Abstract
The immunoglobulin heavy chain locus (IgH) undergoes multiple changes along B-cell differentiation. In progenitor B cells, V(D)J assembly allows expression of μ heavy chains. In mature B cells, class switch recombination may replace the expressed constant (C)μ gene with a downstream CH gene. Finally, plasma cell differentiation strongly boosts IgH transcription. How the multiple IgH transcriptional enhancers tune these changes is unclear. Here we demonstrate that deletion of the whole IgH 3′ regulatory region (3′RR) allows normal maturation until the stage of IgM/IgD expressing lymphocytes, but nearly abrogates class switch recombination to all CH genes. Although plasma cell numbers are unaffected, we reveal the role of the 3′RR into the transcriptional burst normally associated with plasma cell differentiation. Our study shows that transcriptional changes and recombinations occurring after antigen-encounter appear mainly controlled by the 3′RR working as a single functional unit.
Introduction
The immunoglobulin heavy chain locus (IgH) undergoes multiple changes along B-cell differentiation, affecting transcription and accessibility to V(D)J or class switch recombination (CSR). The iEμ enhancer upstream of Cμ mostly promotes V(D)J recombination,1 whereas IgH 3′RR enhancers (hs3a, hs1,2, hs3b, and hs4) have controversial roles. A role in CSR was suggested by replacing mouse hs1,2 with a neomycin resistance gene, thus affecting germline transcription and CSR to γ2a, γ2b, γ3, and ϵ.2 However, deletion of this neo cassette restored CSR.3 hs3a, hs3b, and hs4 also proved individually dispensable for CSR.3-5 Enhancer redundancies might explain that their individual deletion only results in minor effect. Indeed, joint hs3b/hs4 deletion impaired germline transcription and CSR to most isotypes except μ and γ1.6 Reporter genes also demonstrated synergies between 3′RR enhancers,7 which altogether promote CSR into large transgenes.8 The 3′RR is followed with DNase hypersensitive sites (hs5-7) lacking enhancer activity but binding CCCTC-binding factor and constituting the 3′ locus boundary.9 To reconcile the controversial phenotypes of focal mutations, potentially attenuated by functional redundancies, we evaluated IgH expression and CSR after deleting the whole 30-kb extent of the 3′RR.
Methods
Experimental details are available in the supplemental data (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Studies have been reviewed and approved by Centre National de la Recherche Scientifique and University review committee. Generation of 3′RR-deficient mice is described in supplemental Figure 1. B-cell compartments, B-cell proliferation and apoptosis, and CSR were investigated by flow cytometry.4,5,10 Germline and hybrid transcripts were detected by reverse-transcribed polymerase chain reaction (RT-PCR).6 Amounts of the various Ig classes were assessed by enzyme-linked immunosorbent assay.4-6 Mice immunizations were as reported.11
Results and discussion
Generation of 3′RR-deficient mice
The gene-targeting vector replaced the 30-kb genomic fragment encompassing the 3′RR with a loxP/neoR cassette. Neo mutant mice allowed the derivation of 3′RR-deficient animals after cre-deletion of neo (supplemental Figure 1).
B-cell development and activation in 3′RR-deficient mice
In contrast to the iEμ deletion impairing B-cell differentiation,1 progenitor and peripheral B-cell compartments appeared normal in 3′RR-deficient animals (supplemental Figure 2). Surface IgM was normal, contrary to hs4- and hs3b/hs4-deficient mice,5,6 suggesting that the complete deletion might remove some inhibitory elements left after the hs4 deletion. Lipopolysaccharide (LPS) or anti-CD40-induced proliferation, and apoptosis were not affected either (supplemental Figure 3).
Ig synthesis in 3′RR-deficient mice
After in vitro stimulation with LPS and appropriate cytokines, 3′RR-deficient B cells showed an 85% reduced IgM secretion and more than or equal to 99% decrease for other isotypes (Table 1). This was not related to a defect in plasma cell maturation because amounts of Blimp/Xbp1 transcripts and CD138+ plasmablasts after 3 days in vitro LPS activation were normal (supplemental Figure 3). Similar amounts of CD138+ cells in spleen of 3′RR-deficient mice and wild-type animals suggested that the deletion did not impede plasma cell differentiation (supplemental Figure 2), whereas μ IgH transcripts were globally decreased in splenocytes from 3′RR-deficient mice (supplemental Figure 4). Probably because of variable half-lives, serum Ig levels were heterogeneously affected, most strongly lowered, whereas IgG1 and IgG2a were preserved (Table 1). However, investigation of specific antibody levels on challenge with thymus-dependent (ovalbumin) or -independent (DNP-Ficoll) antigens confirmed strong IgM reduction, whereas all 4 IgG subclass antibodies were undetectable (supplemental Figures 5-6).
Hybrid and germline transcripts in 3′RR-deficient mice
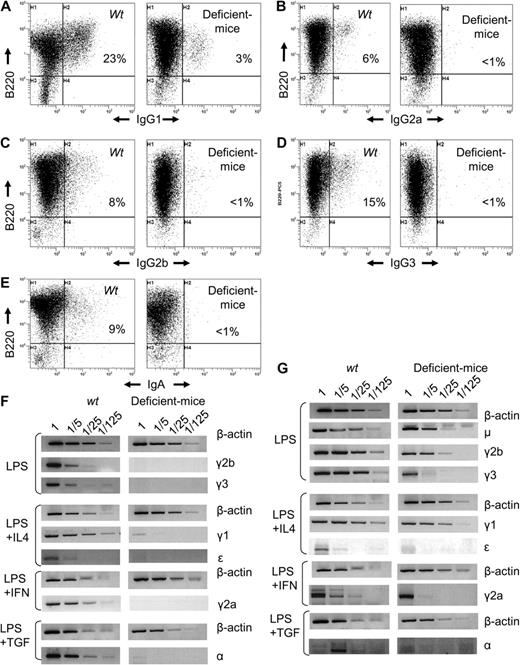
Overall, 3′RR-deficient mice exhibited a globally decreased Ig secretion, culminating in a blockade for class-switched isotypes. To determine whether this resulted from decreased CSR or only from decreased expression of class-switched genes, we appreciated the number of cells switching to a particular isotype after in vitro stimulation. 3′RR-deficient mice splenocytes showed decreased switching to all isotypes (Figure 1A-E). Parallel quantification of Iμ-Cx hybrid transcripts (x being any constant gene) showed a more than 100-fold decrease of Iμ-Cγ2b and Iμ-Cγ3 transcripts in LPS-treated splenocytes, of Iμ-Cϵ and Iμ-Cγ1 on LPS/IL-4 stimulation, of Iμ-Cα on LPS/TGF-β-stimulation, and of Iμ-Cγ2a on LPS/interferon-γ stimulation (Figure 1F). 3′RR-deficient mice thus exhibited a severe blockade of CSR. Germline transcription of CH gene, a known prerequisite of CSR, finally showed a more than 5-fold decrease of Iμ-Cμ, Iγ1-Cγ1, Iγ2a-Cγ2a, Iγ2b-Cγ2b, and a more than 25-fold decrease of Iγ3-Cγ3, Iϵ-Cϵ, and Iα-Cα (Figure 1G). Although semiquantitative RT-PCR may be imperfect and although its extent varied between isotypes, a global decrease of germline transcription was observed and did not spare μ and γ1 as in hs3b/hs4-deficient mice.6 Interestingly, and by contrast to previous focal alterations of the 3′RR, the Iμ-Cμ transcription unit, including the donor Sμ region, was also affected. Confirming CSR defect, excised circle transcripts were lowered in 3′RR-deficient mice compared with wild-type animals (supplemental Figure 7).
Altered class-switch recombination in IgH 3′RR-deficient mice. B cells were cultured for 4 days with LPS plus or minus cytokines at 1 × 106 cells/mL and stained with anti-B220-PC5 and anti-isotype fluorescein isothiocyanate antibodies: anti-IgG1 (A), anti-IgG2a (B), anti-IgG2b (C), anti-IgG3 (D), and anti-IgA (E). Representative results from 6 IgH 3′RR-deficient mice and wild-type mice are shown. (F) Iμ-Cx hybrid transcripts (x being any constant gene after CSR) induced in stimulated splenocytes from wild-type and IgH 3′RR-deficient mice. Total RNA was isolated on day 3. RT-PCR for Iμ → γ2b, Iμ → γ3 was performed on LPS-induced splenocytes RNA; Iμ → γ1 and Iμ → ϵ on LPS plus IL4, Iμ → α on LPS plus TGF-β; and Iμ → γ2a on LPS plus interferon-γ. Fivefold dilutions of cDNA were used per assay. β-Actin amplification was used as control. (G) Germinal transcription in stimulated splenocytes from wild-type and IgH 3′RR-deficient mice. Total RNA was isolated on day 3. RT-PCR for germline μ, γ2b, γ3 transcripts was performed on LPS-induced splenocytes RNA; germline γ1 and ϵ on LPS plus IL4; and germline α on LPS plus TGF-β. Fivefold dilutions of cDNA were used per assay. β-Actin.
Altered class-switch recombination in IgH 3′RR-deficient mice. B cells were cultured for 4 days with LPS plus or minus cytokines at 1 × 106 cells/mL and stained with anti-B220-PC5 and anti-isotype fluorescein isothiocyanate antibodies: anti-IgG1 (A), anti-IgG2a (B), anti-IgG2b (C), anti-IgG3 (D), and anti-IgA (E). Representative results from 6 IgH 3′RR-deficient mice and wild-type mice are shown. (F) Iμ-Cx hybrid transcripts (x being any constant gene after CSR) induced in stimulated splenocytes from wild-type and IgH 3′RR-deficient mice. Total RNA was isolated on day 3. RT-PCR for Iμ → γ2b, Iμ → γ3 was performed on LPS-induced splenocytes RNA; Iμ → γ1 and Iμ → ϵ on LPS plus IL4, Iμ → α on LPS plus TGF-β; and Iμ → γ2a on LPS plus interferon-γ. Fivefold dilutions of cDNA were used per assay. β-Actin amplification was used as control. (G) Germinal transcription in stimulated splenocytes from wild-type and IgH 3′RR-deficient mice. Total RNA was isolated on day 3. RT-PCR for germline μ, γ2b, γ3 transcripts was performed on LPS-induced splenocytes RNA; germline γ1 and ϵ on LPS plus IL4; and germline α on LPS plus TGF-β. Fivefold dilutions of cDNA were used per assay. β-Actin.
In conclusion, there have been several previous focal genomic alterations of the 3′RR.2-5 We generated mice lacking the whole 3′RR, thus extending and clarifying several findings from these previous models. Mice developed normally abundant B cells in bone marrow, spleen, and blood, in agreement with the concept that 3′IgH enhancers play their most crucial role at late stages of B-cell maturation. Functional redundancies of 3′RR enhancers have long kept their role quite elusive. The complete 3′RR deletion dramatically affects CSR and Ig secretion for all isotypes. Hybrid transcripts from class switched loci were more strongly reduced than germline transcripts, suggesting that the 3′RR promotes CSR not only by fostering germline transcription. BAC transgene models also showed the 3′RR to control CSR, except to the Cα gene.8 3′RR-deficient mice demonstrate that only Cγ1 transcription and CSR are partly independent from this control. Similar observations were made after deletion of the distal part of the 3′RR region (hs3b/hs4),6 altogether suggesting that elements other than the 3′RR (such as the hs5-7 sites lying downstream?) are supported by γ1 transcription. As suggested by Wuerffel et al,12 Eμ enhancer might also contribute. How the 3′RR affects CSR can be inferred from 3C experiments showing that S-S synapsis was promoted by the close spatial proximity of Eμ and 3′RR elements forming a unique chromosomal loop configuration.12,13 The germline transcript promoter association with the Eμ-3′RR complex might create an architectural scaffolding, promoting S-S synapsis and further CSR.12 Although Eμ appeared as dispensable, integrity of the 3′RR was mandatory for such conformations of the locus as judged from experiments with B cells lacking the 2 distal 3′RR enhancers.12 Although plasma cells normally differentiated in 3′RR-deficient animals, the IgH defect resulted in depressed secretion of all Ig, including IgM. Although Cμ is more than or equal to 200 kb upstream of the 3′RR, Cμ transcripts severely dropped. Finally, the 3′RR deletion strongly alters CSR and afflicts expression of all IgH genes in plasma cells, including Cμ. The remaining IgH expression, CSR, and γ1 germline transcription suggest that additional elements cooperate with the 3′RR to stimulate IX transcription in activated B cells and pVH transcription in plasma cells. The iEμ enhancer is a suitable candidate because its deletion slightly impacts CSR and Ig synthesis1 and because physical interactions have been documented between Eμ and the 3′RR.12,13 Double mutant lacking both Eμ and the 3′RR would be the best tool for addressing this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank S. Desforges and B. Remerand for help with animal care.
This work was supported by Ligue Contre le Cancer (comité départemental de la Haute-Vienne et de la Corrèze), Conseil Régional du Limousin, and Lions Club de la Corrèze, Zone 33 district 103 Sud. C.V.-F. was supported by Association pour la Recherche sur le Cancer.
Authorship
Contribution: C.V.-F., R.F., V.T., E.P., and Y.D. actively participated in the experimental design of the study (vector construction, embryonic stem targeting, embryonic stem screening, cell proliferation analysis, apoptose analysis, flow cytometric analysis, Ig measurements, CSR experiments, RT-PCR analysis); M.C. and Y.D. participated in the scientific discussion for manuscript writing and obtained financial grants and agreement of the ethics committee of our institution to perform the study; and N.C. generated transgenic animals and played a key role in genotyping mice.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michel Cogné, Centre National de la Recherche Scientifique Unité Mixte de Recherche 6101, Laboratoire d'Immunologie, 2 rue du Dr Marcland, 87025 Limoges Cedex, France; e-mail: cogne@unilim.fr; or Yves Denizot, Centre National de la Recherche Scientifique Unité Mixte de Recherche 6101, Laboratoire d'Immunologie, 2 rue du Dr Marcland, 87025 Limoges Cedex, France; e-mail: yves.denizot@unilim.fr.