease relapse/progression, death, or last contact. Overall survival was measured from the date of transplantation to death from any cause. Event-free survival, progression-free survival, and overall survival were estimated by the method of Kaplan-Meier. The cumulative incidence of graft failure and relapse/progression was estimated with death considered a competing event. The cumulative incidence of grade II-IV acute and chronic GVHD was estimated with graft failure, relapse/progression, and death considered competing events.
Results and discussion
Twenty-three patients were enrolled. Baseline characteristics are presented in Table 1 (additional details are provided in supplemental Table 2, available on the Blood website; see the Supplemental Materials link at the top of the online article). At the 1 mg/m2 bortezomib dose level, 1 of 5 patients experienced low donor chimerism (3%) by day +45, reported as a DLT. The patient developed acute myeloid leukemia (AML)/myelodysplastic syndrome blasts within 2 weeks thereafter, indicating malignant disease relapse/progression as a more probable etiology. At the 1.3 mg/m2 bortezomib dose level, 0 of 3 patients had a DLT. At the 1.5 mg/m2 bortezomib dose level, 2 of 5 patients had low donor chimerism by day +45, reported as DLTs. One patient had 0% donor chimerism and persistent chronic lymphocytic leukemia. The other patient with previously untreated myeloproliferative disease (MPD) did not engraft (0% donor chimerism). No other DLTs were noted. In an expansion cohort to further assess toxicity, 10 patients received bortezomib at the 1.3 mg/m2 dose level.
Posttransplantation cytopenias were limited in the 22 engrafting patients: 9 patients did not experience either neutrophil or platelet nadir, 14 patients did not experience a neutrophil nadir, and 10 patients did not experience a platelet nadir. For 8 patients with a neutrophil nadir (ANC < 500/μL), median engraftment time was 15 days (range, 11-19 days). For 13 patients with a platelet nadir (< 20=000/μL), median engraftment time was 20 days (range, 17-27 days). Nonhematologic toxicity was limited. Four patients experienced Common Toxicity Criteria grade 3 or 4 toxicity by day +45. One patient each had febrile neutropenia (grade 3), parainfluenza pneumonitis (grade 3), cerebrovascular accident with paresthesias (grade 3), and hyperglycemia (grade 4). These were deemed nonattributable to bortezomib by the treating physician.
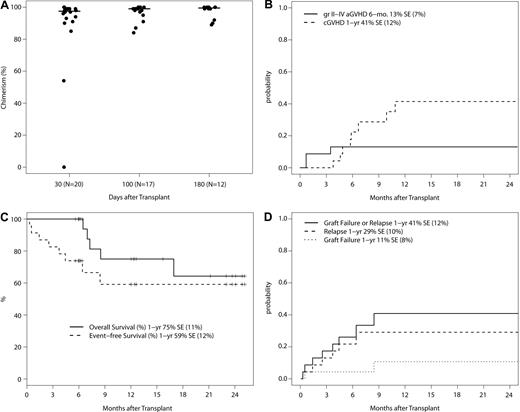
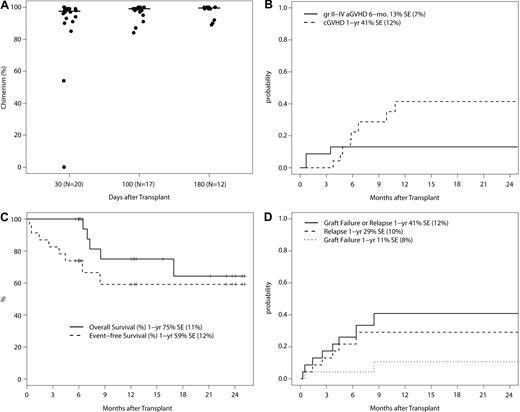
Donor engraftment was robust, with a median chimerism of 97.5% (range, 0%-100%), 99% (range, 84%-100%), and 99.5% (range, 89%-100%) around day +30, +100, and +180, respectively (Figure 1A). As noted above in this section, patients with low donor chimerism often had persistent/progressive disease. However, one patient with MPD at the 1.5 mg/m2 bortezomib dose level failed to engraft. On additional follow-up, one patient with AML at the 1.3 mg/m2 bortezomib dose level had graft failure at 9 months without disease relapse and remains AML-free with endogenous count recovery at 15 months after alloSCT. The 1-year cumulative incidence of graft failure is 11% (SE, ± 8%).
Phase 1 trial outcomes. (A) Total donor chimerism at days +30, +100, and +180 after transplantation in patients without evidence of disease relapse/progression. Donor chimerism analysis was not mandated beyond day +100. The 2 patients with low initial donor chimerism of 54% and 0% had MPD. The protocol was subsequently amended to exclude MPD. (B) Cumulative incidence of grade II-IV acute GVHD and chronic GVHD with graft failure, relapse/progression, and death as competing events. (C) Overall and event-free survival (N = 23). Events include graft failure, relapse/progression, and death. (D) Cumulative incidence of graft failure and relapse/progression with death as a competing event.
Phase 1 trial outcomes. (A) Total donor chimerism at days +30, +100, and +180 after transplantation in patients without evidence of disease relapse/progression. Donor chimerism analysis was not mandated beyond day +100. The 2 patients with low initial donor chimerism of 54% and 0% had MPD. The protocol was subsequently amended to exclude MPD. (B) Cumulative incidence of grade II-IV acute GVHD and chronic GVHD with graft failure, relapse/progression, and death as competing events. (C) Overall and event-free survival (N = 23). Events include graft failure, relapse/progression, and death. (D) Cumulative incidence of graft failure and relapse/progression with death as a competing event.
With regard to MPD and engraftment, both patients enrolled had low donor chimerism. The first patient, at the 1.5 mg/m2 bortezomib dose level, failed to engraft by day +45. The second patient with MPD, at the 1.3 mg/m2 bortezomib dose level, had borderline donor chimerism (53%) by day +45, then developed biopsy-proven toxic epidermal necrolysis that was considered Bactrim-related, subsequently experienced MPD progression with 7% residual donor granulocyte chimerism, and died of sepsis from infected toxic epidermal necrolysis lesions at 6 months after alloSCT. The protocol was subsequently amended to exclude patients with MPD.
The 23 patients on study have a median follow-up of 12.0 months (range, 5.6-25.1 months). Grade II-IV acute GVHD occurred in 3 patients (1 with grade II; 2 with grade III acute GVHD), a 180-day cumulative incidence of 13% (SE, ± 7%; Figure 1B). Chronic GVHD occurred in 9 patients (4 with limited and 5 with extensive chronic GVHD), a 1-year cumulative incidence of 41% (SE, ± 12%). Six patients relapsed/progressed, and 5 deaths occurred, all with relapse before death. The 1-year nonrelapse mortality was zero, the cumulative incidence of relapse/progression was 29% (SE, ± 10%), and overall, progression-free, and event-free survival was 75% (SE, ± 11%), 64% (SE, ± 14%) and 59% (SE, ± 12%), respectively (Figure 1C-D).
In conclusion, acute GVHD prophylaxis with bortezomib, tacrolimus, and methotrexate after busulfan/fludarabine-based RICalloSCT appears safe, convenient, and well tolerated thus far. Phase 2 accrual is ongoing. The incidence of 13% grade II-IV acute GVHD in the HLA-mismatched URD context is encouraging. It is within the 11% to 17% grade II-IV acute GVHD incidence reported after matched-related donor RIC alloSCT.14 Bortezomib is a promising novel immunomodulatory agent in allogeneic transplantation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank study nurses Mary Kelly, Susan Stephenson, and Mildred Pasek and study clinical research coordinator Bhavjot Bindra for their help.
This study was supported in part by Millennium Pharmaceuticals Inc and by the National Institutes of Health (P01 CA142106).
National Institutes of Health
Authorship
Contribution: J.K., J.R., and E.P.A. designed research; J.K., V.T.H., P.A., C.C., J.H.A., R.J.S., and E.P.A. cared for patients; J.K., K.E.S., H.T.K., and M.G. collected data; J.K., K.E.S., and H.T.K. performed statistical analysis; J.K. prepared the manuscript; and J.K., K.E.S., H.T.K., M.G., V.T.H., P.A., C.C., J.R., J.H.A., R.J.S., and E.P.A. edited and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John Koreth, Division of Hematologic Malignancies, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA 02115; e-mail: jkoreth@partners.org.