Abstract
New treatment options are required for primary systemic AL amyloidosis (AL). This phase 1 dose-escalation component of a phase 1/2 study in relapsed AL aimed to determine the maximum tolerated dose (MTD) of bortezomib once weekly (0.7-1.6 mg/m2; days 1, 8, 15, and 22; 35-day cycles) and twice weekly (0.7-1.3 mg/m2; days 1, 4, 8, and 11; 21-day cycles) and assess preliminary hematologic responses. Thirty-one patients with relapsed AL were enrolled across 7 cohorts. Dose-limiting toxicity included grade 3 congestive heart failure in 2 patients (1 at once weekly, 1.6 mg/m2, and 1 at twice weekly, 1.0 mg/m2). MTD was not defined for either schedule; the maximum doses of 1.6 mg/m2 (once weekly) and 1.3 mg/m2 (twice weekly) are being used in phase 2 evaluation. Most commonly reported toxicities on both schedules included gastrointestinal events, fatigue, and nervous system disorders. Discontinuations and dose reductions for toxicity were reported in 12 and 4 patients, respectively. No treatment-related deaths occurred. Hematologic responses occurred in 15 (50%) of 30 evaluable patients, including 6 (20%) complete responses. Median time to first response was 1.2 months. Once-weekly and twice-weekly bortezomib appear generally well tolerated in relapsed AL, with promising hematologic responses. This study is registered with http://ClinicalTrials.gov under identifier NCT00298766.
Introduction
Primary systemic AL amyloidosis (AL) is a protein conformational disorder due to a clonal plasma cell dyscrasia that is related to multiple myeloma.1 In AL, abnormal monoclonal light-chain immunoglobulins form insoluble fibrils that are deposited in various organs and tissues throughout the body, commonly including the kidneys, heart, liver, and peripheral nervous system.1-3 This process can lead to multiple organ dysfunction and death.1 The annual incidence of AL is estimated to be 6 to 9 per million.4-6 Prognosis is poor, with a median survival without therapy of approximately 13 months from diagnosis7 and a 10-year survival rate of 5%8 ; however, survival may be prolonged with effective treatment.9-12
The aim of treatment for AL is to suppress the plasma cell dyscrasia and thereby reduce the production of abnormal light-chain fragments.5,13 Elimination of these amyloid-forming precursors may result in resorption of amyloid deposits and restoration of organ function.5 Therapy is generally based on that used for multiple myeloma.5,13,14
Rapid response to treatment is important.13 More aggressive therapy, for example with high-dose melphalan therapy plus stem cell transplantation (HDT-SCT), is therefore often considered preferable if tolerable. HDT-SCT has been shown to be effective in AL15 ; however, toxicities and treatment-related mortality may be high due to the involvement of multiple organs, necessitating a risk-adapted approach to patient selection.15,16 Consequently, many (39%-82%) AL patients are ineligible for HDT-SCT, depending on the selection criteria.17,18 Less intensive treatment options include thalidomide plus dexamethasone and oral melphalan plus dexamethasone.5,13,14,16 Hematologic response rates of 67%, including 33% complete responses (CRs), with melphalan plus dexamethasone,12 and 48%, including 19% CR, with thalidomide plus dexamethasone19 have been reported. However, median time to response was relatively delayed at 4.5 months12 and 3.6 months,19 respectively, and thalidomide plus dexamethasone in particular was associated with considerable toxicity.19 Therefore, there is an urgent need for better treatment options for AL with substantial, rapid activity and good tolerability, particularly in the relapsed setting, in which no standard treatment exists.14
Bortezomib (VELCADE; Millennium Pharmaceuticals, Inc, and Johnson & Johnson Pharmaceutical Research & Development, LLC) is a potent, specific, and reversible inhibitor of the 26S proteasome that is approved for the treatment of multiple myeloma. Given the proven sensitivity of malignant plasma cells to bortezomib20 and the demonstrated activity of this agent in multiple myeloma,21-23 it has been hypothesized that bortezomib may also be active against AL. Data from 1 multicenter analysis and 2 single-center patient series have provided evidence of promising activity with bortezomib, alone or in combination with dexamethasone, in patients with previously untreated and relapsed/refractory AL.24-26
Here we present findings from the first prospective study of single-agent bortezomib in patients with relapsed AL. We report the results from the phase 1 dose-escalation component of this phase 1/2 study investigating once-weekly and twice-weekly schedules of bortezomib administration. The primary aim of this portion of the study was to determine the maximum tolerated dose (MTD) of bortezomib using each dosing schedule to define the recommended doses for use in the phase 2 component of this trial. We also report preliminary data on hematologic responses.
Methods
Patients
Patients 18 years or older with a diagnosis of AL, including measurable hematologic and amyloid-related end-organ involvement, who had been previously treated and required further treatment due to persistent clonal disease, were eligible. The diagnosis of AL required a histologic tissue diagnosis of amyloid deposition proven by Congo red staining of a tissue biopsy, plus proof of plasma cell dyscrasia (measurable M-protein in serum or urine by immunofixation or electrophoresis, and/or elevated free light chain [FLC] higher than 10 mg/dL and an abnormal FLC ratio). If these parameters were insufficient to establish a diagnosis, DNA analysis and/or amyloid fibril sequencing, as well as electron or immunoelectron microscopy, or DNA-based polymerase chain reaction assays of tissue biopsies, could be used. Amyloid-related end-organ involvement was defined as follows: renal—albuminuria higher than 0.5 g/day in 24-hour urine analysis; cardiac—presence of a mean left ventricular wall thickness on echocardiogram more than 11 mm in the absence of a history of hypertension or valvular heart disease, or unexplained low voltage (< 0.5 mV) on electrocardiogram; hepatic—hepatomegaly on physical examination with alkaline phosphatase higher than 200 U/L; gastrointestinal—gastrointestinal bleeding confirmed by tissue biopsy; autonomic or peripheral neuropathy—based on clinical history, autonomic dysfunction with orthostasis, symptoms of nausea or dysgeusia, gastric atony by gastric emptying scan, diarrhea or constipation, or abnormal sensory and/or motor findings on neurologic examination; soft tissue and lymphatic—based on physical examination findings (macroglossia, shoulder pad sign, raccoon eyes, carpal tunnel syndrome, synovial enlargement, or firm enlarged lymph nodes) or biopsy.
If a patient underwent HDT-SCT as initial therapy, 6 months must have elapsed since transplantation. Patients also required the following: Karnofsky performance status (KPS) of 70% or higher; less than 30% plasma cells in bone marrow aspirate and biopsy; echocardiographic ejection fraction of 40% or higher; platelet count 100 × 109/L (100 000/μL) or higher and absolute neutrophil count 1.5 × 109/L (1500/μL) or higher within 72 hours before the first dose; total bilirubin level 34.2 μM/L (2.0 mg/dL) or less; alkaline phosphatase 4× the upper limit of normal (ULN) or less; alanine transaminase (ALT) or aspartate transaminase (AST) 3× ULN or less; and creatinine clearance .6667 mL/s (40 mL/min) or higher based on 24-hour urine collection.
Patients were excluded if they had received prior treatment with bortezomib; failed to recover from the effects of prior chemotherapy; an uncontrolled infection; New York Heart Association (NYHA) classification III or IV; enzyme-documented myocardial infarction within 6 months before enrollment; chronic atrial fibrillation; grade 2 or 3 atrioventricular block; sustained or recurrent nonsustained ventricular tachycardia; supine systolic blood pressure less than 90 mmHg or symptomatic orthostatic hypotension; grade 3 or higher diarrhea not controllable with medication; grade 2 or higher neuropathy or painful peripheral neuropathy; factor X level 20% or lower; clinically overt multiple myeloma; or presence of other active malignancy (except nonmelanoma skin cancer, cervical cancer, or treated early-stage prostate cancer). Concomitant chemotherapy, immunotherapy, radiotherapy, investigational therapy, digoxin, and anticoagulation with warfarin were not permitted. All patients provided written, informed consent in accordance with the Declaration of Helsinki.
Study design
Patients were enrolled in the phase 1 component of this open-label, dose-escalation phase 1/2 study (http://ClinicalTrials.gov: NCT00298766) at 6 sites in Canada, the United States, and Europe between June 22, 2005, and September 18, 2007. The primary objectives of this study were to determine the MTD (phase 1 component) and safety (phase 2 component) for both once-weekly and twice-weekly administration of bortezomib. The secondary objective was to determine hematologic response rate. The study was carried out in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki, and was approved by the Institutional Review Board/Independent Ethics Committee of all participating centers.
Patients were enrolled in 7 cohorts, initially to receive bortezomib once weekly and subsequently twice weekly. Gradual treatment intensification was used for this first prospective evaluation of bortezomib in AL due to the potentially frail nature of patients with significant pre-existing organ dysfunction. Cohorts 1 to 4 received bortezomib on a once-weekly schedule (days 1, 8, 15, and 22, of 35-day treatment cycles) at planned doses of 0.7, 1.0, 1.3, and 1.6 mg/m2. Cohorts 5 to 7 received bortezomib on a twice-weekly schedule (days 1, 4, 8, and 11, of 21-day treatment cycles) at planned doses of 0.7, 1.0, and 1.3 mg/m2. Patients could receive up to 8 cycles of treatment according to the protocol; prolonged treatment was permitted for patients showing clear evidence of clinical benefit. Dose reductions (1.6 to 1.3, to 1.0, to 0.7, to 0.5 mg/m2) were required for grade 3 or higher neutropenia with fever, grade 4 neutropenia lasting more than 7 days, platelet count less than 25 000/μL, or any grade 3 or higher nonhematologic toxicity considered related to bortezomib. Bortezomib-related neuropathic pain or peripheral sensory neuropathy was managed according to established dose-modification guidelines.27 No dose modifications were permitted during cycle 1, except for dose-limiting toxicity (DLT), defined in “Determination of MTD.”
Patients discontinued treatment if they had unacceptable toxicity (for example, toxicity requiring dose reduction that did not resolve); showed progression of markers of plasma cell dyscrasia; or had a clinically important decrease in KPS with organ function deterioration. Discontinuation could also occur by patient/investigator decision. Patients completing protocol-specified treatment, or discontinuing treatment early for reasons other than progressive disease (PD), were followed up every 6 weeks until PD; post-PD patients were followed every 3 months until study completion (defined as when the final patient has received 8 cycles or discontinued treatment).
Determination of MTD
A standard 3 + 3 dose-escalation design was used to determine the MTD first for once-weekly and then twice-weekly bortezomib administration. Dose escalation proceeded according to the occurrence of DLT. DLT was defined as any grade 4 thrombocytopenia or neutropenia, or any grade 3 or higher nonhematologic toxicity (in particular, worsening neuropathy, life-threatening ventricular arrhythmia or atrial arrhythmia with hemodynamic instability, unresolved fluid retention, symptomatic congestive heart failure, and hypotension) determined by the investigator to be related to bortezomib, that occurred during cycle 1 of treatment.
Three patients were initially enrolled in a cohort (starting with the lowest once-weekly dose cohort). If none of these patients experienced DLT, 3 patients were to be enrolled in the next highest dose cohort. However, if 1 of the first 3 patients experienced DLT, 3 additional patients were enrolled in that cohort; if none of these additional patients experienced DLT, dose escalation continued. The MTD was defined as the dose cohort immediately below that in which 2 or more patients experienced DLT. Upon determination of the MTD for once-weekly dosing, dose escalation proceeded to the twice-weekly schedule, starting with cohort 5. The MTD cohorts for once-weekly and twice-weekly dosing, or the maximumrecommended dose cohorts (1.6 and 1.3 mg/m2, respectively) if the MTD was not defined, were to be expanded for phase 2 investigation.
Assessments
Baseline assessments and procedures included a complete physical examination, assessment of KPS, amyloid-related organ assessment, chest radiographs, 12-lead electrocardiogram, 24-hour Holter monitoring, 2-D echocardiogram, laboratory assessments for hematology and clinical chemistry, serum/urine M-protein analysis, FLC analysis, bone marrow aspirate and biopsy, and pulmonary function tests. These were repeated at the end-of-treatment visit and during treatment and follow up as clinically required. Adverse events (AEs) were recorded throughout the study and until 30 days after the last dose of bortezomib.
Hematologic response was assessed using serum/urine M-protein and FLC analysis during the rest period of each treatment cycle, at the end-of-treatment visit, and then every 6 weeks until PD. Response was determined according to established consensus criteria,28 but without confirmatory bone marrow assessment for CR. Briefly, a CR required absence of M-protein in serum and urine by immunofixation, or a normal FLC ratio in the absence of measurable serum and urine M-protein at baseline. Partial response (PR) required 50% reduction in serum (if baseline > 0.5 g/dL) and urine (if > 100 mg/24 hours) M-protein, and FLC (if > 10 mg/dL). PD was defined as a 50% increase in serum M-protein (to > 0.5 g/dL from nadir/baseline), or urine M-protein (to > 200 mg/24 hours, with a visible peak), or FLC (to > 10 mg/dL from nadir/baseline). PD after CR required reappearance of M-protein by immunofixation or abnormal FLC ratio (light chain must double). Stable disease (SD) defined the response in patients who did not meet the criteria of CR, PR, or PD. Responses of CR, PR, or SD required confirmation at 6 weeks. Organ response data and data on cardiac biomarkers for patients in this phase 1 component were not available at data cutoff for the present report and will be presented together with those for patients in the phase 2 component.
Statistical methods
Based on the dose-escalation scheme, up to 42 patients could be enrolled in the phase 1 component of this study. The safety population included all patients who received at least 1 dose of bortezomib. The efficacy-evaluable population comprised patients who received 1 cycle of treatment and had evaluable response assessments.
Results
Patient characteristics
A total of 31 patients with relapsed AL were enrolled in 7 cohorts in the phase 1 component of this study. Baseline demographics and disease characteristics by cohort and overall are shown in Table 1. Overall median age was 59 years (range, 38-77 years), more than half of patients had involvement of 2 or more organs, and the plasma cell dyscrasia was λ in 26, κ in 4, and biclonal in 1. Eleven (35%) patients had grade 1 sensory neuropathy at baseline. Per protocol, all patients had received prior systemic therapy for AL, including 13 (42%) who had undergone HDT-SCT; median time from diagnosis of AL was 32 months. Patient disposition by cohort is shown in Table 2. At the time of this analysis, 11 (35%) patients had completed treatment, whereas treatment was ongoing in 2 (6%). Among the 18 (58%) patients who terminated the study early, the primary reasons were occurrence of AEs (9 patients, including 4 in the highest twice-weekly dose cohort), patient choice (3 patients), deterioration in overall condition (2 patients) or KPS and organ function (1 patient), progression of amyloid markers, death, and other reasons (each 1 patient).
DLT and determination of MTD
Only 2 patients experienced DLT. One of the first 3 patients enrolled in cohort 4 (bortezomib 1.6 mg/m2 once weekly) experienced grade 3 worsening congestive heart failure that was reported as a serious AE. The patient had cardiac and gastrointestinal organ involvement at baseline. The AE was considered bortezomib related and led to treatment discontinuation. Consequently, a further 3 patients were enrolled in this cohort; none experienced DLT. In addition, 1 of the first 3 patients enrolled in cohort 6 (bortezomib 1.0 mg/m2 twice weekly) also experienced grade 3 congestive heart failure, which resolved after interruption of therapy; bortezomib was recommenced with a dose reduction. This patient had a history of hypertension but with no proven cardiac organ involvement. A further 3 patients were enrolled in this cohort; none experienced DLT. Although none of the first 3 patients enrolled in cohort 7 experienced DLT, another 3 were enrolled to further characterize safety; 1 patient missed 2 doses in cycle 1 and a seventh patient was enrolled as a replacement.
Thus, the MTD was not defined for either bortezomib dosing schedule, and the maximum doses of 1.6 mg/m2 for the once-weekly schedule and 1.3 mg/m2 for the twice-weekly schedule were to be investigated further in the phase 2 component of the study.
Treatment exposure and safety
Exposure to bortezomib by cohort is shown in Table 3. Median dose intensity increased with starting dose in the once-weekly cohorts (1-4) from 2.8 to 6.1 mg/m2 per cycle. In the twice-weekly cohorts, dose intensity increased with dose from cohort 5 to cohort 6, but then remained similar for cohort 7. Cohort 7 was associated with higher incidences of dose modifications.
All 31 patients were evaluable for safety. The most common AEs are summarized in Table 4 by cohort and overall. The most commonly reported AEs of any grade by organ class were gastrointestinal events (27 patients, 87% overall), with nausea and vomiting appearing to be more common in the higher-dose cohorts; general disorders such as fatigue and asthenia (23 patients, 74%); infections (21 patients, 68%; only nasopharyngitis—6 patients, 19%—was reported in more than 5 patients); and nervous system disorders (20 patients, 65%). Within the latter category, headache and paresthesia were each reported in 6 patients (19%), and peripheral neuropathy not elsewhere classified (NEC; high-level term) was reported in 5 patients (16%), comprising individual AEs reported as peripheral sensory neuropathy in 3 patients (10%; 1 each in cohorts 3, 4, and 7), neuropathy in 2 patients (6%, 1 each in cohorts 4 and 7, including 1 patient who was also reported as having peripheral sensory neuropathy), and peripheral motor neuropathy in 1 patient (3%) in cohort 7. Grade 3/4 AEs were predominantly reported in patients in the higher-dose cohorts (cohorts 4, 6, and 7; Table 5).
Discontinuation of bortezomib due to AEs was reported in 12 patients (39%), including 5 of 7 patients in cohort 7 (Table 5). The only AE leading to discontinuation reported in more than 1 patient was fatigue, reported in 3 patients (1 each in cohorts 4, 6, and 7). AEs leading to dose reductions were reported in 4 patients (13%), including 2 in cohort 4 (diarrhea, neuropathy, and paresthesia), 1 in cohort 6 (congestive cardiac failure and peripheral edema), and 1 in cohort 7 (fatigue). The only AEs leading to doses being withheld reported in more than 1 patient were diarrhea (2 patients, 1 each in cohorts 2 and 7) and vomiting (2 patients in cohort 7). The most commonly reported AEs leading to cycle delays were diarrhea (9 patients, 29%), nausea, fatigue (each 8 patients, 26%), constipation, and vomiting (each 7 patients, 23%). At data cutoff for this report, 4 patients had died (1 each in cohorts 3, 4, 6, and 7). Death was due to PD in 2 patients and progression of prostate cancer in 1 patient who had been treated for this malignancy more than 10 years before enrollment and had no evidence of active disease at study entry; all of these deaths occurred more than 30 days after the last dose of bortezomib. The other death was due to an AE of interstitial lung disease considered possibly related to treatment in 1 patient.
Efficacy
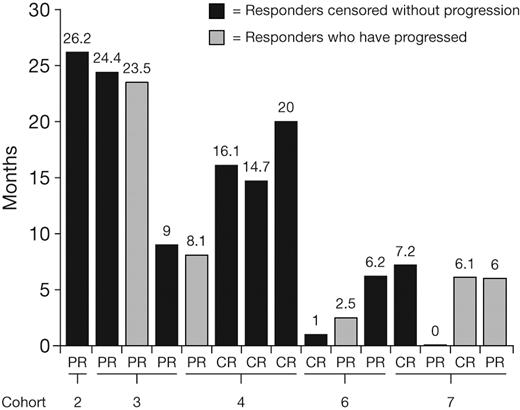
Thirty patients were included in the response-evaluable population; 1 patient in cohort 4 who had DLT and discontinued therapy during cycle 1 was excluded. Best hematologic responses to date are summarized in Table 6. These preliminary data show an overall response rate (CR + PR) of 50% (15/30 patients), including a 20% CR rate (6/30 patients). Median time to first response was 1.2 months (range, 0.4-6.0 months), and median time to CR was 2.3 months (range, 1.2-5.0 months). Responses were seen mainly in the higher-dose cohorts for both once-weekly and twice-weekly dosing. At data cutoff for this report, 9 (30%) of 30 patients had progressed. Although the data are insufficiently mature to characterize duration of response, 5 responders have progressed, with response durations of 2.5 to 23.5 months, and the longest response duration is currently censored at 26.2 months (Figure 1).
Current durations of CR or PR in relapsed AL patients treated with bortezomib, at data cutoff for this report.
Current durations of CR or PR in relapsed AL patients treated with bortezomib, at data cutoff for this report.
Discussion
The results from this phase 1 dose-escalation component of our phase 1/2 study of bortezomib in previously treated AL patients indicate that once-weekly and twice-weekly administration of bortezomib generally has a good safety profile and is well tolerated in this highly selected patient population; to enable a comprehensive characterization of the safety of bortezomib in AL, patients with poor prognosis were excluded. The MTD was not determined for either dosing schedule, with only 2 patients experiencing DLT. Therefore, the maximum recommended doses of 1.6 mg/m2 for the once-weekly schedule and 1.3 mg/m2 for the twice-weekly schedule were selected and are currently being used in the ongoing phase 2 component of this study.
The safety profile of bortezomib in AL reported here appears generally similar to the well-characterized profile seen in studies in relapsed multiple myeloma.29-31 The most common AEs were fatigue, various gastrointestinal events, back pain, and dizziness, with only fatigue, congestive heart failure, thrombocytopenia, and vomiting being reported as grade 3/4 in more than 1 patient. One of the episodes of congestive heart failure, in a patient receiving bortezomib 1.0 mg/m2 twice weekly, represented 1 of the 2 DLTs seen in this study; the event resolved after a temporary cessation of bortezomib therapy, which was recommenced at a lower dose. The other DLT was also a case of congestive heart failure in a patient receiving bortezomib 1.6 mg/m2 once weekly who had cardiac involvement at baseline; the DLT was considered related to treatment and led to bortezomib discontinuation.
The incidences of hematologic toxicities, including anemia, neutropenia, and thrombocytopenia, and of peripheral neuropathy, particularly grade 3/4 events, appeared lower in the present study compared with studies in multiple myeloma.29-31 However, this may reflect the lower dose/dose intensity of bortezomib received by the majority of our AL patients. Bortezomib-associated peripheral neuropathy is a cumulative dose-related toxicity,27,32 and so the apparent difference in incidence may reflect the lower doses received by most AL patients in the present study, together with the limited extent of exposure to treatment in the highest twice-weekly dose cohort. It may also be associated with differences in the underlying disease-related neuropathy of multiple myeloma33 and AL.13,33 The phase 2 component of our study will further characterize the hematologic toxicity and peripheral neuropathy with bortezomib in AL at the maximum recommended doses of 1.6 mg/m2 for once-weekly and 1.3 mg/m2 for twice-weekly dosing.
Hematologic response in AL has been shown to be associated with improved outcome,6,11,12,34 with higher quality of response resulting in prolonged survival.10,35 Therefore, improving overall response and especially CR rates is an important aim of novel therapies for AL. Preliminary data from the present study suggest that bortezomib has promising activity in this highly selected population of patients with relapsed AL, with a hematologic response rate of 50%, including 20% CR. Our patient population excluded those with poor prognosis, and it remains to be seen whether these findings are reflected in less selected patients or the general AL patient population. However, our preliminary results appear to reflect the activity reported recently in 3 case series analyses of bortezomib alone or in combination with dexamethasone in less selected patients with previously untreated and relapsed AL, including populations with poor prognostic factors such as higher rates of cardiac involvement.24-26 For example, in a multicenter series of 94 AL patients, bortezomib with or without dexamethasone resulted in a 71% hematologic response rate, including 25% CR, plus a 30% organ response rate, including 22% cardiac, 17% renal, and 19% hepatic responses.24 Median time to any progression was 13.3 months; estimated 1- and 2-year survival rates were 78% and 71%, respectively.24
A particularly important aspect of the hematologic responses seen with bortezomib is the generally short time to response. The median times to first response and to CR in our preliminary data were 1.2 months and 2.3 months, respectively. This compares with median times of 3.5 to 6.2 months reported with other therapies.9,12,19,36,37 A rapid hematologic response may be of importance in AL to ensure timely therapeutic benefit of treatment in terms of organ responses.13 Further investigation in the phase 2 component of the present study and prolonged follow up in the other studies will be required to determine whether these rapid hematologic responses with bortezomib are sustained and translate into durable organ responses.
Mechanistically, the activity of bortezomib in AL and multiple myeloma may result from similar effects, primarily inhibition of nuclear factor-kappa B transcription and the induction of endoplasmic reticulum stress in immunoglobulin-secreting cells.6 Importantly, endoplasmic reticulum stress may be exacerbated in AL by the production of abnormal amyloid light chains, increasing the sensitivity of amyloidogenic plasma cells to bortezomib.6 The mechanisms of activity of bortezomib have also been shown to enhance the activity of other agents including dexamethasone20 and melphalan38 in preclinical studies in multiple myeloma.
These preclinical findings and the preliminary clinical data provide the rationale for further investigation of bortezomib in AL both alone and in combination. In addition to the ongoing phase 2 component of the present study, investigating once-weekly bortezomib 1.6 mg/m2 and twice-weekly bortezomib 1.3 mg/m2, 2 ongoing phase 2 studies are investigating bortezomib, melphalan, and dexamethasone in patients with AL or light-chain deposition disease, and bortezomib plus dexamethasone as post–HDT-SCT therapy in patients with previously untreated AL; initial data from the latter appear promising in terms of improved hematologic responses and overall survival.39 The results of these studies will help in further defining the promising activity and tolerability of bortezomib-based therapy in the AL patient population.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dixie-Lee Esseltine, MD, of Millennium Pharmaceuticals Inc, who was instrumental in organizing and finalizing the study. The authors also thank Steve Hill and Jane Saunders of FireKite for writing and editorial assistance in the development of this manuscript, which was supported by Millennium Pharmaceuticals Inc.
G.M. and G.P. are supported by the EURAMY Systemic Amyloidoses in Europe project (European Community's Sixth Framework Programme) and by the NOBEL project (Cariplo [Cassa di Risparmio delle Provincie Lombarde] Foundation, Milan, Italy).
A complete list of VELCADE CAN2007 study group investigators is published in the supplemental Appendix (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Authorship
Contribution: D.E.R., Y.A.E., and A.C. designed the research; D.E.R., V.S., U.H., G.M., G.P., J.-P.F., R.A.V., and R.L.C. performed the research; Y.A.E. and A.C. collected data; D.E.R., V.S., U.H., G.M., G.P., J.-P.F., R.A.V., and R.L.C. analyzed and interpreted data; X.L. and A.C. performed statistical analysis; and D.E.R., Y.A.E., and A.C. wrote the paper. All authors reviewed and approved the paper.
Conflict-of-interest disclosure: D.E.R. has received honorarium, consultancy, and research funds from Millennium Pharmaceuticals, Johnson & Johnson, and Ortho Biotech. V.S. has received research funds from Johnson & Johnson and Celgene and is a member of the speakers bureau for Ortho Biotech LLP. G.M. has received an honorarium from Millennium Pharmaceuticals. R.A.V. has received research funds and is a member of the speakers bureau for Millennium Pharmaceuticals and Celgene. X.L., Y.A.E., and A.C. are employees of Johnson & Johnson Oncology Research & Development. R.L.C. is a scientific advisory board member for Millennium Pharmaceuticals and Celgene, and has received research funds from Millennium Pharmaceuticals, Johnson & Johnson, and Celgene. The remaining authors declare no competing financial interests.
Correspondence: Donna E. Reece, Department of Medical Oncology/Hematology, Princess Margaret Hospital, University Health Network, 610 University Ave, Suite 5-207, Toronto, ON, Canada M5G 2M9; e-mail: donna.reece@uhn.on.ca.