Abstract
Neogenin, a deleted in colorectal cancer (DCC) family member, has been identified as a receptor for the neuronal axon guidance cues netrins and repulsive guidance molecules repulsive guidance molecules (RGM). RGMc, also called hemojuvelin (HJV), is essential for iron homeostasis. Here we provide evidence that neogenin plays a critical role in iron homeostasis by regulation of HJV secretion and bone morphogenetic protein (BMP) signaling. Livers of neogenin mutant mice exhibit iron overload, low levels of hepcidin, and reduced BMP signaling. Mutant hepatocytes in vitro show impaired BMP2 induction of Smad1/5/8 phosphorylation and hepcidin expression. Neogenin is expressed in liver cells in a reciprocal pattern to that of hepcidin, suggesting that neogenin functions in a cell nonautonomous manner. Further studies demonstrate that neogenin may stabilize HJV, a glycosylphosphatidylinositol-anchored protein that interacts with neogenin and suppresses its secretion. Taken together, our results lead the hypothesis that neogenin regulates iron homeostasis via inhibiting secretion of HJV, an inhibitor of BMP signaling, to enhance BMP signaling and hepcidin expression. These results reveal a novel mechanism underlying neogenin regulation of HJV-BMP signaling.
Introduction
Iron, a component of many metalloproteins, plays a crucial role in various physiologic processes, such as oxygen sensing and transport, mitochondrial electron transfer in the respiratory chain, and catalytic activity of diverse enzymes. Dysregulation of iron homeostasis leads to either iron deficiency, such as anemia, or iron overload characteristic of hereditary hemochromatosis, a relatively common inherited disorder associated with progressive organ dysfunction.1 Cellular iron overload is toxic and causes cell death, at least in part due to free radical formation and lipid peroxidation.
Hemojuvelin (HJV) is a member of a family of glycosylphosphatidylinositol (GPI)–linked cell surface proteins. It has an essential role in iron homeostasis.2 HJV contains a putative N-terminal signal peptide, a tri-amino acid motif (the RGD site), a partial von Willebrand factor type D domain, and a C-terminal GPI anchor consensus sequence.2,3 It is predominately expressed in the skeletal muscle, heart, and liver.3,4 Mutations in the HJV gene is a leading cause of juvenile hemochromatosis,5-8 and patients have low levels of hepcidin,5,8 a crucial hormone secreted by the liver for iron homeostasis. HJV mutant mice exhibit reduced hepcidin expression and iron overload,5,6 resembling patients with HJV mutations. Consistently, expression of HJV increases, while suppression of HJV decreases, hepcidin expression in cultured cells.9
The mechanisms underlying HJV regulation of hepcidin expression and iron homeostasis are beginning to be elucidated. HJV was shown to function as a coreceptor for bone morphogenetic proteins (BMP). It binds to BMP2/4/6 and forms a complex with BMP receptors (type I and II).10-12 HJV deficient mice/cells exhibit impaired BMP signaling (eg, phosphorylation of smad1/5/8).12 BMP2/4/6 up-regulates hepatic hepcidin expression, which is enhanced by HJV coexpression but blunted in HJV-deficient hepatocytes.12-14 These observations suggest that HJV regulates hepcidin expression and iron homeostasis via BMP signaling.
Soluble HJV (sHJV), like other GPI-linked membrane proteins, is released by proteolytic cleavage or phospholipase cleavage of the GPI anchor moiety, or both. A recent study has demonstrated the presence of sHJV in human serum.15 Of interest is that while HJV induces hepcidin expression in transfected cells, sHJV competitively inhibits this event in culture and in animals,9,13,14 suggesting a critical role of sHJV. However, how HJV cleavage and secretion are regulated remains largely unknown.
Neogenin and its related protein, DCC, contain a large extracellular domain with 4 immunoglobin-like domains and 6 fibronectin type III repeats, a single transmembrane domain, and a cytoplasmic region with 3 conserved domains, namely P1, P2, and P3.16 Neogenin, but not DCC, is a binding partner of repulsive guidance molecules (RGMa).17,18 RGMa-induced growth cone repulsive responses can be blocked by anti-neogenin antibodies or soluble neogenin ectodomain fusion proteins.17 Subsequent studies demonstrate that neogenin indeed interacts with HJV/RGMc.19,20 However, its role in HJV-mediated hepcidin expression and iron homeostasis, and its relationship(s) to HJV and BMP signaling have remained unclear.
In this article, we present evidence for a critical role of neogenin in iron homeostasis. Neogenin-deficient mice exhibit iron overload and reduced expression of hepcidin in the liver. A possible mechanism may be an increased HJV secretion that reduces BMP signaling and hepcidin induction, as we demonstrate that neogenin is coexpressed with HJV in perivenous hepatocytes and neogenin is crucial for prevention of HJV secretion. These results suggest that neogenin regulates iron homeostasis, probably by modulating HJV secretion and BMP-induced hepcidin expression.
Methods
Reagents and animals
Rabbit polyclonal anti-neogenin was generated using the GST-C-terminus of neogenin fusion protein as the antigen as described previously.21 We also generated rabbit polyclonal anti-HJV/RGMc antibody as described in supplemental Figure 2 (available on the Blood website; see the Supplemental Materials link at the top of the online article). In addition, rabbit polyclonal anti-neogenin and goat polyclonal anti-RGMc were also purchased from Santa Cruz Biotechnology Inc. Anti-ferritin (GeneTex Inc), anti–hepcidin-25, anti-TfR2, anti-HFE, and anti-ferroportin (Fpn; Alpha Diagnostic International Inc), anti–phospho-Smad1/5/8 (Cell Signaling Inc), anti–glutamine synthetase (GS; BD Transduction Laboratories) were used. Other chemicals and reagents used in this study were of analytical grade.
Neogenin mutant mice, kindly provided by Dr Sue Ackerman (The Jackson Laboratory), were generated by Bay Genomics. In brief, KST265 embryonic stem (ES) cells that contain the neogenin gene disrupted by gene trapping using the pGT1TMpt vector (see Figure 1A) were obtained from Bay Genomics. ES cell clones were transferred into mouse blastocysts. Chimeras generated from ES cells were crossed to C57/BL6 mice for more than 6 generations, and the resulting heterozygous animals were crossed to produce homozygous mice. Mice were maintained on a standard rodent diet (Harlan Tekled S-2335) containing approximately 0.16 g/kg of iron. Control littermates (wild-type or heterozygote) were processed in parallel for each experiment. Neogenin mutation was confirmed by genotyping by polymerase chain reaction (PCR) and by the loss of the neogenin expression by Western blot analysis. All experimental procedures were approved by the Animal Subjects Committee at the Medical College of Georgia, according to US National Institutes of Health guidelines.
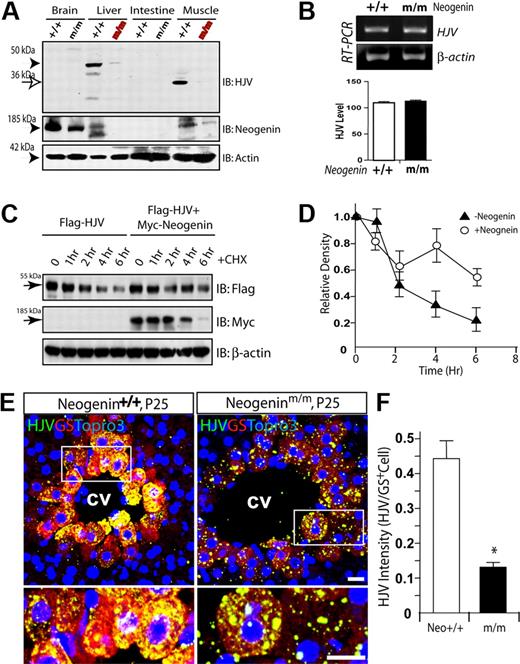
Iron accumulation in periportal hepatocytes of neogenin mutant mice. (A) Diagram of the retrotranspon insertion in neogenin gene. Exons 7 and 8, surrounding intron, and location of genotyping primers are shown diagrammatically. Primer sequences are also indicated. (B) Genotyping and decreased neogenin expression in neogenin mutant mice. Livers of indicated genotype were lysed and resulting lysates were subjected to immunoblotting with anti-neogenin antibody. The top panel shows PCR genotyping. The 327-bp fragment was detected in homozygous mice where the 169-bp fragments in wild-type mice. (C-D) Histologic detection of iron content on cryostat sections of liver (C) and spleen (D) of wild-type and neogenin mutant (neogeninm/m) mice. Five different wild-type and mutant mice were examined, and representative images imaged by Axiophot microscope (Zeiss) and processed using Adobe Photoshop CS2 software are shown. Note iron accumulation was observed in the periportal (portal tract [PT]), but not central vein (CV), regions of P25-old neogeninm/m mice and absence thereof in the red pulp of the spleen. (E) Western blot analysis of liver and spleen lysates from indicated genotype with anti-ferritin-H antibodies. Molecular weights are indicated. (F) Quantitative determination of iron content (μmol/g dry weight; see “Tissue iron staining and quantification”) in various organs of P25-old wild-type (white), neogenin+/m (purple), and neogeninm/m (blue) mice. Means ± SEM of 5 samples for each group were presented. **,#Significant changes (P < .01) in neogeninm/m mice compared with the wild-type control.
Iron accumulation in periportal hepatocytes of neogenin mutant mice. (A) Diagram of the retrotranspon insertion in neogenin gene. Exons 7 and 8, surrounding intron, and location of genotyping primers are shown diagrammatically. Primer sequences are also indicated. (B) Genotyping and decreased neogenin expression in neogenin mutant mice. Livers of indicated genotype were lysed and resulting lysates were subjected to immunoblotting with anti-neogenin antibody. The top panel shows PCR genotyping. The 327-bp fragment was detected in homozygous mice where the 169-bp fragments in wild-type mice. (C-D) Histologic detection of iron content on cryostat sections of liver (C) and spleen (D) of wild-type and neogenin mutant (neogeninm/m) mice. Five different wild-type and mutant mice were examined, and representative images imaged by Axiophot microscope (Zeiss) and processed using Adobe Photoshop CS2 software are shown. Note iron accumulation was observed in the periportal (portal tract [PT]), but not central vein (CV), regions of P25-old neogeninm/m mice and absence thereof in the red pulp of the spleen. (E) Western blot analysis of liver and spleen lysates from indicated genotype with anti-ferritin-H antibodies. Molecular weights are indicated. (F) Quantitative determination of iron content (μmol/g dry weight; see “Tissue iron staining and quantification”) in various organs of P25-old wild-type (white), neogenin+/m (purple), and neogeninm/m (blue) mice. Means ± SEM of 5 samples for each group were presented. **,#Significant changes (P < .01) in neogeninm/m mice compared with the wild-type control.
Tissue iron staining and quantification
Tissues including liver, intestine, and spleen were harvested from 3- to 4-week-old wild-type and mutant mice. Iron was detected in cryostat sections using the Prussian Blue Iron Stain Kit (Sigma-Aldrich) for nonheme iron as previously described.22
Tissue nonheme iron was quantified by the bathophenanthroline assay as previously described.23 In brief, tissues were lyophilized overnight, weighed, and 10 to 15 mg dry weight was digested in 0.5 mL of acid mixture (3M HCl/10% trichloroacetic acid) at 65°C overnight. After cooling to room temperature, 5 μL was placed in 995 μL of bathophenathroline sulfonate chromogen (1 vol of stock bathophenathroline sulfonate chromogen [0.1% bathophenanthroline sulfonate/1% thioglycolic acid] and 10 vol of 2.5M sodium acetate) and vortexed. To generate a standard curve, serial dilutions of a ferric iron standard (FeCl3) were used. Color was allowed to develop for 15 minutes, and absorbance at 535 nm was determined spectrophotometrically.
In situ hybridization
Liver sections from wild-type and/or mutant mice were examined for hepcidin and HJV expression by in situ hybridization. Briefly, wild-type and/or neogenin mutant mice were deeply anesthetized and transcardially perfused. Livers were removed from perfused mice and refixed with 4% paraformaldehyde before cutting. Liver sections cut by a freezing microtome were then subjected to the treatment for the hybridization. The digeoxigenin (DIG)-labeled riboprobes (sense and antisense) for hepcidin and HJV were generated by in vitro transcription using their cDNA templates in the presence of DIG-UTP (DIG RNA Labeling Mix, Roche). Primers for hepcidin and HJV riboprobes are listed in supplemental Table 1. DIG was visualized with the anti-DIG antibody coupled to the alkaline phosphatase using nitro blue tetrazolium/bromo-chloro-indolyl phosphate as a chromogen/substrate system.
RT-PCR and real-time RT-PCR
Reverse transcriptase (RT)-PCR was performed as previously described.24 Briefly, total RNAs were isolated from the liver of wild-type and mutant mice using Trizol (Invitrogen). Three micrograms of RNA were reverse-transcribed into cDNA using Superscript III first-strand synthesis system (Invitrogen). One microliter of cDNA was subjected to PCR amplification with the sequence specific primers (see supplemental Table 1). PCR reactions were performed in triplicate for each cDNA and normalized to endogenous GADPH transcripts. Quantitative real-time RT-PCR was carried out using the Bio-Rad iCycler according to the manufacturer's instructions.
β-Gal
Liver sections derived from wild-type, heterozygotes, and homozygote of neogenin mice were fixed and subjected to the reaction for the measurement of β-Gal activity in vivo as described previously.25
Immunohistochemical staining and confocal microscopy
Livers of age-matched wild-type and mutant mice were fixed in 10% buffered formalin and embedded in paraffin. Deparaffinized tissue sections were used for immunohistochemical analysis as described previously.24
Primary hepatocyte and muscle culture
Primary hepatocytes were isolated by collagenase digestion of livers from 20- to 25-day-old wild-type mice and neogenin mutant mice as described previously26 with modifications. Briefly, mice were anesthetized and perfused through the inferior vena cava with Ca2+, Mg2+-free Hanks balanced salt solution (Mediatech) containing 0.5mM EDTA and 50mM HEPES (pH 7.3) for 4 minutes, followed by perfusion with collagenase solution [1 mg/mouse Liberase Blendzyme 3 (Roche) in Lebowitz L15 (Gibco) with 5mM Ca2+]. After enzymatic digestion, hepatocytes were isolated and filtered through a 70-μm BD Falcon mesh cell strainer (BD Biosciences), centrifuged, and resuspended in culture medium (1:1 Dulbecco modified Eagle/Ham F12 medium [Gibco] supplemented with 100 IU/mL penicillin, 100 μg/mL streptomycin, 18mM HEPES, 1mM sodium pyruvate, 10% fetal bovine serum [FBS; HyClone], 2mM l-glutamine, and 0.1mM nonessential amino acids [Gibco]). Cells were seeded on collagen-coated (Sigma-Aldrich) at 5 × 105 per 60-mm dish. Four hours after plating, hepatocytes were washed, serum-starved with culture medium without FBS for 6 hours, and then treated with recombinant human BMP-2 (100 ng/mL) for varying times. Cells were then harvested for Western blot analysis.
Primary cultures of mouse skeletal muscle cells were prepared from postnatal day 15 (P15) wild-type and neogenin mutant mice. Briefly, muscle tissues dissected from the hind limbs of mice were minced, incubated with collagenase for 15 minutes at 37°C, and grown in 60-mm plates in medium containing F12:Dulbecco modified Eagle medium (1:1), 10% FBS, 2.5% horse serum, 100 U/mL penicillin, and 10 mg/mL streptomycin. Two days after plating, fibroblast growth was inhibited with cytosine arabioside (5mM) that was maintained for 24 hours. For differentiation, the growth medium was changed to serum-free medium at day 4 of culture. Five-day-old cultures were used for the experiments.
HEK293 and HepG2 cell culture and transfection
HEK293 and HepG2 cells were maintained in Dulbecco modified Eagle medium supplemented with 10% fetal calf serum, and 100 units/mL of penicillin G and streptomycin (Gibco). For transfection, HEK293 cells were plated at a density of 106 cells per 10-cm culture dish and allowed to grow for 12 hours before transfection using the calcium phosphate precipitation method.27 Thirty-six hours after transfection, cells were lysed in modified radio immunoprecipitation assay buffer (50mM Tris-HCl, pH 7.4, 150mM sodium chloride, 1% NP40, 0.25% sodium-deoxycholate, and proteinase inhibitors).28 Lysates and medium were subjected to immunoblotting analyses.
Results
Iron overload in neogenin-deficient mouse liver
To analyze the function of neogenin, we took advantage of neogenin-deficient mice generated by retrotransposon-mediated “gene trapping” by Bay Genomics Inc. (Figure 1A). Retrotransposon insertion into the intron of the neogenin gene (between exons 7 and 8; Figure 1A) resulted in approximately 90% reduction in neogenin protein (Figure 1B); thus, the homozygotes were named as neogeninm/m. In view of the known interaction between neogenin and HJV, we determined whether neogenin was required for iron homeostasis. Iron overload was determined by Prussian blue staining of liver samples from wild-type and neogeninm/m mice. Starting at approximately postnatal day 20/25, iron accumulation in portal tracts of mutant, but not wild-type, livers was observed (Figure 1C-F). In contrast, spleens from mutant animals exhibited reduced staining (Figure 1D-F); presumably due to lower iron levels in macrophages. Such a phenotype was further confirmed by Western blotting using antibody against ferritin (heavy chain), an iron storage protein whose concentration correlates with tissue iron content (Figure 1E). These phenotypes resemble those of human juvenile hemochromatosis with hepatic iron overload associated with macrophage iron depletion. Our results thus far support possible involvement of neogenin in the regulation of iron homeostasis.
Reduced hepcidin and increased ferroportin in neogenin mutant mice
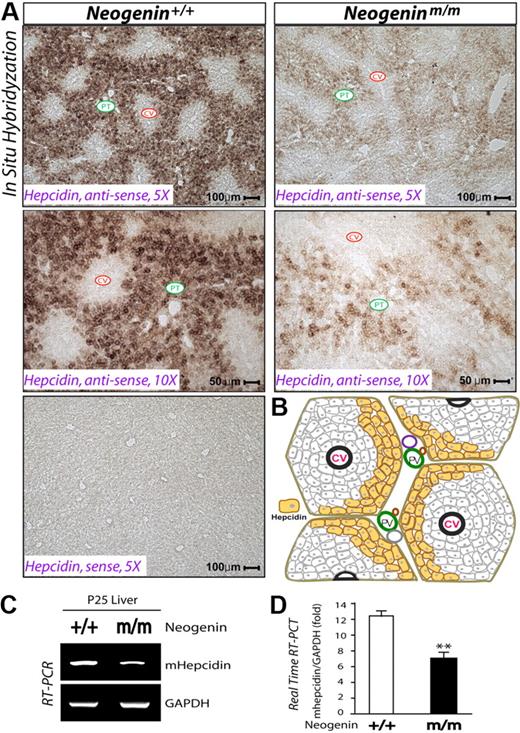
Considering the role of hepcidin in iron homeostasis,29 we examined its expression in livers of neogeninm/m mice. In wild-type animals, hepcidin mRNA was highly expressed and largely restricted to hepatocytes surrounding the portal tracts of the liver (Figure 2A-B), where iron overload was observed in livers of neogeninm/m mice (Figure 1C). Remarkably, hepcidin expression was reduced in neogeninm/m livers by in situ hybridization (Figure 2A), RT-PCR (Figure 2C), and real-time PCR analyses (Figure 2D). These results demonstrate a “zonal distribution” pattern for hepcidin expression and suggest a positive role of neogenin in hepcidin expression.
Reduced hepcidin expression in periportal hepatocytes of neogenin mutant liver. (A) In situ hybridization analysis of hepcidin expression in wild-type and neogenin mutant liver at age P25. The experiments were repeated 3 times, and representative images are shown. Note that only antisense, but not sense, probes showed signals, demonstrating the specificity. Central veins (CV) and portal tract (PT) are indicated. (B) Illustration of the “zonal distribution” of hepcidin transcripts in mouse liver. Hepcidin transcripts were detected mainly in periportal hepatocytes, labeled by yellow color. RT-PCR (C) and real-time PCR (D) analyses of hepcidin expression in neogenin+/+ and neogeninm/m livers (P25). Means ± SEM of 3 samples for each group are shown. **Significant difference (P < .01) from the wild-type control.
Reduced hepcidin expression in periportal hepatocytes of neogenin mutant liver. (A) In situ hybridization analysis of hepcidin expression in wild-type and neogenin mutant liver at age P25. The experiments were repeated 3 times, and representative images are shown. Note that only antisense, but not sense, probes showed signals, demonstrating the specificity. Central veins (CV) and portal tract (PT) are indicated. (B) Illustration of the “zonal distribution” of hepcidin transcripts in mouse liver. Hepcidin transcripts were detected mainly in periportal hepatocytes, labeled by yellow color. RT-PCR (C) and real-time PCR (D) analyses of hepcidin expression in neogenin+/+ and neogeninm/m livers (P25). Means ± SEM of 3 samples for each group are shown. **Significant difference (P < .01) from the wild-type control.
Hepcidin regulates iron homeostasis via reducing Fpn, a hepcidin receptor essential for iron release from macrophages and iron uptake from enterocytes.30-32 We thus examined the expression and distribution of Fpn in neogenin mutant mice in tissues implicated in iron homeostasis, including intestine, liver, spleen, and muscle. Indeed, Fpn protein level was increased in the lung and spleen of mutant mice by Western blot analysis (supplemental Figure 1A-B). The increase in intestine and spleen was confirmed by immunohistochemical staining analysis (supplemental Figure 1C). By contrast, levels of HFE and transferrin receptor 2 (TfR2), 2 other proteins involved in iron homeostasis,33 appeared to be unchanged in the mutant mice (data not shown). These results provide evidence for a deficient hepcidin-ferroportin pathway that may contribute to iron overload.
Requirement of neogenin for BMP-induced Smad1/5/8 phosphorylation and hepcidin expression in mouse liver and in hepatocyte culture
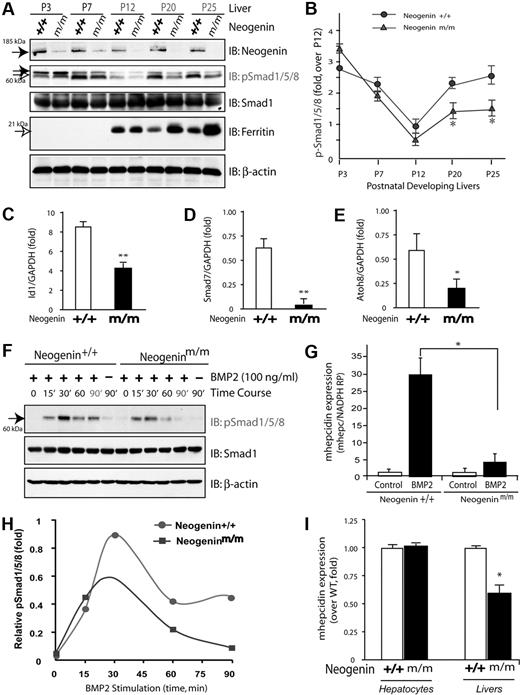
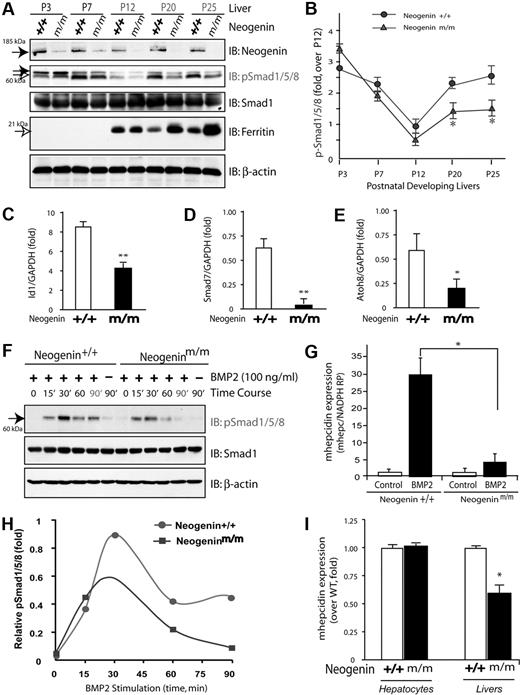
To understand how neogenin regulates hepcidin expression, we asked whether neogenin is required for BMP signaling and BMP-induced hepcidin expression in hepatocytes, as BMP6 has been demonstrated to be essential for hepcidin induction in response to iron.34,35 To this end, we first examined phosphorylation of Smad1/5/8 (p-Smad1/5/8) in wild-type and neogenin mutant liver lysates at different stages of development. Levels of p-Smad1/5/8 appeared to be regulated in a developmentally specific manner: high at birth, decreased at P12, and then increased again at P20 (Figure 3A-B). Such inductions of p-Smad1/5/8 (Figure 3B) resemble the profile of hepcidin expression during development.5 However, this developmental induction of p-Smad1/5/8 after P12 appeared to be blocked in livers from neogeninm/m mice (Figure 3A-B). No change was observed in the level of total Smad1 protein in both wild-type and mutant livers during development (Figure 3A). Notably, ferritin level appeared to be developmentally regulated as well (Figure 3A), consistent with its role in iron metabolism. In support of a role for neogenin in control of BMP signaling, hepcidin expression, and tissue iron levels, livers from neogeninm/m mice displayed an increased ferritin at P20 and P25 (Figure 3A). In addition, expression of other BMP target genes (Id1, Smad7, and Atoh8) was also reduced in the liver of neogeninm/m mice (Figure 3C-E). These results suggest a developmental regulation of BMP signaling and a role for neogenin in modulating BMP signaling in liver or in vivo.
Reduction of BMP signaling and hepcidin expression in neogenin mutant livers and in mutant hepatocyte culture. (A) Western blot analyses of liver lysates derived from wild-type (+/+) and neogenin mutant (m/m) mice during development using indicated antibodies; P3 indicates postnatal day 3. Note that the up-regulation of pSmad1/5/8 after P12 was blocked in neogenin mutant mice. (B) Quantification analysis of data from panel A. Three different mice from each age group were analyzed. Mean ± SEM is shown (n = 3). *P < .01, significant difference from the wild-type control. (C-E) Real-time PCR analysis of the expression of Id1, Smad7, and Atoh8 in wild-type and neogenin mutant livers. The expression was normalized to the reference gene NADPH mRNA. Mean ± SEM shown (n = 3). *P < .01, significant difference from the wild-type control. (F) Western blot analysis of lysates of primary cultured hepatocytes derived from wild-type and neogeninm/m mice. Primary hepatocytes cultured for 5 days were treated with BMP2 (100 ng/mL) for the indicated times. Equal amounts of total proteins were immunoblotted with the indicated antibodies. (G) Quantitative analysis of data from panel A. Mean value was shown, which was from 2 independent experiments. (H-I) Real-time RT-PCR analysis of BMP2 induced (H) and basal (I) hepcidin expression in primary hepatocytes and in livers (D) derived from wild-type and neogenin mutant mice. Primary hepatocytes isolated from perfused livers of wild-type (C57BL/6) and neogenin mutant mice were cultured in collagen coated 12-well plates for 2 hours and treated with BMP2 (100 ng/mL) for 15 hours. The expression of hepcidin and NADPH (as a normalization gene) was assayed by quantitative real-time RT-PCR using TagMan probes. All samples were processed in triplicate, and a graph of mean values ± SEM from 3 independent experiments is shown. *P < .01, significant difference from wild-type control (t test).
Reduction of BMP signaling and hepcidin expression in neogenin mutant livers and in mutant hepatocyte culture. (A) Western blot analyses of liver lysates derived from wild-type (+/+) and neogenin mutant (m/m) mice during development using indicated antibodies; P3 indicates postnatal day 3. Note that the up-regulation of pSmad1/5/8 after P12 was blocked in neogenin mutant mice. (B) Quantification analysis of data from panel A. Three different mice from each age group were analyzed. Mean ± SEM is shown (n = 3). *P < .01, significant difference from the wild-type control. (C-E) Real-time PCR analysis of the expression of Id1, Smad7, and Atoh8 in wild-type and neogenin mutant livers. The expression was normalized to the reference gene NADPH mRNA. Mean ± SEM shown (n = 3). *P < .01, significant difference from the wild-type control. (F) Western blot analysis of lysates of primary cultured hepatocytes derived from wild-type and neogeninm/m mice. Primary hepatocytes cultured for 5 days were treated with BMP2 (100 ng/mL) for the indicated times. Equal amounts of total proteins were immunoblotted with the indicated antibodies. (G) Quantitative analysis of data from panel A. Mean value was shown, which was from 2 independent experiments. (H-I) Real-time RT-PCR analysis of BMP2 induced (H) and basal (I) hepcidin expression in primary hepatocytes and in livers (D) derived from wild-type and neogenin mutant mice. Primary hepatocytes isolated from perfused livers of wild-type (C57BL/6) and neogenin mutant mice were cultured in collagen coated 12-well plates for 2 hours and treated with BMP2 (100 ng/mL) for 15 hours. The expression of hepcidin and NADPH (as a normalization gene) was assayed by quantitative real-time RT-PCR using TagMan probes. All samples were processed in triplicate, and a graph of mean values ± SEM from 3 independent experiments is shown. *P < .01, significant difference from wild-type control (t test).
We next asked if neogenin is required for BMP2-induced phosphorylation of Smad1/5/8 in primary cultured hepatocytes. Exposure to BMP2 increased p-Smad1/5/8 that remained high after 90 minutes in a wild-type hepatocyte culture (Figure 3F-G). However, in hepatocyte cultures from neogenin mutant mice, the BMP2 response was transient and disappeared by 60 minutes of stimulation (Figure 3F-G). These observations suggest a defect in sustained activation of pSmad1/5/8 in livers of neogeninm/m mice.
To determine whether such sustained activation of p-Smad1/5/8 is necessary for hepcidin induction by BMP2, we compared BMP2-induced hepcidin expression in wild-type and neogenin mutant hepatocytes. Treatment with BMP2 (100 ng/mL) for 12 to 16 hours resulted in approximately 30-fold increase of hepcidin expression in wild-type cultures (Figure 3H), consistent with previous report.36 However, in neogenin mutant culture, hepcidin induction was significantly reduced (Figure 3H). Note that although basal levels of hepcidin in unstimulated mutant hepatocytes were comparable with those in wild-type, they were reduced in the mutant liver in vivo (Figure 3I). These results provide a further support for a role of neogenin in regulation of BMP2-induced hepcidin expression.
Reciprocal expression pattern of neogenin and HJV to hepcidin in mouse liver
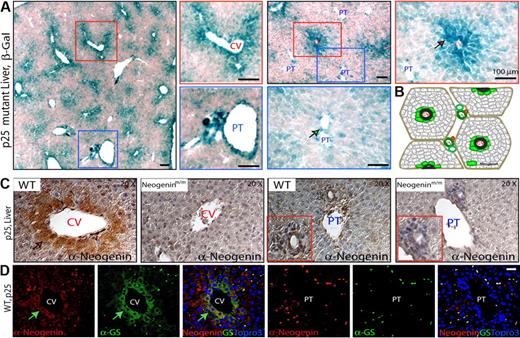
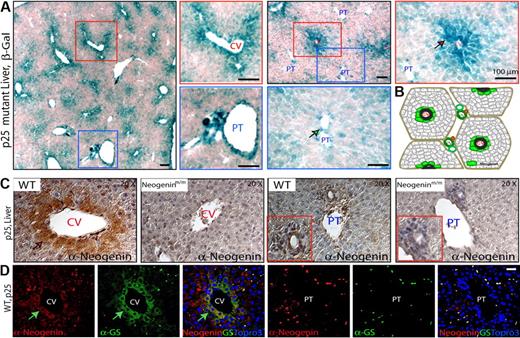
Neogenin was expressed at a moderate level through mouse liver development by Western blot analysis (supplemental Figure 2A). To study neogenin expression at cellular level, we took the advantage of the neogenin mutant mice, where LacZ was “knocked in” in the intron of neogenin gene (Figure 1A); thus, its expression could be used as a reporter for endogenous neogenin expression. LacZ activity was largely restricted at both central vein (CV) and large portal tract (PT) regions of P25 livers of heterozygotes (neogenin+/mu; supplemental Figure 2B) and homozygote (neogeninmu/mu; Figure 4A). Higher-power images suggest that LacZ-positive cells were perivenous hepatocytes in the CV regions (Figure 4A), whereas the LacZ activity in the major PT regions appeared to be associated with cells in vessels but not periportal hepatocytes (Figure 4A-B). Similar results were obtained in staining with anti-neogenin antibody (Figure 4C). Note that antibody staining was diminished in the livers of the mutant mice, demonstrating specificity (Figure 4C). Moreover, neogenin-positive cells expressed glutamine synthetase (GS; Figure 4D), a marker for perivenous hepatocytes,37 indicating that neogenin is expressed predominantly in prevenous hepatocytes surrounding the CVs.
Neogenin expression in mouse liver. (A) Detection of enzymatic lacZ activity in livers from P25-old neogeninm/m mice. (B) Illustration of zonal distribution of neogenin protein in hepatocytes close to central veins and nonhepatocytes align with portal vein (green color). (C) Immunohistochemical detection of neogenin in livers from P25 neogenin+/+ and neogeninm/m mice. (D) Confocal images of coimmunohistochemical analysis in liver sections of P25 wild-type mouse using antibodies against neogenin (polyclonal, red) and GS (monoclonal, green). Arrows point to neogenin-expressing hepatocytes. CV indicates central vein; and PT, portal tract. Bars represent 100 μm. In panels A, C, and D, 3 to 5 different mice for each indicated group were examined, and representative images are shown.
Neogenin expression in mouse liver. (A) Detection of enzymatic lacZ activity in livers from P25-old neogeninm/m mice. (B) Illustration of zonal distribution of neogenin protein in hepatocytes close to central veins and nonhepatocytes align with portal vein (green color). (C) Immunohistochemical detection of neogenin in livers from P25 neogenin+/+ and neogeninm/m mice. (D) Confocal images of coimmunohistochemical analysis in liver sections of P25 wild-type mouse using antibodies against neogenin (polyclonal, red) and GS (monoclonal, green). Arrows point to neogenin-expressing hepatocytes. CV indicates central vein; and PT, portal tract. Bars represent 100 μm. In panels A, C, and D, 3 to 5 different mice for each indicated group were examined, and representative images are shown.
The neogenin distribution was apparently different from that of hepcidin, which is expressed in hepatocytes proximal to the PTs (Figure 2A). To compare the expression of HJV and neogenin, we next examined HJV expression by in situ hybridization. HJV mRNA was detected in hepatocytes proximal to both the PT and CV regions in the P25 liver (Figure 5A-B). On the other hand, HJV mRNA was detected predominantly in hepatocytes proximal to the CV regions of mouse livers at P60 and P90 (Figure 5A), suggesting a developmental regulation. To examine HJV protein distribution in liver, a polyclonal anti-HJV antibody was generated that specifically recognized HJV/RGMc but not RGMa (supplemental Figure 3). Coimmunofluorescence staining analysis demonstrated that HJV protein codistributed with the GS protein in perivenous hepatocytes (Figure 5C-D). These observations suggest that HJV and neogenin are coexpressed in perivenous hepatocytes, implicating a cis interaction between HJV and neogenin.
HJV/RGMc expression in mouse liver. (A-B) In situ hybridization analysis of HJV mRNA expression in mouse (C57BL/6) livers at different age. Note that only antisense (A), but not sense (B), HJV probes showed signals, demonstrating the specificity. (C) Confocal images of coimmunohistochemical detection of both HJV and GS, a marker of hepatocytes surrounding pericentral regions, in liver sections of P25 wild-type mouse. Arrows point to HJV-expressing hepatocytes. Central veins (CVs) and portal tract (PT) are indicated; bars represent 100 μm[b]. (D) Illustration of HJV “zonal distribution” pattern (pink color). In panels A through C, 3 different wild-type mice for indicated age were examined, and representative images are shown.
HJV/RGMc expression in mouse liver. (A-B) In situ hybridization analysis of HJV mRNA expression in mouse (C57BL/6) livers at different age. Note that only antisense (A), but not sense (B), HJV probes showed signals, demonstrating the specificity. (C) Confocal images of coimmunohistochemical detection of both HJV and GS, a marker of hepatocytes surrounding pericentral regions, in liver sections of P25 wild-type mouse. Arrows point to HJV-expressing hepatocytes. Central veins (CVs) and portal tract (PT) are indicated; bars represent 100 μm[b]. (D) Illustration of HJV “zonal distribution” pattern (pink color). In panels A through C, 3 different wild-type mice for indicated age were examined, and representative images are shown.
Neogenin regulation of HJV protein level and distribution
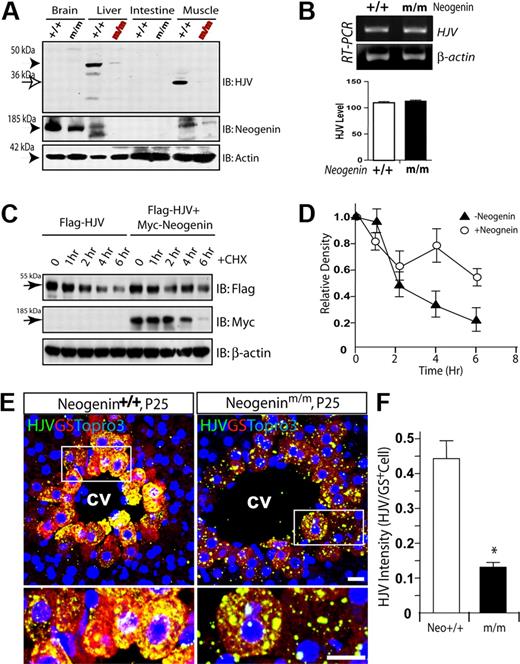
The coexpression of neogenin and HJV in perivenous hepatocytes suggested that neogenin may, via HJV, regulate hepcidin expression in trans or in a cell-nonautonomous manner. To test this hypothesis, we examined the effect of neogenin on HJV expression. As shown in Figure 6A, HJV was expressed abundantly in liver and skeletal muscles, consistent with previous reports.38 Intriguingly, HJV protein in neogenin mutant liver and muscle was significantly reduced (Figure 6A), suggesting a role for neogenin in HJV expression or stability. RT-PCR analysis of P20 muscle RNA demonstrated no difference at HJV mRNA levels between wild-type and mutant mice (Figure 6B), suggesting that neogenin regulation is posttranscriptional. This notion was supported by increased levels of HJV protein in cells coexpressing neogenin (Figure 6C-D). This result also suggests that neogenin may increase HJV protein stability and/or suppress HJV secretion. In addition, neogenin appeared to regulate HJV distribution or trafficking. As shown in Figure 6E-F, in the neogenin mutant, HJV staining appeared to be puncta distributed inside as well as outside of perivenous hepatocytes, whereas in the wild-type liver sections, HJV was evenly distributed in perivenous hepatocytes.
Reduced HJV protein level and altered HJV cellular distribution in neogenin mutant mice. (A) Western blot analysis of HJV protein in different tissue lysates derived from P25 neogenin+/+ and neogeninm/m mice. (B) RT-PCR analysis to assess expression of transcripts for HJV from P20 muscles of neogenin+/+ and neogeninm/m mice. (C-D) Neogenin increases HJV protein level/stability. HEK293 cells were transfected Flag-HJV with or without neogenin. Cells were treated with CHX (an inhibitor of protein synthesis; 100 μg/mL) for indicated times. Lysates were probed with indicated antibodies. Blots were scanned with an Epson scanner and analyzed by National Institutes of Health ImageJ software. Quantitative analysis is shown in panel D. (E) Confocal images of coimmunohistochemical staining analysis of HJV protein distribution in wild-type and neogenin mutant liver at P25. Samples from 3 different mice were examined, and representative images examined by confocal microscope (Zeiss LSM 510 META, 40×/1.00 oil DIC) and processed using Adobe Photoshop CS2 software are shown. HJV (rabbit polyclonal, green), GS (monoclonal, red), and Topro3 (to label nuclei, blue) are indicated. The boxed areas were magnified and are shown in the bottom panels. The scale bars represent 100 μm. (F) Quantification analysis of data from panel E. The HJV immunoflourescence intensity per GS-positive cell is presented as means ± SEM (10 images from 3 different animals). *P < .01 compared with the wild-type liver.
Reduced HJV protein level and altered HJV cellular distribution in neogenin mutant mice. (A) Western blot analysis of HJV protein in different tissue lysates derived from P25 neogenin+/+ and neogeninm/m mice. (B) RT-PCR analysis to assess expression of transcripts for HJV from P20 muscles of neogenin+/+ and neogeninm/m mice. (C-D) Neogenin increases HJV protein level/stability. HEK293 cells were transfected Flag-HJV with or without neogenin. Cells were treated with CHX (an inhibitor of protein synthesis; 100 μg/mL) for indicated times. Lysates were probed with indicated antibodies. Blots were scanned with an Epson scanner and analyzed by National Institutes of Health ImageJ software. Quantitative analysis is shown in panel D. (E) Confocal images of coimmunohistochemical staining analysis of HJV protein distribution in wild-type and neogenin mutant liver at P25. Samples from 3 different mice were examined, and representative images examined by confocal microscope (Zeiss LSM 510 META, 40×/1.00 oil DIC) and processed using Adobe Photoshop CS2 software are shown. HJV (rabbit polyclonal, green), GS (monoclonal, red), and Topro3 (to label nuclei, blue) are indicated. The boxed areas were magnified and are shown in the bottom panels. The scale bars represent 100 μm. (F) Quantification analysis of data from panel E. The HJV immunoflourescence intensity per GS-positive cell is presented as means ± SEM (10 images from 3 different animals). *P < .01 compared with the wild-type liver.
Neogenin inhibition of HJV secretion
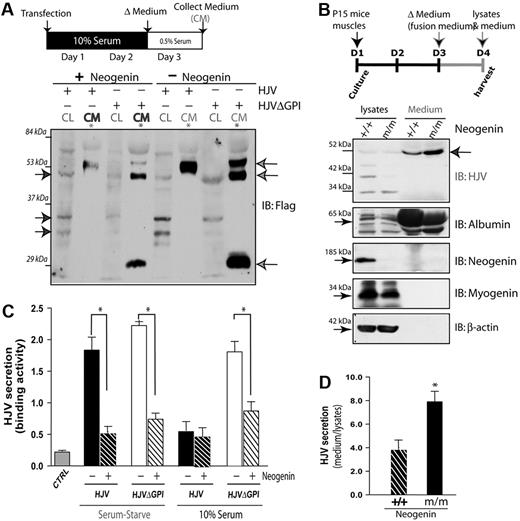
Next, we investigated whether neogenin regulates HJV secretion. Wild-type HJV was barely detected in the culture medium of transfected HEK293 cells in the presence of 10% fetal bovine serum (supplemental Figure 4A). As control, HJVΔGPI, a truncation mutant present in patients of juvenile hemochromatosis,39 was readily detectable (supplemental Figure 4A). In serum-starved culture, secretion of wild-type HJV to the medium was significantly increased (supplemental Figure 4B-C). Interestingly, HJV secretion was inhibited by coexpression of neogenin (Figure 7A-B). These results suggest an inhibitory effect of neogenin on HJV secretion in transfected HEK293 cells.
Neogenin inhibition of HJV secretion. (A-B) Neogenin inhibition of HJV secretion in serum-starved culture. Transfected HEK293 cells were cultured in the presence of 0.5% of serum and the cell lysates and medium were subjected to Western analysis (A). The HJV secretion in the collected medium was also measured by ELISA analysis (B). The mean ± SEM of HJV secretion (binding to the antibodies) was presented. *P < .01, significant difference from the HJV expression alone. (C) Illustration of P15 muscle culture and Western blot analysis of cell lysates and medium from wild-type and neogenin mutant muscle culture with indicated antibodies. Note that HJV was increased in the medium, but reduced in cell lysates, of neogenin mutant culture. (D) Quantification analysis of data from panel C. The data were quantified by National Institutes of Health ImageJ program from 3 different blots and presented as means ± SD. *P < .01, significant difference from the wild-type control.
Neogenin inhibition of HJV secretion. (A-B) Neogenin inhibition of HJV secretion in serum-starved culture. Transfected HEK293 cells were cultured in the presence of 0.5% of serum and the cell lysates and medium were subjected to Western analysis (A). The HJV secretion in the collected medium was also measured by ELISA analysis (B). The mean ± SEM of HJV secretion (binding to the antibodies) was presented. *P < .01, significant difference from the HJV expression alone. (C) Illustration of P15 muscle culture and Western blot analysis of cell lysates and medium from wild-type and neogenin mutant muscle culture with indicated antibodies. Note that HJV was increased in the medium, but reduced in cell lysates, of neogenin mutant culture. (D) Quantification analysis of data from panel C. The data were quantified by National Institutes of Health ImageJ program from 3 different blots and presented as means ± SD. *P < .01, significant difference from the wild-type control.
To determine whether neogenin suppresses HJV secretion in a physiologic condition, we examined primary muscle cells derived from wild-type and neogenin mutant mice using HJV specific antibody that recognizes HJV but not RGMa (an HJV-related protein; supplemental Figure 3). Muscle cells were used because they express high levels of HJV and neogenin and survived well in serum-starved culture condition, thus making analysis easier. Neogeninm/m muscle cells were morphologically similar to those of wild-type control (data not shown). However, the expression of myogenin, a protein for muscle differentiation, was reduced (Figure 7C), suggesting a potential role of neogenin in muscle differentiation, consistent with previous reports.40 The amount of HJV was also reduced in mutant muscle cells (Figure 7C-D). In contrast, the medium of mutant muscle cells exhibited higher levels of HJV than that of wild-type control (Figure 7C-D). These results support the notion for neogenin in preventing HJV secretion in vivo.
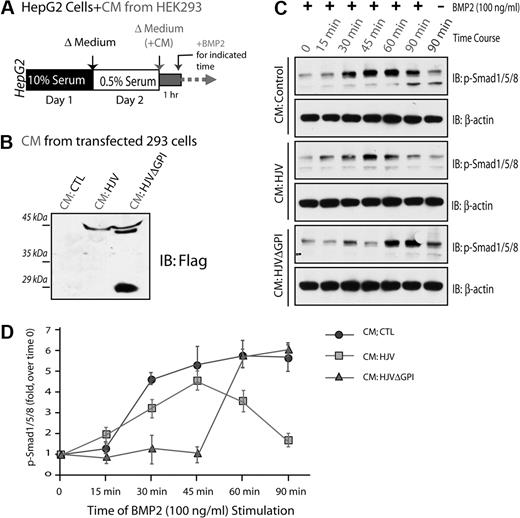
Extracellular HJV, an inhibitor of BMP2 signaling
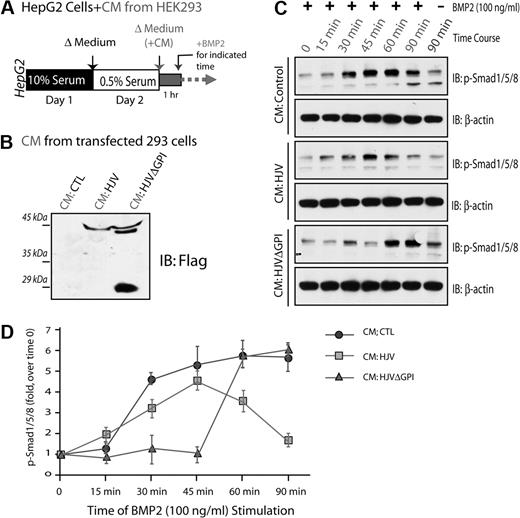
HJV is believed to be a coreceptor of BMPs, regulating hepcidin expression,12 whereas soluble HJV (sHJV) may act as an antagonist in this event.9,13 We thus tested if sHJV inhibits BMP2-induced Smad1/5/8 phosphorylation, a major BMP activated signaling pathway that induces hepcidin expression.41 HepG2 cells were preincubated with the condition medium of HEK293 cells expressing control vector, HJV, or HJVΔGPI (Figure 8B), and then treated with BMP2 for different times (Figure 8A). BMP2 induced Smad1/5/8 phosphorylation in a time-dependent manner in HepG2 cells that were preincubated with the control medium: it began to increase at 15 minutes, peaked at 45 minutes, and remained at high level at 90 minutes of BMP2 stimulation (Figure 8C-D). However, preincubation with the condition medium of HJV or HJVΔGPI-transfected cells reduced and/or delayed phosphorylation of Smad1/5/8 (Figure 8C-D). These results suggest that both secreted HJV and HJVΔGPI are capable of inhibiting BMP signaling.
Inhibition of BMP2 induced Smad1/5/8 phosphorylation by secreted HJV. HepG2 cells were serum-starved for 1 day, preincubated with condition medium (CM) collected from HEK293 cells transfected with indicated plasmids, and then treated with BMP2 (100 ng/mL) for various times (A). CM containing secreted HJV or HJVΔGPI mutant was revealed by Western blot analysis (B). BMP2-induced Smad1/5/8 phosphorylation in HepG2 cells was analyzed by Western blot analysis as shown in panel C. (D) Quantification analysis of data from panel C. Data were quantified by National Institutes of Health ImageJ program from 3 different experiments. Means ± SD are presented.
Inhibition of BMP2 induced Smad1/5/8 phosphorylation by secreted HJV. HepG2 cells were serum-starved for 1 day, preincubated with condition medium (CM) collected from HEK293 cells transfected with indicated plasmids, and then treated with BMP2 (100 ng/mL) for various times (A). CM containing secreted HJV or HJVΔGPI mutant was revealed by Western blot analysis (B). BMP2-induced Smad1/5/8 phosphorylation in HepG2 cells was analyzed by Western blot analysis as shown in panel C. (D) Quantification analysis of data from panel C. Data were quantified by National Institutes of Health ImageJ program from 3 different experiments. Means ± SD are presented.
Discussion
The present study provides in vivo evidence for neogenin regulation of iron homeostasis. Molecular and biochemical results suggest a working hypothesis that neogenin suppresses HJV secretion, enhances BMP signaling and hepcidin expression, and regulates iron homeostasis.
The phenotypes of neogenin mutant mice are remarkable similar to those in HJV−/− or recently published BMP6 mutant mice: hepatic iron overload, reduced hepcidin expression, and defective BMP signaling.5,6,35,42,43 However, there is some difference among the 3 mutant mice. An earlier-onset phenotype (eg, iron overload and hepcidin reduction at P25) was observed in neogenin mutant mice compared with that in HJV or BMP6 mutants, in which the reduction of hepcidin expression and iron overload appeared to be approximately 2- to 3-month-old mutant mice.5,6,35,42,43 A relatively weaker reduction of hepcidin in P25 neogenin mutant liver was noted compared with that in HJV or BMP6 mutant mice.5,6,35,42,43 A more severe reduction of BMP signaling in neogenin mutant mice/livers than that in cultured hepatocytes was also found. In addition, neogenin mutant mice have reduced body weight, decreased bone density, and early postnatal death (at ∼ P30; data not shown), apparently caused by decreased BMP signaling in other tissues/cells (data not shown), in agreement with the model presented in this article.
We speculate that several reasons may account for the difference among neogenin, HJV, and BMP6 mutant mice. First, multiple BMP signaling defects appeared to be associated with neogenin mutant mice, whereas BMP6 or HJV mutant shows relatively more selective defect on BMP6 signaling. Second, there is a lack of soluble HJV generation in HJV-null mice, but it was increased in neogenin mutant. The soluble HJV in neogenin mutant mice may inhibits multiple BMPs induced signaling, including BMP2, 4, 6, and 9, in a cell nonautonomous manner. Finally, the different genetic background between HJV (in 129Svj background) and neogenin (C57/BL6) mutants may also contribute to the difference of the phenotypes observed.
Although hepcidin reduction in P25 neogenin mutant livers is relatively weaker, it is possible that the reduction of hepcidin may have started out even lower in younger mutant mice, which may be increased by the severe iron overload in P25 mutant mice. Our studies support a crucial role of neogenin in enhancing BMP-induced hepcidin expression. Neogenin mutant livers show reduced hepcidin expression (Figure 2). Increased Fpn protein in neogenin mutant spleen and intestine (supplemental Figure 1) supports the notion for a loss of hepcidin protein function in the mutant. Neogenin mutant hepatocytes exhibit impaired BMP induction of hepcidin expression (Figure 3H). Together, these results support for a defective BMP-hepcidin pathway in neogenin mutant mice contribute to the hepatic iron overload.
How does neogenin regulate hepcidin expression? Neogenin may regulate hepcidin expression by modulating HJV-BMP signaling for the following reasons. Loss of neogenin function both in mice and in cultured hepatocytes leads to an impaired BMP2-induced phosphorylation of Smad1/5/8 and reduced hepcidin expression. In addition, a reduced HJV protein level, altered HJV distribution, and increased HJV secretion observed in neogenin mutant liver and muscle suggest a role for neogenin in preventing HJV protein secretion, thus enhancing BMP signaling and hepcidin induction.
As hepcidin is highly regulated in response to multiple stimuli: up-regulated when iron levels are increased or by inflammation, and down-regulated in response to anemia or hypoxia,1,29,44,45 it remains to be investigated if neogenin plays a role in iron- or inflammation-associated hepcidin induction.
How does neogenin modulate HJV-BMP signaling and BMP-induced hepcidin expression? One hypothesis is that neogenin may be a component of the “super-receptor complex” previously thought to consist of HJV and BMP receptors,12 modulating BMP signaling. This hypothesis is in line with the observations that neogenin, HJV, and BMP receptors are coexpressed in various cells, including perivenous hepatocytes (Figures 6–7) and muscles,38,46,47 that BMP2 signaling was reduced in primary cultured hepatocytes of neogenin mutant mice (Figure 3F-G), and that suppression of HJV expression by siRNA inhibits BMP signaling in hepatoma cell lines.12 This hypothesis would suggest that neogenin and HJV may regulate BMP signaling and hepcidin expression in a cell-autonomous manner. However, using in situ hybridization, we have shown that hepcidin is expressed in periportal hepatocytes but not perivenous hepatocytes where neogenin and HJV are mainly expressed (Figures 2, 4,Figure 5–6). These results are intriguing and suggest that hepcidin expression may be also regulated by neogenin in trans or in a cell nonautonomous manner in vivo. In this “trans” model, neogenin may enhance hepcidin expression in periportal hepatocytes by maintaining HJV protein level and inhibiting HJV secretion from perivenous hepatocytes and/or from muscles. This is in line with the observations that sHJV acts as an inhibitor of BMP-induced hepcidin expression in vitro and in vivo,9,13 both neogenin and HJV are largely coexpressed in same types of cells, and that neogenin is involved in regulation of HJV protein level and secretion.
Our results from in vitro and in vivo studies have pointed to a function of neogenin in suppressing HJV secretion. It was reported that overexpression of HJV induces hepcidin promoter activity in Hep3B cells in a neogenin-independent manner.48 This result suggests that neogenin may not be a HJV downstream receptor in mediating HJV induction of hepcidin expression, excluding a cell-autonomous function for neogenin in this event. However, recently it has been reported that neogenin is required for HJV-induced hepcidin expression in transfected HepG2 cell lines, suggesting a cell-autonomous function by neogenin.49 The controversial reports may be due to different cell lines used, and they are not contradictory to our model. However, studies by Zhang et al15 have suggested that neogenin promotes HJV/RGMc shedding and sHJV production, in contradiction to our studies. The reason underlying this difference is unclear and remains to be further investigated.
In summary, the present study supports a role for neogenin in hepcidin expression and iron homeostasis. Neogenin regulation of hepcidin expression may be due to its regulation of HJV protein trafficking and secretion, thereby enhancing BMP-induced hepcidin expression.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Sue Ackerman (The Jackson Laboratory) for providing neogenin mutant mice and Dr Xiao-Ming Yin (University of Pittsburgh) for providing protocol and advice on primary culture of mouse hepatocytes.
This study was supported in part by grants from the American Heart Association Southeastern (GIA 0755561B to W.C.X.) and the National Institutes of Health (to L.M. and W.C.X.).
National Institutes of Health
Authorship
Contribution: D.-H.L. and X.-J.Z. designed and performed the experimental work; Z.Z., J.-X.X., J.-U.J., Y.L., and C.-X.X. performed part of experimental work; L.M. provided reagents and helped to analyze data; and W.-C.X. designed experiments, analyzed the data, and wrote the article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wen-Cheng Xiong, PhD, Institute of Molecular Medicine & Genetics and Department of Neurology, Medical College of Georgia, Augusta, GA 30912; e-mail: wxiong@mcg.edu.
References
Author notes
D.-H.L., L.-J.Z., Z.Z., and J.-X.X. contributed equally to this work.

![Figure 1. Iron accumulation in periportal hepatocytes of neogenin mutant mice. (A) Diagram of the retrotranspon insertion in neogenin gene. Exons 7 and 8, surrounding intron, and location of genotyping primers are shown diagrammatically. Primer sequences are also indicated. (B) Genotyping and decreased neogenin expression in neogenin mutant mice. Livers of indicated genotype were lysed and resulting lysates were subjected to immunoblotting with anti-neogenin antibody. The top panel shows PCR genotyping. The 327-bp fragment was detected in homozygous mice where the 169-bp fragments in wild-type mice. (C-D) Histologic detection of iron content on cryostat sections of liver (C) and spleen (D) of wild-type and neogenin mutant (neogeninm/m) mice. Five different wild-type and mutant mice were examined, and representative images imaged by Axiophot microscope (Zeiss) and processed using Adobe Photoshop CS2 software are shown. Note iron accumulation was observed in the periportal (portal tract [PT]), but not central vein (CV), regions of P25-old neogeninm/m mice and absence thereof in the red pulp of the spleen. (E) Western blot analysis of liver and spleen lysates from indicated genotype with anti-ferritin-H antibodies. Molecular weights are indicated. (F) Quantitative determination of iron content (μmol/g dry weight; see “Tissue iron staining and quantification”) in various organs of P25-old wild-type (white), neogenin+/m (purple), and neogeninm/m (blue) mice. Means ± SEM of 5 samples for each group were presented. **,#Significant changes (P < .01) in neogeninm/m mice compared with the wild-type control.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/15/10.1182_blood-2009-11-251199/4/m_zh89991049780001.jpeg?Expires=1765020340&Signature=Uqse8b6IN17XE7S068C-OBvNydNfWvyYifr6gFWkiLhl~Yj7MKIR6APgzzYHlV8cXvpgqCX1dvKQ7CuRPeL72D0SjDMn3qy81fqxM0eL7GY7K8cR1r4-i5LG9PxE14BHR1N8vGjsUD6qCxeIxnqnaVxQRE8hPymb0A2Hs99sna3gQqP-EzJE655H0mbtR3bc2pSrbaoScpdEtaNeDowumkNXbWP28y-m1CtD8s7zNo3VYG7KFu4IkHI2b4pXQN4xDXE4rNtafr9jVaeuTmV7teQwA3GV09xH3Jm3P-nDxTBVyWoUpGrOT96vzTbxrfDTCWMr~aFpebjV378~8-1mAA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. HJV/RGMc expression in mouse liver. (A-B) In situ hybridization analysis of HJV mRNA expression in mouse (C57BL/6) livers at different age. Note that only antisense (A), but not sense (B), HJV probes showed signals, demonstrating the specificity. (C) Confocal images of coimmunohistochemical detection of both HJV and GS, a marker of hepatocytes surrounding pericentral regions, in liver sections of P25 wild-type mouse. Arrows point to HJV-expressing hepatocytes. Central veins (CVs) and portal tract (PT) are indicated; bars represent 100 μm[b]. (D) Illustration of HJV “zonal distribution” pattern (pink color). In panels A through C, 3 different wild-type mice for indicated age were examined, and representative images are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/15/10.1182_blood-2009-11-251199/4/m_zh89991049780005.jpeg?Expires=1765020341&Signature=nohX-0ZuzRvBFKYljvorUvWqOPXaClKTbq8VvhK5pOolUUJ4UupFnOP0tV5-Jn9ivW~ljwDrWOTgHuRc3LqNMwEMHTrRKh7eecyHySvr1v1ec68Q0R0AaqFOaEAv0DwUUnqnXJ9czyKCCY3anC9P4onu7tQ3gIiDhAC0OTJ-aMW~-rfq9K8QmFi-rLdPF980rA3oGouipezN2Z8Ak3o5Sr33x9vxemRK1d8WEKdgrVy~Jv8M8SA4ca9zy2Bz6tn2ngGEt05524aO0tJss6uJldxwzCTfuq3voV4Ihj8pI96EyQld1~X-XQSAdLI3NFNZF6-X78lXoV55VaWzKKYPmg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 1. Iron accumulation in periportal hepatocytes of neogenin mutant mice. (A) Diagram of the retrotranspon insertion in neogenin gene. Exons 7 and 8, surrounding intron, and location of genotyping primers are shown diagrammatically. Primer sequences are also indicated. (B) Genotyping and decreased neogenin expression in neogenin mutant mice. Livers of indicated genotype were lysed and resulting lysates were subjected to immunoblotting with anti-neogenin antibody. The top panel shows PCR genotyping. The 327-bp fragment was detected in homozygous mice where the 169-bp fragments in wild-type mice. (C-D) Histologic detection of iron content on cryostat sections of liver (C) and spleen (D) of wild-type and neogenin mutant (neogeninm/m) mice. Five different wild-type and mutant mice were examined, and representative images imaged by Axiophot microscope (Zeiss) and processed using Adobe Photoshop CS2 software are shown. Note iron accumulation was observed in the periportal (portal tract [PT]), but not central vein (CV), regions of P25-old neogeninm/m mice and absence thereof in the red pulp of the spleen. (E) Western blot analysis of liver and spleen lysates from indicated genotype with anti-ferritin-H antibodies. Molecular weights are indicated. (F) Quantitative determination of iron content (μmol/g dry weight; see “Tissue iron staining and quantification”) in various organs of P25-old wild-type (white), neogenin+/m (purple), and neogeninm/m (blue) mice. Means ± SEM of 5 samples for each group were presented. **,#Significant changes (P < .01) in neogeninm/m mice compared with the wild-type control.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/15/10.1182_blood-2009-11-251199/4/m_zh89991049780001.jpeg?Expires=1765410405&Signature=plx3WU-dNsbA8XhDyMdMODEqLIH1XGhCnI7y7Aw06EZt-q40RDdCh1U3MVeDz0iNOmesEvqjkhYZO433wMjaPIYqfJ683CsmeQcOZGgVb~jymVcH99tKrK61iAdbH0ITLQQtw7CCVXoPAbUt85HBC64cgIGj4aHqG09M9h0PYYMftfvMlZ1iKx9m6g1d78N9tZ8aCPGbGo3SwnorvugUznzxJtwZHBkFtMrjA8mQnkEkKjCX6VlPleGmZLtDhw6Qq8Hcczb-JrTSyroZdtEQZwqBc9QSsfv6oqv3RgxmyRLtNWBpxIWbz1JlomJ8FB2S8sOqrcOhTYonrM1Z-UOtXQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 5. HJV/RGMc expression in mouse liver. (A-B) In situ hybridization analysis of HJV mRNA expression in mouse (C57BL/6) livers at different age. Note that only antisense (A), but not sense (B), HJV probes showed signals, demonstrating the specificity. (C) Confocal images of coimmunohistochemical detection of both HJV and GS, a marker of hepatocytes surrounding pericentral regions, in liver sections of P25 wild-type mouse. Arrows point to HJV-expressing hepatocytes. Central veins (CVs) and portal tract (PT) are indicated; bars represent 100 μm[b]. (D) Illustration of HJV “zonal distribution” pattern (pink color). In panels A through C, 3 different wild-type mice for indicated age were examined, and representative images are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/15/10.1182_blood-2009-11-251199/4/m_zh89991049780005.jpeg?Expires=1765410405&Signature=0XHsKluQy3OXHyKrAl8jBZi20lq9fSz2Yc3GR4eyJtQuz5PJ22H2SPn9pYwvw9rErz1jHiDV~zPNqkF43Nf5byelwZ4XaFf1L7uBkPbtCsZmJjcCHMHWKLHntXMq5RfomC-dTtU3MGx42ybA4PeTmVbMX-I5SLIM1LEDESakg2rbD97b2kO5jEKlwOgcNO97UI39xNRinZbyIlqSlx4ZE41tJgpIgURTI6HlqQIMxqRWnrjt-pTLovsvorZLon9qk0IcG3K6h3R~obDVn9tcu4ID0jpFKzla~guIt8hqhfCcLdA4tXXdqTvLloa93oMckwB6~ptLRiTY94r-1siOQw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)