Abstract
Previous studies of follicular lymphoma (FL) patients treated heterogeneously have suggested that decreased numbers of regulatory T cells correlates with improved survival. We studied advanced-stage FL patients from a single institution phase 2 trial. All patients were treated uniformly with multiagent chemotherapy and radiation. Tissue microarrays were constructed using diagnostic biopsies available in 105 patients and stained with CD4, CD8, CD25, and forkhead/winged helix transcription factor 3 (FOXP3) antibodies. Both cell content and cell distribution were evaluated. For all antibodies, there were cases with a predominant intrafollicular or perifollicular localization of cells (follicular pattern) while others displayed a diffuse pattern. The median follow-up of living patients was 17.1 years. The International Prognostic Index score predicted overall survival (OS; P = .004) but not risk of transformation (RT). Cell content did not impact survival, while immunoarchitectural patterns of CD4/CD8 were significant for progression-free survival (PFS; P = .056), CD25 for both PFS and OS (P = .002 and P = .024, respectively), and FOXP3+ predicted PFS, OS, and RT (P = .001, P < .001 and p = .002, respectively). A Cox multivariate model showed both International Prognostic Index score and FOXP3+ pattern were independent predictors of OS (P = .008 and P < .001, respectively), while only FOXP3+ pattern predicted RT (P = .004). We conclude that FOXP3+ cell distribution significantly predicts survival and RT in FL.
Introduction
Follicular lymphoma (FL) is the most common indolent non-Hodgkin lymphoma worldwide.1 It is characterized by a significant degree of clinical heterogeneity.2 Transformation into aggressive lymphoma, usually diffuse large B cell lymphoma is a dominant clinical event frequently associated with inferior survival.3 Clinical prognostic variable scoring systems such as the International Prognostic Index (IPI) can be used to assess patient risk and guide therapeutic decisions.4 However, such clinical variables represent only surrogate markers for the underlying biology. Although desirable, no consistent biologic markers have been identified that predict survival or, equally importantly, risk of transformation. The majority of candidate biomarkers studied in the past have focused on the malignant cells, including morphologic features, cytogenetic abnormalities, molecular aberrations, and altered protein expression. However, the lack of large, uniformly treated patient cohorts and the low frequency of several of these molecular events have hampered their translation into clinical practice.
Recent gene expression studies have revealed that both survival and progression of disease were significantly associated with non-neoplastic immune cell gene signatures.5,6 On the strength of these observations we have shown in FL treated uniformly with multiagent chemotherapy (bleomycin, cisplatin, etoposide, doxorubicin, cyclophosphamide, vincristine, and prednisone [BP-VACOP]) and involved region radiotherapy, lymphoma-associated macrophage (LAM) content significantly predicted survival. Using tissue microarrays and immunohistochemistry, we found that in a minority of tumors (12%) a high density of macrophages infiltrating the tumor correlated with inferior survival.7 Several other subsequent studies have found similar results.8-11
Regulatory T cells (Tregs) have the important role of suppressing effector T cells and preventing reactivity to self-antigens.12 These cells recognize specific tumor antigens and differentiate into cells capable of suppressing naive and CD4+ Th1 antitumor effector cells.13 It has been shown that tumor immunosurveillance is more efficient when Tregs are depleted.14,15 In human cancer, effective antitumor responses are also affected by the presence of Tregs. These cells have been identified in increased frequency in the peripheral blood of patients with several different tumor types.16-20 Furthermore, increased density of Tregs within carcinoma biopsies is predictive of poor survival.20
Tregs differ from other lymphocytes in origin, phenotype, and mode of action. In humans, they constitute 5% to 10% of CD4+ T cells and arise as a distinct lineage within the thymus, so-called “natural” Tregs, but regulatory function can also be acquired peripherally when uncommitted CD4+ cells receive antigenic stimulation.12 These cells also express other surface molecules associated with activated/memory cells such as CD25, glucocorticoid-inducible tumor necrosis factor receptor (GITR), CD62L, and cytotoxic T lymphocyte–associated antigen 4, and these cells can secrete transforming growth factor-beta (TGFβ) and interleuking-10 (IL-10). However, none of these proteins is unique to these cells. The forkhead/winged helix transcription factor 3 (FOXP3) is a transcriptional factor shown to be the key control gene in the development and function of Tregs both in mice and humans.21-23 Although multiple T-cell subsets (eg, Tr1, Th3, Th1, Th17, Tfh) have been shown to exert negative immunoregulatory effects by producing immunomodulatory cytokines, such as TGFβ and IL-10, FOXP3+ T cells represent the major Treg population critical for immune homeostasis.
In lymphoma, FOXP3 is expressed by a subset of adult T-cell leukemia/lymphoma cases that tend to have a worse prognosis.24 In FL, FOXP3 expression is only detected in the background reactive T cells present at variable frequency. Importantly, intratumoral FOXP3+ T cells have been shown to migrate or be induced in response to chemokines produced by malignant B cells and they display the ability to suppress the function of other infiltrating T cells.25-29 The mechanisms by which Tregs exert their suppressive function are likely multiple30 but not fully elucidated in FL.
In retrospective studies of FL, increased numbers of Tregs expressing FOXP3 have been associated with improved overall survival, a finding that is contradictory to nonhematopoietic cancers.8,31 However, these series are limited by the marked heterogeneity of treatments used, thereby confounding straightforward interpretation of these data. Treatment heterogeneity has been shown to correlate with different, sometimes contradictory results regarding the clinical impact associated with different cells of the microenvironment in FL.9,11,32 We hypothesize that these markedly different treatments are differentially visited on the malignant B cells versus the nonneoplastic immune cells in the tumor microenvironment. These observations and hypotheses prompted us to analyze the role of Tregs as a prognostic marker in FL, both in terms of their numbers and their distribution in the tumoral microenvironment, in a series of uniformly treated patients with long follow-up.
Methods
Patient characteristics
The British Columbia Cancer Agency is the primary referral center for patients diagnosed with lymphoid malignancies in the province of British Columbia. Consecutive patients seen at the British Columbia Cancer Agency between July 1987 and May 1993 were offered enrolment in a phase 2 trial consisting of multiagent chemotherapy (BP-VACOP) followed by involved field irradiation to sites of original nodal involvement. Patients were eligible for enrollment if they were aged 16 to 61 years, were newly diagnosed, treatment-naive, HIV-negative, and had advanced-stage indolent non-Hodgkin lymphoma. Advanced-stage disease was defined as Ann Arbor stage III or IV, or stage II with B symptoms, nonradioencompassable disease, or bulk 10 cm or larger in maximum diameter at any individual tumor site. Approval to review, analyze, and publish the data in this study was given by the University of British Columbia–British Columbia Cancer Agency Research Ethics Board. The source of pathology specimens for diagnosis included only lymph node biopsies. One pathologist (R.D.G.) classified all cases as indolent according to the Working Formulation for Clinical Usage and later updated them according to the World Health Organization (WHO) classification.33 For this study, 2 pathologists (R.D.G. and P.F.) re-reviewed all of the FL cases, grading them according to the 2008 WHO criteria. Transformation was defined as biopsy proven or clinically diagnosed diffuse large B cell lymphoma or other aggressive lymphoma as described previously.3
TMA
A tissue microarray (TMA) was constructed using a tissue arrayer device (Beecher Instruments). Of the 126 cases of FL treated with BP-VACOP and radiation, 105 had paraffin blocks with adequate material remaining in the block to be used for the TMA. Duplicate 1.0-mm cores were used to construct 2 TMAs, including both B5 and formalin-fixed cases. The 2 TMAs shared 40% overlapping cases, which provided a valuable internal control. Slides from the TMA block were cut at 4 μm.
Histology and immunohistochemistry
A hematoxylin and eosin stain of the TMA was prepared using routine methods. All cases contained neoplastic follicles (average of 3 follicles per core). A battery of immunohistochemical stains were performed including CD20, CD21, CD4, CD8, CD25, and FOXP3 (see Table 1). Immunostaining was performed using a Dako autostainer and the EnVision polymer detection system. Pressure cooking antigen retrieval was used for all antibodies. A variety of buffers were used depending on the specific antibody to allow optimal detection of the antigens. The chromogen in all cases was diaminobenzidine.
The immunostained TMA slides were screened with CD20 to ensure tumor cell content. For the different T-cell markers (CD4, CD8, CD25, and FOXP3), the numbers of positively stained cells and their patterns of distribution were evaluated. Before counting, the morphologic patterns and cell content were first evaluated for their consistency between duplicate cores and between TMAs (B5 vs formalin).
The total number of positive cells (FOXP3 and CD25) was counted per core (× 1000 magnification). Immunoarchitectural patterns of distribution of the different T-cell markers were determined in relation to the neoplastic follicles. The “follicular” pattern was characterized by an intrafollicular and perifollicular predominance of positive cells, whereas in the “diffuse” pattern the cells were diffusely distributed with no clear relationship to the follicles. To better classify these patterns a follicular dendritic cell meshwork stain (CD21) was used. Whenever the majority of positive cells were within the follicles in each core, cases were labeled as having a follicular pattern. From the remaining cases with predominance of positive cells outside the follicles, those with a predominant perifollicular rim of positive cells were labeled perifollicular. For purposes of analysis, both groups, those with a follicular distribution of positive cells and those with a perifollicular distribution, were grouped together as having a follicular pattern. Assignment of the CD4 pattern and the combined CD4 versus CD8 pattern was done similarly to our previous study.7 Both were scored semiquantitatively and in the CD4 versus CD8 pattern determination, both stains were evaluated using sequential sections by overlaying both slides on the microscope. Cases with a follicular predominance of CD4 versus CD8 cells were identified as having a follicular pattern, whereas cases with a similar distribution of CD4 versus CD8 cells were defined as “diffuse.”
Statistics and survival analysis
Progression-free survival (PFS) was defined as the interval between diagnosis and progression of lymphoma or death due to lymphoma or toxicity of treatment. Overall survival (OS) was defined as the interval from date of diagnosis until death from any cause, and risk of transformation was defined as the interval from diagnosis until clinical or biopsy-confirmed transformation into aggressive disease. The variables IPI score, histologic grade, age, gender, and each of the 4 new biomarkers (see Table 2) were evaluated for prognostic significance. Survival estimates were calculated using the Kaplan-Meier method.34 Significance levels, estimates of hazard ratios (HR), and their 95% confidence intervals (CI) were calculated using the proportional hazards regression model.35
Results
Clinical characteristics and outcome
A total of 102 patients were included in this study, representing the FL patients from the original clinical cohort (n = 126) with available blocks, sufficient tissue remaining in the blocks, and successful TMA construction and interpretation (FOXP3). The median age of the evaluable patients was 45 years (range, 19-61 years). There were 50 women and 52 men. The distribution of IPI scores included 62 patients with IPI scores of 0/1 (group 1), 39 with IPI score of 2/3 (group 2), and only 1 patient with an IPI score of 4/5 (group 3). For analysis purposes, the single patient in IPI group 3 was included with IPI group 2 to eliminate a clinical group with only 1 patient. The median follow-up of living patients (n = 51) was 17.1 years. The median OS was 15.5 years. The IPI was highly significant as a predictor of OS (HR = 2.4, 95% CI = 1.4-4.2, P = .001) and PFS (HR = 2.0, 95% CI = 1.2-3.3, P = .006), but not risk of transformation.
Pathology variables
Some cores (< 6%, variable by biomarker) were lost during the preparation of the sections or were not interpretable due to poor fixation and/or inadequate staining. Histologic grading of FL subtypes according to the WHO classification included 80 grade 1, 16 grade 2, and 6 grade 3A FL. Histologic grade, the presence of marginal zone differentiation, the proliferation rate (Ki-67), and the patterns of T cells (total CD3, total CD7, CD4 vs CD8 predominance, CD57 content, and architectural distribution) and follicular dendritic cell patterns had no effect on survival (data previously published).7 Results of the new biomarkers (CD4 vs CD8 distribution, CD25, and FOXP3) are summarized in Table 2.
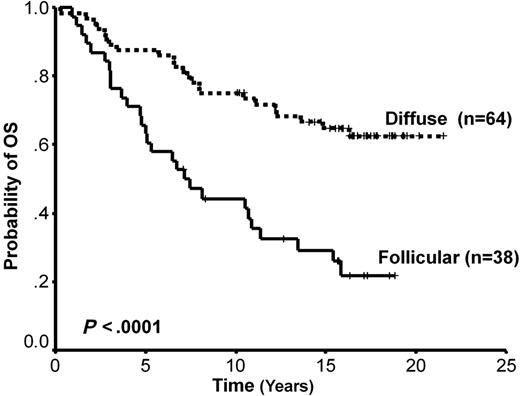
The total number of any individual T-cell population analyzed as well as the CD4 pattern showed no impact on survival. Follicular versus diffuse pattern of distribution of CD4 versus CD8+ cells was associated with PFS only (HR = 0.54, 95% CI = 0.29-1.0, P = .05), and CD25 pattern was significant for both PFS (HR = 2.4, 95% CI = 1.4-3.9, P = .001) and OS (HR = 1.8, 95% CI = 1.1-3.1, P = .024) but not risk of transformation. Only the FOXP3 score had a significant impact on PFS, OS, and risk of transformation (Table 2). As described in “Methods,” cases with either a follicular pattern (n = 20) or a perifollicular pattern (n = 18) were grouped together as Follicular FOXP3, and the remaining cases were classified as Diffuse (n = 64; see Figure 1). Cases with a follicular versus diffuse FOXP3+ pattern of distribution had a median OS of 7.13 years and not reached, respectively (HR = 3.1, 95% CI = 1.8-5.4, P < .001; Figure 2), and a median PFS of 2.2 and 8.8 years, respectively (HR = 2.7, 95% CI = 1.6-4.6, P = .001). Finally, cases with follicular versus diffuse patterns had median risks of transformation of 13.3 years and not reached, respectively (HR = 3.7, 95% CI = 1.5-8.9, P = .004; Figure 3). Histologic grade, IPI, and bone marrow involvement were evenly distributed between these 2 groups (Table 3). Interestingly, all but 1 case with a high content of LAM had a FOXP3+ follicular pattern and a follicular pattern of CD25+ cells. The pattern of CD25+ and FOXP3+ cells was significantly correlated (Table 3). A multivariate proportional hazards model that included the FOXP3+ immunoarchitectural pattern, the IPI, and the LAM content showed that FOXP3+ pattern and IPI are independent predictors of OS (HR = 2.9, 95% CI = 1.7-5.1, P = .001 and HR = 2.1, 95% CI = 1.1-3.7, P = .008, respectively). Interestingly, in the multivariate Cox model for risk of transformation only FOXP3 positivity showed significance (HR = 3.9, 95% CI = 1.5-9.9, P = .004).
Representative tissue microarray cores of follicular lymphoma stained for FOXP3. The images on the left and center show the follicle-based patterns with FOXP3 cells present mostly within the follicle or around the follicle and in the mantle zone, respectively (n = 38). On the right, the image shows a diffuse case with no follicle-centered pattern defined (n = 64). Microscope: Nikon Eclipse E600; digital camera: Dxm1200.
Representative tissue microarray cores of follicular lymphoma stained for FOXP3. The images on the left and center show the follicle-based patterns with FOXP3 cells present mostly within the follicle or around the follicle and in the mantle zone, respectively (n = 38). On the right, the image shows a diffuse case with no follicle-centered pattern defined (n = 64). Microscope: Nikon Eclipse E600; digital camera: Dxm1200.
Overall survival curve based on FOXP3 immunoarchitectural patterns. The top curve represents cases with a diffuse pattern and the bottom curve those cases with a follicular pattern.
Overall survival curve based on FOXP3 immunoarchitectural patterns. The top curve represents cases with a diffuse pattern and the bottom curve those cases with a follicular pattern.
Risk of transformation curve based on FOXP3 immunoarchitectural patterns. The top curve represents cases with a follicular pattern and the bottom curve those cases with a diffuse pattern.
Risk of transformation curve based on FOXP3 immunoarchitectural patterns. The top curve represents cases with a follicular pattern and the bottom curve those cases with a diffuse pattern.
Discussion
It has been almost a century since immunosurveillance was proposed as a mechanism responsible for the repression of cancer growth.36 Since then, our understanding of tumor immunobiology has increased indicating that immunosurveillance represents 1 dimension of the complex relationship between the immune system and cancer.37 It is now a widely held idea that the growth of tumors is influenced by nonneoplastic cells of the immune system. These cells are thought to exert selective pressure on the malignant cells. Ultimately, the malignant cells escape immune recognition and destruction and much like tolerance to self-antigens, tolerance to tumor-associated antigens may arise and permit or even promote disease progression.37
Tregs were initially defined as a subset of suppressor T cells that mediate immune tolerance by inhibiting autoreactive T cells.38 Naturally occurring Tregs play a vital role in maintenance of tolerance to self-antigens and immunologic homeostasis through the antigen-specific suppression of effector CD4+ and CD8+ T cells.12 However, mechanisms whereby Tregs, including peripherally induced cells, bring about the inhibition of other T-cell subtypes are still not well understood.30 They include direct suppression by cell contact and/or cytokine production, as well as inhibition of antigen-presenting cell function. The T-cell receptor repertoire of Treg cells is as broad and diverse as that of other CD4+ T cells, but it is skewed toward recognizing complexes of self-peptides and major histocompatibility complex.
Many of the tumor-associated antigens recognized by autologous T cells in cancer are antigenically normal self constituents. This suggests that Tregs normally engaged in the maintenance of self-tolerance may also suppress immunosurveillance against autologous tumor cells. Depletion of these Treg cells before a tumor challenge encourages effective immune responses to tumors in mouse models.39 In mice with advanced tumors, including lymphoma, the administration of anti-CD25 or intratumoral agonistic anti-GITR antibodies reduces tumor-infiltrating Foxp3+CD25+CD4+ Tregs and results in enhanced antitumor immunity with a significant decrease of the tumor burden without causing autoimmune disease.40,41 In humans, T cells reactive to tumor-associated antigens can expand and become detectable in the peripheral blood when Tregs are first depleted.13,42 Tregs accumulate within different types of tumor-draining lymph nodes and the peripheral blood.16-20 FOXP3+ T cells present in the tumor microenvironment contribute to the growth of the tumor, and their presence is associated with unfavorable prognosis in patients with ovarian carcinoma.20 This study by Curiel et al highlights the importance of the chemokine network in the recruitment and function of these cells. Decreased CCL22 produced by the tumor cells and/or tumor-associated macrophages reduced the recruitment of the Tregs that characteristically express the CCL22 cognate receptor CCR4.
In reactive lymphoid tissues, FOXP3+ Tregs are found mostly at the borders between T and B cells and within germinal centers where they suppress T cells as well as B-cell responses through both inhibition of immunoglobulin class switch recombination43 or generation of “Treg epitopes” identified in the Fc region of the immunoglobulin molecule itself.44
Intratumoral CD4+ T cells (CD25+ or CD25−) expressing FOXP3 are increased in FL compared with reactive lymph nodes.25 These FOXP3+ cells are either attracted (CCL22) or induced locally by lymphoma cells through cell contact without stimulation of TCR but by expressing CD70.25,28,45 These FOXP3+ cells suppress intratumoral CD4+ or CD8+ T cells either through TCR activation, cell contact, or by producing TGFβ.25,27,28 In a small series of FL cases purified using fluorescence-activated cell sorting, we found that CCL22 was one of the chemokine genes most highly expressed by the malignant B cells (R.D.G., unpublished observation, 2008). Moreover, Tregs have also been shown to induce alternatively activated macrophages, the so-called M2 type,46 which are also associated with protumoral immunity. Finally, in combination with CpG vaccination, antibody-mediated T cell modulation, mostly by depletion of Tregs through antifolate receptor 4 antibodies or functionally blocking them using anti-GITR, anti–cytotoxic T lymphocyte–associated antigen 4, or antiprogrammed death-ligand 1 antibodies, correlates with a significant reduction of tumor burden both in mouse models and a phase 2 clinical trial in patients with recurrent FL.47,48
In concordance with most published data, including mouse models and several different primary human tumors as well as in vitro and functional data in FL, where Tregs are associated with a protumoral immunity, we found a follicular pattern of FOXP3+ Tregs to be associated with inferior survival in FL. Importantly, this follicular pattern also predicted an increased risk of transformation. Superficially, this finding appears to contradict previous retrospective studies of the survival impact of Tregs in FL using TMAs and immunohistochemistry.8,31 However, in contrast to those studies, our analysis found the distribution of Tregs to be more important than their numbers. In addition, our patients received uniform therapy, thus holding this important variable constant. Recent data convincingly demonstrate the dramatically variable impacts that different therapies have on the nonneoplastic immune cells in the microenvironment.7,9,11,32 Indeed, studies of the microenvironment in FL become nearly impossible to interpret when patients receive very heterogeneous treatments, because the specific chemotherapeutic agents, radiation, and biologic agents differentially impact immune cells. As was shown in several recent series studying the impact of LAM in FL, the specific therapeutic regimens used decisively influenced the prognostic impact of nonneoplastic cells in the microenvironment.7,9-11,32
In FL, where the content and distribution of various immune-related cells is markedly heterogeneous, we reasoned that the topographic distribution of Tregs relative to the neoplastic follicle might reflect their function and thus their clinical impact. Qualitative (architectural patterns) or semiquantitative biomarkers (cell counts) such as those measured in this study are difficult to define precisely and may be difficult to reproduce.49 However, the use of TMAs and immunohistochemistry at least partially overcomes this difficulty and has been shown to provide a reliable strategy to assess large numbers of clinical samples and validate novel biomarkers discovered by gene expression profiling.7,50
In this study we analyzed Tregs within FL biopsies using 3 markers known to be characteristically expressed by Tregs: CD4, CD25, and FOXP3. As previously described, Tregs belong to the CD4+CD25+ T-cell subset. However, Tregs can be CD25−, and CD8+CD25+ T cells can exhibit regulatory activity.51 FOXP3 is the single best marker of activation of Tregs.21 Simple enumeration of cells identified using these markers showed no impact on survival or risk of transformation. However, we found that a follicular distribution of these CD25+ or/and FOXP3+ cells strongly correlates with outcome (Table 3). Interestingly, the survival associations in our cohort followed a pattern linked to specificity for the regulatory phenotype: CD4/8 pattern had an impact just on PFS, CD25 showed significance for PFS and OS, and FOXP3 had an impact on PFS, OS, and, importantly, risk of transformation.
This impact of either follicular or perifollicular FOXP3+ cells suggests a functional suppression of immunosurveillance within the tumor microenvironment that is present at the time of diagnosis. The malignant cells may have the capacity to “recruit” or “skew” Treg activity toward the suppression of antitumoral T cells, thus allowing the malignant cells to “escape” from the regulatory activity.37 Interestingly, 11 of 12 cases with a high content of LAMs in this series had a follicular FOXP3 pattern. Both Tregs and macrophages may cooperate, in a subset of cases, providing the neoplastic B cells with trophic and survival signals that promote tumor progression, the accumulation of further genetic damage, and transformation.
In summary, the immunoarchitectural pattern of Tregs within lymph nodes involved with follicular lymphoma predicts both survival and risk of transformation in patients treated uniformly with an aggressive combination regimen including multiagent chemotherapy (BP-VACOP) and involved region radiation. These findings require validation in other patient cohorts, but they will likely only be meaningful if treatment is held constant. Previous publications analyzing patients treated with heterogeneous regimens may have shown discrepant results because of the differential effect of these therapies on neoplastic B cells versus nonneoplastic immune cells in the microenvironment. Moreover, these findings will need to be validated in the current era of immunochemotherapy, again requiring uniform treatment and lengthy follow-up best performed in the context of phase 3 clinical trials.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank all of the physicians of the British Columbia Cancer Agency Center for Lymphoid Cancer for allowing us to include their patients, and Jane Donaldson and Suman Singh for help with data gathering and analysis. We thank Nadia Gale for technical expertise in performing immunohistochemistry. In addition, we greatly appreciate the cooperation from all of the pathologists throughout British Columbia for their support of the provincial lymphoma pathology program.
This work was supported in part by Terry Fox Foundation Program Project Award 019001 (R.D.G. and J.M.C.), the Turner Family Lymphoma Outcome Fund, the Mary Toye Memorial Fund, and unrestricted educational grants from Roche Canada, Berlex Canada, Berlex US, and AG Schering. A molecular pathology fellowship (P.F.) was also partially supported by the Canadian Institute of Health Research (CIHR STP-53912) and by the Fundação para a Ciência e Tecnologia (FCT BD13230/2003), Portugal.
Authorship
Contribution: P.F. designed and performed research, analyzed data, and wrote the paper.; A.A.-T., K.G., and R.K. designed and performed research; and J.M.C. and R.D.G. designed and performed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Randy D. Gascoyne, Department of Pathology and Advanced Therapeutics, Rm 5-113, BC Cancer Agency and BC Cancer Research Centre, 675 W 10th Ave, Vancouver, BC V5Z 1L3, Canada; e-mail: rgascoyn@bccancer.bc.ca.
References
Author notes
Presented in part at the 49th Annual Meeting of the American Society of Hematology, December 11, 2007, Atlanta, GA.