Abstract
Responses can be achieved with dasatinib or nilotinib after failure of 2 prior tyrosine kinase inhibitors (TKIs). We report on 48 chronic myeloid leukemia patients sequentially treated with 3 TKIs: 34 with dasatinib after imatinib/nilotinib failure and 14 with nilotinib after imatinib/dasatinib failure. Before the third TKI, 25 patients were in chronic phase (CP), 10 in accelerated phase (AP), and 13 in blast phase (BP). Best response to third TKI in CP was 5 major molecular responses (MMR), 3 complete cytogenetic (CCyR), 2 partial cytogenetic (PCyR), 3 minor cytogenetic (mCyR), 6 complete hematologic responses (CHR), and 6 with no response (NR). In AP, 1 patient achieved MMR, 1 CCyR, 2 PCyR, 1 mCyR, 4 CHR, and 1 NR. In BP, 1 achieved MMR, 2 CCyR, 1 PCyR, 1 mCyR, 2 returned to CP, and 6 NR. Median CCyR duration was 16.3 months; 3 CP patients achieving CCyR had a response more than 12 months. Median failure-free survival was 20 months for patients in CP, 5 months in AP, and 3 months in BP. Use of second-generation TKI after failure to 2 TKIs may induce responses, but these are usually not durable except in some CP patients. New treatment options are needed.
Introduction
Most patients with chronic-phase chronic myeloid leukemia (CP CML) have a sustained response to imatinib. However, some can develop resistance to imatinib via various mechanisms, including point mutations in the Abl kinase domain and overexpression of Bcr-Abl.1,2 Mutations have been reported in many different amino acids, each conferring different levels of resistance.3,4 In addition, Src-related kinases are up-regulated in some cases of imatinib resistance, a phenomenon that is thought to contribute to leukemogenesis.5-8
Second-generation tyrosine kinase inhibitors (TKI), such as nilotinib and dasatinib, have shown increased inhibitory potency against Bcr-Abl kinase and have shown efficacy in treating patients with many of the Bcr-Abl kinase domain mutations that develop on imatinib; T315I is the one mutation clearly resistant to second-generation TKI.9-13 Nilotinib (Tasigna, AMN107; Novartis) is structurally related to imatinib but has 30-fold higher potency and increased selectivity against Bcr-Abl.9 Dasatinib (Sprycel, BMS-354825; Bristol-Myers Squibb) has 300-fold increased potency against Bcr-Abl compared with imatinib and also has Src-inhibitory activity.14 Both nilotinib and dasatinib have been approved for the treatment of patients with CML after imatinib failure.
With the availability of imatinib, nilotinib, and dasatinib, a scenario seen with increasing frequency is that of patients who have failed imatinib and one of the second-generation TKIs. The other second-generation TKIs are usually considered viable alternatives for therapy, and preliminary results suggest that some patients may indeed respond to a second-generation TKI used as third-line therapy.15,16 However, the long-term benefit of such an approach is largely unknown. In this study, we report the response rates and long-term results of using a second-generation TKI after failure to imatinib and another second-generation TKI.
Methods
Study group
Patients with CML who were sequentially treated with 3 different TKIs at M. D. Anderson Cancer Center between September 2004 and July 2008 were included in this analysis. Doses were adjusted for toxicity as previously described.12 Patients were followed with complete blood counts, cytogenetic analysis, bone marrow aspirations, and real-time reverse transcription-polymerase chain reaction every 3 months. Mutational analysis by direct sequencing was performed on each patient after imatinib failure and before the start of both second and third TKIs.
Patients were switched to second- or third-line TKI when they had a treatment failure. Treatment failure was defined as failure to achieve a complete hematologic response (CHR), (CP only), or any hematologic response (accelerated phase [AP] or blast phase [BP]) after 3 months of therapy, persistence of 100% Philadelphia chromosome (Ph)–positive metaphases after 6 months of therapy, or 35% or more after 12 months, transformation to AP or BP, or loss of cytogenetic response or CHR at any time during the course of therapy.17 Patients who were unable to continue therapy because of toxicity (ie, intolerant) were recorded as having treatment failure.
All patients were registered in studies approved by the M. D. Anderson Cancer Center Institutional Review Board, and informed consent was provided in accordance with the Declaration of Helsinki.
Definition of response
Response criteria were as previously described.18 CHR was defined as a normal white blood cell count with normal differential and platelet count less than 10 × 109/L, and no signs or symptoms of leukemia, including resolution of splenomegaly. Cytogenetic response assessment was based on karyotype analysis of at least 20 metaphases and defined as complete (CCyR, 0% Ph+), partial (PCyR, 1%-35%, Ph+), and minor (mCyR, 36%-95% Ph+). A major cytogenetic response (MCyR) included CCyR and PCyR (ie, ≤ 35% Ph+). Molecular response was assessed by real-time TaqMan-based quantitative polymerase chain reaction as previously described. Major molecular response (MMR) was defined as bcr-abl/abl ratio of less than or equal to 0.05%19
Statistical analysis
Event-free survival was considered from the time the third TKI was started to loss of major hematologic response, loss of cytogenetic response, transformation to AP or BP phase, or death. Failure-free survival was considered from the start of the third TKI to development of an event as defined for event-free survival, or loss of CCyR, or discontinuation because of toxicity. Overall survival was considered from the start of the third TKI to death.
Results
Patient characteristics
A total of 48 patients were treated with sequential TKI: 34 with dasatinib after imatinib and nilotinib failure, and 14 with nilotinib after imatinib and dasatinib failure (Table 1). The median age was 52 years (range, 18-70 years). Before starting imatinib, 27 (56%) patients received interferon-α, 5 (10%) received homoharringtonine (HHT), 14 (29%) received single-agent cytarabine, and 2 (4%) had undergone an allogeneic stem cell transplantation (SCT). Twenty patients (42%) received imatinib as first-line therapy. At the start of imatinib, 39 (81%) patients were in CP, 8 (17%) in AP, and 1 (2%) in BP; 5 in AP in the group who went on to nilotinib as second-line therapy and 3 AP/1 BP in the group who went on to dasatinib as second-line therapy. The median time on imatinib was 36.3 months, and the median dose was 400 mg daily. Best response to imatinib was 4 MMR, 4 CCyR, 10 mCyR, 10 CHR, and 1 no response (NR); 19 patients did not have data available as they presented to our hospital after having failed imatinib. Forty-seven patients (98%) were taken off of imatinib because of resistance and 1 (2%) because of intolerance (grade 3 rash). Twenty-three patients (48%) were in either AP or BP on starting a second TKI.
Response to therapy with a second TKI
At the start of nilotinib as second-line TKI treatment, 17 patients were in CP (50%), 10 in AP (29%), and 7 in BP (21%). Fifteen patients (44%) received a starting dose of 400 mg twice daily. Other patients received 600 mg twice daily (n = 6), 400 mg once daily (n = 5), 800 mg once daily (n = 2), 100 mg once daily (n = 2), and 1 patient each 1200 mg once daily, 100 mg twice daily, 50 mg once daily, and 200 mg once daily. Twenty patients (59%) in this group had a mutation before starting nilotinib (Table 2). Twenty patients (59%) received no other treatment between the time of imatinib failure and the start of nilotinib, whereas 3 (9%) received anagrelide, 2 (6%) tipifarnib, 2 (6%) decitabine, 3 (9%) chemotherapy with cytarabine and idarubicin, and 1 patient each (3%) HHT, 6-mercaptopurine, topotecan, single-agent cytarabine, gemtuzumab ozogamicin, etoposide, mitoxantrone, all-trans retinoic acid, and allogeneic SCT. The best response to the second-line treatment with nilotinib was 5 MMR (15%), 3 CCyR (9%), 4 PCyR (12%), 11 mCyR (32%), 10 CHR (29%), and 1 (3%) NR. Thirty patients (88%) eventually failed nilotinib therapy because of resistance, whereas 4 (12%) were considered intolerant (dyspnea/pulmonary edema, gynecomastia, atrial fibrillation, and non–Q-wave myocardial infarction in a patient with a history of stent placement). Among the 30 patients who developed resistance to nilotinib, 17 (57%) had mutations identified before starting nilotinib. In 14 (82%), the same mutation persisted after failure to nilotinib, whereas in 1 (6%) a new mutation was observed. In 2 patients (12%), the baseline mutation disappeared (Table 2).
Among the 14 patients treated with dasatinib as second-line treatment, 8 patients were in CP (57%), 3 in AP (21%), and 3 in BP (21%). Seven patients (50%) received a starting dose of 70 mg twice daily. Other patients received doses of 50 mg twice daily (n = 3), 100 mg once daily (n = 2), 180 mg once daily (n = 1), and 50 mg once daily (n = 1). Four patients (29%) had baseline mutations before starting dasatinib (Table 2). Eleven patients (79%) did not receive interval treatment between the discontinuation of imatinib and start of dasatinib, whereas 1 patient received HyperCVAD followed by allogeneic SCT, 1 received anagrelide, and 1 received several sequential treatments, including cytarabine, interferon-α, decitabine, HHT, and anagrelide. The best response to dasatinib included 2 CCyR (14%), 1 PCyR (7%), 5 mCyR (36%), 4 CHR (29%), and 2 NR (14%). Nine patients (64%) eventually failed dasatinib treatment because of resistance and 5 (36%) were taken off because of intolerance (pleural effusion in 2 patients, thrombocytopenia in 2, and protein-losing enteropathy in 1). Of 9 (64%) patients who failed dasatinib because of resistance, 1 (11%) had a mutation identified before the start of therapy with this agent, and this mutation persisted at the time resistance developed (Table 2).
The median time on second-line TKI (dasatinib or nilotinib) was 8.3 months (range, 1.1-42 months). Nineteen of 34 patients (56%) in the nilotinib group and 5 of the 14 (36%) in the dasatinib group had either AP or BP before starting the third-line TKI (Table 2).
Response to therapy with a third TKI
At the start of third TKI, 25 patients (52%) were in CP, 10 (21%) in AP, and 13 (27%) in BP. Twenty-four of 34 patients (71%) on dasatinib and 8 of 14 (57%) on nilotinib as third-line TKI had mutations before treatment initiation (Table 2).
The best response to the third TKI in CP was 5 MMR, 3 CCyR, 2 PCyR, 3 mCyR, 6 CHR, and 6 NR. In AP, 1 patient achieved MMR, 1 CCyR, 2 PCyR, 1 mCyR, 4 CHR, and 1 NR. In BP, there were 1 MMR, 2 CCyR, 1 PCyR, 1 mCyR, 2 return to CP (RCP), and 6 NR (Table 3). In the dasatinib group, 7 patients (21%) discontinued treatment because of toxicity despite an acceptable response, including 2 patients who discontinued because of pleural effusion, and 1 each for gastrointestinal bleeding, neutropenia, renal failure, atrial fibrillation, and myalgias. None of the 14 patients on third-line nilotinib was taken off because of intolerance (Table 4).
The median CCyR duration for the 11 patients (2 patients with MMR did not have CCyR) who achieved this response was 16 months (range, 2-49 months); only 3 patients in CP who achieved CCyR (2 also attained MMR) had a response lasting longer than 12 months (28.6+ months, 16.3+ months, and 18.7+ months). These included 1 patient treated with nilotinib after imatinib and dasatinib failure who had mutations V299L and F486S at the start of nilotinib, and 2 patients treated with dasatinib after imatinib and nilotinib failure (1 with H396R mutation and 1 with E459G mutation at the start of dasatinib). Nine of 34 patients (26%) on dasatinib and 3 of the 14 patients (21%) on nilotinib had disease transformation while on therapy with the third TKI.
After a median follow-up of 16 months (range, 3-34 months), 13 patients continue on therapy (10 CP, 2 AP, 1 BP; 7 on nilotinib, 6 on dasatinib; Tables 5–6). Of the 10 patients in CP, 5 continue with their best response after a median follow-up of 15 months (range, 3-34 months): 2 with sustained CCyR, 1 PCyR, and 2 mCyR. Four other patients have lost their best response: 1 patient lost a CHR, 1 lost a CCyR (now in PCyR), and 2 lost a MMR (1 in CHR, 1 in mCyR); 1 patient has not responded after 10 months. Both AP patients are still on therapy after having lost their best response: 1 lost a CHR and 1 lost a MMR (now in PCyR). The patient in BP is still on therapy and remains in MMR.
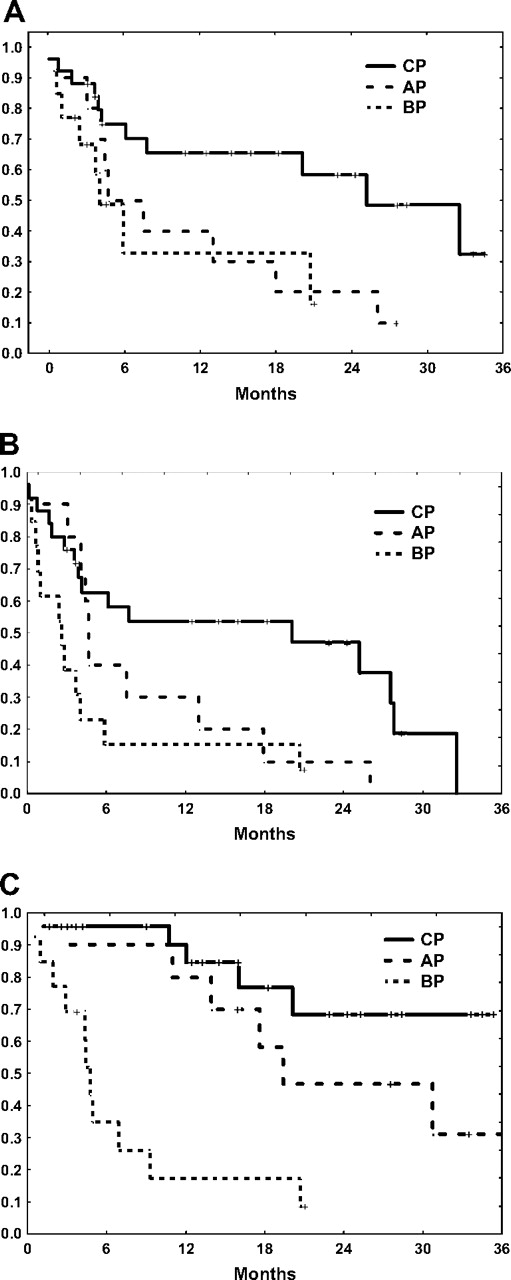
With a median follow-up for the total population of 13 months (range, 0.5-41 months) since the start of the third TKI, 25 patients (31%) are still alive, including 15 treated with dasatinib and 10 with nilotinib for a median overall survival of 20 months. The median event-free survival for the total group is 13 months, and the failure-free survival is 5 months. The outcome varies by stage of the disease, with a median failure-free survival of 20 months for patients in CP at the time the third TKI was commenced, compared with only 5 months for patients treated in AP and 3 months for those in BP (Figure 1).
Patient outcome on third-line TKI. (A) Event-free survival. (B) Failure-free survival. (C) Overall survival.
Patient outcome on third-line TKI. (A) Event-free survival. (B) Failure-free survival. (C) Overall survival.
Discussion
Although imatinib remains the standard treatment for patients with CML, second-generation TKIs have provided additional therapeutic options for patients with CML who fail imatinib. Despite the significant clinical activity demonstrated in clinical trials, the rate of CCyR is only 40% to 50%, leaving a significant number of patients who do not achieve the optimal response; some may eventually lose their response. It has been suggested that patients who do not achieve a MCyR by 12 months from the start of therapy with second-generation TKI or who are still 100% Ph+ by 3 to 6 months have an increased risk of progression.20 These data are frequently used to consider a change to alternative therapies. With the availability of 2 of these agents, nilotinib and dasatinib, it has become an increasingly common practice to use one of these agents when patients have failed imatinib and the other second-generation TKI. Early data have suggested that this strategy may be of benefit to some patients. Another study reported a hematologic and cytogenetic response rate of 57% and 30%, respectively, among 23 patients with CML in all phases treated with dasatinib after having failed imatinib and nilotinib (all resistant to nilotinib).15 Similarly, a recent report indicates that a MCyR was achieved with nilotinib by 32% of patients with CML in CP who had failed prior therapy with imatinib and dasatinib.16 The results of our present analysis confirm the activity of a third-line TKI (either dasatinib or nilotinib) in patients with CML after failure of both imatinib and nilotinib/dasatinib (second-line therapy). Overall, 33% of patients achieved a MCyR (32% in CP, 40% in AP, and 31% in BP), and 65% of patients in AP or BP achieved a CHR. Furthermore, 7 of 48 (15%) patients achieved an MMR. Of particular interest, however, was the transient duration of cytogenetic response for those patients who achieved it. The median CCyR duration was 16 months with only 3 patients (all treated in CP) having a CCyR sustained for more than 12 months.
One reason for the lack of durable cytogenetic remission could be the emergence of new kinase domain mutations as patients are exposed to sequential TKI. Twelve patients had new mutations that emerged after second-line treatment with a TKI; 7 new mutations emerged with nilotinib as second-line therapy (1 patient each developed A276G, Y253F, Y253H, E255V, F311L, F359C, and H396P) and 5 with dasatinib (F317L in 2 instances, Y253H in 1 patient, and 2 patients with 2 coexisting mutations, F359V/F311L, and V299L/F486S). In some instances, the emerging mutation had low in vitro sensitivity to the agent being administered but higher sensitivity to the alternative agent (eg, V299L emerging on dasatinib). In some of these instances, a change of therapy resulted in an adequate response (eg, patient 2 in Table 2). Interestingly, however, some of the mutations have been reported to have relatively good in vitro sensitivity to the agent being administered when they emerged. Two of the 7 mutations (F311L and H396P) that emerged during nilotinib therapy have a reported cellular proliferation IC50 less than 50nM, which suggests adequate in vitro sensitivity. Similarly, 2 of 5 mutations (Y253H and F359V/F311L) emerging on dasatinib as second-line therapy have a cell proliferation IC50 less than 3nM suggesting good in vitro sensitivity to dasatinib.11 For some mutations (eg, A276G and F359C), in vitro sensitivity to second-generation TKI has not been reported.
Of particular concern is the emergence of T315I as this mutant is not inhibited by any of the available TKIs. It could be that consecutive treatment with the available TKI may cause selection of this mutation. In our series, 3 patients had T315I at the start of the second TKI, and this remained detectable before the start of the third TKI. As expected, none of these patients achieved an MCyR. Importantly, none of the patients developed the T315I mutation during third-line treatment.
Several reports have suggested that there is little cross-intolerance between imatinib and dasatinib21 or nilotinib.22 In our series, 7 of 48 (15%) patients (all on dasatinib) discontinued third-line TKI treatment because of grade 2 or 3 side effects, including myalgia, renal failure, gastrointestinal bleeding, and pleural effusions. Of these 7 patients, 2 also were taken off of nilotinib as second-line therapy because of side effects (one developed a pleural effusion with dasatinib and ECG abnormalities on second-line therapy with nilotinib, whereas another patient had a pleural effusion on dasatinib and had pulmonary edema on second-line therapy with nilotinib). None of these 7 patients had intolerance to imatinib. The relatively high incidence of side effects while on the dasatinib arm as third-line TKI could be because these patients were heavily pretreated before starting dasatinib (median, 5 prior therapies; range, 2-8 prior therapies). Cytogenetic data are available for 6 of these 7 patients as 1 patient discontinued dasatinib because of side effects (myalgias) shortly after the start of therapy. Of these 6 patients, 2 achieved a MCyR with therapy, and it was sustained in 1 patient at the time therapy had to be discontinued. The one patient with a durable MCyR response was given a starting dose of 50 mg twice daily. The other patients received 70 mg twice daily (n = 3), 140 mg once daily (n = 2), and 100 mg once daily (n = 1). It is possible that, in more heavily treated patients, alternative doses and schedules should be investigated to maximize safety and efficacy.
The long-term efficacy of TKI therapy is frequently expressed in terms of event-free survival or duration of MCyR. However, these definitions frequently underestimate other reasons for failure, such as intolerance or lack of cytogenetic response. To paint a more realistic picture of the true benefit of this strategy, we analyzed the failure-free survival where all of these endpoints were considered as failure in addition to the more conventional definition of loss of MCyR or CHR, transformation, or death. When analyzed in this way, the strategy of using a second-generation TKI as third-line therapy results in minimal value for most patients as the time to failure is only 3 to 5 months for patients in advanced stages and 20 months for those in CP. The transient benefit may be valuable to serve as a bridge to SCT where an improved hematologic and cytogenetic status may be of benefit. Indeed, two-thirds of the patients treated in this series in AP or BP achieved a CHR. This strategy cannot be relied on as a long-term solution for these patients. Allogeneic SCT should be considered in all patients who fail 2 TKI who are adequate candidates for this approach. For others, alternative strategies are required.
In conclusion, the use of a second-generation TKI after failure of 2 TKIs may induce responses in some patients, but these are usually not durable except in occasional patients in CP. There remains no clear difference between the different sequences explored in our study (ie, imatinib → dasatinib → nilotinib, vs imatinib → nilotinib → dasatinib). Patients who fail 2 TKIs should be offered allogeneic SCT if they are eligible candidates, and new strategies are needed for all others.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: R.J.G. collected, analyzed, and interpreted the data, performed the research, and wrote the manuscript; H.K., S.O., A.Q.-C., S.F., and Z.E. treated the patients and edited and reviewed the manuscript; and J.C. designed and performed the research, analyzed and interpreted the data, performed the statistical analysis, treated the patients, and helped write the manuscript.
Conflict-of-interest disclosure: J.C. and H.K. receive research support from Novartis and Bristol-Myers Squibb. The remaining authors declare no competing financial interests.
Correspondence: Jorge Cortes, Department of Leukemia, Box 428, University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: jcortes@mdanderson.org.