Abstract
Forkhead transcription factors form a superfamily (19 subclasses FOX-A to –S) of evolutionary conserved proteins that function in diverse physiological processes including cellular differentiation, tumor suppression, metabolism, cell cycle arrest, resistance and apoptosis. The members of the FOXO family (FOXO 1, 3, 4, 6), are involved with chromosomal translocations in many tumors including leukemia, and have been shown to be tumor suppressors. Their function interacts with several signal transduction pathways (Ras-Mek-Erk , PI3K-AKT) shown to be deregulated and prognostic in AML. Phosphorylation leads to inactivation via a shift from nuclear to cytoplasmic localization and increased ubiqination and protein degradation. Loss of function could therefore arise from under expression or phosphorylation.
To assess the importance of FOXO3A, in AML we measured the level of total and phosphoprotein (pFOXO3A) expression, by reverse phase protein array (RPPA) in 511 newly diagnosed AML patients.
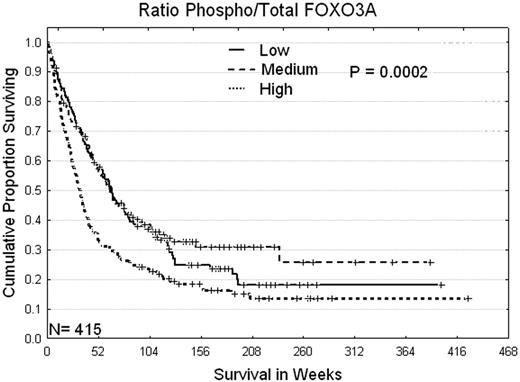
Expression of total and pFOXO3A was in the same range as normal CD34+ cells but the mean expression was significantly lower for both ( P = 0.0001 and 0.0025) Expression in 140 paired blood and marrow samples were similar (total p=0.12, pFOXO3A p=.022). Levels of total FOX3A were higher at relapse compared to diagnosis (p=0.0007). Levels of pFOXO3A or the ratio of phospho to total (PT) were higher in FAB M5 (p=0.000008) and lower in FAB M2 (p=0.00002). Levels of p- or PT-FOXO3A were not associated with karyotpe but were significantly higher in patients with FLT3 mutations (p=0.000004). Higher levels of p- or PT-FOXO3A were associated with increased proliferation evidenced by strong correlation with higher WBC, percent marrow and blood blasts (all p <0.00001) and by correlation with higher levels of Cyclins B1, D1 and D3, pGSK3, LYN, pMTOR pPKCδ, pPKCγand pStat5 (all with p <0.0000004). PT-FOXO levels were also strongly correlated with apoptosis proteins Bax and BAK and inactivated Bad-pSerine136. Patients with High levels of p- or PT-FOXO3A had higher rates of primary resistance and shorter remission durations which combine to cause an inferior survival experience (median survival 33 weeks (See figure, p = 0.0002) compared to patients with low or middle levels (median survival 60 and 64 weeks). A high PT-FOXO3A level was adverse for patients with intermediate (p=0.034) and unfavorable (p=0.024) cytogenetics with a similar but not significant trend noted for those with favorable cytogenetics. This was independent of the presence or absence of a FLT3-ITD or D835 mutation. In multivariate analysis PT-FOXO3A was a statistically significant independent predictor (p = 0.008) along with Age (<0.000001), cytogenetics(<0.000001) albumin (<0.000003) FLT3 mutation (0.005) and gender ( p = 0.03).
Patients with high ratios of PT-FOXO3A have a higher proliferation rate, higher rates of primary resistance, shorter remission durations and significantly shorter survival. High levels of phosphorylation of FOXO3A are a therapeutically targetable, independent adverse prognostic factor in AML.
Character count 3519
No relevant conflicts of interest to declare.
This icon denotes an abstract that is clinically relevant.
Author notes
Asterisk with author names denotes non-ASH members.