Abstract
The clinical hallmark of paroxysmal nocturnal hemoglobinuria (PNH) is chronic intravascular hemolysis that is a consequence of unregulated activation of the alternative pathway of complement (APC). Intravascular hemolysis can be inhibited in patients by treatment with eculizumab, a monoclonal antibody that binds complement C5 thereby preventing formation of the cytolytic membrane attack complex of complement. However, in essentially all patients treated with eculizumab, persistent anemia, reticulocytosis, and biochemical evidence of hemolysis are observed; and in a significant proportion, their PNH erythrocytes become opsonized with complement C3. These observations suggest that PNH patients treated with eculizumab are left with clinically significant immune-mediated hemolytic anemia because the antibody does not block APC activation. With a goal of improving PNH therapy, we characterized the activity of anti-C3b/iC3b monoclonal antibody 3E7 in an in vitro model of APC-mediated hemolysis. We show that 3E7 and its chimeric-deimmunized derivative H17 block both hemolysis and C3 deposition on PNH erythrocytes. The antibody is specific for the APC C3/C5 convertase because classical pathway–mediated hemolysis is unaffected by 3E7/H17. These findings suggest an approach to PNH treatment in which both intravascular and extravascular hemolysis can be inhibited while preserving important immune functions of the classical pathway of complement.
Introduction
Although commonly regarded as a type of hemolytic anemia, paroxysmal nocturnal hemoglobinuria (PNH) is actually a hematopoietic stem cell disorder that arises as a result of nonmalignant clonal expansion of 1 or several hematopoietic stem cells that have an acquired somatic mutation of the X-chromosome gene PIGA (reviewed in Parker1 ). As a consequence of mutant PIGA, progenies of affected stem cells (erythrocytes, granulocytes, monocytes, platelets, and lymphocytes) are deficient in all glycosyl phosphatidylinositol–anchored proteins (GPI-APs) that are normally expressed on hematopoietic cells. The clinical manifestations of PNH are hemolytic anemia, thrombophilia, and bone marrow failure, but only the hemolytic anemia is unequivocally a consequence of somatic mutation of PIGA because the 2 membrane proteins, CD55 and CD59, that are primarily responsible for controlling complement activation on erythrocytes are both GPI anchored.
The chronic intravascular hemolysis of PNH is mediated by the alternative pathway of complement (APC), a component of innate immunity. This ancient system evolved to protect the host against invasion by pathogenic microorganisms.2,3 Unlike the classical pathway of complement (CPC) that requires antibody for initiation of activation, the APC is in a state of continuous activation, always armed to protect the host. The APC cascade can be divided into 2 functional components, the amplification C3 and C5 convertases and the cytolytic membrane attack complex (MAC). The C3 and C5 convertases are enzymatic complexes that initiate and amplify the activity of the APC and ultimately generate the MAC. The MAC is the common cytolytic subunit of the classical and lectin pathways of complement as well as the APC.
Because the APC is always primed for attack, elaborate mechanisms for self-recognition and for protection of the host against APC-mediated injury have evolved. Both fluid-phase and membrane-bound proteins are involved in these processes. Normal human erythrocytes are protected against APC-mediated cytolysis primarily by decay-accelerating factor (CD55) and membrane inhibitor of reactive lysis (CD59).4-7 These proteins act at different steps in the complement cascade. CD55 regulates the formation and stability of the C3 and C5 convertases, whereas CD59 blocks formation of the MAC. Deficiency of both CD55 and CD59 on PNH erythrocytes is the pathophysiologic basis of the direct antibody test (DAT)–negative, intravascular hemolysis that characterizes the disease in its untreated state.
The complement-mediated intravascular hemolysis of PNH can be inhibited by blocking formation of the MAC that consists of complement components C5b, C6, C7, C8, and multiple molecules of C9. Eculizumab (Soliris; Alexion Pharmaceutics Inc) is a humanized monoclonal antibody that binds C5, preventing its activation to C5b and thereby inhibiting MAC formation. In 2007, eculizumab was approved by both the US Food and Drug Administration and the European Union Commission for treatment of the hemolysis of PNH (reviewed in Parker8 ). Treatment with eculizumab reduces transfusion requirements, ameliorates the anemia, and improves quality of life by resolving the debilitating constitutional symptoms (fatigue, lethargy, asthenia) associated with chronic complement-mediated intravascular hemolysis.9,10 After treatment, serum lactate dehydrogenase concentration, a surrogate marker for intravascular hemolysis, returns to near normal, but anemia, hyperbilirubinemia, and reticulocytosis persist in essentially all treated patients, with only approximately half achieving transfusion independence.9,10 The known pathophysiology of the disease predicts that CD55 deficiency would result in ongoing extravascular hemolysis of PNH erythrocytes as a consequence of C3 opsonization, as eculizumab does not block the activity of the APC C3 convertase. Support for this hypothesis is provided by recent studies of Risitano et al who showed that, in patients treated with eculizumab, a portion of the PNH erythrocytes (ie, the CD59-deficient population) had C3 fragments bound.11 Those studies also confirmed the DAT-negative designation of PNH, as no C3 was found bound to PNH erythrocytes before initiation of treatment with eculizumab, suggesting that, in the untreated state, PNH erythrocytes upon which complement has been activated are destroyed directly as a consequence of MAC-mediated cytolysis. Thus, the studies of Risitano et al provide a plausible explanation for the persistent hemolytic anemia observed in PNH patients treated with eculizumab. By inhibiting formation of the MAC, eculizumab prevents direct cytolysis of PNH erythrocytes, allowing the manifestations of CD55 deficiency to become apparent in the form of aberrant regulation of the APC and the consequent deposition of activated C3 on the cell surface. Covalently bound activation and degradation products of C3 then serve as opsonins that are recognized by specific receptors on reticuloendothelial cells, resulting ultimately in clinically significant extravascular hemolysis. These observations suggest that treatment of the hemolysis of PNH can be improved by blocking the APC and thereby preventing extravascular and intravascular hemolysis by inhibiting both C3 deposition and MAC formation.
We developed and extensively characterized the complement-inhibitory activity of monoclonal antibody (mAb) 3E7 and its chimeric-deimmunized derivative H17. Our experiments demonstrated that 3E7/H17 blocks the APC in vitro based on its capacity to prevent C3b deposition on the surface of a variety of APC activators including zymosan and Sepharose.12 We also showed that 3E7/H17 blocks C3b deposition and complement-mediated hemolysis in whole human serum using the powerful APC activator, rabbit erythrocytes, as the indicator cells and that mAb 3E7 blocks hematin-mediated mediated APC activation in an in vitro model of malaria.13 Together, these experiments suggest that 3E7/H17 can block initiation of the APC regardless of the activating stimulus. With the ultimate goal of improving therapy for PNH, we initiated studies designed to determine whether 3E7/H17 can inhibit hemolysis and C3 deposition on PNH erythrocytes in an in vitro model of APC activation.
Methods
Patient blood samples
Samples of peripheral blood from patients with PNH or chronic cold agglutinin disease (CCAD) or from healthy donors were obtained according to protocols approved by the institutional review boards of the University of Utah and the University of Virginia after written informed consent was obtained in accordance with the Declaration of Helsinki. Blood samples were obtained from 5 patients with PNH with patients 1, 2, and 5 being actively treated with eculizumab. The diagnosis of PNH was made based on flow cytometric analysis of expression of CD55 and CD59 on peripheral blood granulocytes and erythrocytes.
Monoclonal antibodies and reagents
mAbs specific for human C3 fragments (1H8, 3E7/H17, 7C12) were previously described.12,14-16 mAb 5G9, specific for human C3b/iC3b, was prepared and characterized following published procedures.12,14,15 mAb 5G9 inhibits CPC activation, as determined by assay with hemolysin-opsonized sheep red blood cells17 (J. D. Lambris and R.P.T., unpublished observations, April 2009). mAbs were labeled with Alexa (Al) dyes (Invitrogen) according to the manufacturer's directions. mAb HD1A, specific for CD55,18 was the kind gift from Drs Paul Morgan and Claire Harris (University of Wales College of Medicine, Cardiff, United Kingdom). Phycoerythrin (PE)–conjugated mAb anti-CD59 was purchased from Caltag. The specificities of all mAbs used in this study are provided in Table 1. C5-deficient human sera were purchased from Complement Technology.
PNH erythrocytes and hemolysis experiments
In all cases, erythrocytes were centrifuged, the buffy coat was aspirated, and the cells were thoroughly washed in gelatin veronal buffer (GVB) before each experiment.12,19 Most reported experiments were performed using erythrocytes from patients 1 and 2, because we obtained more frequent blood samples from those patients. However, similar results were obtained with the blood of the other patients, and representative experiments are illustrated. Tests for the susceptibility of erythrocytes to APC-mediated lysis followed previously described methods.19 In brief, ABO-matched normal human sera were diluted with GVB containing 0.15mM CaCl2 and 0.5mM MgCl2 (GVB+2) and acidified to pH 6.4 (acidified normal human serum [aNHS]) and used to reconstitute the erythrocytes to a hematocrit of .016 (1.6%) in 50% aNHS. The mixtures were incubated at 37°C, and after 1 hour, the erythrocytes were pelleted by centrifugation, and the optical density at 405 nm of an aliquot of the recovered supernate was used to calculate the percentage lysis. Samples reconstituted in acidified serum–EDTA (ethylenediaminetetraacetic acid) were processed similarly and used to define background non–complement-mediated lysis (typically less than 3%). Complete lysis (100%) was determined after incubating the erythrocytes in distilled water.
Erythrocytes from healthy persons are not lysed by incubation in aNHS. Therefore, erythrocytes from healthy donors were reacted with aminoethylisothiouroinium bromide (AET; Sigma) exactly as described by Ezzell et al, to generate an aNHS-sensitive PNH-like phenotype.20 In most experiments, the AET-treated erythrocytes were also treated with mAb HD1A, which specifically blocks the function of CD55.18
Serum from a patient with chronic cold agglutinin disease (CCAD) was used to opsonize PNH erythrocytes with immunoglobulin M (IgM).21 Briefly, ABO-matched PNH erythrocytes were washed 3 times with GVB, and 4 μL of packed erythrocytes was mixed with 20 μL of CCAD serum containing 10mM EDTA. After incubation at 30°C for 30 minutes to allow binding of the IgM antibody, the erythrocytes were washed once with GVB prewarmed to 30°C and incremental concentrations of mAb 3E7 or a fixed concentration of mAb 5G9 was added in 80 μL of 50% NHS diluted with GVB+2. After incubation at 37°C for 60 minutes, erythrocytes were pelleted by centrifugation, and percentage lysis was determined. Serum chelated with EDTA was substituted for normal serum to control for non–complement-mediated lysis.
Measurement of C3 activation fragment deposition on ghosts and on intact erythrocytes
Flow cytometry was used to analyze deposition of C3 activation fragments on intact and lysed PNH erythrocytes (ghosts) that were generated after incubation in aNHS. The ghosts and intact erythrocytes were pelleted by centrifugation at 11 500g for 5 minutes and, after washing, were reconstituted in bovine serum albumin–phosphate-buffered saline containing 2 mg/mL normal mouse IgG and probed, as appropriate, with Al or PE-labeled mAbs specific for C3b/iC3b (7C12), C3b/iC3b/C3dg (1H8), C3b/iC3b (H17/3E7), or CD59. The 3 mAbs, H17/3E7, 1H8, and 7C12, all bind to separate, nonoverlapping epitopes on C3 fragments.12 In experiments where indicated, ghosts were distinguished from intact erythrocytes based on differences in light-scattering properties. Alternatively, differential centrifugation was used to separate the 2 cell populations using the following method. After the initial high-speed centrifugation step that pelleted both erythrocytes and ghosts, the cells were reconstituted in bovine serum albumin–phosphate-buffered saline and centrifuged at 150g for 5 minutes to pellet the intact erythrocytes but leave the ghosts in the supernatant fluid. Next, the 2 cell populations were isolated by aspirating the supernatant fluid, probed separately with the appropriate mAb, and subsequently evaluated by flow cytometry using a modified light-scattering gate. In certain experiments, calibrated fluorescent beads were used to convert mean fluorescence intensities to molecules of equivalent soluble fluorochrome (MESF) to allow for quantitative evaluations and comparisons of C3 fragment deposition on erythrocytes and ghosts.12 To mimic the effects of treatment with eculizumab, C5-deficient NHS was substituted for NHS in the acidified serum lysis assay.
Results
mAb H17/3E7 blocks APC- but not CPC-mediated lysis of PNH erythrocytes
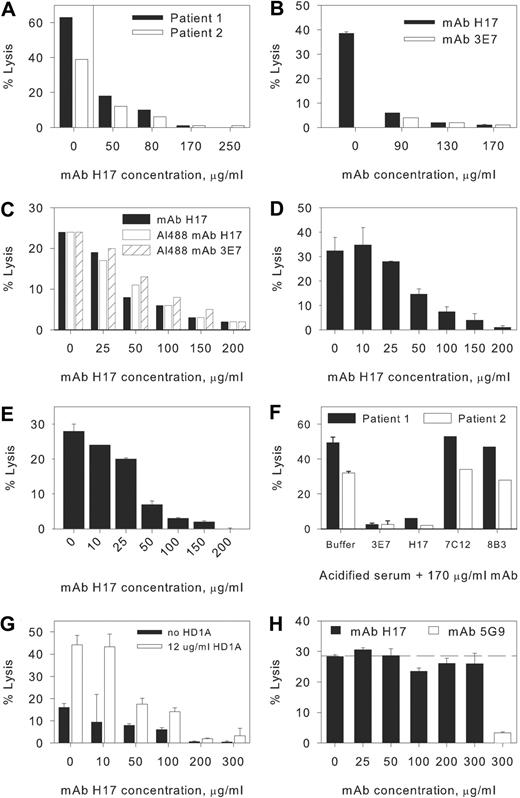
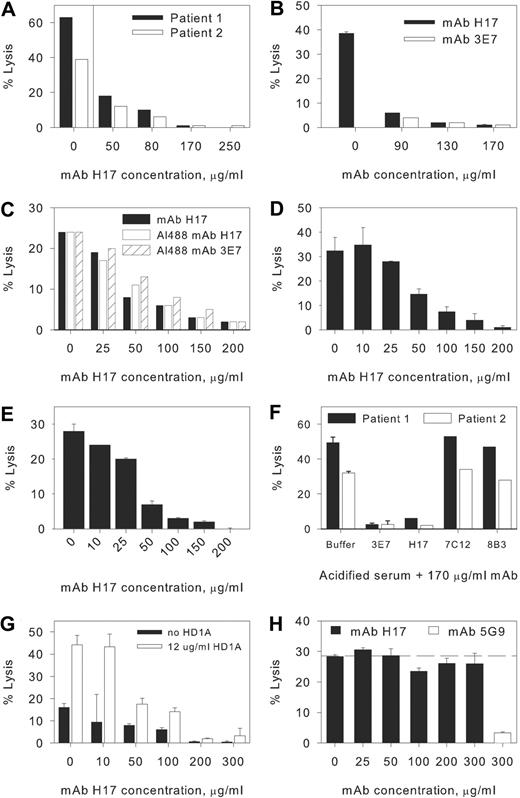
Acidification of NHS to pH 6.4 initiates brisk activation of the APC and induces lysis of PNH erythrocytes from patients 1 and 2 (Figure 1A-F). Both murine mAb 3E7 and its chimeric counterpart H17 inhibit acidified serum lysis of PNH erythrocytes in a concentration-dependent fashion with essentially complete inhibition at a concentration of 170 μg/mL (Figure 1A-E). The inhibitory activity of 3E7/H17 is specific, as neither mAb 7C12, which recognizes an epitope expressed on C3b/iC3b distinct from that recognized by 3E7/H17, or an isotype-matched mAb with irrelevant specificity, 8B3 (Table 1), has any effect on hemolysis (Figure 1F). We also note that the Alexa 488 derivatives of H17 and 3E7 have inhibitory activity comparable with that of unlabeled H17 (Figure 1C). In PNH patient 3, we found that hemolysis in acidified serum was reduced from 27% (± 0.5%) to 3.5% (± 1%; n = 2) when the acidified serum contained 150 μg/mL mAb H17.
Effects of mAbs on hemolysis of PNH erythrocytes. Concentration-response effect of monoclonal antibodies (mAbs) H17 and 3E7. Erythrocytes from paroxysmal nocturnal hemoglobinuria (PNH) patients 1 and 2 were incubated in aNHS in the presence of incremental concentrations of the mAbs, and hemolysis was subsequently quantified. Experiments for separate blood samples obtained from these patients (all in 2009) in March (A,F), April (B-C, patient 2), and June (D, patient 1; E, patient 2) are shown. Sample standard deviations for duplicate or triplicate determinations are provided. In certain experiments only single points were evaluated, and in these instances, error bars are absent. (A) A concentration-dependent effect was observed for mAb H17 with near complete inhibition observed at a concentration of 170 μg/mL mAb H17. (B-E) Comparison of the effects of H17, 3E7, and Al488 H17 or Al488 3E7 on lysis in acidified serum. Similar dose responses for inhibition of lysis are evident. Each experiment illustrated in panels A through F is representative of at least 1 other comparable experiment. (F) Specificity of 3E7/H17. Erythrocytes from PNH patients 1 and 2 were incubated in aNHS in the presence of a fixed concentration of mAb, and hemolysis was subsequently quantified. The murine IgG1 mAb 3E7, which is specific for C3b/iC3b and inhibits APC C3 convertase formation, and H17 (chimeric-deimmunized 3E7) block acidified serum lysis, but another murine IgG1 mAb, 7C12, which recognizes an epitope expressed on C3b/iC3b distinct from that of H17/3E7, and a mAb of irrelevant specificity, 8B3 (mouse IgG1), have no effect. (G) Normal human erythrocytes reacted with AET and additionally blocked with anti-CD55 mAb HD1A are lysed in aNHS, and mAb H17 blocks lysis. Representative of 5 similar experiments. (H) Effects of mAbs on CPC-mediated lysis of PNH erythrocytes. Erythrocytes from patient 2 were incubated with EDTA-chelated serum from a patient with CCAD to allow binding of the IgM antibody. After washing, the IgM-bearing cells were incubated with NHS and incremental concentrations of mAb H17 or 300 μg/mL mAb 5G9 (specific for the CPC). The results of 1 experiment in duplicate, representative of 3 experiments, are illustrated. The dashed line is set at the percentage lysis observed in the absence of mAb H17 (0 μg/mL). mAb H17 has no effect on hemolysis of PNH erythrocytes mediated by the CPC.
Effects of mAbs on hemolysis of PNH erythrocytes. Concentration-response effect of monoclonal antibodies (mAbs) H17 and 3E7. Erythrocytes from paroxysmal nocturnal hemoglobinuria (PNH) patients 1 and 2 were incubated in aNHS in the presence of incremental concentrations of the mAbs, and hemolysis was subsequently quantified. Experiments for separate blood samples obtained from these patients (all in 2009) in March (A,F), April (B-C, patient 2), and June (D, patient 1; E, patient 2) are shown. Sample standard deviations for duplicate or triplicate determinations are provided. In certain experiments only single points were evaluated, and in these instances, error bars are absent. (A) A concentration-dependent effect was observed for mAb H17 with near complete inhibition observed at a concentration of 170 μg/mL mAb H17. (B-E) Comparison of the effects of H17, 3E7, and Al488 H17 or Al488 3E7 on lysis in acidified serum. Similar dose responses for inhibition of lysis are evident. Each experiment illustrated in panels A through F is representative of at least 1 other comparable experiment. (F) Specificity of 3E7/H17. Erythrocytes from PNH patients 1 and 2 were incubated in aNHS in the presence of a fixed concentration of mAb, and hemolysis was subsequently quantified. The murine IgG1 mAb 3E7, which is specific for C3b/iC3b and inhibits APC C3 convertase formation, and H17 (chimeric-deimmunized 3E7) block acidified serum lysis, but another murine IgG1 mAb, 7C12, which recognizes an epitope expressed on C3b/iC3b distinct from that of H17/3E7, and a mAb of irrelevant specificity, 8B3 (mouse IgG1), have no effect. (G) Normal human erythrocytes reacted with AET and additionally blocked with anti-CD55 mAb HD1A are lysed in aNHS, and mAb H17 blocks lysis. Representative of 5 similar experiments. (H) Effects of mAbs on CPC-mediated lysis of PNH erythrocytes. Erythrocytes from patient 2 were incubated with EDTA-chelated serum from a patient with CCAD to allow binding of the IgM antibody. After washing, the IgM-bearing cells were incubated with NHS and incremental concentrations of mAb H17 or 300 μg/mL mAb 5G9 (specific for the CPC). The results of 1 experiment in duplicate, representative of 3 experiments, are illustrated. The dashed line is set at the percentage lysis observed in the absence of mAb H17 (0 μg/mL). mAb H17 has no effect on hemolysis of PNH erythrocytes mediated by the CPC.
To further generalize our observations, we reacted normal human erythrocytes with AET to disrupt the conformation and activity of the erythrocyte complement control proteins and thereby generate a PNH-like phenotype.20 A fraction of the AET-treated erythrocytes are lysed in aNHS, and lysis is particularly effective when the residual protective activity of CD55 is blocked by addition of anti-CD55 mAb HD1A18 (Figure 1G). Addition of mAb H17 to this system substantially inhibits lysis, and the dose-response experiment indicates that the concentration of H17 that blocks lysis of the AET-reacted cells is in the same range as that required to protect PNH erythrocytes from lysis.
The specificity of mAb H17 for the APC was demonstrated in experiments in which PNH erythrocytes were sensitized with an IgM antibody from a patient with CCAD and incubated in NHS as the complement source. Under these conditions, hemolysis is mediated by the CPC (Figure 1H). No inhibition of CPC-mediated hemolysis was observed at concentrations of mAb H17 that are sufficient to completely inhibit APC-mediated hemolysis. In contrast, a mAb (5G9) that recognizes an epitope expressed by C3b/iCb distinct from that recognized by 3E7/H17 markedly inhibits the CPC-mediated lysis of PNH erythrocytes (Figure 1H). Together, these experiments demonstrate the activity and specificity of 3E7/H17.
mAb H17/3E7 blocks APC-mediated deposition of C3 activation fragments
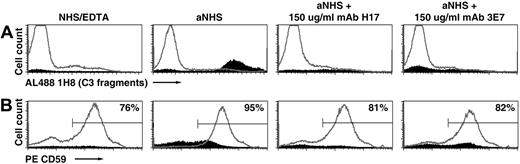
To investigate the mechanism whereby H17/3E7 inhibits hemolysis of PNH erythrocytes in acidified serum, cells were incubated in acidified serum in the absence and presence of a fixed concentration of mAb H17 or mAb 3E7 (Figure 2). Both lysed and unlysed cells were recovered and analyzed separately by selectively gating on the intact and lysed (ghost) cells based on their characteristic and distinct forward and side scatter properties. After incubation in acidified serum, unlysed cells (Figure 2A gray line histograms) had little or no C3 fragments bound (Figure 2A, aNHS). The erythrocytes of PNH are a mosaic of GPI-AP–deficient and phenotypically normal cells (Figure 2B, NHS-EDTA). Incubation in acidified serum eliminated the population of cells with absent or low CD59 expression (Figure 2B, aNHS compared with NHS-EDTA). In the presence of mAb H17 or 3E7, however, the population of CD59-deficient cells was preserved (Figure 2B second from right and far right panels). Together, these findings demonstrate that mAb H17 prevents lysis of the complement-sensitive population of PNH erythrocytes by blocking APC activation on the cell surface. Analysis of the lysed cells (ghosts) showed evidence of deposition of activation and degradation products of C3 on the CD59-deficient population, confirming that lysis in aNHS was mediated by APC activation on the surface of the GPI-AP–deficient cells (Figure 2A-B solid histograms, aNHS). Quantitation of C3 fragment deposition on the ghosts, based on binding mAb 1H8 and expressed as molecules of equivalent soluble fluorochrome (MESF), gave a signal of 18 300 MESF, whereas the corresponding signal for the unlysed cells from the same incubation was 434 MESF (Figure 2A, aNHS, solid histogram compared with gray histogram). Few C3 fragments were detected on the small population of ghosts recovered from the samples incubated either with aNHS chelated with EDTA to prevent complement activation or with aNHS containing an inhibitory concentration mAb H17 or 3E7 (Figure 2A), and binding of anti-CD59 to these populations was indistinct. These findings suggest that the small percentage of ghosts in these samples resulted in part from non–complement-mediated lysis. Three comparable experiments, using the erythrocytes of patient 4, produced a similar outcome with the results of a representative experiment as follows: after reaction in aNHS, the level of binding of mAb 1H8 to ghosts was 9400 (± 300) MESF (n = 3), and binding of mAb 1H8 to the unlysed erythrocytes in the same samples gave an MESF value of 160 (± 4; n = 3).
Inhibition of C3 deposition on PNH erythrocytes by mAb 3E7/H17. PNH erythrocytes from patient 2 were incubated in aNHS chelated with EDTA (to prevent complement activation; NHS/EDTA, left panel), with aNHS (45% lysis; middle panel), or with aNHS containing 150 μg/mL mAb H17 or 3E7 (4% and 5% lysis, respectively; right panels). Both lysed and unlysed cells were recovered by high-speed centrifugation, washed, and incubated with a combination of fluorescently labeled 1H8 (Al488 1H8; Table 1), a mAb that recognizes an epitope expressed on C3b, iC3b, and C3dg and phycoerythrin-labeled anti-CD59 (PE CD59). Gating based on forward scatter and side scatter characteristics was used to analyze the unlysed and lysed (ghost) cells separately. Erythrocytes are denoted in gray histograms; ghosts, in solid histograms. The percentage of ghosts in the samples was as follows: 5% in the sample incubated in NHS-EDTA; 31% in the sample incubated in aNHS; 9% in the sample incubated in aNHS containing 150 μg/mL mAb H17; and 17% in the 3E7 sample. (A) Binding of C3 activation and degradation products on the unlysed cells (erythrocytes) and on the ghosts. (B) Expression of CD59 on the unlysed cells and on the ghosts. The percentage of erythrocytes contained in the area indicated by the mark is shown in the upper right corner of each panel. The results of 1 experiment, representative of 5 experiments, are illustrated. After incubation in aNHS, CD59-deficient (low or absent) cells are hemolyzed and C3 fragments are deposited on the CD59-deficient ghosts (aNHS; A-B), and these processes are inhibited by both mAb H17 and 3E7.
Inhibition of C3 deposition on PNH erythrocytes by mAb 3E7/H17. PNH erythrocytes from patient 2 were incubated in aNHS chelated with EDTA (to prevent complement activation; NHS/EDTA, left panel), with aNHS (45% lysis; middle panel), or with aNHS containing 150 μg/mL mAb H17 or 3E7 (4% and 5% lysis, respectively; right panels). Both lysed and unlysed cells were recovered by high-speed centrifugation, washed, and incubated with a combination of fluorescently labeled 1H8 (Al488 1H8; Table 1), a mAb that recognizes an epitope expressed on C3b, iC3b, and C3dg and phycoerythrin-labeled anti-CD59 (PE CD59). Gating based on forward scatter and side scatter characteristics was used to analyze the unlysed and lysed (ghost) cells separately. Erythrocytes are denoted in gray histograms; ghosts, in solid histograms. The percentage of ghosts in the samples was as follows: 5% in the sample incubated in NHS-EDTA; 31% in the sample incubated in aNHS; 9% in the sample incubated in aNHS containing 150 μg/mL mAb H17; and 17% in the 3E7 sample. (A) Binding of C3 activation and degradation products on the unlysed cells (erythrocytes) and on the ghosts. (B) Expression of CD59 on the unlysed cells and on the ghosts. The percentage of erythrocytes contained in the area indicated by the mark is shown in the upper right corner of each panel. The results of 1 experiment, representative of 5 experiments, are illustrated. After incubation in aNHS, CD59-deficient (low or absent) cells are hemolyzed and C3 fragments are deposited on the CD59-deficient ghosts (aNHS; A-B), and these processes are inhibited by both mAb H17 and 3E7.
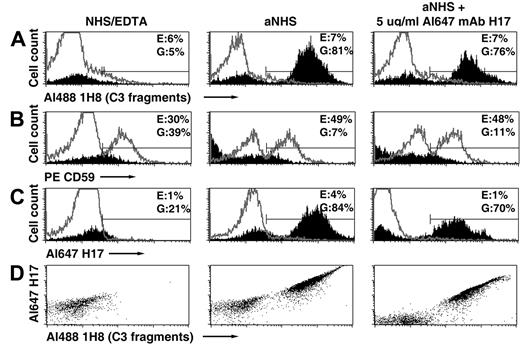
APC activation on PNH erythrocytes is initiated when nascent C3b binds covalently to glycophorin A on the cell surface.22 Bound C3b then serves as the nidus for formation of the amplification APC C3 convertase (C3bBbP).8,23,24 We hypothesize, that at high concentrations, mAb H17/3E7 inhibits C3 deposition and hemolysis by binding to cell-associated C3b, thereby preventing formation of the APC C3 convertase and subsequent amplification of C3b deposition by blocking binding of factor B to C3b. To investigate this hypothesis, binding of fluorescently labeled H17 (Al647 mAb H17) to PNH erythrocytes incubated in acidified serum was investigated (Figure 3). In these experiments, 2 methods for analyzing binding of Al647 mAb H17 to PNH erythrocytes were used. In one case, cells were incubated in acidified serum, and the lysed and unlysed cells were separated by differential centrifugation. Next, the 2 samples were probed with a combination of fluorescently labeled H17 (Al647 mAb H17), 1H8 (Al488 1H8), and anti-CD59 (anti-CD59 PE) and analyzed by flow cytometry. In the second case, PNH erythrocytes were incubated in acidified serum in the presence of a concentration (5 μg/mL) of Al647 mAb H17 insufficient to inhibit hemolysis but sufficient to observe binding. Under these later conditions, H17 likely binds to the C3b on the cells after decay of the APC C3b convertase and dissociation of Bb or naive factor B. The lysed and unlysed cells were separated by differential centrifugation, probed with fluorescently labeled 1H8 and anti-CD59, and analyzed by flow cytometry.
Binding of mAb H17 to PNH erythrocytes incubated in acidified NHS. Erythrocytes from patient 1 were incubated in aNHS chelated with EDTA (to prevent complement activation; NHS/EDTA, left panel), with aNHS (middle panel), or with aNHS containing 5 μg/mL fluorescently labeled mAb H17 (Al647 mAb H17, right panel). Unlysed erythrocytes and ghosts were separated by differential centrifugation. In the case of the cells initially incubated with NHS-EDTA or with aNHS, the unlysed and lysed populations were incubated with a combination of fluorescently labeled mAb 1H8 (Al488 1H8), mAb H17 (Al647 H17), and anti-CD59 (PE CD59). In the case of the cells initially incubated with aNHS containing 5 μg/mL Al647 mAb H17, the unlysed and lysed populations were incubated with a combination of fluorescently labeled mAb 1H8 (Al488 1H8) and phycoerythrin-labeled anti-CD59. Erythrocytes are denoted in gray histograms; ghosts, in solid histograms. (A) Erythrocytes and ghosts analyzed for bound C3 fragments based on probing with Al488 mAb 1H8. (B) Analysis for CD59 expression. (C) Analysis for binding of Al647 mAb H17. The percentage of erythrocytes (E) or ghosts (G) contained in the area indicated by the mark is shown in the upper right corner of each panel. (D) Ghosts analyzed for binding of both mAbs 1H8 and H17. Neither mAb 1H8 nor mAb H17 binds to intact erythrocytes after incubation in aNHS, indicating that the APC was not activated on these cells. The gate used to analyze antibody binding to ghosts includes some debris, likely accounting for the fraction of ghosts that are not opsonized with C3 fragments after reaction in aNHS, as this pattern is also present in the controls (NHS/EDTA). All samples were analyzed in duplicate, and 1 representative flow cytometry experiment is illustrated.
Binding of mAb H17 to PNH erythrocytes incubated in acidified NHS. Erythrocytes from patient 1 were incubated in aNHS chelated with EDTA (to prevent complement activation; NHS/EDTA, left panel), with aNHS (middle panel), or with aNHS containing 5 μg/mL fluorescently labeled mAb H17 (Al647 mAb H17, right panel). Unlysed erythrocytes and ghosts were separated by differential centrifugation. In the case of the cells initially incubated with NHS-EDTA or with aNHS, the unlysed and lysed populations were incubated with a combination of fluorescently labeled mAb 1H8 (Al488 1H8), mAb H17 (Al647 H17), and anti-CD59 (PE CD59). In the case of the cells initially incubated with aNHS containing 5 μg/mL Al647 mAb H17, the unlysed and lysed populations were incubated with a combination of fluorescently labeled mAb 1H8 (Al488 1H8) and phycoerythrin-labeled anti-CD59. Erythrocytes are denoted in gray histograms; ghosts, in solid histograms. (A) Erythrocytes and ghosts analyzed for bound C3 fragments based on probing with Al488 mAb 1H8. (B) Analysis for CD59 expression. (C) Analysis for binding of Al647 mAb H17. The percentage of erythrocytes (E) or ghosts (G) contained in the area indicated by the mark is shown in the upper right corner of each panel. (D) Ghosts analyzed for binding of both mAbs 1H8 and H17. Neither mAb 1H8 nor mAb H17 binds to intact erythrocytes after incubation in aNHS, indicating that the APC was not activated on these cells. The gate used to analyze antibody binding to ghosts includes some debris, likely accounting for the fraction of ghosts that are not opsonized with C3 fragments after reaction in aNHS, as this pattern is also present in the controls (NHS/EDTA). All samples were analyzed in duplicate, and 1 representative flow cytometry experiment is illustrated.
As illustrated in Figure 3, the amount of H17 was subinhibitory, as the extent of lysis of the CD59-deficient population was similar whether or not H17 was present in the aNHS incubation mixture (Figure 3B left panel compared with middle and right panels). Analysis of the lysed cells showed that the binding pattern for H17 was similar to that of 1H8 (solid histograms in Figure 3A,C and dot plot in Figure 3D), supporting the hypothesis that H17 inhibits hemolysis of PNH erythrocytes in acidified serum by binding to C3b on the GPI-AP–deficient population of cells (Figure 3C right panel) and thereby inhibiting formation of the APC C3 convertase. Quantitative results for these experiments confirm the specific nature of the binding of the antibodies to lysed cells compared with the unlysed cells (Table 2). Less Al647 mAb H17 was bound to lysed cells when it was present during reaction in aNHS (2100 MESF compared with 13 000 MESF, Table 2). This result may reflect competition for binding to C3b by factor B or Bb. Some binding of mAb 1H8 (Table 2) may be because of recognition of cell-associated C3dg; the epitope recognized by H17 is expressed by C3b and iC3b but not by C3dg (Table 1). Binding of these antibodies to ghosts is clearly evident, suggesting that mAb H17 recognizes the C3b constituent of the APC C3 convertase but that the concentration of mAb H17 is insufficient to prevent APC activation and subsequent lysis of the PNH erythrocytes. The small population of lysed cells without C3 deposition is probably not generated by complement-mediated lysis, as the same population is observed in samples recovered from the incubation mixture containing EDTA (Figure 3A,C-D left panels). No binding of 1H8 or H17 to unlysed cells was observed whether or not H17 was present in the aNHS incubation mixture (Figure 3A,C gray line histograms), indicating that PNH I cells, which express GPI-AP at normal levels, are protected against APC activation by constitutively expressed decay-accelerating factor (CD55).8
C3 activation fragments are deposited on PNH erythrocytes incubated in acidified C5-deficient NHS
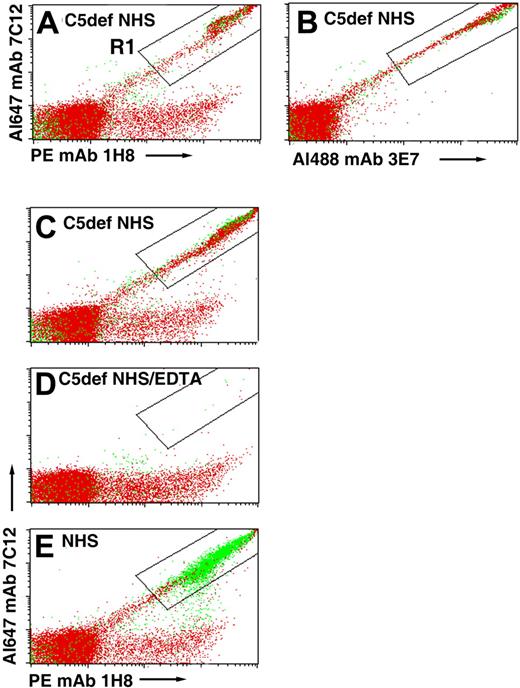
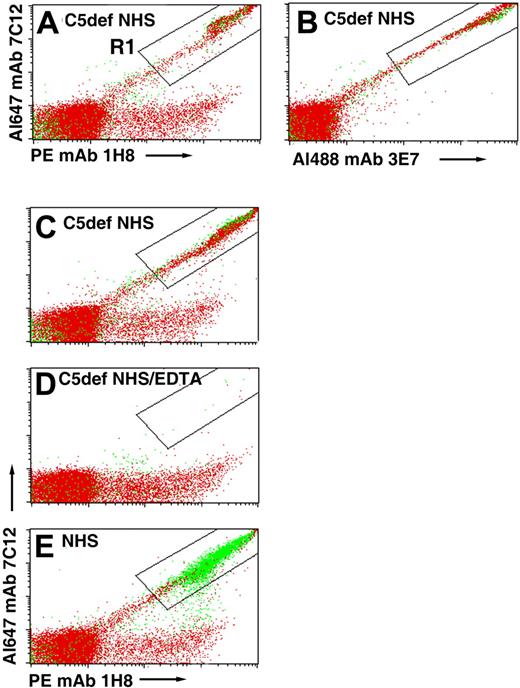
We hypothesized that patients with PNH treated with eculizumab develop an immune-mediated hemolytic anemia because the drug blocks MAC-induced lysis, thus allowing the CD55/CD59-deficient cells to become opsonized with activation and degradation products of C3 as a consequence of unrestricted activation of the APC. If this hypothesis were correct, PNH erythrocytes incubated in acidified serum should be opsonized with C3 fragments when C5 is inhibited by eculizumab. To investigate this hypothesis, we tried to obtain eculizumab but were unable to do so. As an alternative approach, we analyzed deposition of C3 fragments on PNH cells after incubation in acidified serum lacking C5 (Figure 4). In these experiments, lysis is not observed because the MAC cannot form in the absence of C5, however, C3 deposition is apparent as evidenced by the strong fluorescence intensity observed in a diagonal pattern in the samples incubated with a combination of fluorescently labeled anti-C3 antibodies that recognize nonoverlapping epitopes expressed by C3b, iC3b, and C3dg (Figure 4A-C). When acidified NHS was substituted for C5-deficient serum, almost all of the C3 deposition was on lysed erythrocytes (Figure 4E compared with 4A-C). That the C3 deposition is complement mediated is evidenced by the results for the EDTA-chelated sample that showed no florescence along the diagonal (Figure 4D). These experiments support the hypothesis that APC activation in the absence of functional C5 leads to opsonization of PNH erythrocytes.
Binding of C3 fragments to PNH erythrocytes incubated in acidified C5-deficient NHS. PNH erythrocytes from patient 2 were incubated in acidified C5-deficient human serum (C5def NHS; A-C), in acidified C5-deficient human serum chelated with EDTA (D), or in aNHS (E). After washing, the cells were stained with a combination of fluorescently labeled mAbs specific for activation and degradation products of C3 (Table 1) and subsequently analyzed by flow cytometry. In panels A and B, the cells were stained with a cocktail that contained 3 mAbs: Al488 mAb 3E7, PE mAb 1H8, and Al647 mAb 7C12. In panels C through E, only 2 mAbs, PE mAb 1H8 and Al647 mAb 7C12, were used to stain the cells. Cells that are doubly positive for both anti-C3 antibodies are contained in the area circumscribed by the rectangular area labeled R1. The readouts are similar in both panels A (all 3 mAbs used to stain, including 3E7) and C (only 2 mAbs used to stain), indicating that mAb 3E7 does not interfere with binding of mAbs 7C12 and 1H8. Dot plots show both erythrocytes (red) and ghosts (green). In the case of the cells incubated in acidified C5-deficient serum, the fluorescently labeled anti-C3b antibodies bound to intact erythrocytes because lysis did not occur, whereas in the case of the cells incubated in aNHS, the fluorescently labeled anti-C3b antibodies bound to ghost because the GPI-AP–deficient cells are hemolyzed. The low level of binding of PE mAb 1H8 to the erythrocytes observed along the horizontal axis of panels A and C through E is likely due to the presence of C3dg that was deposited on these cells in vivo, as the patient whose cells were used in this experiment was being treated with eculizumab. The results of 1 experiment, representative of 2, are illustrated.
Binding of C3 fragments to PNH erythrocytes incubated in acidified C5-deficient NHS. PNH erythrocytes from patient 2 were incubated in acidified C5-deficient human serum (C5def NHS; A-C), in acidified C5-deficient human serum chelated with EDTA (D), or in aNHS (E). After washing, the cells were stained with a combination of fluorescently labeled mAbs specific for activation and degradation products of C3 (Table 1) and subsequently analyzed by flow cytometry. In panels A and B, the cells were stained with a cocktail that contained 3 mAbs: Al488 mAb 3E7, PE mAb 1H8, and Al647 mAb 7C12. In panels C through E, only 2 mAbs, PE mAb 1H8 and Al647 mAb 7C12, were used to stain the cells. Cells that are doubly positive for both anti-C3 antibodies are contained in the area circumscribed by the rectangular area labeled R1. The readouts are similar in both panels A (all 3 mAbs used to stain, including 3E7) and C (only 2 mAbs used to stain), indicating that mAb 3E7 does not interfere with binding of mAbs 7C12 and 1H8. Dot plots show both erythrocytes (red) and ghosts (green). In the case of the cells incubated in acidified C5-deficient serum, the fluorescently labeled anti-C3b antibodies bound to intact erythrocytes because lysis did not occur, whereas in the case of the cells incubated in aNHS, the fluorescently labeled anti-C3b antibodies bound to ghost because the GPI-AP–deficient cells are hemolyzed. The low level of binding of PE mAb 1H8 to the erythrocytes observed along the horizontal axis of panels A and C through E is likely due to the presence of C3dg that was deposited on these cells in vivo, as the patient whose cells were used in this experiment was being treated with eculizumab. The results of 1 experiment, representative of 2, are illustrated.
Discussion
The purpose of these studies was to characterize the effects of mAb 3E7 and its humanized counterpart H17 on C3 deposition and hemolysis when complement is activated by the APC. Our experiments showed that 3E7/H17 inhibits hemolysis of both PNH (Figure 1A-F) and AET-reacted (Figure 1G) erythrocytes in a concentration-dependent manner, and that at a concentration of antibody sufficient to abrogate hemolysis, C3 deposition onto PNH erythrocytes is completely inhibited (Figure 2). The activity of 3E7/H17 is unique as a different mAb that recognizes a different epitope expressed by C3b lacks the capacity to block APC-mediated hemolysis of PNH erythrocytes in acidified serum (Figure 1F). Moreover, 3E7/H17 is specific for the APC, as the mAb has no inhibitory activity against CPC-mediated hemolysis (Figure 1H).
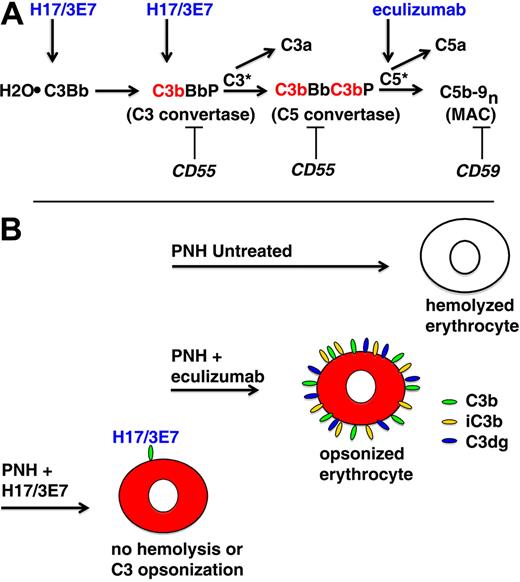
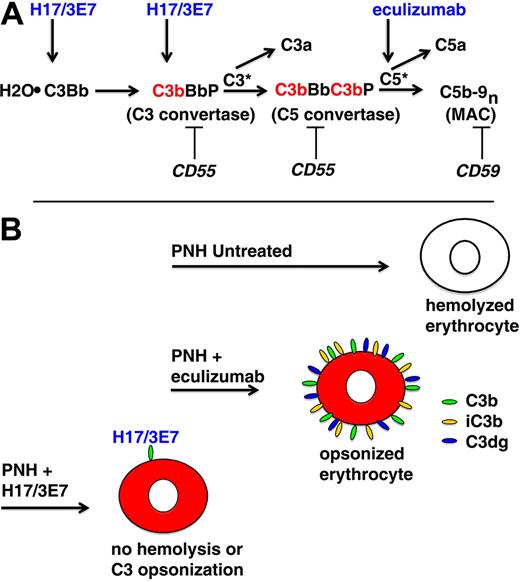
In vivo, PNH erythrocytes undergo complement-mediated lysis because activation of the APC and consequent generation of the MAC is not restricted because of deficiency of GPI-anchored complement regulatory proteins, CD55 and CD59 (Figure 5A). PNH is classified as a DAT-negative hemolytic anemia because evidence of complement activation on surviving PNH erythrocytes in the form of membrane-bound C3 activation (C3b) and degradation (iC3b and C3dg) products is not observed. That is, complement-sensitive cells are destroyed by the lytic process and complement-insensitive cells inhibit APC activation and are not lysed because they are CD55/CD59 sufficient (Figure 2). However, when the lytic process is blocked by eculizumab, evidence of APC activation on PNH erythrocytes is observed because the cells are protected against direct MAC-induced lysis (Figure 5B).11 Activation of C3 to C3b on the surface of erythrocytes by the APC C3 convertase results in exposure of an internal thioester bond, resulting in covalent binding of C3b to glycophorin A via ester (primarily) and amide bonds.22 Bound C3b is rapidly degraded to iC3b and then to C3dg by the concerted actions of membrane (CR1, CD35) and plasma (factor H and factor I) proteins.25,26 As evidenced by the hemolytic anemia of CCAD, these covalently bound activation and degradation products of C3 act as opsonins that mediate extravascular hemolysis as a consequence of interaction with complement receptor–expressing cells (macrophages and B lymphocytes) that are resident in the liver and spleen. This process accounts for the immune-mediated, extravascular hemolysis that develops in patients treated with eculizumab (Figure 5B), and its clinical significance is suggested by the observation that essentially all treated patients have persistent anemia with laboratory evidence of ongoing hemolysis.10 Moreover, approximately 50% of patients treated with eculizumab fail to achieve transfusion independence. These observations suggest that treatment of PNH could be improved by blocking APC activation, a process that would inhibit both C3 deposition and hemolysis.
APC activation on PNH erythrocytes. (A) The hemolytic anemia of PNH is mediated by the antibody-independent alternative pathway of complement (APC). In vivo, the APC is in a state of continuous activation as a consequence of low-grade, fluid-phase hydrolysis of the internal thioester bond of C3 (C3·H2O) in a process called the tick-over phenomenon. Nascent C3·H2O can bind factor B, and upon cleavage of factor B by factor D, an unstable C3 convertase (H2O·C3Bb) is formed. This complex can convert a small amount of C3 to C3b before it decays, and if an APC activator surface is in close proximity, the nascent C3b can bind covalently and form the nidus for the cell-bound APC C3 convertase, consisting of activated C3 (C3b), activated factor B (Bb, the enzymatic subunit of the complex), and factor P (a protein that stabilizes the complex, formally called properdin). The C5 convertase has the same components as the C3 convertase except that 2 C3b molecules are required to bind and position C5 for cleavage by activated factor B (Bb). C3a and C5a are bioactive peptides that are generated by cleavage of C3 and C5, respectively, by their specific activation convertases. The surface-bound C3 and C5 convertases greatly amplify complement activation by cleaving multiple substrate molecules (C3* and C5*). The membrane attack complex (MAC) consists of activated C5 (C5b), C6, C7, C8, and multiple molecules of C9 (C9n). The MAC is the cytolytic unit of the complement system. On normal erythrocytes, the GPI-anchored complement regulatory protein CD55 restricts formation and stability of both the C3 and the C5 amplification convertases by destabilizing the interaction between activated factor B (Bb) and C3b, whereas GPI-anchored CD59 blocks formation of the MAC by inhibiting the binding of C9 to the C5b-8 complex. These 2 membrane proteins are deficient in PNH, allowing unregulated activation of the APC and leading to MAC formation and hemolysis. Inhibition of MAC formation by the humanized monoclonal anti-C5 antibody eculizumab (arrow) ameliorates the intravascular hemolysis of PNH. The current studies show that hemolysis of PNH erythrocytes can also be inhibited by mAb H17/3E7. These antibodies bind to C3·H2O and C3b (arrows) and inhibit the tick-over phenomenon and block formation of the APC C3 convertase. (B) Untreated, PNH erythrocytes are lysed when the APC is activated on the membrane surface. Treatment with eculizumab blocks direct (intravascular) hemolysis of PNH erythrocytes by inhibiting formation of the MAC, but the cells become opsonized with activation and degradation products of C3 (C3b, iC3b, and C3dg) because eculizumab has no effect on the APC C3 convertase. The opsonized PNH erythrocytes are recognized by specific receptors on reticuloendothelial cells, resulting in extravascular hemolysis. mAb H17/3E7 blocks formation of the APC C3 convertase by binding to activated C3b, thereby preventing both opsonization and hemolysis of PNH erythrocytes in vitro.
APC activation on PNH erythrocytes. (A) The hemolytic anemia of PNH is mediated by the antibody-independent alternative pathway of complement (APC). In vivo, the APC is in a state of continuous activation as a consequence of low-grade, fluid-phase hydrolysis of the internal thioester bond of C3 (C3·H2O) in a process called the tick-over phenomenon. Nascent C3·H2O can bind factor B, and upon cleavage of factor B by factor D, an unstable C3 convertase (H2O·C3Bb) is formed. This complex can convert a small amount of C3 to C3b before it decays, and if an APC activator surface is in close proximity, the nascent C3b can bind covalently and form the nidus for the cell-bound APC C3 convertase, consisting of activated C3 (C3b), activated factor B (Bb, the enzymatic subunit of the complex), and factor P (a protein that stabilizes the complex, formally called properdin). The C5 convertase has the same components as the C3 convertase except that 2 C3b molecules are required to bind and position C5 for cleavage by activated factor B (Bb). C3a and C5a are bioactive peptides that are generated by cleavage of C3 and C5, respectively, by their specific activation convertases. The surface-bound C3 and C5 convertases greatly amplify complement activation by cleaving multiple substrate molecules (C3* and C5*). The membrane attack complex (MAC) consists of activated C5 (C5b), C6, C7, C8, and multiple molecules of C9 (C9n). The MAC is the cytolytic unit of the complement system. On normal erythrocytes, the GPI-anchored complement regulatory protein CD55 restricts formation and stability of both the C3 and the C5 amplification convertases by destabilizing the interaction between activated factor B (Bb) and C3b, whereas GPI-anchored CD59 blocks formation of the MAC by inhibiting the binding of C9 to the C5b-8 complex. These 2 membrane proteins are deficient in PNH, allowing unregulated activation of the APC and leading to MAC formation and hemolysis. Inhibition of MAC formation by the humanized monoclonal anti-C5 antibody eculizumab (arrow) ameliorates the intravascular hemolysis of PNH. The current studies show that hemolysis of PNH erythrocytes can also be inhibited by mAb H17/3E7. These antibodies bind to C3·H2O and C3b (arrows) and inhibit the tick-over phenomenon and block formation of the APC C3 convertase. (B) Untreated, PNH erythrocytes are lysed when the APC is activated on the membrane surface. Treatment with eculizumab blocks direct (intravascular) hemolysis of PNH erythrocytes by inhibiting formation of the MAC, but the cells become opsonized with activation and degradation products of C3 (C3b, iC3b, and C3dg) because eculizumab has no effect on the APC C3 convertase. The opsonized PNH erythrocytes are recognized by specific receptors on reticuloendothelial cells, resulting in extravascular hemolysis. mAb H17/3E7 blocks formation of the APC C3 convertase by binding to activated C3b, thereby preventing both opsonization and hemolysis of PNH erythrocytes in vitro.
The APC is in a state of continuous activation (called the tick-over phenomenon) as a consequence of low-grade hydrolysis of the internal thioester bond of C3.27 Nascent C3·H2O has the capacity to bind factor B, the enzymatic subunit of the APC C3/C5 convertase (Figure 5A). Cleavage by factor D activates the factor B zymogen, resulting in an unstable APC C3 convertase (H2O·C3Bb) that can convert a few molecules of C3 to C3b before the complex rapidly decays. If an APC activator surface (such as bacteria or a PNH erythrocyte) is in close proximity, nascent C3b generated by a H2O·C3Bb C3 convertase can bind to the cell surface and initiate APC activation (Figure 5A). Acidification of serum exaggerates the normal activation of the APC because formation of the APC C3 convertase occurs optimally at pH 6.4.19,20,28 We used acidified serum lysis in our model system to test the efficacy of 3E7/H17 for 2 reasons. First, by inducing fluid-phase activation of the APC, acidification of serum mimics the mechanism by which PNH erythrocytes are lysed in vivo. Second, acidification of serum induces brisk APC activation, thereby presenting a strong challenge to the inhibitory capacity of 3E7/H17. Our experiments (Table 2), and those of others,29 demonstrate that PNH erythrocytes incubated in acidified serum have C3 fragments bound in high density (> 104 molecules/cell). That 3E7/H17 can completely inhibit lysis and C3 deposition on PNH erythrocytes incubated in the powerful APC activator, acidified serum, suggests that the antibody will be effective in vivo where it will be necessary to prevent only low-grade, tick-over APC activation. Furthermore, our previously published studies demonstrated that 3E7/H17 also binds C3·H2O.12,13 Therefore, in vivo, it is likely that 3E7/H17 will block the tick-over phenomenon directly and prevent APC activation before it can be initiated on the surface of PNH erythrocytes. However, we also showed that 3E7/H17 binds to C3b on PNH erythrocytes (Figure 3, Table 2). Therefore, any nascent C3b that escapes fluid-phase inactivation and binds to the cell surface will be inactivated by 3E7/H17, and this process will be supported in vivo by factor H (Figure 5).30 Together, these observations suggest that the concentration of 3E7/H17 required to inhibit lysis of PNH erythrocytes in vivo will be substantially lower than that required to completely inhibit acidified serum lysis (∼ 170 μg/mL, Figure 1).
We hoped to compare the efficacy of 3E7/H17 with that of eculizumab, but we were unable to obtain the antibody. Nonetheless, using normal human serum depleted of C5, we showed that, although absence of functional C5 prevents hemolysis of PNH erythrocytes, it does not reduce APC activation on the surface of PNH erythrocytes (Figure 4). These findings support the hypothesis that the C3 deposition on PNH erythrocytes observed in patients treated with eculizumab is a consequence of unregulated APC activation and not CPC activation by antibody.11
Our ultimate goal is to improve treatment for patients with PNH by developing a pharmacologic reagent that blocks both C3 deposition and hemolysis. For such a reagent to be useful clinically, it must be safe. Continuous blockade of the APC could put patients at risk for microbial infection, but we are reassured that our approach to treatment of PNH will be safe by observations in factor B knockout mice.31-34 The phenotype of the factor B knockout is expected to be similar to that of chronic APC suppression by 3E7/H17 as the antibody blocks factor B binding to C3b (Figure 5). Homozygous factor B knockout mice have birthrates, developmental characteristics, and reproductive rates identical to those of wild-type littermates. They do not appear to be at increased risk for spontaneous infection, although when severely challenged (directly inoculated) with high concentrations of some fungi and bacteria they show greater morbidity and mortality than their wild-type counterparts.33,34 Humans heterozygous for mutant factor B have no distinct phenotype, and humans homozygous for mutant factor B have not been reported.32 However, it seems unlikely that homozygous factor B deficiency is embryonically lethal based on the mouse studies. Although rare and associated with early mortality, homozygous C3 deficiency (resulting in absence of APC, CPC, and lectin pathway function) has been observed in humans.35 Conceivably, homozygous factor B deficiency in humans has not been reported because there is no clinical phenotype. In support of this hypothesis, most of the pathophysiology attributable to aberrant APC regulation is a consequence of uncontrolled activation rather than to abnormalities that inactivate function.3,36 Nonetheless, the effects of continuous, long-term inhibition of the APC remain speculative, and carefully designed safety studies are required to address this issue. The unique properties of 3E7/H17, however, will allow us to perform some of these investigations using a nonhuman primate model.15
The present studies demonstrated (Figure 1H) that mAb 3E7/H17 does not inhibit the function of the CPC, and previously we reported that, at concentrations that completely block the APC, 3E7/H17 has no inhibitory activity with respect to CPC-mediated lysis of sheep erythrocytes.12 Moreover, we and others showed that mAb 3E7/H17 increases CPC-mediated lysis of rituximab-opsonized B cells.15,37 mAb 3E7/H17 binds to C3b at, or very close, to the site that would otherwise be bound by either factor B or factor H. By blocking binding of factor B, the mAb 3E7/H17 abrogates activation of the APC on the cell surface. However, by blocking binding of factor H, 3E7/H17 increases the activity of C3b generated by the CPC by stabilizing C3b against factor H–dependent, factor I–mediated enzymatic degradation, leaving cell-bound C3b intact to serve as the binding site for C5 in the CPC C5 convertase. The CPC C3 convertase is not affected by 3E7/H17 because it consists of components C4b (the binding site for C3) and C2a (the enzymatic subunit), neither of which is recognized by the mAbs. This feature of 3E7/H17 (specific inhibition of the APC while enhancing the activity of the CPC) is important clinically as a functional CPC is needed for immune-complex solubilization and protection against infection by microorganisms in the setting of chronic APC blockade. We demonstrated this important activity of 3E7 in vivo using a nonhuman primate model. In those studies, we observed that when cynomolgus monkeys are infused with the therapeutic mAb rituximab, the CPC is activated on circulating B cells, resulting in deposition of C3b on these rituximab-targeted cells. Infused mAb 3E7 binds to CPC-generated cell-bound C3b on monkey B lymphocytes, and by competing with factor H for binding to C3b, enhances CPC activity by preserving the integrity of C3b.15
Together, the studies reported here showed that 3E7/H17 inhibits both APC-mediated hemolysis and C3 deposition without compromising the activity of the CPC, suggesting important advantages over currently available therapy (Figure 5). These studies support further testing of 3E7/H17 as a potential therapeutic agent for treatment of patients with PNH. mAb 3E7/H17 recognizes both human and monkey C3b,15 and we observed that mAb H17 blocks the APC in the serum of both rhesus and cynomolgus monkeys. Therefore, it should be possible to directly test the efficacy and safety of mAb H17 in an in vivo primate model of APC activation.13,38,39
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.A.L. conceived and designed the study, was responsible for experimental work, interpreted the data, and wrote the paper; A.W.P. and E.M.P. were responsible for experimental work; K.H. provided essential material for the experiments; and R.P.T. and C.J.P. conceived and designed the study, interpreted the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ronald P. Taylor, Department of Biochemistry and Molecular Genetics, Box 800733, University of Virginia Health Sciences Center, Charlottesville, VA 22908-0733; e-mail: rpt@virginia.edu; or Charles J. Parker, Hematology and Bone Marrow Transplant, University of Utah School of Medicine, 30N 1900 East, Salt Lake City, UT; e-mail: charles.parker@hsc.utah.edu.