Abstract
The BCR–ABL T315I mutation represents a major mechanism of resistance to tyrosine kinase inhibitors (TKIs). The objectives of this retrospective observational study were to estimate overall and progression-free survival for chronic myeloid leukemia in chronic-phase (CP), accelerated-phase (AP), or blastic-phase (BP) and Philadelphia chromosome—positive (Ph)+ acute lymphoblastic leukemia (ALL) patients with T315I mutation. Medical records of 222 patients from 9 countries were reviewed; data were analyzed using log-rank tests and Cox proportional hazard models. Median age at T315I mutation detection was 54 years; 57% cases were men. Median time between TKI treatment initiation and T315I mutation detection was 29.2, 15.4, 5.8, and 9.1 months, respectively, for CP, AP, BP, and Ph+ ALL patients. After T315I mutation detection, second-generation TKIs were used in 56% of cases, hydroxyurea in 39%, imatinib in 35%, cytarabine in 26%, MK-0457 in 11%, stem cell transplantation in 17%, and interferon-α in 6% of cases. Median overall survival from T315I mutation detection was 22.4, 28.4, 4.0, and 4.9 months, and median progression-free survival was 11.5, 22.2, 1.8, and 2.5 months, respectively, for CP, AP, BP, and Ph+ ALL patients. These results confirm that survival of patients harboring a T315I mutation is dependent on disease phase at the time of mutation detection.
Introduction
BCR-ABL kinase domain mutations have been identified as the major mechanisms of resistance to tyrosine kinase inhibitors (TKIs) in Philadelphia chromosome—positive (Ph+) leukemias. The BCR-ABL T315I mutation affects a common Abl kinase contact residue and confers high-level cross-resistance to all current approved Abl kinase inhibitors (imatinib, dasatinib, and nilotinib).1-4 The reported T315I mutation frequency in imatinib-resistant chronic myeloid leukemia (CML) patients ranges between 2% and 20%,2,5-8 with variability related to detection methods as well as patient cohort characteristics and treatment. T315I mutation frequency appears to be greater in Ph+ acute lymphoblastic leukemia (ALL) patients2 and likely increases with the continuation of TKI treatment.3
Although hematologists are now increasingly aware of the importance of the T315I mutation, published literature focusing on survival information for CML patients with T315I mutation remains very limited. A multicenter French study of 27 T315I+ CML patients suggested that the presence of a T315I mutation is associated with significantly worse survival than other mutations and is more strongly prognostic in chronic phase (CP) compared with accelerated phases (APs) of CML.8,9 Conversely, a more recent study10 from the M. D. Anderson Cancer Center, also of 27 patients with the T315I mutation, suggested no statistically significant survival difference between patients with T315I mutation and those with other mutations. Because of limited sample size, neither of these studies had adequate statistical power to query survival information for different phases of CML (CP, AP, or blastic phase [BP]), and no information has been published to date on the survival of Ph+ ALL patients harboring the T315I mutation.
Several investigational agents have been reported to demonstrate activity against the T315I mutation. Giles et al11 reported 3 patients with T315I+ CML or Ph+ ALL who achieved clinical responses to the aurora kinase inhibitor MK-0457 without associated adverse events, whereas Legros et al12 reported that the T315I mutation disappeared in a CML patient treated with homoharringtonine. In addition, it has been reported that some CML patients harboring a T315I mutation may have a transient response to second-generation TKIs in vivo.10 Several other agents have demonstrated activity against T315I mutation-bearing leukemic cells in preclinical models; however, none of these agents has been clinically proven to be safe and effective in the treatment of CML and Ph+ ALL patients with T315I mutations.
It can be anticipated that the number of CML and Ph+ ALL patients with T315I mutation will increase as the use of TKIs increases. Given the lack of information regarding the response to treatment and subsequent survival of patients who develop a T315I mutation while on TKIs, we conducted a multicenter epidemiologic study and collected detailed demographic, clinical, treatment, and mutation detection information for patients identified as harboring the BCR-ABL T315I mutation.
Methods
Study population
Eligible patients were those with CML and Ph+ ALL identified as possessing the T315I mutation between the years 1999 and 2008 by the use of any available validated technique. Patients were 18 years of age or older when their T315I mutation was detected, had received treatment with first- (imatinib) or second-generation (dasatinib or nilotinib) TKIs, and had documented hematologic or cytogenetic resistance, either primary or secondary (acquired), as described in the European LeukemiaNet Guidelines.8,13,14 The minimum follow-up time from the date of T315I mutation detection for patients who were still alive was 3 months; there was no required minimum follow-up time for patients who were deceased. A total of 9 countries from 3 regions participated in this study, including Europe (Denmark, France, Germany, Italy, United Kingdom), Asia (Japan, South Korea, Singapore), and North America (United States). Eligible patients were identified either through a national CML database (ie, French group of CML, Fi-LMC group), central laboratory databases (ie, Italian and German patients), or single hospital-based databases. It is notable that information on a small proportion of patients has been published.8-10 The study was approved by the institutional review board/ethics review committee for each participating site/country. Written informed consent for voluntary participation in the study was obtained from the patient or his or her legal representative, where required, in accordance with the Declaration of Helsinki.
Data collection
Uniform case report forms (CRFs) were designed to collect detailed demographic, clinical, treatment, mutation detection, and current survival information from medical records and/or clinical databases at each site. The same criteria for different phases of CML and Ph+ ALL, treatment response, disease progression, and TKI resistance were used for all sites. T315I mutation was detected by the use of different methods, including direct sequencing, polymerase chain reaction (PCR)–based methods, and denaturing high-performance liquid chromatography. Detailed information on each mutation test (irrespective of T315I presence or absence) was collected, including dates of sample collection and analysis, biologic sample type, techniques used for mutation detection, and other mutated clones detected. T315I mutation detection date was defined as the sample collection date in which a T315I mutation was first identified, and the predominant clone was defined as the one present in the highest percentage among all detected clones, whenever this information was available.
Survival measurement
Overall survival (OS) and progression-free survival (PFS) were the 2 primary end points for this study. OS was calculated from different starting points (from the date of first TKI treatment initiation, date of first identification of TKI resistance, and date of T315I mutation detection) to date of death or most recent date that the patient was known to be alive, stratified by the corresponding disease phase at different starting points. TKI resistance and treatment failure and disease progression were defined on the basis of the criteria of the European LeukemiaNet14 and the pivotal International Randomized Study of Interferon Versus STI571 (ie, IRIS) clinical trial.15
Quality control
Two levels of quality control were performed to ensure the quality of the data. At each site, a minimum of 25% of patients identified for chart abstraction or at least one medical chart (if n < 4) was reviewed by a second, independent staff member at the site. Identification of suboptimal CRF completion resulted in data management retraining and repeat abstraction of data. In addition, site monitors checked the completion of each CRF and performed source data verification on 6 critical data points: date of diagnosis, disease phase at diagnosis, date of first TKI resistance, date of T315I mutation detection, phase at T315I mutation detection, and date of death or most recent date that the patient was known to be alive.
Statistical analysis
Median follow-up time was calculated for all patients. Kaplan-Meier plots and log-rank tests were used for survival analysis for different phases of CML (CP, AP, and BP) and Ph+ ALL patients, starting from the date of first TKI treatment initiation, date of first TKI resistance, and date of T315I mutation detection. Separate Cox proportional hazard models on OS from T315I mutation detection were performed for each phase of CML and Ph+ ALL patients. Candidate covariates include demographics (eg, age, sex, race, country), performance status, and mutation detection (eg, techniques and biologic samples used for T315I mutation detection, whether the T315I BCR-ABL mutation was predominant, whether other mutations were detected before T315I or concurrently with T315I). Because of the retrospective study design and the researchers' inability to collect adequate information on dosing, drug therapies could not be included in the Cox proportional hazard models. If the 2-sided P value for a certain covariate was less than .10 in crude analysis, this covariate was included in the adjusted Cox model for further analysis by the use of backward stepwise methods.16
Results
Demographic information
A total of 222 patients were included in this study (Table 1). The median age at time of T315I mutation detection was 54 years (range, 18-84 years). A total of 126 (57%) patients were men. The majority of patients were white (75%), and 22% were Asian, likely the result of disproportionate accrual from 1 site in South Korea. Most of the patients were from France (32%; 13 sites), Italy (21%; 2 sites), South Korea (15%, 1 site), and the United States (12%, 3 sites).
The majority of CML patients were in CP at the time of diagnosis (70%) or TKI initiation (60%); however, at the time of T315I mutation detection, many patients in CP had progressed to AP and BP, such that only 82 patients (37%) remained in CP, whereas 38 (17%) progressed to AP and 56 (25%) progressed to BP, in addition to the 46 (21%) patients who were Ph+ ALL at the time of T315I mutation detection. Of the patients in BP, 16 were lymphoid, 32 were myeloid, and 8 were either biphenotypic or missing phenotype.
Advanced-phase CML (AP and BP) and Ph+ ALL patients were usually treated with TKIs shortly after diagnosis (median time between diagnosis and TKI initiation: AP, 1.1 months; BP, 0.5 months; Ph+ ALL, 0.4 months vs CP, 11.7 months) and developed TKI resistance within a shorter period of time than CP patients (median time between TKI initiation and TKI resistance: AP, 9.4 months; BP, 4.7 months; and Ph+ ALL, 8.8 months vs CP, 17.4 months). The median time between TKI treatment start and T315I mutation detection was 29.2 months, 15.4 months, 5.8 months, and 9.1 months, respectively in CP, AP, BP, and Ph+ ALL patients.
Treatment information
Treatment before TKI initiation.
A minority of patients (n = 60, 27%) had TKIs as the first line of treatment after diagnosis, most of whom received imatinib as first-line treatment (56 of 60). A total of 162 (73%) patients had other treatment(s) before TKIs, including hydroxyurea (n = 118, 53%), interferon-α (IFN-α, n = 77, 35%), cytarabine (n = 43, 19%), and stem cell transplantation (n = 14, 6%; including 7 autologous and 7 allogeneic transplantations).
Treatment between TKI initiation and T315I mutation detection.
All 222 patients received TKI therapy before T315I detection. Between TKI treatment initiation and T315I mutation detection, 97% of patients received imatinib for a median duration of 13 months (range, 0.3-77.0 months); 50% received a second-generation TKI for a median duration of 3.6 months (range, 0.1-31.2 months); 32% were treated with hydroxyurea (median duration 2.5 months [range, 0.1-49.4 months]); 27% with cytarabine (median duration 0.5 months [range, 0.1-20.9 months]); 10% underwent stem cell transplantation, including 7% allogeneic transplantation; and 8% were treated with IFN-α (median duration 3.8 months [range, 0.4-19.8 months]).
Treatment after T315I mutation detection.
Treatment information after T315I detection was available for 216 patients (Table 2); overall, 125 (58%) patients were treated with second-generation TKI (dasatinib or nilotinib), including 58 (27%) who were continuing treatment that had commenced before T315I detection (carry-over); 87 (40%) with hydroxyurea, including 13 (6%) carry-over; 78 (36%) with imatinib, including 50 (23%) carry-over; 57 (26%) with cytarabine (all new treatment, no carry-over); 37 (17%) with stem cell transplantation, including 31 (14%) allogeneic transplantations; 25 (12%) with MK-0457; 26 (12%) with other investigational agent(s); and 14 (6%) with IFN-α.
After the detection of T315I, the first therapy delivered was a second-generation TKI for 88 (41%) patients (81 [38%] as monotherapy), imatinib in 72 (33%) patients (59 [27%] as monotherapy), and hydroxyurea in 38 (18%) patients (18 [8%] as monotherapy). A total of 151 patients received a second-line therapy after T315I detection: 50 (33%) were treated with hydroxyurea, 34 (23%) with second-generation TKI (25 [17%] as monotherapy), and 31 (21%) with cytarabine (4 [3%] as monotherapy). Additional treatment information after T315I detection is summarized in Table 2.
T315I mutation information
Other mutations before T315I mutation detection.
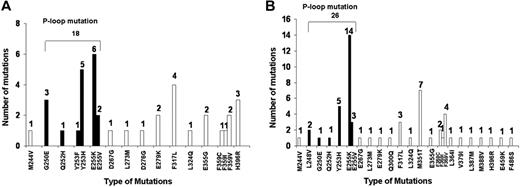
Fifty-eight (26%) of 222 patients had a BCR-ABL mutation test before T315I mutation detection, and the median time between the first mutation test and T315I mutation detection was 7 months (range, 0.5-35 months). Before T315I mutation detection, 62% (36/58) of patients had other mutations detected, and 2 patients had 2 mutations detected in different tests (one patient with G250E + L273M and the other patient with Y253H + F317L). A total of 18 P-loop mutations were detected (Figure 1A), with the most frequent being E255K (n = 6) and Y253H (n = 5).
BCR-ABL mutations detected before and concomitantly to the T315I mutation detection. (A) Other BCR-ABL mutations detected before the T315I mutation detection (n = 36; 16%). Two patients had 2 mutations detected in different tests (one with G250E/L273M and one with Y253H/F317L). (B) Other BCR-ABL mutations detected at the time of T315I detection (n = 52; 23%). Five patients had 2 additional mutations detected. *Solid black bars are different types of P-loop mutations.
BCR-ABL mutations detected before and concomitantly to the T315I mutation detection. (A) Other BCR-ABL mutations detected before the T315I mutation detection (n = 36; 16%). Two patients had 2 mutations detected in different tests (one with G250E/L273M and one with Y253H/F317L). (B) Other BCR-ABL mutations detected at the time of T315I detection (n = 52; 23%). Five patients had 2 additional mutations detected. *Solid black bars are different types of P-loop mutations.
T315I mutation detection.
Most patients (n = 162; 73%) had T315I mutation detected after TKI resistance, with a median “lead time” (time from first TKI resistance until collection of sample that first identified a T315I mutation) of 6.4 months (range, 0.1-73.2 months); 14% had simultaneous categorization of TKI resistance and the presence of the T315I mutation; and 13% of patients had the T315I mutation detected before meeting criteria for clinical resistance, with a median “lead time” (time from collection of sample which first identified a T315I mutation until first TKI resistance) of 1.2 months (range, 0.1-20.7 months previously). In addition, within 1 month either before or after TKI resistance, 114 (51%) patients had mutation analysis performed, and 90 (41%) were identified as harboring the T315I.
Other mutations found at the time of T315I mutation detection.
A total of 52 (23%) patients had other mutations detected concomitantly with T315I detection. By disease phase, 18% (15/82) of CML CP and 26% (37/140) of non-CML CP patients had other mutations detected in addition to T315I. Five patients had 2 other mutations detected in addition to T315I, including G250E plus F317L, Y253H plus M351T, E255K plus V, L273M plus F317L, and M351T plus L387M, totaling 57 mutations among the 52 patients (Figure 1B). Twenty-five patients (11%) had a P-loop mutation detected, the most frequent being E255K (n = 14).
Clonal predominance at the time of T315I mutation detection.
T315I was the predominant clone among 194 (87%) patients when first detected, as reported by each individual participating site. Nine patients had a predominant P-loop mutant clone, including Y253H (n = 1), E255K (n = 6), and E255V (n = 2), and 6 patients had other predominant clones identified, including D267G (n = 1), L273M (n = 1), M351T (n = 2), F359C (n = 1), and F359V (n = 1). Six patients had wild-type BCR-ABL as the predominant clone. The predominant clone could not be determined for 7 patients. Among the 194 patients whose predominant clone was T315I, 30 patients also had other mutations (including 2 patients with 2 other mutations, 1 with G250E + F317L, and 1 with M351T + L387M).
Techniques and biologic samples used for T315I mutation detection.
Of the various techniques used to detect the T315I mutation, direct sequencing was the most frequently used method (55% patients underwent the direct sequencing method only and 12% patients underwent both direct sequencing and various PCR-based methods). Other techniques included reverse transcription-PCR/denaturing high-performance liquid chromatography and sequencing (20%), and a PCR-based method only (13%). Among the 12% of patients in whom mutation analysis was done by both direct sequencing and PCR-based method, there was no discordance between the different techniques. Peripheral blood was the most frequently used sample type (73% used blood samples only and 5% used both blood and bone marrow samples). The majority of the T315I mutant samples (n = 172, 77%) were analyzed within 1 month of collection.
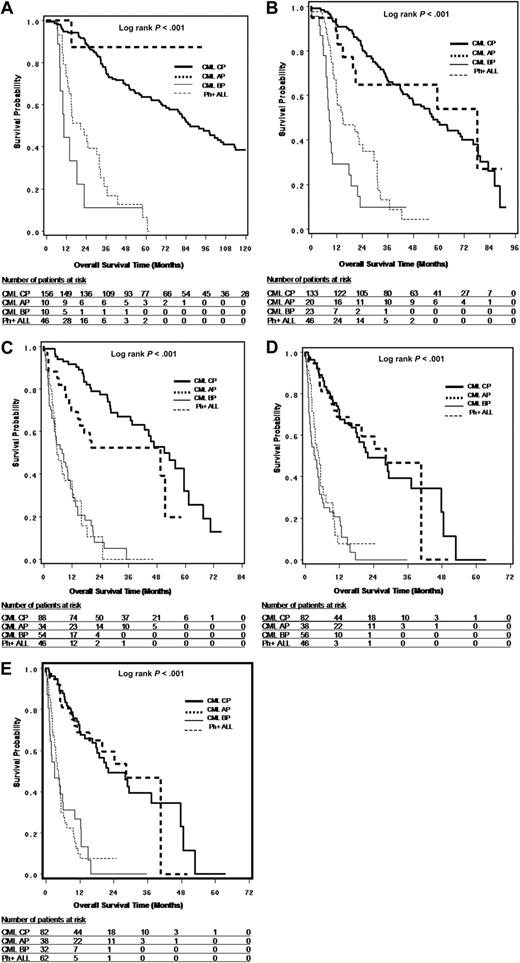
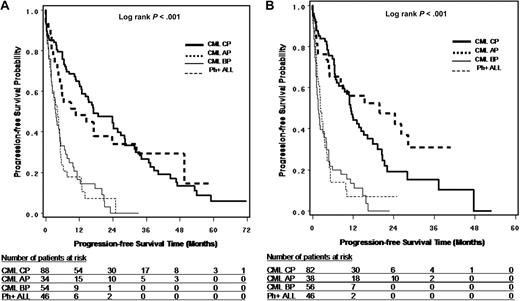
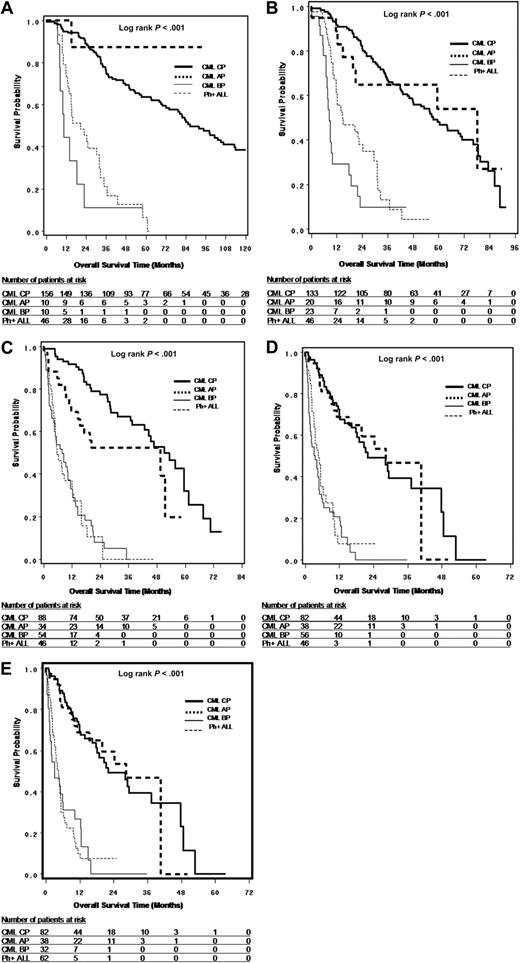
OS and PFS
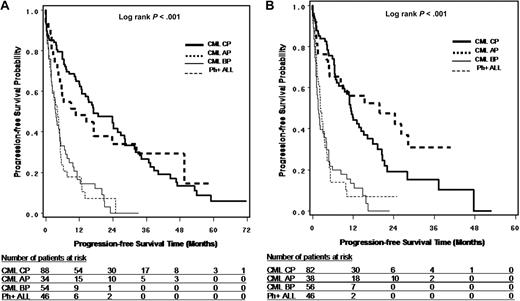
Survival from leukemia diagnosis (OS only), TKI treatment initiation (OS only), first time of TKI resistance (OS and PFS), and T315I mutation detection (OS and PFS) are shown in Figures 2A through D and 3A and B and summarized in Table 3. The results suggest that OS is dependent on disease phase at corresponding starting points and that survival is similar between CP and AP and between BP and Ph+ ALL. For survival from T315I detection, the median OS was 22.4, 28.4, 4.0, and 4.9 months, and median PFS was 11.5, 22.2, 1.8, and 2.5 months, respectively, for CP, AP, BP, and Ph+ ALL patients. It should be noted that the sample sizes for AP and BP patients were limited, especially at the time of leukemia diagnosis and TKI treatment initiation.
Survival analysis in patients with T315I BCR-ABL mutation. (A) OS from leukemia diagnosis by disease phases at the time of leukemia diagnosis. (B) OS from TKI treatment start by disease phase at the time of TKI treatment start. (C) OS from first TKI resistance by disease phase at the time of first TKI resistance. (D) OS from first T315I mutation detection by disease phase at the time of T315I mutation detection. (E) OS from first T315I mutation detection by disease phase at the time of T315I mutation detection, where CML LBP were combined with Ph+ ALL (n = 214)
Survival analysis in patients with T315I BCR-ABL mutation. (A) OS from leukemia diagnosis by disease phases at the time of leukemia diagnosis. (B) OS from TKI treatment start by disease phase at the time of TKI treatment start. (C) OS from first TKI resistance by disease phase at the time of first TKI resistance. (D) OS from first T315I mutation detection by disease phase at the time of T315I mutation detection. (E) OS from first T315I mutation detection by disease phase at the time of T315I mutation detection, where CML LBP were combined with Ph+ ALL (n = 214)
Progression-free survival in patients with T315I BCR-ABL mutation. (A) PFS from first TKI resistance by disease phase at the time of first TKI resistance. (B) PFS from T315I mutation detection by disease phase at the time of T315I mutation detection.
Progression-free survival in patients with T315I BCR-ABL mutation. (A) PFS from first TKI resistance by disease phase at the time of first TKI resistance. (B) PFS from T315I mutation detection by disease phase at the time of T315I mutation detection.
To further understand the OS from T315I mutation detection for CP patients (n = 82), we classified the CP patients into 4 subgroups: patients who had never progressed to AP or BP during their lifetime (n = 34, median OS = 48.9 months); patients who had progressed to AP or BP before T315I mutation detection while remaining CP from the time of T315I mutation detection (n = 13, median OS = 22.1 months); patients who were constant CP before T315I mutation detection and progressed to AP or BP after T315I mutation detection (n = 28, median OS = 29.5 months); and patients who had progressed to AP or BP both before and after T315I mutation detection, although they were classified as CP at the time of T315I mutation detection (n = 7, median OS = 8.7 months). The results suggested that if CP patients had progressed to AP or BP either before or after T315I mutation, their survival was much worse than those who had never progressed to AP or BP during their lifetime.
In an exploratory analysis combining the 16 lymphoid CML BP(LBP) patients with de novo Ph+ ALL patients, the median OS from the time of T315I mutation detection was 4.8 months (95% confidence interval [CI], 1.6-10.0; n = 32) for CML myeloid blastic phase, and 4.5 months (95% CI, 3.5-5.4; n = 62) for LBP/Ph+ ALL (Figure 2E).
Cox proportional hazard model for OS from T315I mutation detection
Cox proportional hazard models on OS from T315I mutation detection were performed for different phases of CML and Ph+ ALL patients. As stated previously, treatment with different drugs were not included in the models because of the retrospective collection of the treatment information. In the crude analysis, older age, female sex, patients from Asian sites, detection of T315I mutation by direct sequencing, detection of T315I mutation by the use of blood samples, worse performance status, and additional clones at the time of T315I mutation detection demonstrated a trend of associating with worse survival across different phases of CML and Ph+ ALL patients. In the adjusted Cox model, however, only the following covariates were either statistically significantly (P < .05) or borderline significantly associated with worse OS (adjusted hazard ratio; 95% CI): older age (by median, 2.30; 1.04-5.09) in Ph+ ALL patients, female sex in BP (1.73; 0.96-3.10); worse performance status in Ph+ ALL (1+ vs 0, 2.18; 1.02-4.68); and detection of T315I mutation by direct sequencing (vs other methods) in AP (3.03; 0.89-10.29) and Ph+ ALL (2.33; 1.06-5.12).
Discussion
We observed a strong link between the emergence of the T315I mutation and disease progression, with a shift in disease phase noted from time of diagnosis/TKI initiation (70%/60% in CP, respectively) to time of T315I mutation identification (37% in CP), with 48% (84 of 176 CML patients) having progressed to AP or BC. In this cohort advanced-phase cases displayed rapid TKI resistance and across all phases the rapidity of identifying a T315I mutation increased incrementally with phase: median time between TKI treatment start and T315I detection was 29.2 months for CP, 15.4 months for AP, 5.8 months for BP, and 9.1 months for Ph+ ALL. This finding suggests that emergence of kinase domain mutants may be a function of clonal instability and the proliferation rate, both of which increased in advanced disease. In addition, exposure of Ph+ ALL and CML AP/BP patients to TKI therapy (≤1 month for median time between diagnosis and TKI treatment start) was much more rapid than CML CP patients (11.7 months), potentially contributing to emergence of T315I clones.
Regarding the use of TKIs after identification of the T315I mutation, 27% (58 of 125) of patients continued (“carried over”) dasatinib or nilotinib, and an additional 31% (the remaining 67 of 125) were switched to dasatinib or nilotinib. Although the use of more potent Abl inhibitors might accentuate T315I clonal selection, this has not been noted to affect survival.9 An additional proportion of patients was maintained on imatinib (23% carry-over) or switched to imatinib (13%). Outside of currently approved TKIs, 12% of cases were treated after T315I with MK-0457 during its period of investigation (as well as another 12% with other investigational drugs, including homoharringtonine and unspecified drugs), often after multiple (eg, 3 or 4) lines of previous therapy; 40% were moved to hydroxyurea, 26% to cytarabine, and 6% to IFN-α, which is not surprising given the timeframe of the study predates clinical trial options for T315I-targeted agents. Finally, 17% of patients moved on to stem cell transplantation, an option currently still advised given lack of approval or proven efficacy for the BCR-ABLT315I active agents in development.17
Regarding mutation detection overall, 62% (36/58) of cases with testing results before T315I detection were found to harbor other kinase domain mutations, consistent with data that initial mutations often are associated with secondary mutations and sequential treatment resistance.18 T315I was determined to be “dominant” in most (87%) of cases and thus “driving” resistance; whether such cases represented compound mutations in single clones or singular mutations in multiple clones is unknown. In 73% of the cases, T315I mutation was identified after a median of 6 months of TKI resistance. In addition, within 1 month either before or after TKI resistance, 114 (51%) patients had mutation analysis performed, and 90 (41%) were identified as harboring the T315I, suggesting that the time from clinical TKI resistance and identifying the T315I mutation, when it occurs, is rather short.
Cox proportional hazard modeling of the study population was limited in its ability to identify predictors of worse outcome. In cases of Ph+ ALL, older age and poorer performance status (likely influence choice and tolerance of treatment), and for unclear reasons detection of T315I by direct sequencing were all associated with worse outcome. When BP patients were regrouped according to myeloid or lymphoid phenotype (myeloid BP vs lymphoid BP and Ph+ ALL), rates of OS were not different from when cases were divided broadly as either BP or Ph+ ALL, addressing the previously unanswered question of difference in natural history of T315I+ lymphoid and myeloid disease.
The similar survival rates observed in CP and AP likely reflects the difficulty in defining precisely late-CP and true AP patients, rather than a unique biology of T315I-associated progression in the limited number of cases reported as AP, as well as the fact that only a minority of patients in this analysis represent de novo primary imatinib-treated patients with the majority representing post-interferon, “late chronic phase” patients with known inferior response rates to imatinib,19 and potentially different disease biology with regard to genesis of genetic instability, as evidenced by greater rates of clonal evolution in Ph− cells. Additional analysis suggested that that if CP patients had progressed to AP or BP either before or after T315I mutation, their survival (median OS, 8.7-29.5 months) is much worse than those who had never progressed to AP or BP during their lifetime (median OS, 48.9 months). Therefore, it is very important to collect and understand the whole disease history of this group of patients.
This study represents the first large-scale assessment of the current natural history and implications of the T315I mutation in Ph+ leukemias. Although cases were culled from a broad range of global CML centers/hospitals, one limitation may be the inclusion of a large fraction of Asian patients (22%; 15% of patients from the South Korean site) and that approximately 50% of cases were from France/Italy (including the majority of Ph+ ALL cases) if different patterns of resistance, response, or survival are geographically or ethnically based. Although not observed to any great degree in other studies, there are suggestions of differential ethnic tolerance and efficacy of TKIs.20,21 Despite its obvious limitations, including retrospective study design and selective populations, this study confirmed that survival of patients harboring a T315I mutation remains dependent on the disease phase at T315I mutation detection, as well as the disease history during patients' lifetime. The fact that we did not observe clear treatment pattern after T315I mutation detection highlights the need for early detection of this mutation in clinically TKI-resistant patients to optimally manage this selective and still elusive resistance pattern with current and emerging therapeutics.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank all the centers and scientific groups worldwide that participated in this study, including the French group of CML (Fi-LMC group) and the German CML study group, Drs John Di'Persio, Hans Hasselbalch, Giuseppe Saglio, Tomoki Naoe, and Yasushi Miyazaki. We also thank the following Merck employees for scientific and operational support: Elisabeth Gachard, Staci Grayson, Jason B. Clark, Jing Su, Jonathan Harris, Greg Hocking, Alexis Anne Serapilio, and Lou Ann Eader.
F.E.N. and C.P. participated on behalf of the French group of CML (Fi-LMC group); M.C.M. and A.H. participated on behalf of the German CML Study Group.
This study was supported by research funding from Merck Research Laboratories.
Authorship
Contribution: F.E.N., M.J.M., G.M., D-W.K., A.H, J.C., J.D.P., S.P, C.R., F.G., and W.Z. designed and performed research; F.E.N., M.J.M., G.M., D-W.K., S.S., M.C.M, A.H., J.C., C.C., I.H.D., J.F.A., F.Y., and C.P. performed research; F.E.N., M.J.M., G.M., D-W K., A.H., J.C., J.F.A., F.Y., J.D.P., S.P., C.R., F.G., J.M.G., and W.Z. analyzed and/or interpreted data; F.E.N., M.J.M., S.S., M.C.M., and W.Z. wrote the manuscript; and G.M., D-W.K., A.H, J.C., C.C., I.H.D., J.F.A., F.Y., J.D.P., S.P, C.R., C.P., F.G., and J.M.G. critically reviewed and approved the manuscript.
Conflict-of-interest disclosure: J.D.P., S.P., C.R., and W.Z. are employees of Merck & Co Inc, who may potentially own stock and/or hold stock options in the Company; F.E.N. received research support from Novartis Pharma France and Chemgenex Inc, honoraria from MSD France, and serves as scientific advisor for Novartis Pharma France and Europe and Bristol-Myers Squibb France; M.J.M. was on the Research and Speaker's Bureau for Novartis Oncology and on the Research, Consultant, and Speaker's Bureau of Bristol-Myers Squibb; D.-W.K. received research support from Novartis, Ilyang Co, and Merck & Co Inc; A.H. received some research support from MSD, Novartis, and Bristol-Myers Squibb; J.C. received research support from MSD, Novartis, and Bristol-Myers Squibb; C.C. received honoraria from Bristol-Myers Squibb Asia-Pacific and serves as scientific advisor for Bristol-Myers Squibb Asia-Pacific and Novartis Pharma Asia-Pacific; and F.G. received research support from Merck. The remaining authors declare no competing financial interests.
Correspondence: Franck E. Nicolini, MD, PhD, Hematology Department, E Pavilion, Hôpital Edouard Herriot, 5 place d'Arsonval, 69437 Lyon, Cédex 03, France; e-mail: franck-emmanuel.nicolini@chu-lyon.fr.
References
Author notes
F.E.N. and M.J.M. contributed equally to this work.