Abstract
Ischemia exists in many diseased tissues, including arthritic joints, atherosclerotic plaques, and malignant tumors. Macrophages accumulate in these sites and up-regulate hypoxia-inducible transcription factors (HIFs) 1 and 2 in response to the hypoxia present. Here we show that the gene expression profile in primary human and murine macrophages changes markedly when they are exposed to hypoxia for 18 hours. For example, they were seen to up-regulate the cell surface receptors, CXCR4 and GLUT1, and the potent, tumor-promoting cytokines, vascular endothelial growth factor A, interleukin (IL)-1β and IL-8, adrenomedullin, CXCR4, and angiopoietin-2. Hypoxia also stimulated their expression and/or phosphorylation of various proteins in the nuclear factor-κB (NF-κB) signaling pathway. We then used both genetic and pharmacologic methods to manipulate the levels of HIFs-1α and 2α or NF-κB in primary macrophages to elucidate their role in the hypoxic induction of many of these key genes. These studies showed that both HIF-1 and -2, but not NF-κB, are important transcriptional effectors regulating the responses of macrophages to such a period of hypoxia. Further studies using experimental mouse models are now warranted to investigate the role of such macrophage responses in the progression of various diseased tissues, such as malignant tumors.
Introduction
Cells experience sustained periods of hypoxia in diseased tissues, such as malignant tumors, atherosclerotic plaques, and arthritic joints.1-3 The predominant transcription factors mediating the effects of hypoxia on gene expression are hypoxia-inducible factors (HIFs) 1 and 2.4,5 These consist of distinct, hypoxia-responsive α subunits and an identical, constitutively expressed β subunit. In the presence of oxygen, the α subunits are hydroxylated by oxygen-sensitive enzymes called prolyl hydroxylases, which targets them for degradation by a ubiquitin-proteasomal pathway.4 In hypoxia, HIFα subunits accumulate and translocate to the nucleus, couple with the HIF-1β subunit, and bind to hypoxic response elements (HREs) in the promoters of various genes, activating their transcription.4,5
Macrophages accumulate in most ischemic diseased sites, including tumors,6-9 where they accumulate both HIF-1α and 2α10,11 and up-regulate HIF target genes, such as the potent proangiogenic growth factor, vascular endothelial growth factor A (VEGFA).12 There are conflicting views of the relative contribution of each HIF to the regulation of hypoxic gene expression in these cells. Some studies suggest that the main form of HIF up-regulated by tumor-associated macrophages (TAMs) is HIF-2,11,13 and overexpression of HIF-2α in normoxic human macrophages up-regulates various proangiogenic genes.14 However, human macrophages also markedly up-regulate HIF-1α when exposed to hypoxia in vitro and in tumors,10 and HIF-1α–deficient murine macrophages express lower levels of such HIF-regulated genes as VEGF and the glucose receptor GLUT1 in hypoxia than their wild-type counterparts.15
Interestingly, the exact contribution of HIFs-1 and -2 to the regulation of hypoxic gene expression appears to vary between different cell types. HIF-1, for example, mediates the induction of virtually all hypoxia-activated genes in mouse embryonic fibroblasts and human breast tumor cells,16,17 whereas HIF-2 performs this function in renal tumor cells.17 This depends partly on the cell type–specific expression of other transcription factors, such as Elk-l, which bind to the promoters of some genes conferring HIF-2 target specificity on them.18,19
Hypoxia may also employ another transcription factor, nuclear factor-κB (NF-κB), as 2 major components of canonical NF-κB signaling, κB kinase β (IKK-β) and p65 (RelA) are activated when murine macrophages experience short-term (< 4 hours) hypoxia. This then up-regulates their expression of both HIF-1α and various HIF target genes.20-22
In the present study, we show that exposure to hypoxia for 18 hours markedly up-regulates a broad array of tumor-promoting genes in primary macrophages, and then investigated the role of HIFs-1 and -2 and NF-κB in this phenomenon.
Methods
Cells
Two forms of primary macrophages were used in this study: macrophages differentiated in vitro from human peripheral blood (monocyte-derived macrophages [MDMs]) and bone marrow–derived macrophages (BMDMs) derived from bone marrow progenitors isolated from control (ie, wild-type or +/−) mice or mice bearing myeloid cell-targeted deletions in either the HIF-1α or HIF-2α gene. Mouse studies were approved and conducted by the Abramson Family Cancer Research Institute.
Isolation and culture of human MDMs.
Monocytes were isolated from Buffy coats (National Blood Service, Sheffield, United Kingdom) as previously described.10 A total of 50 × 106 mononuclear cells were seeded in Iscove Modified Dulbecco Media (BioWhittaker UK Ltd) with 5% human AB serum (neat AB serum contains ∼ 1 ng/mL human CSF-1) and 2 mM L-glutamine (all from Sigma-Aldrich) and incubated at 37°C, 5% CO2. After 2 hours, adherent cells were washed and cultured for 7 days to allow differentiation into MDMs.
Isolation and culture of murine BMDMs.
As previously described,22 BMDMs were isolated from the bones of control mice or mice bearing a targeted deletion of (1) the HIF-1α gene in myeloid cells (2loxP/1loxP, LysM Cre/+ mice15 ) or (2) the HIF-2α gene in myeloid cells (2loxP/1loxP, LysM Cre/+ mice; H.Z.I. and M.C.S., manuscript submitted, April 2009).
Bone marrow aspirates were washed and resuspended in medium with 10% heat-inactivated fetal calf serum (BioWhittaker UK Ltd), 2 mM L-glutamine (Sigma-Aldrich), 100 IU/mL penicillin and 100 μg/mL streptomycin (BioWhittaker UK Ltd), and murine macrophage colony-stimulating factor (M-CSF; PeproTech Ltd), and cultured at 37°C, 5% CO2 for 7 days to allow macrophage differentiation. Their purity was assessed after 7 days using an F4/80 antibody. Only BMDM cultures of more than 90% purity were used in subsequent experiments.
Successful deletion of HIFs-1 or 2α has been demonstrated previously using Southern and/or immunoblotting assays of extracts from hypoxic BMDMs from the HIF-1α LysM-Cre mice23 and HIF-2α LysM-Cre (H.Z.I. and M.C.S., manuscript submitted April 2009) mice used in this study.
Normoxic and hypoxic cell cultures
Human MDMs or murine BMDMs were subjected to severe hypoxia (< 0.5% O2) or normoxia (20.9% O2) in 5% CO2 humidified multigas incubators (Heto) for 18 hours.
siRNA treatment of human MDMs in vitro
siRNA duplexes for HIF-1α or HIF-2α were synthesized by Eurogentec Laboratories. A randomly scrambled duplex was synthesized as a negative control. The HIF-1α siRNA duplex sequences were composed of: sense, 5-CUGAUGACCAGCAACUUGAdTdT-3; and antisense, 5-UCAAG-UUGCUGGUCAUCAGdTdT-3. The HIF-2α siRNA duplex sequences were: sense, 5-CAGCAUCUUUGAUAGCAGUdTdT-3; and antisense, 5-ACUGCUAUCAAAGAUGCUGdTdT-3. The scrambled nonspecific duplex sequences were: sense, 5-AGUUCAACGACCAGUAGUCdTdT-3; and antisense, 5-GACUACUGGUCGUUGAdTdT-3. Transient siRNA transfections were carried out using RNAifect as described by the manufacturer's instructions (QIAGEN). Five-day human MDMs were washed and incubated in 100 μL siRNA complex for 48 hours. Cells were then washed, fresh media added, and cells incubated in normoxia or hypoxia for 18 hours as described earlier.
RNA and protein extraction from human MDMs
Total RNA was prepared using RNeasy kit (QIAGEN) according to the manufacturer's instructions and stored at −80°C. For protein extraction, cells were lysed with lysis buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1% Triton X-100, and 1 protease inhibitor tablet, Roche). Protein levels were measured using the bicinchoninic acid (BCA) protein assay (Sigma-Aldrich).
RNA and protein extraction from murine BMDMs
Total RNA and protein isolation was prepared using NucleoSpin RNA/Protein kit (Macherey-Nagel) and stored at −80°C for RNA and −20°C for protein. For HIF-2α–deficient BMDMs, whole cell extracts were prepared using radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 1% NP40, 0.1% sodium dodecyl sulfate, 0.25% deoxycholate, 1 mM ethylenediaminetetraacetic acid) containing phosphotase inhibitors (0.1 mM sodium fluoride, 1 mM sodium orthovanadate, 2 mM sodium pyrophosphate, and 10 mM β-glycerophosphate). Again, protein extracts were stored at −20°C until used for immunoblotting.
Transcriptional profile analysis
Human Genome U133A plus 2.0 gene chip arrays (Affymetrix UK) that detect 47 000 transcripts were used. Total RNA was reverse-transcribed to generate cDNA libraries using oligo dT and superscript II (Invitrogen). cDNA was amplified using MEGscript T7 kit and cleaned using GeneChip Cleanup (both Affymetrix). Labeled cRNA was synthesized using GeneChip IVT kit and then hybridized to the arrays after the manufacturer's instructions (Affymetrix). Gene chips were processed using an Affymetrix GeneChip scanner 3000.
To verify the results obtained using Affymetrix arrays, total RNA was extracted from 2 separate experiments, reverse-transcribed, amplified, and hybridized to Sentrix HumanRef-8_V2 Bead Chip from Illumina according to the manufacturer's protocols. After washing and drying, the Beadarray was scanned using an Illumina Bead Station 500X, which uses SentrixScan Application, Version 2.7.2 software. Illumina BeadStudio software was used for quality control assessment and normalization of data using the LOESS normalization method from BioConductor R packages.
Genes that were up-regulated in both arrays by more than 1.5-fold or down-regulated by less than 0.67-fold in hypoxia relative to normoxia were considered differentially expressed. One Affymetrix and an Illumina microarray were conducted on RNA isolated from separate experiments. Their combined use was considered to be the first level of screening for the most robust hypoxia robust genes in human macrophages. Only mRNA species regulated by hypoxia on all arrays were considered to be reproducibly regulated by hypoxia and worthy of further study. Using this criterion, 148 genes were up-regulated and 60 genes down-regulated by hypoxia. A panel of selected genes was then further analyzed using real-time polymerase chain reaction (PCR).
Real-time PCR
cDNAs was prepared from 1 μg total RNA using SuperScript Synthesis kit (Invitrogen) and amplified with TaqMan gene expression Master Mix and predesigned gene probes using a ABI 7900HT Sequence Detection System (Applied Biosystems). The human TaqMan gene expression assay probes used were VEGF, interleukin-1β (IL-1β), IL-6, CXCL8, CXCR4 (chemokine C-X-C receptor 4), adrenomedullin (ADM), STAT4, adenosine receptor 2A (ADORA2A), intercellular adhesion molecule 1 (ICAM1), heme oxygenase 1 (HMOX1), prolyl hydroxylase 2 (PHD2), CITED2, heat shock 70-kDa protein 1B (HSPA1B), ADAM metallopeptidase domain 8 (ADAM8), ERO1-like (ERO1L), matrix metalloproteinase 7 (MMP7), glucose transporter 1 (GLUT-1), and β-2-microglobulin as the endogenous control (Applied Biosystems). The murine TaqMan probes used for murine homologs of these were also supplied by Applied Biosystems. Real-time PCR cycling conditions for both human and murine samples were 2 minutes at 50°C and then 95°C for 10 minutes followed by 40 cycles of 15 seconds at 95°C followed by 1 minute at 60°C. In addition, the human NF-κB signaling genes were analyzed using SyBr green real-time PCR. The primer sequences used were as follows: NFKBIA, forward, TCGCAGTGGACCTGCAAAAT; reverse, TGAGCTGGTAGGGAGAATAGC; IKK-α, forward, CACCATCCACACCTACCCTG; reverse, CTTATCGGGGATCAACGCCAG; IKK-γ, forward, CGTACTGGGCGAAGAGTCTC; reverse, GGCTGGCTTGGAAATGCAG; NFKB1 (p50), forward, TGCCAACAGATGGCCCATAC; reverse, TGTTCTTTTCACTAGAGGCACCA; and Rel A, forward, TTGAGGTGTATTTCACGGGACC; reverse, GCACATCAGCTTGCGAAAAGG. Real-time PCR was done using SyBr Green PCR Master Mix, detected by ABI-Prism 5700 Sequence Detector, and data processed using GeneAmp software (Applied Biosystems) The murine TaqMan probes used for murine homologs of Rel A and IKK-β were also supplied by Applied Biosystems. The threshold cycle (Ct) of all human and murine data was normalized against their respective endogenous controls (unaltered by hypoxia). Real-time PCR was analyzed in RNA extracts generated in 3 to 5 independent experiments and then fold changes in expression relative to normoxic cells calculated with ΔCt values of the sample and reference gene using the formula 2−ΔΔCt.
Immunoblotting studies
Immunoblotting for human HIFs-1α and 2α was conducted as described previously10,11 using 1:1000 antihuman HIF-1α monoclonal antibody supplied by BD Biosciences or 1:1000 antihuman HIF-2α monoclonal antibody from Novus. Both blots were incubated with horseradish peroxidase-conjugated antimouse antibody (Dako Denmark) and protein bands visualized using an enhanced chemilluminescence detection system (GE Healthcare). In all cases, expression of β-actin was used as a loading control. For NF-κB immunoblotting assays, an antihuman phospho-NF-κB p65, total NF-κB p65, phospho-IKK-α/IKK-β, or total IKK-α/β (Cell Signaling Technology) was used at a dilution of 1:500 or 1:1000 and incubated overnight at 4°C.
Cytokine release assay
Cell supernatants were centrifuged for 5 minutes at 400g and filtered to eliminate cell debris and then stored at −20°C. The levels of VEGF, IL-8, and IL-1β in these supernatants were measured using a BD FACSArray bioanalyzer (BD Biosciences).
Role of NF-κB in hypoxic gene regulation in primary macrophages
This was investigated in 2 ways. First, human MDMs were exposed to a specific NF-κB inhibitor, 4-methyl-N1-(3-phenyl-propyl)-benzene-1,2-diamine (JSH-23; Merck Chemicals), which blocks translocation of phophorylated NF-κB (p65) to the nucleus of cells and its subsequent activation of NF-κB gene targets.24 MDMs were exposed to medium alone or medium containing 40 μM JSH-23 (or the equivalent amount of the vehicle for JSH-23, dimethyl sulfoxide [DMSO]) for 1.5 hours, washed, and incubated in normoxia or hypoxia for 18 hours. Normoxic MDMs were also exposed to 10 ng/mL recombinant human tumor necrosis factor-α (TNF-α; PeproTech) for 18 hours as a positive control for NF-κB activation. RNA and nuclear proteins were then extracted from parallel cultures of MDMs after these treatments for real-time reverse-transcribed (RT) PCR and immunoblot analysis, respectively. Some cells were also fixed in 3% formaldehyde in phosphate-buffered saline for 15 minutes, washed and permeabilized with ice-cold 100% methanol for 10 minutes, and blocked with 5% goat serum in 0.3% Triton X-100/phosphate-buffered saline solution for 1 hour. NF-κB p65 was detected using a rabbit anti–mouse antibody (1:25, Cell Signaling Technology) followed by addition of goat anti–rabbit Alexa-488 secondary antibody (Invitrogen; 1:250 dilution). Cells were counterstained with 300 nM 4,6-diamidino-2-phenylindole (DAPI; Invitrogen) and then photographed on a confocal fluorescent microscope (original magnification ×400). Twelve areas of cells were photographed for each treatment group and the degree of nuclear p65 immunofluorescence (ie, Alexa-488 labelling) in each DAPI-stained nuclei quantified using Analysis D software (Olympus). The proportion of green fluorescence per nuclei was then calculated for all nuclei in 5 fields of view/treatment. The number of all MDMs in each field of view containing Alexa-488–labeled (p65+) nuclei was also counted. To confirm JSH-23 inhibition of NF-κB activity in hypoxic MDMs, electrophoretic mobility shift assays for NF-κB binding to an NF-κB DNA consensus site were conducted as described previously by us25 on lysates from MDMs exposed to nomoxia, hypoxia, or hypoxia plus JSH-23 (all in the presence of DMSO as the vehicle for JSH-23). Protein extracts from parallel cultures of MDMs were also immunoblotted for HIFs-1 and -2α (as described above in “Immunoblotting studies”).
The second approach was to infect MDMs with an adenovirus expressing a dominant negative inhibitor of IKK-β to block phosphorylation/activation of p65/RelA. After 4 days in culture, MDMs were exposed to 50 ng/mL recombinant human M-CSF for 24 hours to stimulate up-regulation of integrin αVβ5 (required for adenovirus infection of macrophages26 ). The adv-IKK-βDN and control adv (Adv-GFP; a gift from Dr Thorsten Hagemann, Barts and The London Cancer Centre, London, United Kingdom) were E1/E3-deleted, of the Ad5 serotype, and used to transfect MDMs as described previously.27 MDMs were infected for 2 hours with 100 multiplicity of infection of either adenovirus in serum-free medium. The adenovirus was then removed and fresh medium containing 2% antibody serum added. MDMs were maintained for a further 2 days in culture and then exposed to hypoxia or normoxia for 18 hours. This infection protocol markedly reduces the activity of p65/RelA in human MDMs27 and human endothelial cells.28
Immunofluorescent labeling of IL-1β expressed by TAMs in hypoxic areas of murine 4T1 mammary tumors
Frozen sections of 4T1 murine mammary tumors were generated in a previous study.29 These had been grown in the mammary fat pads of female BALB/c mice and removed and snap frozen 2 hours after injection of mice with the hypoxic cell marker, pimonidazole.29 Sections (7 μM) were blocked with FcR Blocking Reagent (Miltenyi Biotec) in Tris-buffered saline-0.05% Tween 20 for 30 minutes at room temperature and then incubated with rat anti–F4/80-Alexa 488 (1 μg/mL, clone CI:A3-1; AbD Serotec), goat anti–mouse IL-1β (15 μg/mL; R&D Systems), and rabbit anti-PIMO (1:4000, a gift from James Raleigh) for 30 minutes at room temperature. Negative controls included substitution of primary antibodies with species-matched, nonspecific antibodies. Sections were then washed twice and incubated in donkey anti–goat-Alexa 568 (8 μg/mL; Invitrogen) or Alexa 647–conjugated goat anti–rabbit (8 μg/mL; Invitrogen) secondary antibodies for 30 minutes at room temperature in the dark and 30 nM DAPI (Invitrogen) for 2 minutes.
Gene set enrichment analysis
Gene set enrichment analysis (GSEA) was performed as described previously30 on gene lists ranked by level of hypoxic gene induction (hypoxia/normoxia fold induction) separately for both the Affymetrix and Illumina gene expression datasets. Correlations to the predefined curated and transcription factor target gene set collections were analyzed with the GSEA preranked tool using 1000 permutations. Further information regarding the gene sets used in these analyses is available in the Molecular Signatures Database (MSigDB; www.broad.mit.edu/gsea/msigdb).
Statistics
All experiments were repeated 3 to 6 times. Statistical analyses were performed using the 1- or 2-tailed Student t test to determine statistical significance after checking the data for normality (as appropriate). P values less than .05 were considered statistically significant. All data are expressed as mean plus or minus SEM.
Results
Evidence of distinct transcriptional signaling in primary human macrophages experiencing hypoxia
Hypoxic MDMs up-regulated both HIF-1α and HIF-2α, and this was markedly inhibited by prior treatment with siRNA to either HIFα (Figure 1A). As in previous publications,31,32 genes were defined as being differentially regulated in hypoxia if they exhibited more than 1.5-fold increase in gene expression (Table 1) or down-regulated if they showed less than 0.67-fold change (Table 2) compared with normoxic cultures. A comparison of our human MDM microarray results (Tables 1–2) with those obtained previously for related human myeloid cell types exposed to hypoxia (monocytes and monocyte-derived dendritic cells31,32 ) shows that some genes were seen to be regulated by all 3 cell types (up-regulated: VEGFA, CXCR4, TNF-α, TIMP1, PHD3, aldolases A and C, enolase 2, TREM1, NCF1; down-regulated: cathepsin C). However, some genes regulated by hypoxia in MDMs are not similarly regulated by hypoxia in these other 2 cell types, such as IL-1β, IL-12p40, Ang-2, endothelin 1, STATS 4 and 6, CCLs 3 and 5, CCR7, HMOX1, and hsp70 (up-regulated) and CD36, PECAM1 (CD31), HIF-2α, and MHCII DMβ; down-regulated; Tables 1–2).
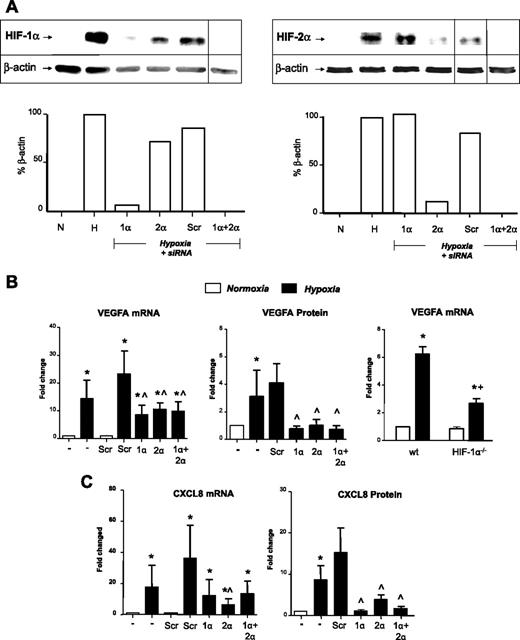
Role of HIF-1α and -2α in the hypoxic induction of VEGFA and CXCL8: insights from siRNA knockdown studies and use of macrophages bearing a deletion in the HIF-1α gene. (A) Immunoblots of HIF-1α or HIF-2 α in MDM lysates after their exposure to normoxia (20.9% O2; N) or hypoxia (0.1% O2; H) for 18 hours, or hypoxia for 18 hours after exposure to siRNA for HIF-1α (1α), HIF-2 (2α), both HIFs-1α and 2α together (1α + 2α), or a scrambled control (Scr). Loading controls were β-actin. Vertical lines have been inserted to indicate repositioned lanes from the same gel. Below each gel picture is the densitometric analysis of HIF expression relative to its β-actin loading control. (B-C) Effects of HIF-1α and -2α knockdown on the hypoxic induction of VEGFA (B) and CXCL8 (IL-8; C) mRNA and protein. In the case of VEGF, gene expression was also assayed in normoxic and hypoxic BMDMs from mice bearing a myeloid cell-specific knockout of the HIF-1 α gene (HIF-1α−/−) in vitro by quantitative RT-PCR (B right panel). It was not possible to do this for CXCL8, as this gene is not expressed in mice. Pooled data from 6 replicate experiments are shown. *P < .05 compared with corresponding normoxic group. ∧P < .05 compared with the scr siRNA/hypoxia group. +P < .05 compared with macrophages from wild-type mice exposed to hypoxia.
Role of HIF-1α and -2α in the hypoxic induction of VEGFA and CXCL8: insights from siRNA knockdown studies and use of macrophages bearing a deletion in the HIF-1α gene. (A) Immunoblots of HIF-1α or HIF-2 α in MDM lysates after their exposure to normoxia (20.9% O2; N) or hypoxia (0.1% O2; H) for 18 hours, or hypoxia for 18 hours after exposure to siRNA for HIF-1α (1α), HIF-2 (2α), both HIFs-1α and 2α together (1α + 2α), or a scrambled control (Scr). Loading controls were β-actin. Vertical lines have been inserted to indicate repositioned lanes from the same gel. Below each gel picture is the densitometric analysis of HIF expression relative to its β-actin loading control. (B-C) Effects of HIF-1α and -2α knockdown on the hypoxic induction of VEGFA (B) and CXCL8 (IL-8; C) mRNA and protein. In the case of VEGF, gene expression was also assayed in normoxic and hypoxic BMDMs from mice bearing a myeloid cell-specific knockout of the HIF-1 α gene (HIF-1α−/−) in vitro by quantitative RT-PCR (B right panel). It was not possible to do this for CXCL8, as this gene is not expressed in mice. Pooled data from 6 replicate experiments are shown. *P < .05 compared with corresponding normoxic group. ∧P < .05 compared with the scr siRNA/hypoxia group. +P < .05 compared with macrophages from wild-type mice exposed to hypoxia.
The 2 full sets of array data (Affymetrix and Illumina) have been deposited in NCBI's Gene Expression Omnibus and are accessible through GEO Series accession numbers GSE15949 and GSE16099, respectively.33,34
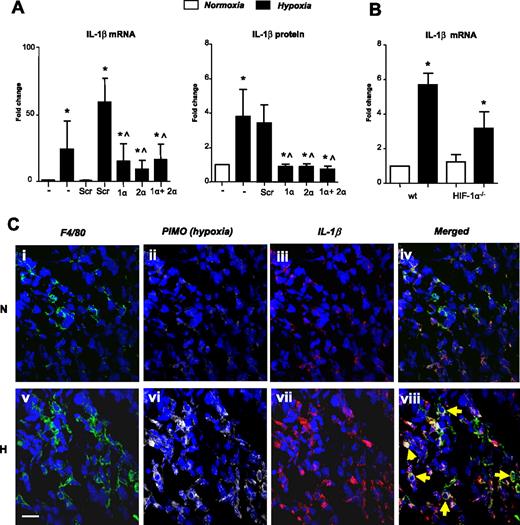
Several key genes were selected and their up-regulation confirmed using quantitative RT-PCR (Table 1). Macrophages were also shown to express abundant IL-1β protein in pimonidazole-stained (hypoxic) areas of murine 4T1 mammary tumors (Figure 2C).
Hypoxic up-regulation of IL-1β by human MDMs in vitro and by TAMs in hypoxic areas of murine mammary tumors: role of HIF-1 and -2. (A) IL-1β mRNA levels and protein release by human MDMs after their exposure to normoxia (20.9% O2; N) or hypoxia (0.1% O2; H) for 18 hours, or hypoxia for 18 hours after exposure to siRNA for HIF-1 α (1 α), HIF-2α (2 α), both HIFs-1α and -2α together (1α + 2α), or a scrambled control (Scr). (B) Hypoxic induction of IL-1β mRNA by BMDMs from wild-type mice and mice bearing a myeloid cell-specific knockout of the HIF-1 α gene. (C) Up-regulated expression of IL-1β by F4/80+ macrophages in pimonodazole-stained (ie, hypoxic; H) compared with pimonodazole-unstained (ie, normoxic; N) areas of murine mammary (4T1) tumors (yellow arrows on the merged H image). Pooled data from 3 replicate experiments are shown. *P < .05 compared with corresponding normoxic group. ∧P < .05 compared with the Scr siRNA/hypoxia group. +P < .05 compared with macrophages from wild-type mice exposed to hypoxia.
Hypoxic up-regulation of IL-1β by human MDMs in vitro and by TAMs in hypoxic areas of murine mammary tumors: role of HIF-1 and -2. (A) IL-1β mRNA levels and protein release by human MDMs after their exposure to normoxia (20.9% O2; N) or hypoxia (0.1% O2; H) for 18 hours, or hypoxia for 18 hours after exposure to siRNA for HIF-1 α (1 α), HIF-2α (2 α), both HIFs-1α and -2α together (1α + 2α), or a scrambled control (Scr). (B) Hypoxic induction of IL-1β mRNA by BMDMs from wild-type mice and mice bearing a myeloid cell-specific knockout of the HIF-1 α gene. (C) Up-regulated expression of IL-1β by F4/80+ macrophages in pimonodazole-stained (ie, hypoxic; H) compared with pimonodazole-unstained (ie, normoxic; N) areas of murine mammary (4T1) tumors (yellow arrows on the merged H image). Pooled data from 3 replicate experiments are shown. *P < .05 compared with corresponding normoxic group. ∧P < .05 compared with the Scr siRNA/hypoxia group. +P < .05 compared with macrophages from wild-type mice exposed to hypoxia.
Genetic manipulation of HIFs-1 and -2α demonstrates the coregulation of genes in primary human macrophages experiencing hypoxia
The hypoxic accumulation of both HIFs-1 and -2α was ablated after transfection with siRNA for either α subunits. Both VEGFA mRNA and protein were markedly increased by hypoxia, and this was significantly inhibited by siRNA for either HIFα subunit (Figure 1B left and middle panels). It may appear that the hypoxic induction of VEGFA mRNA is higher in hypoxic macrophages treated with the scrambled control siRNA than in the “no siRNA” group. However, this failed to reach statistical significance. This was also the case for these 2 groups in Figure 3E,G,I, and K.
Role of HIFs-1α and -2α in the hypoxic induction of other key genes by macrophages. (A,C,E,G,I,K) Hypoxic induction of mRNA for CXCR4, GLUT-1, ADM, STAT4, ADORA2A, and ICAM1 (as measured by quantitative RT-PCR) after exposure of primary human macrophages to normoxia (20.9% O2; N) or hypoxia (0.1% O2; H) for 18 hours, or hypoxia after exposure to siRNA for HIF-1 α (1 α), HIF-2 α (2 α), both HIFs-1 α and -2 α together (1 α + 2 α), or a scrambled control (Scr). (B,D,F,H,J,L) Hypoxic induction of the same genes in wild-type or HIF-1α–deficient murine BMDMs. Pooled data from 3 replicate experiments are shown. *P < .05 compared with corresponding normoxic group. ∧P < .05 compared with the Scr siRNA/hypoxia group. +P < .05 compared with macrophages from wild-type mice exposed to hypoxia.
Role of HIFs-1α and -2α in the hypoxic induction of other key genes by macrophages. (A,C,E,G,I,K) Hypoxic induction of mRNA for CXCR4, GLUT-1, ADM, STAT4, ADORA2A, and ICAM1 (as measured by quantitative RT-PCR) after exposure of primary human macrophages to normoxia (20.9% O2; N) or hypoxia (0.1% O2; H) for 18 hours, or hypoxia after exposure to siRNA for HIF-1 α (1 α), HIF-2 α (2 α), both HIFs-1 α and -2 α together (1 α + 2 α), or a scrambled control (Scr). (B,D,F,H,J,L) Hypoxic induction of the same genes in wild-type or HIF-1α–deficient murine BMDMs. Pooled data from 3 replicate experiments are shown. *P < .05 compared with corresponding normoxic group. ∧P < .05 compared with the Scr siRNA/hypoxia group. +P < .05 compared with macrophages from wild-type mice exposed to hypoxia.
CXCL8 mRNA and protein release were also up-regulated in hypoxic MDMS (Figure 1C); and although both HIFα siRNA treatments reduced hypoxia-induced CXCL8 mRNA, only the effect of HIF-2α siRNA reached significance. However, both HIFα siRNA species significantly reduced CXCL8 protein release (Figure 1C). The inhibitory effect of HIF siRNA on the hypoxic induction of both VEGFA and CXCL8 appeared to be slightly greater at the protein than the mRNA level.
Hypoxia also up-regulated IL-1β mRNA and protein, and this was significantly inhibited by exposure to siRNA for either HIF α subunit (Figure 2A). We then investigated the role of HIFs-1 and -2 in the hypoxic regulation of several other genes listed in Table 1. The hypoxic up-regulation of mRNA for CXCR4, GLUT1, adrenomedulin (ADM), and STAT-4 was significantly (P < .05) reduced by HIF-1α or 2α siRNA (Figure 3A,C,E,G). In contrast to the other genes investigated, the hypoxic induction of adenosine A2a receptor (ADORA2A) and ICAM1 mRNA was significantly (P < .05) inhibited only by HIF-2α siRNA (Figure 3I,K).
Transcriptional signaling in primary human MDMs experiencing hypoxia for 18 hours is independent of NF-κB
Gene set enrichment analysis.
To assess the likelihood of NF-κB playing a role in hypoxic signal transduction in human macrophages, we first searched our data for correlations with several published gene sets relating to hypoxia-regulated regulated genes in other cell types (eg, the “HYPOXIA_REVIEW” gene set35 ; Figure 4 top panels). This highlighted a significant degree of enrichment of known hypoxia-regulated genes in our array data, showing that hypoxia-induced gene expression changes in MDMs follow a consensus hypoxia gene expression profile (Figure 4 top panels). This was evident for both the Affymetrix array data (Normalized Enrichment Score [NES]: 2.2; False Discovery Rate, q < 0.001) and the Illumina data (NES: 2.24; q < 0.001). Table 1 shows that many genes up-regulated by hypoxic MDMs have previously been identified as NF-κB target genes. In the GSEA analysis, the hypoxic MDMS array data also correlated significantly with several NF-κB-related gene sets (eg, the V$NFKAPPAB_01 gene set36 ; Figure 4 bottom panels). Again, this was evident for both the Affymetrix data (NES: 1.69, q = 0.02) and the Illumina data (NES: 1.67; q = 0.12).
Gene enrichment analysis to compare key genes up-regulated by hypoxia in human MDMs and known NF-κB–regulated genes. Hypoxia up-regulated genes identified in 2 separate macrophage cultures using Affymetrix or Illumina microarrays were ranked by level of hypoxia-mediated induction. The ranked gene lists were then compared with both a previously published gene set for hypoxia-regulated genes in tumor cells (the hypoxia-regulated gene set, top row) or genes previously shown to be NF-κB target genes (the NF-κB target gene set, bottom row) by GSEA. The hypoxia-regulated gene set (top row) was significantly enriched in the hypoxic macrophage gene set identified on both the Affymetrix (Array 1; NES = 2.2, q < 0.001) and the Illumina (Array 2; NES = 2.24, q < 0.001) arrays. The NF-κB target gene set (lower row) was also enriched in the hypoxic macrophage gene set on both Affymetrix (NES = 1.69, q = 0.02) and Illumina (NES = 1.67, q = 0.12) arrays.
Gene enrichment analysis to compare key genes up-regulated by hypoxia in human MDMs and known NF-κB–regulated genes. Hypoxia up-regulated genes identified in 2 separate macrophage cultures using Affymetrix or Illumina microarrays were ranked by level of hypoxia-mediated induction. The ranked gene lists were then compared with both a previously published gene set for hypoxia-regulated genes in tumor cells (the hypoxia-regulated gene set, top row) or genes previously shown to be NF-κB target genes (the NF-κB target gene set, bottom row) by GSEA. The hypoxia-regulated gene set (top row) was significantly enriched in the hypoxic macrophage gene set identified on both the Affymetrix (Array 1; NES = 2.2, q < 0.001) and the Illumina (Array 2; NES = 2.24, q < 0.001) arrays. The NF-κB target gene set (lower row) was also enriched in the hypoxic macrophage gene set on both Affymetrix (NES = 1.69, q = 0.02) and Illumina (NES = 1.67, q = 0.12) arrays.
Hypoxic up-regulation of NF-κB signaling in human macrophages: role of HIF-1 and -2.
The effect of exposure of human MDMs to hypoxia for 18 hours on NF-κB signaling was then assessed. IKK-β and γ, IκBα, NF-κB1 (p50), and p65/RelA mRNA levels were up-regulated (and IKK-α mRNA slightly down-regulated) in MDMs exposed to 0.1% O2 for 18 hours. This hypoxic regulation (with the exception of IKKs α and γ) was inhibited using siRNA to knock down either HIF-1 or 2α (Figure 5A). Figure 5C shows that, although there was a small hypoxic induction of total IKK-β protein, the hypoxic up-regulation of p65/RelA mRNA was not mirrored by a similar up-regulation of total p65/RelA protein, suggesting a differential effect of hypoxia on mRNA vs protein expression for p65/RelA. By contrast, the phosphorylation of both IKK-α/β and p65/RelA was up-regulated in hypoxic human MDMs (Figure 5C).
Effect of hypoxia on the expression and/or phosphorylation of components of the canonical NF-κB signaling pathway in MDMs: regulation by HIF-1α and -2α. (A) Fold induction (hypoxia, 0.1% O2/normoxia, 20.9% O2) of mRNA levels for individual NF-κB signaling proteins in primary human MDMs. The contribution of both HIFs-1 and -2 to the regulation of many of these genes was also assessed using siRNA to knock down the expression of each α subunit in MDMs. (B) Effect of normoxia (N) or hypoxia (0.1% O2; H) for 18 hours on the expression of IKK-β and p65 mRNA by murine BMDMs from wild-type or HIF-1–deficient mice. *P < .05 compared with corresponding normoxic group. +P < .05 compared with macrophages from wild-type mice exposed to hypoxia. (C) Immunoblots showing the effect of exposure to normoxia or hypoxia (0.1% O2) for 18 hours on the levels of total and phosphorylated IKK-β and p65/RelA in primary human MDMs. Vertical lines have been inserted to indicate repositioned lanes from the same gel. (D) Effects of normoxic (N) or hypoxic (0.5% O2; H) culture on the level of total or phosphorylated p65 protein in control (+/−) or HIF-2α–deficient murine BMDMs. Similar results were obtained using wild-type or HIF-1α–deficient murine BMDMs (data not shown).
Effect of hypoxia on the expression and/or phosphorylation of components of the canonical NF-κB signaling pathway in MDMs: regulation by HIF-1α and -2α. (A) Fold induction (hypoxia, 0.1% O2/normoxia, 20.9% O2) of mRNA levels for individual NF-κB signaling proteins in primary human MDMs. The contribution of both HIFs-1 and -2 to the regulation of many of these genes was also assessed using siRNA to knock down the expression of each α subunit in MDMs. (B) Effect of normoxia (N) or hypoxia (0.1% O2; H) for 18 hours on the expression of IKK-β and p65 mRNA by murine BMDMs from wild-type or HIF-1–deficient mice. *P < .05 compared with corresponding normoxic group. +P < .05 compared with macrophages from wild-type mice exposed to hypoxia. (C) Immunoblots showing the effect of exposure to normoxia or hypoxia (0.1% O2) for 18 hours on the levels of total and phosphorylated IKK-β and p65/RelA in primary human MDMs. Vertical lines have been inserted to indicate repositioned lanes from the same gel. (D) Effects of normoxic (N) or hypoxic (0.5% O2; H) culture on the level of total or phosphorylated p65 protein in control (+/−) or HIF-2α–deficient murine BMDMs. Similar results were obtained using wild-type or HIF-1α–deficient murine BMDMs (data not shown).
Role of NF-κB in the transcriptional responses of human macrophages to hypoxia.
Figure 6A and B illustrates the effects of the NF-κB inhibitor JSH-23 on the hypoxic induction of various genes in human MDMs. This shows that immunoreactive p65 was cytoplasmic in normoxic MDMs but transported to the nucleus on exposure to TNF-α or 18 hours of hypoxia. In both cases, this was significantly (P < .05) inhibited by prior exposure to JSH-23. EMSA assays confirmed the induction of NF-κB DNA binding in hypoxic MDMs and the inhibition of this by JSH-23 (Figure 6C). JSH-23–treated cells also exhibited slightly lower levels of HIFs-1 and -2α (particularly HIF-1α) than MDMs exposed to hypoxia alone (Figure 6C). We then investigated the effect of JSH-23 inhibition of NF-κB activity on the induction of 8 hypoxia-regulated genes listed in Table 1. Exposure to TNF-α for 18 hours significantly (P < .05) increased the expression of VEGFA, CXCL8, and STAT4, in a manner that was inhibited by JSH-23 (Figure 6). Hypoxia significantly (P < .05) up-regulated all 8 genes studied (Figure 6D) in a manner that was not reduced by prior exposure of cells to JSH-23.
Inhibition of nuclear translocation of p65 has no effect on the hypoxic induction of various genes in human MDMs in vitro. (A-B) Effect of the p65 inhibitor JSH-23 (or its vehicle, DMSO) on the nuclear translocation of p65 induced by TNF-α or hypoxia by human MDMs. N indicates normoxia; H, hypoxia (0.1% O2); % nuclear p65 immunofluorescence, the percentage of the total, DAPI-stained (blue) area of MDM nuclei that was GFP+ (green). The figures at the base or just above each bar represent the average percentage of all MDM nuclei immunofluorescent for p65 (B). *P < .05 with respect to normoxia alone group. +P < .05 with respect to TNF-α + DMSO group. ∧P < .05 with respect to hypoxia + DMSO group. (C) Effect of JSH-23 on NF-κB binding and accumulation of HIFs-1 and -2α in hypoxic MDMs. N indicates normoxia; H, hypoxia, H + JSH-23, hypoxia after JSH.23 treatment. All 3 groups received the vehicle for JSH-23, DMSO. A vertical line has been inserted to indicate repositioning of lanes from the same gel; (1) left panel: EMSA showing NF-κB binding to a DNA consensus sequence, and (2) right panel: immunoblots for HIFs-1 and -2α. (D) Effect of JSH-23 blockade of p65 function on the fold induction of VEGFA, CXCL8, IL-1β, CXCR4, GLUT-1, STAT4, ADM, and ADORA2A by TNF-α or hypoxia. *P < .05 with respect to normoxia with DMSO alone. **P < .05 with respect to group indicated. $P < .05 with respect to TNF + DMSO group. Pooled data from 3 replicate experiments are shown.
Inhibition of nuclear translocation of p65 has no effect on the hypoxic induction of various genes in human MDMs in vitro. (A-B) Effect of the p65 inhibitor JSH-23 (or its vehicle, DMSO) on the nuclear translocation of p65 induced by TNF-α or hypoxia by human MDMs. N indicates normoxia; H, hypoxia (0.1% O2); % nuclear p65 immunofluorescence, the percentage of the total, DAPI-stained (blue) area of MDM nuclei that was GFP+ (green). The figures at the base or just above each bar represent the average percentage of all MDM nuclei immunofluorescent for p65 (B). *P < .05 with respect to normoxia alone group. +P < .05 with respect to TNF-α + DMSO group. ∧P < .05 with respect to hypoxia + DMSO group. (C) Effect of JSH-23 on NF-κB binding and accumulation of HIFs-1 and -2α in hypoxic MDMs. N indicates normoxia; H, hypoxia, H + JSH-23, hypoxia after JSH.23 treatment. All 3 groups received the vehicle for JSH-23, DMSO. A vertical line has been inserted to indicate repositioning of lanes from the same gel; (1) left panel: EMSA showing NF-κB binding to a DNA consensus sequence, and (2) right panel: immunoblots for HIFs-1 and -2α. (D) Effect of JSH-23 blockade of p65 function on the fold induction of VEGFA, CXCL8, IL-1β, CXCR4, GLUT-1, STAT4, ADM, and ADORA2A by TNF-α or hypoxia. *P < .05 with respect to normoxia with DMSO alone. **P < .05 with respect to group indicated. $P < .05 with respect to TNF + DMSO group. Pooled data from 3 replicate experiments are shown.
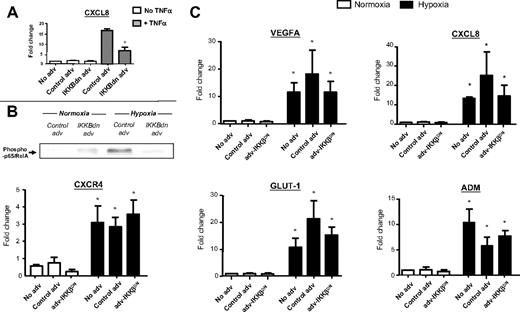
Infection of MDMs with adv-IKK-βDN significantly (P < .05) inhibited their TNF-α–induced expression of CXCL8 mRNA (Figure 7A) as well as the nuclear accumulation of phospho-p65/RelA by MDMS after 18 hours of exposure to hypoxia (Figure 7B). The control adv vector had no such effect. However, adv-IKK-βDN blockade of hypoxia-induced phospho-p65/RelA failed to reduce the hypoxic induction of VEGFA, CXCL8, GLUT-1, CXCR4, or ADM mRNA.
IKK-β inhibition has no effect on the hypoxic induction of various genes in human MDMs in vitro: use of a recombinant adenovirus expressing a dominant negative inhibitor of IKK-β (adv-IKK-βDN). MDM infection with adv-IKK-βDN (but not control adv) significantly inhibited both TNF-α–induced gene expression of CXCL8 (A) and hypoxia-induced nuclear accumulation of phospho-p65/RelA (B) by human MDM. (C) Hypoxia significantly increased the expression of VEGFA, CXCL8, CXCR4, GLUT-1, and ADM mRNA in untreated and adenovirally infected MDM compared with respective normoxic MDM controls. However, there was no significant difference in the expression of these genes between hypoxic MDM infected with adv-IKK-βDN or the control adenovirus. N = 3. *P < .05 with respect to respective normoxic group. +P < .05 with respect to TNF-α + control adenovirus group.
IKK-β inhibition has no effect on the hypoxic induction of various genes in human MDMs in vitro: use of a recombinant adenovirus expressing a dominant negative inhibitor of IKK-β (adv-IKK-βDN). MDM infection with adv-IKK-βDN (but not control adv) significantly inhibited both TNF-α–induced gene expression of CXCL8 (A) and hypoxia-induced nuclear accumulation of phospho-p65/RelA (B) by human MDM. (C) Hypoxia significantly increased the expression of VEGFA, CXCL8, CXCR4, GLUT-1, and ADM mRNA in untreated and adenovirally infected MDM compared with respective normoxic MDM controls. However, there was no significant difference in the expression of these genes between hypoxic MDM infected with adv-IKK-βDN or the control adenovirus. N = 3. *P < .05 with respect to respective normoxic group. +P < .05 with respect to TNF-α + control adenovirus group.
Hypoxic regulation of genes in primary murine macrophages: role of HIFs-1 and -2
HIF-1–deficient BMDMs were only able to mount partial VEGFA and IL-1β responses to hypoxia (Figures 1B right panel, 2B). The hypoxic up-regulation of CXCR4 and STAT4 was lost in BMDMs lacking HIF-1α (Figure 3B,H). This contrasts with our aforementioned human MDMs data showing that these were regulated by both HIFs-1 and -2 (Figure 3A,G). The fact that GLUT1 and ADM were reduced but not ablated in HIF-1α null BMDMs (Figure 3D,F) agrees with our finding that these 2 genes are coregulated by HIFs-1 and -2 in human MDMs (Figure 3C,E). In addition, in agreement with the human MDM data (Figure 3I,K), the hypoxic up-regulation of neither the ADORA2A nor ICAM1 genes was inhibited in hypoxic HIF-1α null BMDMs (Figure 3J,L).
Discussion
Our data show that exposure to hypoxia activates a distinct transcriptional profile in primary human macrophages, including the up-regulation of VEGFA, ILs-1α and β, IL-8, STAT4, and ADM; the receptors, glucose transporter, GLUT1, CXCR4, and the adenosine receptor 2A (ADORA2A). Some were seen to also be regulated by hypoxia in monocytes and immature dendritic cells (VEGFA, GLUT1, and CXCR4).31,32 However, others, such as IL-1β, ADORA2A, and STAT4, were only altered in hypoxic macrophages. These differences could be the result of variations in the severity and/or duration of hypoxia applied to cells31,32 and/or may reflect differences in the transcription factors used by these 3 cell types in hypoxia. For example, hypoxic human monocytes exposed to a similar level and duration of hypoxia as in the current study failed to up-regulate HIFs-1 and -2α but rather other transcription factors, such as ATF-4 and Egr-1.37 Moreover, the ability to regulate hypoxic gene expression via HIFs is maturation-linked in macrophages.38 Although dendritic cells accumulate HIF-1α in hypoxia,39 immature forms of this cell type up-regulate other hypoxia-responsive genes, such as CCL20 via up-regulated p50/p50 NF-κB homodimers rather than HIFs.40 A study of the responses of such related myeloid cell types to identical hypoxic conditions would be interesting but beyond the remit of this study.
As macrophages are known to express receptors for both VEGF41 and IL-1,42 it is possible that that, during such exposure to hypoxia, their hypoxia-induced release might have then stimulated the expression of other genes in macrophages (making it look as if they are also directly up-regulated by hypoxia when, indeed, the effect is indirect). However, hypoxic gene expression by human MDMs is not reduced in the presence of either a neutralizing VEGF antibody or an IL-1 receptor antagonist (H.-Y.F., C.M., R.H., and C.E.L., unpublished observations, March 2009).
We also show, for the first time, that genes encoding the 2 transcription factors, STATs (signal transducers and activators of transcription) 4 and 6, are up-regulated by hypoxia in macrophages. STATs 4 and 6 are known to mediate the marked effects of 2 central immunomodulatory cytokines, IL-12 and IL-4, respectively.43,44 It remains to be seen whether their hypoxic induction could in this way “prime” macrophages to the effects of these cytokines.
Our HIF siRNA studies showed that both HIFs play a part in regulating the hypoxic induction of the known HIF target genes, VEGFA, GLUT1, CXCR4, IL-8, and ADM by MDMs. Furthermore, hypoxic induction of these genes was reduced but not lost in HIF-1α–deficient murine macrophages. Similar results were obtained for the hypoxic up-regulation of VEGFA and ADM in murine HIF-2α–deficient BMDMs (H.Z.I. and M.C.S., manuscript submitted, April 2009).
The pluripotent cytokine, IL-1β, stimulates many steps in tumor progression45 and was up-regulated by hypoxic MDMs. We show that TAMs express abundant IL-1β in hypoxic areas of murine mammary (4T1) tumors. The IL-1β gene promoter bears multiple HREs and is transactivated by HIF-1.46,47 Our HIF siRNA knockdown studies show that HIFs-1 and -2 coregulate the hypoxic induction of IL-1β in macrophages, a finding confirmed by the hypoxic up-regulation of IL-1β being only partially diminished in HIF-1α–deficient (Figure 1) and HIF-2α–deficient BMDMs (H.Z.I. and M.C.S., manuscript submitted April 2009).
It remains to be seen whether HIFs-1 and -2 bind to different HREs on the promoters of the aforementioned coregulated genes or whether other, unknown mechanisms underpin the phenomenon of dual HIF responsiveness. Furthermore, as mentioned previously, this may vary between cell types as HIF-1 has been shown to be the primary regulator of various genes in some cell types,4,16 whereas other cells use HIF-2 or both HIFs in their regulation.18,19,48,49 Interestingly, when just one HIF was inhibited using siRNA, the other did not appear to compensate for its loss and maintain maximal hypoxic induction. It is known that many HIF-target genes have multiple HREs. If, once HIFs-1 and -2 have bound to different HREs in a given promoter they then cooperate, both might be required for maximal gene transcription. This may be similar to the molecular “cooperation” that takes place between HIF-2 and Elk-1 on some gene promoters.18,19
The knockdown of both HIFs-1α and 2α in MDMs failed to completely block the hypoxic up-regulation of most of the HIF-target genes shown in Figures 1 through 3. This suggests that other transcription factors may also be involved in regulating their hypoxic induction. The transcription factor, NF-κB, may be one such factor. This has been shown recently to be activated in macrophages by short-term (2-4 hours) exposure to hypoxia, with the expression and/or phosphorylation of IKK-β, IKBα, and p65/RelA, as well as the nuclear translocation and DNA-binding activity of p65 being up-regulated.20-22 There also appears to be a close interplay between NF-κB and HIF-1 as p65/p50 heterodimers bind to the HIF-1α gene promoter and drive its expression under hypoxia. Interestingly, HIF-2α is not up-regulated by NF-κB in murine macrophages during short-term hypoxia (4 hours).22 The present report shows that p65 protein is phosphorylated and binds DNA in the nuclei of MDMs in hypoxia. Furthermore, we show that both HIFs-1 and -2 contribute to the maintenance of high levels of p65 expression and phosphorylation in such cells.
As many of the genes we found to be markedly up-regulated in human macrophages by hypoxia had previously been identified as potential NF-κB target genes (Table 1; Figure 4), we examined the role of p65 in the hypoxic up-regulation of the most highly up-regulated ones. Studies using the synthetic inhibitor of nuclear translocation of p65, JSH-23,24 or an adenoviral inhibitor of IKK-β showed that NF-κB is not essential for their induction during an 18-hour exposure to hypoxia. The up-regulation of HIF-1α in macrophages exposed to short-term hypoxia (4 hours) is partially dependent on NF-κB,22 so the fact that both HIFs-1 and -2α continued to be up-regulated in JSH-treated MDMs after hypoxia in our study suggests that either p65 inhibition was incomplete or the accumulation of these subunits during a more sustained period (18 hours) of hypoxia is independent of NF-κB.
However, although both forms of NF-κB inhibition resulted in the marked inhibition of TNF-α-induced CXCL8 (as well as other genes examined with JSH-23), it had no detectable effect on the hypoxic expression of any of the genes examined. These data are supported by the recent finding that that the hypoxic induction of several such NF-κB target genes in murine BMDMs does not involve activation of NF-κB (H.Z.I. and M.C.S., manuscript submitted April 2009).
Our data indicate then that NF-κB signaling may not contribute to the induction of these genes by macrophages in response to an 18-hour exposure to hypoxia. At first glance, this appears to contrast with the finding mentioned earlier: that hypoxic induction of HIF-1α and various HIF target genes was diminished in IKK-β–deficient BMDMs after exposure to short-term hypoxia (ie, 4 hours).22 It may be that there is a switch from acute, NF-κB-dependent hypoxic responses in macrophages that are critical for innate immunity (eg, bacterial infection) to an NF-κB-independent, HIF-driven response to the chronic hypoxia present at sites such as tumors. Clearly, a detailed investigation of the role of NF-κB in the temporal and gene-specific responses of macrophages to hypoxia is now warranted.
Taken together, our data show that, when macrophages experience hypoxia for 18 hours, it elicits a profound change in their expression of various tumor-promoting genes. Although this study provides invaluable insights into the basic repertoire of such macrophage responses, it should be remembered that macrophages in hypoxic areas of complex tissues such as tumors are a heterogeneous mix of cells, including immature, monocyte-like cells and mature macrophages.6 Moreover, the responses of these cells to hypoxia will also be influenced by a host of tumor-derived signals, such as cytokines and enzymes. Further in vivo studies are now warranted to investigate the role of hypoxic macrophage responses within the complex milieu of tumors.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Bosco and Varesio (G. Gaslini Institute, Genoa, Italy) and Michael Karin (United States) for their helpful comments in the preparation of this manuscript, Dr Thorsten Hagemann (Institute of Cancer, Barts and The London School of Medicine and Dentistry, London, United Kingdom) for providing the control and IKKBdn adenoviruses, and Drs Sheila Francis and Sarah Walmsley (University of Sheffield Medical School, Sheffield, United Kingdom) for their help with the adenoviral infections and HIF-1−/− BMDM isolations, respectively.
This work was supported by Yorkshire Cancer Research, United Kingdom and Breast Cancer Campaign, United Kingdom.
Authorship
Contribution: H.-Y.F., R.H., C.M., S.B.C., S.K.B., E.F., F.R.G., and J.R. designed and performed research; A.L.H. and C.E.L. contributed vital new reagents or analytical tools, analyzed data, and wrote the paper; R.S.J. and M.C.S. contributed vital new reagents or analytical tools and analyzed data; and H.Z.I. performed research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Claire E. Lewis, Academic Unit of Pathology, Faculty of Medicine, Dentistry and Health, University of Sheffield, Beech Hill Rd, Sheffield, S10 2RX, United Kingdom; e-mail: claire.lewis@sheffield.ac.uk.