Abstract
Natural killer (NK) cells suppress graft-versus-host disease (GVHD) without causing GVHD themselves. Our previous studies demonstrated that allogeneic T cells and NK cells traffic similarly after allogeneic bone marrow transplantation (BMT). We therefore investigated the impact of donor NK cells on donor alloreactive T cells in GVHD induction. Animals receiving donor NK and T cells showed improved survival and decreased GVHD score compared with controls receiving donor T cells alone. Donor T cells exhibited less proliferation, lower CD25 expression, and decreased interferon-γ (IFN-γ) production in the presence of NK cells. In vivo, we observed perforin- and Fas ligand (FasL)–mediated reduction of donor T cell proliferation and increased T cell apoptosis in the presence of NK cells. Further, activated NK cells mediated direct lysis of reisolated GVHD-inducing T cells in vitro. The graft-versus-tumor (GVT) effect was retained in the presence of donor NK cells. We demonstrate a novel mechanism of NK cell–mediated GVHD reduction whereby donor NK cells inhibit and lyse autologous donor T cells activated during the initiation of GVHD.
Introduction
Allogeneic bone marrow transplantation (BMT) has proven to be an effective treatment for hematologic malignancies and some solid tumors.1 However, the high incidence of graft-versus-host disease (GVHD) as a complication of this treatment has limited the overall effectiveness of BMT.2 GVHD is mediated by the activation and proliferation of alloreactive T cells leading to tissue damage in the host, primarily in the gastrointestinal tract, liver, and skin. Thus, there is a need for novel strategies to suppress the development of GVHD, but maintain effective donor T cell–mediated immune responses to provide a graft-versus-tumor (GVT) effect
Previous murine studies have shown that natural killer (NK) cells can suppress the development of GVHD while inducing an antitumor response. The primary effector function of NK cells is to eliminate susceptible target cells and amplify the antitumor immune response by direct cellular lysis and cytokine production,3 and in an allogeneic murine model of BMT, this effect appeared to be due in part to transforming growth factor-β.4,5 Previous studies demonstrated that NK cell lysis of host antigen-presenting cells (APCs) can suppress development of GVHD by ablating the host APCs, which are critical for donor T cell activation in GVHD induction.6
The spatial and temporal dynamics of alloreactive T cell activation, proliferation, and tissue distribution in GVHD are such that the first several days after T cell transplantation are critical in GVHD induction.7,8 T cell activation and proliferation in the lymphoid organs occurs during the first 3 to 4 days after transplantation, followed by migration into target tissues such as the gastrointestinal tract and skin, resulting in tissue damage. The pathophysiology of acute GVHD has been described in 3 major phases, with the second phase of donor T cell activation, proliferation, and differentiation being most critical for the T cell–mediated effects of GVHD.9 NK cell trafficking after BMT shares many spatial and temporal characteristics with that of T cells as NK cells traffic to and proliferate in lymphoid organs, and also reach GVHD target tissues.10 However, the proliferation and in vivo persistence of NK cells is markedly shorter than that of T cells.
Recent data have demonstrated that in addition to their classical role in providing potent antitumor and antiviral immunity, NK cells also have the ability to regulate the T cell arm of the adaptive immune response. In vitro experiments using murine and human cells have demonstrated lysis of activated T cells by autologous NK cells.11,12 These studies demonstrated that up-regulation of NKG2D ligands on activated T cells renders them susceptible to NK-mediated lysis. NKG2D, an activating receptor expressed on a majority of NK cells,13 binds to ligands typically up-regulated on stressed, transformed, or tumor cells.13–16 NK cells also mediate direct cellular lysis by perforin and granzymes, as well as through Fas ligand (FasL) and tumor necrosis factor–related apoptosis-inducing ligand–mediated mechanisms.17–19 Because of the similar trafficking pattern of donor T cells and NK cells after BMT, as well as these recent data indicating that activated T cells are susceptible to NK cell–mediated lysis, we hypothesized that donor NK cells may have a direct impact in vivo on alloreactive T cells in GVHD induction.
Our results demonstrate that donor NK cells regulate syngeneic donor T cells within the allogeneic host at critical stages of T cell activation and proliferation, resulting in reduced severity and delayed progression of GVHD. We show in vivo evidence for the direct lysis of activated, alloreactive GVHD-inducing T cells by activated, autologous donor NK cells. These findings demonstrating a regulatory role of NK cells constitute a novel mechanism of NK cell–mediated reduction of GVHD.
Methods
Mice
FVB/N (H-2q), BALB/c (H-2d), and C57Bl/6 (H-2b, CD45.2) mice from The Jackson Laboratory or Charles River Laboratories, and Fas ligand−/− (FasL−/−), perforin−/−, and interferon-γ−/− (IFN-γ−/−) mice from The Jackson Laboratory, were used between 7 and 16 weeks of age. All experiments were performed with sex-matched animals. Luciferase-expressing (luc+) FVB/N L2G85 mice and C57Bl/6 (H-2b, CD45.1, luc+) mice were created as previously described.20,21 Animal protocols were approved by the Institutional Animal Care and Use Committee at Stanford University.
Flow cytometric analysis
The following antibodies were obtained from BD Pharmingen, eBioscience, and Biolegend: unconjugated CD16/32, CD4 (GK1.5), CD8 (53-6.7), CD45.1 (A20), interleukin-2 (IL-2; JES6-5H4), IFN-γ (XMG1.2), CD25 (PC61), CD69 (H1.2F3), Foxp3 (FJK-16S), Rae1γ (CX1), NKG2D blocking antibody (CX5), and immunoglobulin isotype control antibody (rat immunoglobulin G1). For dead cell analysis, propidium iodide (PI) was used for freshly isolated cells, and ethidium monoazide (Molecular Probes) or Live/Dead Fixable Dead Cell Staining kit (Invitrogen) was used for fixed cells. Analysis was performed on a 4-laser LSRII or 2-laser LSR (Becton Dickinson).
Cell isolation
Splenocytes harvested from donor mice were processed in phosphate-buffered saline (PBS; Invitrogen) with 2% fetal calf serum (FCS; Invitrogen). After red blood cell lysis, cells were blocked with CD16/32, stained with anti-DX5 microbeads (Miltenyi Biotec), and positively selected by manual magnetic-activated cell sorting column selection (Miltenyi Biotec). The DX5+ fraction was sorted for DX5+CD3−PI− cells on a fluorescence activated cell sorter (FACS) Aria II or MoFlow system to greater than 95% purity. Purified NK cells were cultured at 3 × 106 cells per 5 mL of cRPMI with 10% FCS, 2 mM l-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin (all from Invitrogen), and 5 μg/mL 2-mercaptoethanol (Sigma-Aldrich) with 1500 units recombinant human interleukin-2 (IL-2; Chiron) for 48 hours. Bone marrow was flushed from femurs and tibias, and conventional wild-type and luc+ T cells were isolated from spleen, mesenteric lymph nodes (mLNs), and peripheral lymph nodes (pLNs, including inguinal, cervical, and axillary lymph nodes) from donor mice. Cells were stained with anti-CD4+ and CD8+ beads (Miltenyi Biotec) and prepared by magnetic-activated cell sorting–negative or –positive column selection, respectively.
GVHD model
Recipient mice were irradiated with 8 Gy (BALB/c) split into 2 doses of 4 Gy, 4 hours apart. To induce GVHD, irradiated recipients received 5 × 106 donor T cell–depleted bone marrow (TCD-BM) cells on day 0 and 0.8 to 1 × 106 T cells from either FVB/N or C57Bl/6 donors on day 2. Some groups received 1 × 106 highly purified IL-2 cultured or fresh (Figure 2A) donor NK cells on day 0. Mice were kept in autoclaved cages and on antibiotic water (sulfamethoxazole and trimethoprim; Hi-Tech Pharmacal Co Inc) for a minimum of 30 days after transplantation. Animals were humanely sacrificed when they exhibited the euthanasia GVHD criteria.
In vivo and bioluminescence imaging
In vivo bioluminescence imaging was performed as previously described22 with an IVIS100 charge-coupled device imaging system (Xenogen). Images were analyzed with Living Image Software 2.5 (Xenogen) and Igor Pro Carbon (Wavemetrics).
CFSE proliferation analysis
Donor CD4+ and CD8+ cells were labeled with 5 μM Vybrant carboxyfluorescein diacetate, succinimidyl ester Tracer kit (Molecular Probes) in PBS with 2% FCS for 6 minutes at 37°C. The labeling reaction was quenched by addition of cold RPMI (Invitrogen) with 10% FCS, and cells were washed twice with PBS with 2% FCS to remove excess carboxyfluorescein succinimidyl ester (CFSE).
Tumor model
The Balb/c B-cell lymphoma A20 luc+/yfp cell line was created as described.22 Recipient mice were lethally irradiated and received 5 × 106 T cell–depleted bone marrow cells along with 1 × 105 A20 luc+/yfp cells intravenously. One day after irradiation, some groups received 1 × 106 IL-2–activated NK cells, and 2 days later some groups received 1 × 106 T cells. Tumor growth was assessed by bioluminescence imaging (BLI).
51Cr release cytotoxicity assay
Spleen and lymph node cells were reisolated 5 days after T-cell transplantation and were positively selected with CD4+ and CD8+ microbeads and activated in vitro on anti-CD3–coated plates in cRPMI for 48 hours. T cells and A20 control targets were labeled with 300 μCi (11.1 MBq) 51Cr overnight at 37°C and 5% CO2. 51Cr release cytotoxicity assay and antibody blocking was performed as previously described with IL-2–activated NK effector cells at various effector to target ratios.23
Analysis of apoptosis
CD4+ and CD8+ cells reisolated from spleen and lymph nodes of mice that underwent transplantation were fixed and permeabilized (eBioscience), and stained with fluorescein-tagged deoxyuridine triphosphate (dUTP; terminal deoxynucleotidyl transferase dUTP nick-end labeling [TUNEL] method), according to the manufacturer's instructions (In Situ Cell death kit; Roche Diagnostics).
Intracellular cytokine staining
Donor T cells were reisolated from spleen and lymph nodes of mice that underwent transplantation, and stimulated with phorbol myristate acetate (40 ng/mL), ionomycin (2μM), and monensin (2μM; all from Sigma-Aldrich), for 5 hours at 37° and 5% CO2 in complete RPMI. Unstimulated controls were incubated with monensin only. Cells were fixed and permeabilized according to the manufacturer's instructions (BD Biosciences), and intracellularly stained for IL-2 and IFN-γ. Percentage of cells secreting cytokine was calculated by (% of stimulated cells) − (% of unstimulated cells) positive for each cytokine.
Intracellular staining for Foxp3 was performed using Foxp3 fixation and permeabilization buffers (eBioscience) according to the manufacturer's instructions.
Statistical analysis
Differences in GVHD score, luc+ conventional T cell (Tcon) proliferation measured by BLI in vivo and CFSE in vitro, CD25 expression, CD4+ and CD8+ T cell numbers, mean fluorescence intensity (MFI) of TUNEL and Rae1γ, and percentage of specific lysis were analyzed using the 2-tailed Student t test, and animal survival was analyzed using the log-rank test (Graphpad Prism). BLI differences among NK cell effector molecule knockouts were analyzed using a 1-way analysis of variance and Dunnett multiple comparison test (GraphPad Prism).
Results
NK cells reduce GVHD severity and prolong survival in a model of major histocompatibility complex–mismatched BMT
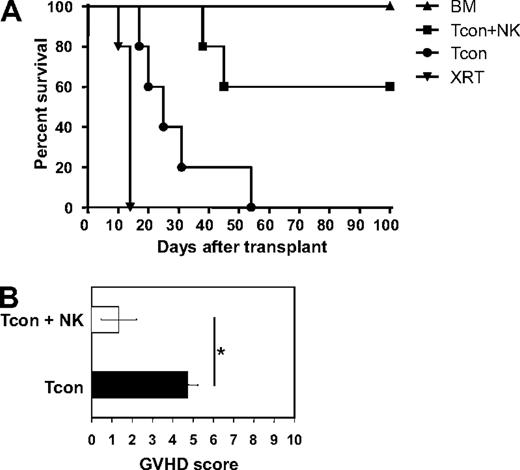
Previous reports have demonstrated the ability of NK cells to suppress GVHD in a major histocompatibility complex–mismatched allogeneic bone marrow transplantation setting.4,5 To investigate this further, donor DX5+CD3− NK cells were sorted to high purity (> 95%), cultured in IL-2 for 2 days, and infused into lethally irradiated allogeneic recipients along with T cell–depleted bone marrow. GVHD was induced by infusing donor CD4+ and CD8+ conventional T cells (Tcons) on day 2 after transplantation. As the donor Tcons were isolated from the same donor strain as the NK cells, the 2 populations are syngeneic, but both are allogeneic to the host. Compared with mice treated with T cells (Tcon) only, mice treated with NK cells in addition to T cells (Tcon + NK) survived significantly longer (P < .05; Figure 1A). Survival of allogeneic bone marrow control mice was 100%. The Tcon group had lower average body weights (data not shown) and also had higher GVHD scores (4.8 ± 0.5 for Tcon compared with 1.3 ± 0.9 for Tcon + NK [P < .05]) when assessed for criteria of GVHD progression, including diarrhea, fur loss, hunched posture, and inflammation24 (Figure 1B). Some of the Tcon + NK mice did succumb and had some evidence of GVHD development (mild fur loss and inflammation), but the progression of disease was reduced and symptoms were less severe. T cell–depleted bone marrow (TCD-BM) control animals regained their body weight and did not show any signs of clinical GVHD, as expected.
Donor NK cells improve survival and reduce GVHD severity after allogeneic BMT. (A) Donor natural killer (NK) cells prolong survival of mice receiving donor conventional T cells (Tcon + NK) compared with Tcon only (Tcon). Mice were sacrificed because of morbidity according to euthanasia criteria at the indicated time points. Mice receiving irradiation plus T cell–depleted bone marrow (TCD-BM) only and mice receiving irradiation only (XRT) are shown as controls. Tcon + NK animals had significantly prolonged survival compared with Tcon mice (P < .05). Data are from 5 mice per group, and are representative of 3 experiments. (B) Mice were scored for GVHD symptoms after transplantation. Tcon + NK mice had significantly lower average graft-versus-host disease (GVHD) scores than Tcon mice (*P < .05). Data are shown for day 20 after T-cell transplantation, and are representative of 2 experiments.
Donor NK cells improve survival and reduce GVHD severity after allogeneic BMT. (A) Donor natural killer (NK) cells prolong survival of mice receiving donor conventional T cells (Tcon + NK) compared with Tcon only (Tcon). Mice were sacrificed because of morbidity according to euthanasia criteria at the indicated time points. Mice receiving irradiation plus T cell–depleted bone marrow (TCD-BM) only and mice receiving irradiation only (XRT) are shown as controls. Tcon + NK animals had significantly prolonged survival compared with Tcon mice (P < .05). Data are from 5 mice per group, and are representative of 3 experiments. (B) Mice were scored for GVHD symptoms after transplantation. Tcon + NK mice had significantly lower average graft-versus-host disease (GVHD) scores than Tcon mice (*P < .05). Data are shown for day 20 after T-cell transplantation, and are representative of 2 experiments.
Reduced CD4+ and CD8+ donor T-cell proliferation in the presence of NK cells
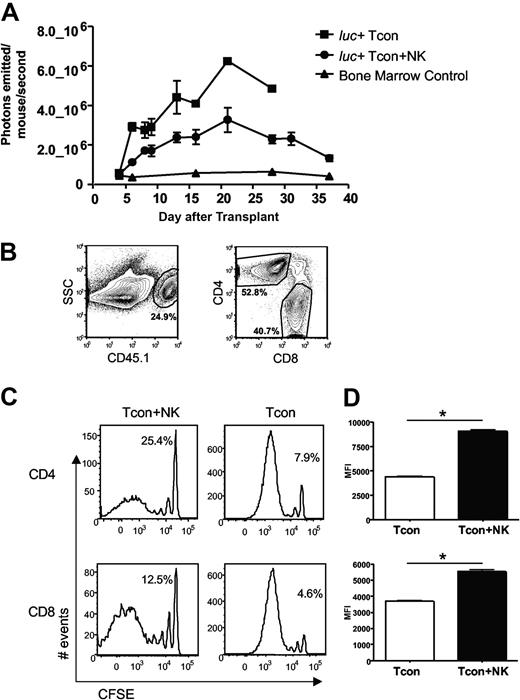
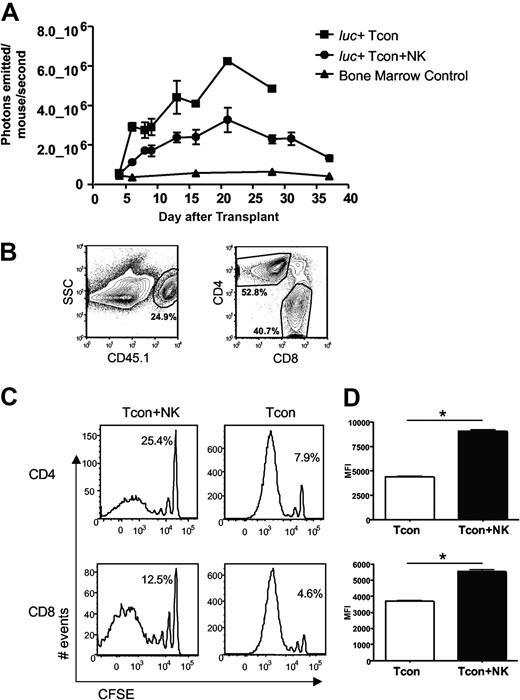
Previous studies demonstrating suppression of GVHD by NK cells have postulated that a mechanism for this observation is donor NK cell–mediated lysis of host APCs, which would otherwise prime donor T cells.5 Previous studies from our laboratory have shown that NK cells and T cells from the same donor home to the same tissues after transplantation in an allogeneic host, including lymphoid organs and GVHD target tissues.10 Thus, we investigated whether the observed reduction in GVHD was due to NK cell regulation of the T cell response. Donor Tcons were isolated from an FVB L2G85 luciferase-transgenic donor mouse to allow in vivo bioluminescence imaging (BLI) of the transplanted T cells in allogeneic recipient mice.20 Quantification of total photons emitted per mouse revealed a significantly decreased bioluminescent signal from donor Tcons in the presence of NK cells (P < .05, Figure 2A). To determine whether this decrease in BLI signal was due to reduced proliferation or reduced accumulation of donor T cells, CFSE-labeled donor Tcons from congenic C57Bl/6 CD45.1+ donor mice were used (Figure 2B). CFSE dilution profiles of donor Tcons reisolated from peripheral lymph nodes (pLNs) on day 4 after T cell transplantation revealed fewer dividing T cells in the Tcon + NK mice compared with the Tcon mice (Figure 2C). Similar results were seen with bromodeoxyuridine incorporation of donor Tcons (data not shown). The percentage of cells in the undivided peak was greater in the Tcon + NK group (Figure 2C). Among donor Tcons, 25% of CD4+ and 13% of CD8+ were undivided in Tcon + NK animals, and 8% and 5%, respectively, were undivided in Tcon animals. The difference in proliferation was more pronounced among the CD4+ T cell subset. In addition, the mean fluorescence intensity of CFSE was significantly lower in the Tcon group, indicating more extensive proliferation of the CD4+ and CD8+ cells (P < .001, Figure 2D). These results demonstrate reduced donor Tcon proliferation in the presence of donor NK cells.
Donor Tcon proliferation is reduced in the presence of donor NK cells. (A) Average photons emitted from luc+ donor T cells in animals receiving TCD-BM, TCD-BM plus luc+ Tcons, or TCD-BM plus luc+ Tcons and donor NK cells; n = 4-5 animals per group, representative of 2 experiments. Signal intensity is higher in luc+ Tcon compared with luc+ Tcon + NK animal (P < .05, day 6). Error bars represent SE. (B) Donor Tcons reisolated from spleen and lymph nodes of mice that underwent transplantation are gated on the donor marker CD45.1, and CD4 and CD8. (C) CFSE histograms of donor Tcons reisolated from pLN on day 4 in the presence (Tcon + NK) or absence (Tcon) of donor NK cells. More CD4+ and CD8+ cells have divided in the Tcon group. Percentage of undivided cells is indicated. One representative FACS of 4 pooled mice is shown, representative of 2 independent experiments. (D) Mean fluorescence intensity of CFSE staining for CD4+ (top) and CD8+ (bottom) cells reisolated from pLN on day 4 in the presence (Tcon + NK) or absence (Tcon) of donor NK cells. A significantly lower mean fluorescence intensity (MFI) (*P < .001 for CD4+ and CD8+) was observed in Tcon groups.
Donor Tcon proliferation is reduced in the presence of donor NK cells. (A) Average photons emitted from luc+ donor T cells in animals receiving TCD-BM, TCD-BM plus luc+ Tcons, or TCD-BM plus luc+ Tcons and donor NK cells; n = 4-5 animals per group, representative of 2 experiments. Signal intensity is higher in luc+ Tcon compared with luc+ Tcon + NK animal (P < .05, day 6). Error bars represent SE. (B) Donor Tcons reisolated from spleen and lymph nodes of mice that underwent transplantation are gated on the donor marker CD45.1, and CD4 and CD8. (C) CFSE histograms of donor Tcons reisolated from pLN on day 4 in the presence (Tcon + NK) or absence (Tcon) of donor NK cells. More CD4+ and CD8+ cells have divided in the Tcon group. Percentage of undivided cells is indicated. One representative FACS of 4 pooled mice is shown, representative of 2 independent experiments. (D) Mean fluorescence intensity of CFSE staining for CD4+ (top) and CD8+ (bottom) cells reisolated from pLN on day 4 in the presence (Tcon + NK) or absence (Tcon) of donor NK cells. A significantly lower mean fluorescence intensity (MFI) (*P < .001 for CD4+ and CD8+) was observed in Tcon groups.
T-cell activation and proinflammatory cytokine secretion are reduced in the presence of NK cells
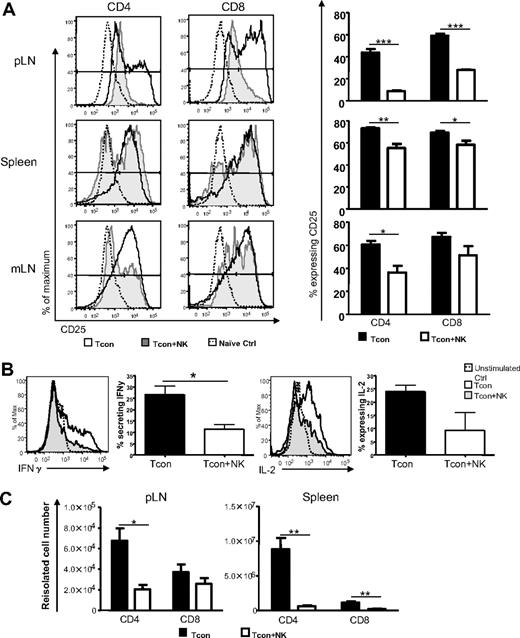
In addition to T cell proliferation, T cell activation and differentiation are hallmarks of the efferent phase of GVHD induction.9 Therefore, we assessed the expression of activation markers and secretion of proinflammatory cytokines among donor Tcons to further assess the impact of donor NK cells. The T cell activation marker CD25 was expressed on a significantly smaller percentage of T cells from Tcon + NK–treated mice than from Tcon-treated mice in the lymph nodes and spleen on day 4 after T cell transplantation (for CD4 and CD8, respectively, P < .01 and P < .05 in spleen, P < .05 and not significant [NS] in mLN, and P < .001 for both cell types in the pLN; Figure 3A). Foxp3+ cells were excluded from the analysis to eliminate the possibility of effects of the regulatory T cell subset. The difference in CD25 expression between the groups was more marked among CD4+ cells. Similar results were seen for the early activation marker CD69 (data not shown). The secretion of Th1 proinflammatory cytokines by donor Tcons has been implicated in GVHD progression.25 Interleukin-2 (IL-2) amplifies GVHD and is secreted primarily by CD4+ T cells26 and during GVHD T cells secrete interferon-γ (IFN-γ), which can up-regulate molecules for antigen presentation9,27 and mediate intestinal and skin damage.28,29 Intracellular cytokine staining for proinflammatory cytokines from donor Tcons revealed a greater percentage of CD4+ cells from the spleens of mice that underwent transplantation secreting IFN-γ at day 3 after T cell transplantation (Figure 3B; IFN-γ P < .05). There was also a trend toward a greater percentage of cells from Tcon animals secreting IL-2 compared with Tcon + NK animals at this time point, but these data were not statistically significant. This difference was not observed at later time points after T cell transplantation (day 10, data not shown), indicating that the primary impact of the NK cells on T cell cytokine secretion occurs within the first few days of GVHD induction. The absolute numbers of donor Tcons reisolated from pLN and spleen were quantified on day 4 after transplantation, and significantly reduced T cell numbers were seen among CD4+ and CD8+ populations in the Tcon + NK mice, with this difference greater among the CD4+ subset (for CD4 and CD8, respectively, P < .05 and NS for pLN; P < .01 for CD4+ and CD8+ from spleen; Figure 3C). These data indicate that the transfer of NK cells results in reduced numbers of activated alloreactive T cells.
NK cells reduce activated and IFN-γ–secreting donor T cells. (A) CD25 expression on CD45.1+ Foxp3− and CD4+ or CD8+ donor T cells reisolated from spleen, pLN, or mLN of Tcon (white histograms) or Tcon + NK mice (gray histograms) on day 4, or a naive control mouse (dotted line). Percentage of T cells from Tcon or Tcon + NK animals expressing CD25 is quantified in the bar graphs. CD25 expression is reduced in Tcon + NK animals in the spleen, mLN, and pLN (*P < .05, **P < .01, ***P < .001). (B) Intracellular cytokine staining demonstrates decreased IFN-γ secretion from Tcon + NK (gray histograms) compared with Tcon (white histograms) animals in the spleen on day 3. Graphic representation of summary statistics with SE appear to the right of histograms (*P < .05, IL-2 was NS). Unstimulated staining controls are depicted in the dotted lines. (C) Quantification of absolute cell numbers reisolated from Tcon and Tcon + NK animals on day 4. Donor CD4+ and CD8+ numbers are reduced in Tcon + NK mice from pLN and spleen (*P < .05, **P < .01). For all, data are averaged from 3 to 4 mice and are representative of 2 to 3 independent experiments. Error bars represent SE.
NK cells reduce activated and IFN-γ–secreting donor T cells. (A) CD25 expression on CD45.1+ Foxp3− and CD4+ or CD8+ donor T cells reisolated from spleen, pLN, or mLN of Tcon (white histograms) or Tcon + NK mice (gray histograms) on day 4, or a naive control mouse (dotted line). Percentage of T cells from Tcon or Tcon + NK animals expressing CD25 is quantified in the bar graphs. CD25 expression is reduced in Tcon + NK animals in the spleen, mLN, and pLN (*P < .05, **P < .01, ***P < .001). (B) Intracellular cytokine staining demonstrates decreased IFN-γ secretion from Tcon + NK (gray histograms) compared with Tcon (white histograms) animals in the spleen on day 3. Graphic representation of summary statistics with SE appear to the right of histograms (*P < .05, IL-2 was NS). Unstimulated staining controls are depicted in the dotted lines. (C) Quantification of absolute cell numbers reisolated from Tcon and Tcon + NK animals on day 4. Donor CD4+ and CD8+ numbers are reduced in Tcon + NK mice from pLN and spleen (*P < .05, **P < .01). For all, data are averaged from 3 to 4 mice and are representative of 2 to 3 independent experiments. Error bars represent SE.
NK cells increase the ratio of splenic donor Tregs to total donor Tcons
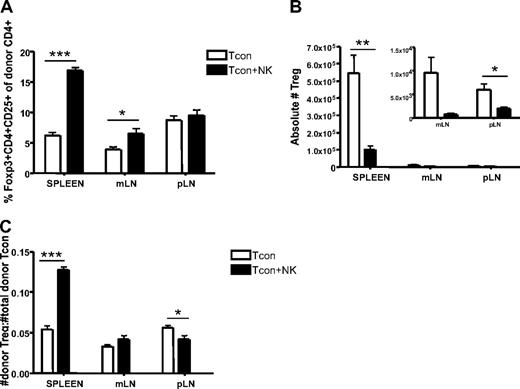
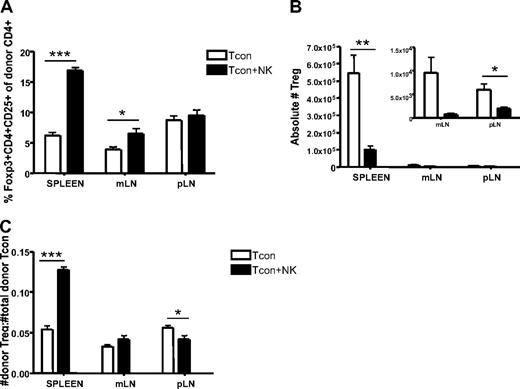
Although regulatory T cells (Tregs) were excluded from the analysis shown in Figure 3 to clearly identify the impact of donor NK cells on donor CD4+ T cells, the question remains whether NK cells would have the same effects on the Treg population as they do on the Tcon population. We therefore compared the percentage of CD4+CD25+Foxp3+ cells reisolated from Tcon and Tcon + NK groups. The spleen and mLN of Tcon + NK animals contained a greater percentage of Tregs (P < .001 and P < .05, respectively; Figure 4A). However, there were still significantly greater absolute numbers of Tregs reisolated from spleen and lymph nodes of Tcon animals, compared with Tcon + NK (P < .01 for spleen, NS for mLN, and P < .05 for pLN; Figure 4B). This suggests that donor Tregs are being decreased by donor NK cells, consistent with our findings of decreased CD4+ and CD8+ cell numbers in Figure 3C, but to a lesser extent than conventional T cells, explaining the increased percentage of Tregs seen in Figure 4A. To address this, we calculated a ratio of donor Tregs to total donor T cells. We found that this ratio was significantly greater in Tcon + NK animals in the spleen and pLN (P < .001 and P < .05, respectively; Figure 4C) but not in the mesenteric lymph node. Taken together, these results suggest that NK cells may increase the ratio of Tregs to donor CD4+ cells, a finding that may be responsible in part for the action in reducing GVHD.
Increased ratio of splenic donor Tregs to total donor Tcons in the presence of NK cells. (A) The percentage of donor Tregs (CD45.1+CD4+CD25+Foxp3+) reisolated from Tcon + NK groups was significantly higher in spleen and mLN (***P < .001 and *P < .05, respectively; pLN was NS) compared with Tcon. (B) Absolute donor Treg cell numbers were higher in the Tcon group compared with the Tcon + NK group (spleen, **P < .01; mLN, NS; pLN, *P < .05). Inset shows the same mLN and pLN data as large graph at higher magnification to visualize relative differences. (C) Ratios of donor Tregs/total donor Tcons (CD4+ and CD8+) were significantly higher in Tcon + NK animals than Tcon animals in the spleen (***P < .001). Results were mixed in the lymph node (mLN, NS; pLN, *P < .05). All data are from day 4 after T-cell transplantation, are averaged from 4 mice, and are representative of 3 independent experiments.
Increased ratio of splenic donor Tregs to total donor Tcons in the presence of NK cells. (A) The percentage of donor Tregs (CD45.1+CD4+CD25+Foxp3+) reisolated from Tcon + NK groups was significantly higher in spleen and mLN (***P < .001 and *P < .05, respectively; pLN was NS) compared with Tcon. (B) Absolute donor Treg cell numbers were higher in the Tcon group compared with the Tcon + NK group (spleen, **P < .01; mLN, NS; pLN, *P < .05). Inset shows the same mLN and pLN data as large graph at higher magnification to visualize relative differences. (C) Ratios of donor Tregs/total donor Tcons (CD4+ and CD8+) were significantly higher in Tcon + NK animals than Tcon animals in the spleen (***P < .001). Results were mixed in the lymph node (mLN, NS; pLN, *P < .05). All data are from day 4 after T-cell transplantation, are averaged from 4 mice, and are representative of 3 independent experiments.
Increased donor T-cell apoptosis and perforin- and FasL-mediated reduction of donor Tcons in the presence of donor NK cells
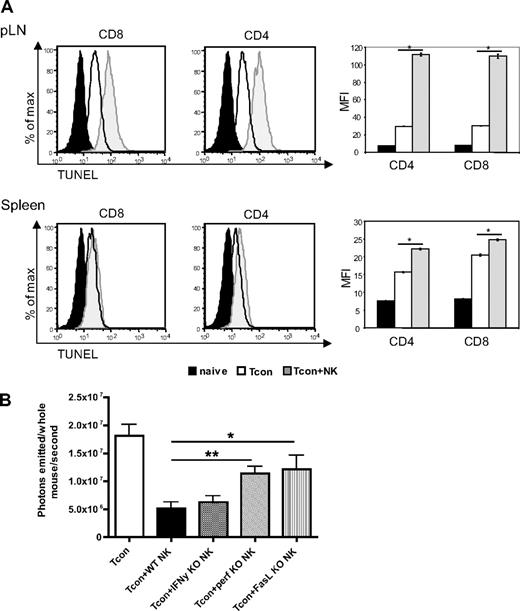
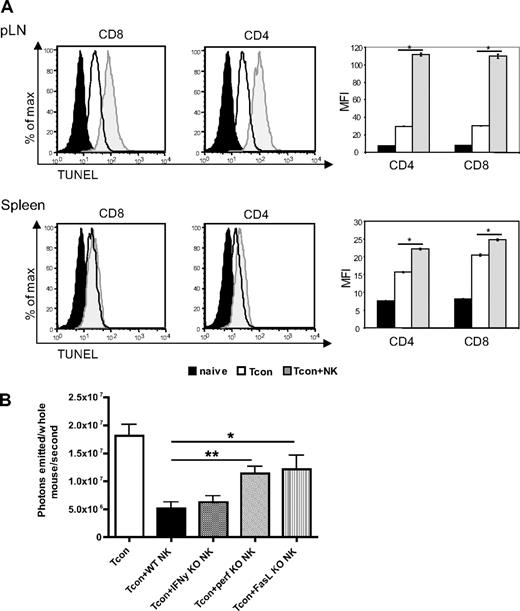
We hypothesized that the effects of NK cells on the GVHD T cell response could be due in part to a direct killing of donor T cells by donor NK cells. To address this, we assessed the extent of apoptosis among donor T cells in secondary lymphoid organs, the priming sites of alloreactive T cells. We observed an increase in terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) staining on T cells reisolated from Tcon + NK–treated mice compared with Tcon-treated mice on day 4 after transplantation (Figure 5A). This increase was significant in the lymph nodes (P < .001) and in the spleen (P < .001), but mean fluorescence values were much higher in the lymph nodes. This was observed in both the CD4+ and CD8+ populations.
Increased donor T-cell apoptosis in lymphoid organs and perforin- and FasL-mediated reduction of donor Tcons in the presence of donor NK cells. (A) FACS-based TUNEL staining on reisolated donor Tcons on day 4 after transplantation. Histograms indicate CD8+ and CD4+ reisolated from pLNs and spleens from Tcon + NK animals (gray filled histograms) stained significantly higher for TUNEL than the Tcon group (white filled histograms) group. TUNEL staining on freshly isolated T cells is shown as a control (black filled histograms). Quantification of the mean fluorescence intensity (right panels) shows significantly increased TUNEL stain in the Tcon + NK group (*P < .001). Error bars indicate SE. Data are pooled from 3 mice and are representative of 3 independent experiments. (B) Average photons emitted from animals receiving luc+ Tcon together with NK cells from wild-type donors or donors deficient for either IFNγ (IFN-γ KO), perforin (perf KO), or FasL (FasL KO) on day 4 after T-cell transplantation. Bioluminescent signal was significantly greater in animals receiving perforin−/− or FasL−/− NK cells compared with wild-type NK cells, implicating these molecules in the mechanism of NK cell–mediated reduction of donor Tcons (*P < .05, **P < .01). One-way analysis of variance was also significant (P = .001) and Dunnett multiple comparison test showed that only the Tcon + WT NK and Tcon + perf KO NK groups were significantly different from control. Data are an average of 5 mice per group, and are representative of 2 experiments.
Increased donor T-cell apoptosis in lymphoid organs and perforin- and FasL-mediated reduction of donor Tcons in the presence of donor NK cells. (A) FACS-based TUNEL staining on reisolated donor Tcons on day 4 after transplantation. Histograms indicate CD8+ and CD4+ reisolated from pLNs and spleens from Tcon + NK animals (gray filled histograms) stained significantly higher for TUNEL than the Tcon group (white filled histograms) group. TUNEL staining on freshly isolated T cells is shown as a control (black filled histograms). Quantification of the mean fluorescence intensity (right panels) shows significantly increased TUNEL stain in the Tcon + NK group (*P < .001). Error bars indicate SE. Data are pooled from 3 mice and are representative of 3 independent experiments. (B) Average photons emitted from animals receiving luc+ Tcon together with NK cells from wild-type donors or donors deficient for either IFNγ (IFN-γ KO), perforin (perf KO), or FasL (FasL KO) on day 4 after T-cell transplantation. Bioluminescent signal was significantly greater in animals receiving perforin−/− or FasL−/− NK cells compared with wild-type NK cells, implicating these molecules in the mechanism of NK cell–mediated reduction of donor Tcons (*P < .05, **P < .01). One-way analysis of variance was also significant (P = .001) and Dunnett multiple comparison test showed that only the Tcon + WT NK and Tcon + perf KO NK groups were significantly different from control. Data are an average of 5 mice per group, and are representative of 2 experiments.
To determine which NK cell effector molecules are responsible for the increased apoptosis in donor T cells, we used NK cells from perforin, FasL, or IFN-γ knockout donors. NK cells isolated from either perforin- (P < .01) or FasL- (P < .05) deficient animals demonstrated an impaired ability to decrease Tcon proliferation compared with wild-type donor NK cells, as a significantly greater BLI signal was seen in these animals at day 4 after Tcon transplantation, a critical time point in GVHD induction (Figure 5B). This indicates that both perforin and FasL, but not IFN-γ, are important for the regulatory role of NK cells in this GVHD model. The significantly higher BLI signal in Tcon animals compared with Tcon + NK corroborates the increase in TUNEL staining at day 4 shown in Figure 5A by an independent technique, and demonstrates the role of specific molecules in the impact of NK cells on Tcons. As expected, neither effector molecule was solely responsible for the reduction of BLI signal.
Direct in vitro NK cell–mediated lysis of activated donor T cells
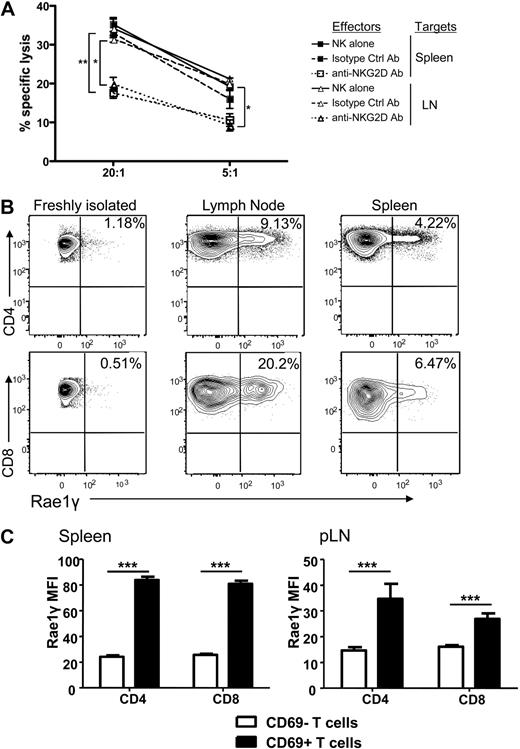
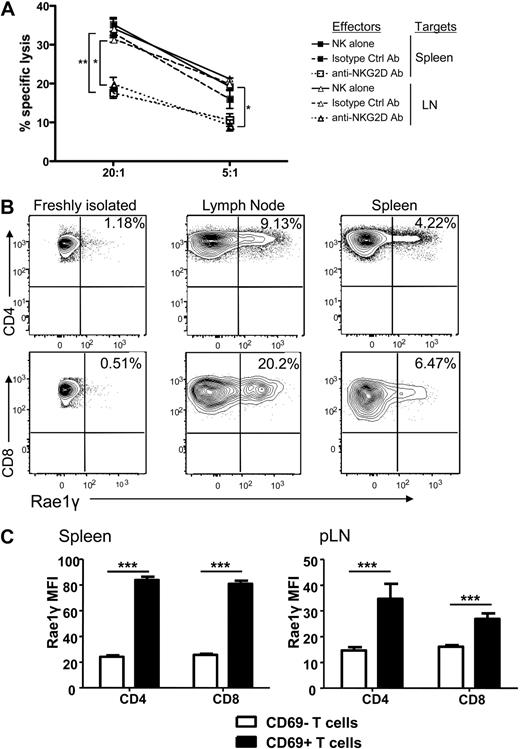
The observed reduction of T cell proliferation, activation, and cytokine secretion as well as increased T cell apoptosis could theoretically be explained by the previously described mechanism of NK cell elimination of APCs,5 thus impacting the allogeneic T cell response indirectly. We therefore determined whether there was direct cellular lysis of the activated donor Tcons by activated syngeneic NK cells. Donor T cells were reisolated from spleen and lymph nodes on day 5 after transplantation and used as targets in a chromium release killing assay. We observed specific lysis of the reisolated donor T cells by donor NK cells (Figure 6A). This effect was decreased in the presence of a blocking antibody for the activating receptor NKG2D compared with an isotype control antibody (P < .001 for spleen and P < .01 for lymph node reisolated cells at a 20:1 effector to target ratio, and P = NS and P < .01 at a 5:1 E/T ratio). Lysis of the NK cell–sensitive A20 target cells and T cells from naive mice served as controls (data not shown).
NK cells directly lyse activated, donor Tcons in an NKG2D-dependent manner. (A) Activated NK cells alone (solid line) or incubated with isotype control antibody (dashed line) lysed donor Tcon reisolated from spleen and lymph node of transplant recipients. Lysis was significantly reduced with NKG2D-blocking antibody at a 20:1 effector to target ratio and for LN at a 5:1 E/T ratio (*P < .01, **P < .001, 5:1 spleen was NS). Data are an average of triplicates. (B) Expression of the NKG2D ligand Rae1γ was higher on donor CD4+ and CD8+ cells reisolated from lymph node and spleen on day 5 than naive, freshly isolated T cells as detected by FACS analysis. Data are representative of 3 experiments. (C) The MFI of Rae1γ NKG2D ligand expression was significantly higher on activated CD69+ than CD69− CD4+ and CD8+ cells from spleen and lymph node (***P < .001). Data are pooled from 3 mice per group and are representative of 2 experiments.
NK cells directly lyse activated, donor Tcons in an NKG2D-dependent manner. (A) Activated NK cells alone (solid line) or incubated with isotype control antibody (dashed line) lysed donor Tcon reisolated from spleen and lymph node of transplant recipients. Lysis was significantly reduced with NKG2D-blocking antibody at a 20:1 effector to target ratio and for LN at a 5:1 E/T ratio (*P < .01, **P < .001, 5:1 spleen was NS). Data are an average of triplicates. (B) Expression of the NKG2D ligand Rae1γ was higher on donor CD4+ and CD8+ cells reisolated from lymph node and spleen on day 5 than naive, freshly isolated T cells as detected by FACS analysis. Data are representative of 3 experiments. (C) The MFI of Rae1γ NKG2D ligand expression was significantly higher on activated CD69+ than CD69− CD4+ and CD8+ cells from spleen and lymph node (***P < .001). Data are pooled from 3 mice per group and are representative of 2 experiments.
Because of the impact of the NKG2D blocking antibody, we examined expression of NKG2D ligands on donor T cells reisolated from mice that underwent transplantation. Up-regulation of the ligand Rae1γ was found on CD4+ and CD8+ donor T cells reisolated from the spleen and lymph nodes (Figure 6B), with the mean fluorescence intensity of the NKG2D ligand Rae1γ expression found to be higher on activated (CD69+) than nonactivated (CD69−) T cells in both the spleen and lymph node (P < .001; Figure 6C). This indicates that upon activation, NKG2D ligands are up-regulated on activated donor T cells in GVHD priming sites in mice that underwent transplantation. This represents one potential, additional mechanism by which T cells are rendered susceptible to autologous, donor NK cell–mediated lysis.
GVT response is preserved in the presence of allogeneic NK cells
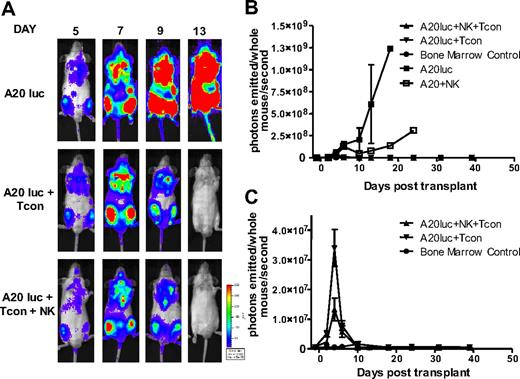
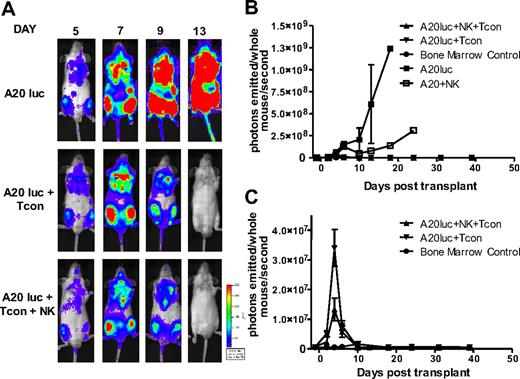
In addition to exploring the potential of NK cells to suppress GVHD, we sought to evaluate their antitumor activity in the same model. As NK cells have inherent antitumor potential, they may be able to destroy remaining cancer cells after chemotherapy or irradiation.30 To exclude the possibility that regulation of alloreactive T cells leads to a diminished GVT response, lethally irradiated mice received a transplant of a luciferase (luc+)/yfp-expressing B-cell lymphoma (A20) as well as rescue bone marrow, and were treated with NK cells plus Tcons, or Tcons alone to measure the GVT activity. Tumor progression was assessed by in vivo BLI. All groups had an early expansion of the tumor, which engrafts in the bone marrow cavities of recipient animals. In contrast to the steady progression of A20 tumor growth in TCD-BM control–treated animals, both the Tcon and Tcon + NK groups cleared the tumor within 2 weeks after transplantation (Figure 7A). This is mirrored in the quantification of the BLI images (Figure 7B-C). Additional antitumor effect was provided by the NK cells, as indicated by the lower BLI signal of the Tcon + NK group at the peak time point of tumor burden (P < .05; Figure 7C). Treatment with NK cells without T cells retarded tumor growth, but all animals in this group ultimately died of tumor progression (Figure 7B). These results show that despite targeting alloreactive T cells and reducing GVHD, the GVT effect is intact and maintained in the presence of NK cells.
NK cells maintain GVT response to A20. (A) Ventral BLI images of animals that received a transplant of TCD-BM plus A20-luc+/yfp, A20-luc+/yfp plus Tcons, or A20-luc+/yfp plus Tcons and NK cells. Both Tcon and Tcon + NK groups reject the tumor within 2 weeks after transplantation. (B) Quantification of BLI images from panel A. Compared with A20 controls, Tcon and Tcon + NK groups have significantly lower BLI signal. NK cells alone provide some antitumor capability but fail to clear the tumor. (C) BLI quantification shown without the A20 control. TCD-BM–only recipients are shown as controls. Tcons + NK have significantly lower BLI at day 4 after T-cell transplantation (P < .05). Data points are average BLI from 4 to 5 mice, and are representative of 2 experiments. Error bars represent SE.
NK cells maintain GVT response to A20. (A) Ventral BLI images of animals that received a transplant of TCD-BM plus A20-luc+/yfp, A20-luc+/yfp plus Tcons, or A20-luc+/yfp plus Tcons and NK cells. Both Tcon and Tcon + NK groups reject the tumor within 2 weeks after transplantation. (B) Quantification of BLI images from panel A. Compared with A20 controls, Tcon and Tcon + NK groups have significantly lower BLI signal. NK cells alone provide some antitumor capability but fail to clear the tumor. (C) BLI quantification shown without the A20 control. TCD-BM–only recipients are shown as controls. Tcons + NK have significantly lower BLI at day 4 after T-cell transplantation (P < .05). Data points are average BLI from 4 to 5 mice, and are representative of 2 experiments. Error bars represent SE.
Discussion
Our results demonstrate a novel mechanism by which donor, allogeneic NK cells can reduce GVHD and maintain GVT in a murine model of allogeneic bone marrow transplantation. We show that donor NK cells decrease T cell proliferation and activation and cytokine secretion, and directly lyse donor T cells, a remarkable observation as the 2 cell populations are syngeneic. We demonstrate that NK cells use the effector molecules perforin and FasL, with potential involvement of the activating receptor NKG2D, to reduce the donor Tcon population. Thus, NK cells can regulate the adaptive arm of the immune response. This study corroborates work by Rabinovich et al11 and Cerboni et al,12 who both showed in vitro susceptibility of murine and human T cells, respectively, to NKG2D-mediated NK cell lysis from syngeneic NK cells. Our work demonstrates this phenomenon in an in vivo model of T cell–mediated GVHD. Other studies have demonstrated a regulatory role for NK cells: these include regulating CD4+ T cells in a model of colitis,31 and regulating experimental autoimmune encephalomyelitis at both the initiation and T-cell effector phases, recently shown to be through the direct killing of syngeneic, myelin antigen–specific T cells.32,33
We demonstrate prolonged survival and decreased GVHD among animals treated with allogeneic NK cells. However, GVHD is not completely abrogated at the given dose of donor NK and T cells. There appears to be an early time window during which NK cells have a beneficial effect in reducing GVHD. Experiments in which Tcon + NK mice were treated with an additional dose of activated donor NK cells 7 days after the initial injection did not demonstrate significantly improved survival (data not shown). In fact, some groups have shown that administration of NK cells after GVHD induction can exacerbate GVHD,4 which is consistent with data showing that IFN-γ can prevent GVHD when given early after BMT, but can exacerbate GVHD when given after GVHD induction.34 Previous work from our laboratory has shown an absence of NKG2D ligands on GVHD target tissues after irradiation,35 representing one explanation for the lack of tissue damage in target tissues by NK cells even though they traffic to these tissues.
We demonstrate a direct interaction between NK cells and activated T cells, where NK cells are capable of directly lysing donor T cells, resulting in depletion of proliferating, activated, IFN-γ–secreting donor Tcons. However, other mechanisms may be involved, as Trivedi et al have shown that rat bone marrow–derived NK cells can inhibit T-cell proliferation by up-regulating the cell cycle inhibitor p21.36 Therefore, both of these NK cell–mediated mechanisms are possibly contributing to the observed effects. Fewer T cells expressing CD25 could be due to decreased proliferation of this subset, or direct lysis of the CD25+ activated T cell subset.
Interestingly, our data indicate that NK cells do have an impact on donor Tregs, with an increased percentage of Tregs and increased ratio of Tregs to total donor Tcons. This is highly relevant information in this GVHD model as Tregs have previously been shown to decrease GVHD while maintaining GVT effects, and may be an important component of the mechanism of suppression of GVHD in vivo.37 Future experiments will aim to dissect this interaction more fully. In addition, although these experiments were performed with 2 different strains of donor mice (C57Bl/6 and FVB), Balb/c recipients were used in both settings. Thus, studies in additional donor-recipient strain combinations are necessary to determine whether these data can be applied more widely to BMT settings.
The addition of NK cells resulted in a reduction in donor T cells secreting IFN-γ at day 3. NK cell regulation of CD4+ and CD8+ T cell IFN-γ secretion has also been shown in a model of murine cytomegalovirus.38 The effect we observed on donor Tcon cytokine secretion was seen primarily at this early time point (day 3) after transplantation during the initiation phase of GVHD induction. Therefore, this correlates with previous data from our laboratory indicating that allogeneic NK cells proliferate primarily in the first week after transplantation, and their persistence in vivo is markedly shorter than donor T cells. Interestingly, although both the CD4+ and CD8+ T cell subsets showed up-regulation of the NKG2D ligand Rae1γ, NK cells had a more pronounced impact on proliferation, CD25 expression, cytokine secretion, and absolute number cell counts in the CD4+ donor T cell subset compared with the donor CD8+ T cell subset. Therefore, it seems likely that additional mechanisms are at play.
The NK cell effector molecules FasL and perforin are both shown to play a role in the reduction of the donor Tcon population, demonstrating effector function redundancy. As expected, neither effector molecule was solely responsible for the reduction of BLI signal. As evidenced by these data, it is most likely that several additive NK cell–mediated mechanisms contribute to the reduction of Tcons. Our in vitro data also suggest a potential role for the activating receptor NKG2D in addition to these NK cell effector molecules in reduction of the donor Tcon population. Direct cellular lysis provides critical evidence that the increase in T cell apoptosis is directly induced by NK cells, rather than an absence of antigen-presenting cells, although the latter mechanism was not specifically investigated in this model. Therefore, this represents an additional, rather than exclusive, mechanism by which NK cells suppress the T cell response in GVHD.
In this model, we did not specifically address the role of Ly49 receptors in missing self-recognition. Donor NK cells should be tolerant to “self” and hence inhibited from lysing syngeneic T cells. We hypothesize that expression of an NKG2D ligand in the inflammatory milieu after transplantation tips the balance of NK signaling in favor of the activating signal coming from NKG2D rather than an inhibitory signal from the Ly49 receptors, rendering these cells susceptible to NK cell–mediated lysis. A recent publication by Regunathan et al demonstrated that expression of the activating NKG2D receptor ligand H60 could overcome inhibitory signals from the receptor Ly49C/I.39 In our model, NK cells isolated from C57 donors express Ly49C/I and are inhibited by H-2b, expressed on donor Tcons also isolated from C57Bl/6 donors. Although we have identified expression of a different NKG2D ligand (Rae1γ), it is thus possible that NKG2D activating signals can overcome inhibitory signals that may be recognizing “self” in the setting of BMT.
Importantly, donor T cell–mediated GVT effects were maintained in the presence of donor NK cells. As the Tcon + NK mice have significantly reduced GVHD but still show some symptoms of the disease, it is possible that NK cells are lysing a percentage of the total donor Tcon population, leaving a percentage capable of mediating the GVT effect. Alternatively, the activated donor Tcons susceptible to NK cell–mediated lysis may represent a population distinct from those donor Tcons that mediate the GVT effect. Furthermore, the A20 tumor cell line used in our model highly expresses NKG2D ligands13 and is therefore itself a target for NK cell lysis. The slightly enhanced NK cell–mediated antitumor effect seen in the Tcon + NK group is likely due to this NKG2D ligand expression.40
In summary, our data demonstrate a novel mechanism by which allogeneic NK cells reduce GVHD while maintaining GVT effects, supporting the ongoing efforts to use adoptive infusions of allogeneic NK cells in clinical allogeneic bone marrow transplantation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank all members of the Negrin laboratory for helpful discussions and technical assistance.
This work was supported by National Institutes of Health grants R01 CA125276 and P01 CA049605.
National Institutes of Health
Authorship
Contribution: J.A.O. designed and performed research, analyzed data, and wrote the paper; D.B.L.-G. and S.G. performed research and helped write the paper; J.B. designed and performed research and helped write the paper; A.B. contributed vital new reagents, contributed ideas, and helped write the paper; and R.S.N. designed experiments, provided overall guidance, and helped write the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for J.A.O. is Department of Laboratory Medicine and Pathology, Center for Immunology, University of Minnesota, Minneapolis, MN.
Correspondence: Robert S. Negrin, Center for Clinical Science Research Bldg, Rm 2205, 269 W Campus Dr, Stanford University, Stanford, CA 94305; e-mail: negrs@stanford.edu.
References
Author notes
D.B.L.-G. and S.G. contributed equally to this study.