Abstract
During infection, chemokines sequestered on endothelium induce recruitment of circulating leukocytes into the tissue where they chemotax along chemokine gradients toward the afflicted site. The aim of this in vivo study was to determine whether a chemokine gradient was formed intravascularly and influenced intraluminal neutrophil crawling and transmigration. A chemokine gradient was induced by placing a macrophage inflammatory protein-2 (MIP-2)–containing (CXCL2) gel on the cremaster muscle of anesthetized wild-type mice or heparanase-overexpressing transgenic mice (hpa-tg) with truncated heparan sulfate (HS) side chains. Neutrophil-endothelial interactions were visualized by intravital microscopy and chemokine gradients detected by confocal microscopy. Localized extravascular chemokine release (MIP-2 gel) induced directed neutrophil crawling along a chemotactic gradient immobilized on the endothelium and accelerated their recruitment into the target tissue compared with homogeneous extravascular chemokine concentration (MIP-2 superfusion). Endothelial chemokine sequestration occurred exclusively in venules and was HS-dependent, and neutrophils in hpa-tg mice exhibited random crawling. Despite similar numbers of adherent neutrophils in hpa-tg and wild-type mice, the altered crawling in hpa-tg mice was translated into decreased number of emigrated neutrophils and ultimately decreased the ability to clear bacterial infections. In conclusion, an intravascular chemokine gradient sequestered by endothelial HS effectively directs crawling leukocytes toward transmigration loci close to the infection site.
Introduction
Chemokine-induced recruitment of circulating leukocytes is fundamental in the immune response to bacterial infections. The leukocyte recruitment cascade is initiated by endothelial cell activation and presentation of chemokines to rolling leukocytes, which, by activating leukocyte integrins, results in leukocyte adhesion to and diapedesis through the vessel wall.1-3 Recently, an additional step in the leukocyte recruitment cascade was detected bridging adhesion and diapedesis, namely, Mac-1–mediated intraluminal crawling.4-7 In these studies, neutrophils were observed to crawl on endothelium in all directions before transmigration through endothelial junctions; and if crawling were disabled, diapedesis was delayed and occurred preferentially through the transcellular pathway.4,5
However, chemokine presence on endothelium is not enough to initiate leukocyte diapedesis; a chemotactic gradient over the vessel wall with a higher extravascular concentration is required.8 Outside the vasculature, leukocytes chemotax along a chemical gradient in the extracellular matrix toward the chemokine source.9,10 During infection, a multitude of chemotactic factors are present simultaneously, and the ability of leukocytes to prioritize between end-target (bacterial peptides) and intermediate (eg, macrophage inflammatory protein-2 [MIP-2]) chemotactic cues is crucial for leukocytes to find the site of infection.11 However, although interstitial leukocyte chemotaxis along a chemotactic gradient has been extensively studied in vitro and in vivo, it is not known whether a chemotactic gradient is established on endothelium within the vasculature. Intraluminal crawling has so far only been investigated during chemokine superfusion, which results in homogeneous extravascular chemokine concentrations.4,5 Whether localized chemokine release influences the direction of intraluminal leukocyte crawling by establishing an intravascular chemokine gradient has not yet been studied.
Chemokines have been suggested to be immobilized on the luminal endothelial cells by heparan sulfate proteoglycans (HSPGs). HSPGs consist of complex polysaccharide chains that are negatively charged (heparan sulfate [HS]) and conjugated to a protein core. HSPGs are expressed on the surface of most cell types but are also secreted and deposited into the extracellular matrix.12 The main HSPGs species expressed by endothelial cells are syndecans, which constitute 50% to 90% of the total endothelial proteoglycans.13,14 Chemokines and a variety of positively charged proteins bind to HS chains through specific and/or electrostatic interactions.12,15-17 During inflammation, endothelial HSPGs serve as ligands to L-selectin on leukocytes, transport chemokines in a basolateral to apical direction across the endothelium, as well as present chemokines at the luminal surface of the endothelium to circulating cells.1,14,18,19 Heparanase is an endo-β-D-glucuronidase responsible for endoglycosidic cleavage of HS. Transgenic overexpression of this enzyme in mice results in structurally modified and significantly shorter HS side chains20 with reduced ability for binding ligands.21
The aim of this in vivo study was to determine whether localized extravascular release of chemokines influences the direction of intraluminal crawling of neutrophils through creation of an endothelial chemokine gradient and whether this gradient is sequestered on endothelial HS. We found that a majority of intravascular neutrophils crawled toward and transmigrated closer to a chemokine-releasing gel that was placed beside the vessel. This directional crawling was absent in heparanase-transgenic (hpa-tg) mice, which expressed shorter HS chains because of overexpression of heparanase, the predominant HS-degrading enzyme. This resulted in random crawling and decreased leukocyte recruitment in the hpa-tg versus wild-type (WT) mice and ultimately a severely reduced ability to clear a bacterial infection. We therefore conclude that a chemokine gradient is formed along HS on the endothelium and that this intravascular gradient effectively directs crawling leukocytes toward transmigration sites closer to the site of infection.
Methods
Animals
WT (B&K Universal), hpa-tg,22 and CX3CR1-GFP (The Jackson Laboratory) mice on C57BL/6 background weighing between 20 and 30 g were used. All procedures were approved by the Uppsala University Ethical Committee for Animal Experiments.
Exposure of cremaster muscle
Mice were anesthetized by spontaneous inhalation of isoflurane (Abbott Scandinavia). The left cremaster muscle was exposed and mounted for intravital microscopic observation of leukocytes in the cremasteric microcirculation and adjacent tissue.4,9,25,26 The muscle was continuously superfused, with a prewarmed (37°C) bicarbonate-buffered saline solution (pH 7.4).
Induction of chemotaxis by MIP-2 superfusion
Recombinant murine MIP-2 (CXCL2, R&D Systems) was added to the bicarbonate buffer to induce nondirectional neutrophil recruitment in the cremaster preparation in a concentration (0.5nM) previously shown to recruit optimal numbers of crawling leukocytes to enable visualization and tracking of each crawling leukocyte within the observed venule.4,5
Induction of chemotaxis by a gradient of chemoattractant
This method has previously been described.9,24,26 In brief, an agarose gel (2% in Hanks balanced salt solution, Sigma-Aldrich) containing MIP-2 (0.5μM), keratinocyte-derived chemokine (KC, CXCL1, 5.2μM, R&D Systems), or WKYMVm (synthetic mimetic of bacterial formyl-methionyl-leucyl-phenylalanine [fMLP], 150μM, Phoenix Europe) was used to induce chemotaxis of leukocytes. The concentration of chemokine in the gel was chosen to attain similar numbers of recruited leukocytes as in the superfusion experiments. Gel without added chemokine failed to induce leukocyte recruitment in this setup (data not shown) as previously described.26 A 1-mm3 piece of the chemokine-loaded gel was placed on the surface of the cremaster using a micromanipulator in a preselected avascular area approximately 400 μm from a postcapillary venule.
Intravital video microscopy
The number of rolling, adherent, and emigrated leukocytes in single unbranched venules (25-40 μm in diameter) before and after addition of the chemokine was determined using video playback analysis.9,24,26 For bright-field imaging, an intravital microscope (Leitz Ortholux II) with a 25×/0.6W (Leitz) or a 40×/0.8W (Carl Zeiss) objective connected to a video camera (Hamamatsu C3077; Apple iMovie HD 6.0.3 acquisition software) was used.
Crawling studies
Images were recorded in time-lapse format (1 frame per second) on the selected section of venule between 30 and 90 minutes after administration of chemoattractant in the superfusate or the gel. Adherent neutrophils and the percentage of cells that started to crawl were calculated. Crawling neutrophils were tracked, and full paths (x-y coordinates), as well as crawling duration were measured.
Intravital and spinning disk fluorescence imaging
MIP-2 conjugated to fluorescein isothiocyanate (FITC; Sigma-Aldrich) was used to induce leukocyte recruitment (1nM for the superfusion; 1μM for the gel). Endothelial cell junctions were labeled with anti-CD31 monoclonal antibody (mAb) (50 μg, clone 390, eBioscience) conjugated to Alexa Fluor 555 (Invitrogen), and neutrophils were visualized with anti–Gr-1 mAb (20 μg, RB6-8C1, eBioscience) conjugated to Alexa Fluor 647 (Invitrogen) or anti-Ly6G mAb (30 μg, 1A8, eBioscience) conjugated to Alexa Fluor 555 administered via the femoral artery. Monocytes were followed in CX3CR1-GFP mice. For in vivo fluorescence detection, either a spinning disk microscope (Olympus BX51 with a 20×/0.95W XLUM Plan Fluor objective, with a Quorum WaveFx spinning disk head, a Hamamatsu C910013 camera and Improvison Volocity 5.2.0 Acquisition software) or an intravital microscope (Leica DM5000B with 20×/0.5W and 40×/0.5W HCS Apo objectives, with a Hamamatsu C10600 camera and Improvison Volocity 5.2.0 Acquisition software) was used.
Ex vivo imaging of cremaster muscles
After in vivo experiments, muscles were incubated in 0.2% Triton X-100, 0.5% bovine serum albumin and 0.5% fetal calf serum in phosphate-buffered saline (PBS) for 2 hours and incubated overnight at 4°C with primary antibodies (anti-FITC, R&D Systems; anti-CD31, anti–Gr-1, or anti–syndecan-1, Invitrogen). Muscles were then incubated for 1.5 hours at room temperature with secondary antibodies conjugated to Alexa Fluor 488, 555, or 647 and mounted in Fluoromount G (Southern Biotechnology). Z-stacks were acquired with Zeiss LSM510 Meta with Plan Apo 10×/0.45 and Plan Fluor 20×/0.75 objectives and Zeiss Zen 2008 AIM software. Control muscles (WT, no chemokine, and no antibodies added) were used as autofluorescence controls.
Intravascular binding of MIP-2
MIP-2 (0.1 μg) conjugated to FITC was administered via the left femoral artery of WT and hpa-tg mice. The chemokine was allowed to circulate for 30 minutes. Chemokine binding to the endothelium of the exposed cremaster muscles was observed by intravital fluorescence microscopy between 30 and 60 minutes after FITC-MIP-2 administration. Intensity of FITC-MIP-2 was quantified from images acquired under constant settings using ImageJ 1.41o (National Institutes of Health). Intravascular MIP-2 was also visualized after intra-arterial injection of chemokine by a subsequent injection of anti-MIP-2 mAb (R&D Systems) conjugated to Alexa Fluor 555. Anti-CD31 mAb conjugated to Alexa Fluor 488 was administered to stain endothelial cell junctions. Fifteen minutes after administration, the muscle was perfused with paraformaldehyde via the femoral artery and dissected for confocal imaging.
In vitro binding assays
The interaction of HS with MIP-2 was assayed using a nitrocellulose filter assay as described earlier.27 Briefly, MIP-2 was incubated with 3H-labeled HS isolated from WT and hpa-tg mice as described.20 Equal mol of HS (50 pmol) from WT and hpa-tg mice or heparin (45 pmol) were mixed with 0.5 μg of MIP-2 in 200 μL of PBS, pH 7.4, and incubated at room temperature for 2 hours. The mixture was passed through a nitrocellulose filter (Sartorius, diameter 25 mm; pore size, 0.45 μm; VWR), followed by washing with 10 mL of PBS. The HS trapped on the filter in a complex with MIP-2 was released by addition of 2M NaCl and measured by scintillation counting. Two or 3 independent assays were performed for each group.
Bacterial clearance
Approximately 106 colony forming units (CFUs) of bioluminescent Staphylococcus aureus Xen29 (Caliper Life Sciences; 10 mg/mL of inert Cytodex beads [Sigma-Aldrich] in PBS used as a carrier) were injected subcutaneously into the back of anesthetized WT and hpa-tg mice. Thirty minutes after inoculation, 2.5 hours after inoculation, and every 24 hours thereafter (for 2 weeks), mice were anesthetized with isoflurane in a bioimaging device (IVIS Spectrum, Caliper Life Sciences) and bioluminescence quantified using Living Image 3.1 software (Caliper Life Sciences). The same settings were used for bioluminescence quantification for all animals and for all time points.11
Statistics
All data are presented as mean plus or minus SEM. Unpaired Student t test or analysis of variance (one-way) with Bonferroni correction were used for comparison between 2 or several groups, respectively. For verifying single variable changes with time and within one group, paired Student t test or analysis of variance (repeated measures) with Bonferroni correction was used. Significance was considered for values of P less than .05.
Results
We investigated how an extravascular chemokine gradient influences intraluminal crawling of neutrophils. To mimic bacterial infection, a chemokine-releasing gel, previously shown to create an extravascular chemokine gradient,9,26 was placed on the cremaster muscle of mice. Intraluminal crawling of neutrophils and chemokine gradients were evaluated by intravital and confocal microscopy.
Directional crawling of neutrophils is induced by a chemotactic gradient on the vascular endothelium
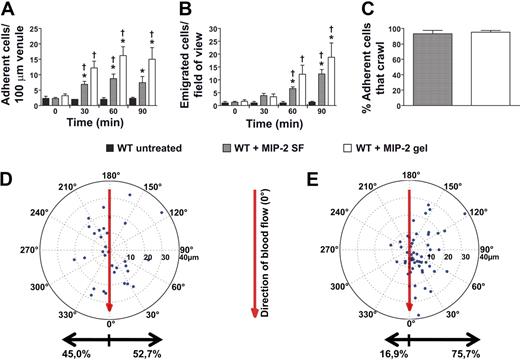
Addition of the chemokine MIP-2 (CXCL2, 0.5nM) to the superfusate or administration of an MIP-2–containing gel (0.5μM) to the cremaster muscle, induced neutrophil recruitment with increased number of adherent and emigrated neutrophils compared with untreated mice (Figure 1A-B; Table 1). The concentration of MIP-2 in the superfusion buffer has previously been used in studies of intraluminal crawling,4,5 and the chemokine concentration in the gel was selected to achieve levels of adherent and emigrated neutrophils comparable with the MIP-2 superfusate (Figure 1A-B). MIP-2 superfusion (90 minutes) was found to recruit 88.1% Ly6G-positive cells, which corresponds to neutrophils, whereas 11.6% were identified as monocytes (CX3CR1-GFP). Analysis of time-lapse recordings of adherent neutrophils within the postcapillary venules revealed that the percentage of adherent neutrophils that started to crawl was similar and approached 100%, regardless of the mode of MIP-2 administration (superfusate or gel, Figure 1C). When crawling displacement was plotted (the center of the circle corresponds to the start of adhesion, and the dots represent the site of transmigration), it became evident that MIP-2 superfusion resulted in equal distribution of crawling neutrophils to each side of the observed venules (Figure 1D). In contrast, the MIP-2 gel clearly influenced crawling directionality as intraluminal crawling occurred predominantly to the side of the venule closest to the MIP-2 gel (76% ± 3% of crawling cells, P < .001, Figure 1E; supplemental Video 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Even though the majority of neutrophils crawled perpendicular to blood flow, a small percentage of cells in each group (7%-8%) was displaced completely with or against the blood flow. Neutrophils crawling toward the MIP-2 gel were displaced on average 14 plus or minus 1 μm before initiating diapedesis. The few neutrophils that crawled away from the gel were not noticeably displaced from where they first started to adhere (8 ± 1 μm). Interestingly, when analyzing the total distance neutrophils crawled before diapedesis, it was evident that the MIP-2 gel caused neutrophils to crawl shorter distances and with lower velocity compared with MIP-2 superfusion (Figure 2). Ultimately, this resulted in a significantly shorter total time between initiation of crawling and transmigration when MIP-2 was administered in the gel compared with in the superfusate (gel, 9 ± 1 minute; superfusion, 15 ± 1 minute, P < .001). This indicates that localized chemokine release accelerates leukocyte recruitment as intraluminal crawling is directed to potential optimal sites for transmigration toward the site of infection.
Localized extravascular chemokine release induces directional intraluminal crawling of neutrophils toward infection site. (A) Number of adherent neutrophils per 100-μm venule and (B) number of emigrated neutrophils per field of view, in untreated (n = 3) WT cremaster muscle or activated by MIP-2 superfusion (n = 7) or an MIP-2-containing gel (n = 5). (C) Percentage of all adherent neutrophils that crawled within the observed vessel section before transmigration (MIP-2 SF, n = 67, 4 mice; MIP-2 gel, n = 100, 5 mice). (D-E) Displacement of crawling neutrophils (with crawling start and endpoints within the field of view) from the adhesion point (the center of the circle) to transmigration site (marked dots) during activation by (D) MIP-2 superfusion (n = 32, 4 mice) or by (E) an MIP-2-containing gel (n = 57, 5 mice) placed at 90 degrees at a distance of 400 μm from the venule. The vertical axis in the plot corresponds to the direction of blood flow (toward 0 degrees). *P < .05 compared with time 0 before addition of chemokine. †P < .05 vs WT untreated.
Localized extravascular chemokine release induces directional intraluminal crawling of neutrophils toward infection site. (A) Number of adherent neutrophils per 100-μm venule and (B) number of emigrated neutrophils per field of view, in untreated (n = 3) WT cremaster muscle or activated by MIP-2 superfusion (n = 7) or an MIP-2-containing gel (n = 5). (C) Percentage of all adherent neutrophils that crawled within the observed vessel section before transmigration (MIP-2 SF, n = 67, 4 mice; MIP-2 gel, n = 100, 5 mice). (D-E) Displacement of crawling neutrophils (with crawling start and endpoints within the field of view) from the adhesion point (the center of the circle) to transmigration site (marked dots) during activation by (D) MIP-2 superfusion (n = 32, 4 mice) or by (E) an MIP-2-containing gel (n = 57, 5 mice) placed at 90 degrees at a distance of 400 μm from the venule. The vertical axis in the plot corresponds to the direction of blood flow (toward 0 degrees). *P < .05 compared with time 0 before addition of chemokine. †P < .05 vs WT untreated.
Localized extravascular chemokine release results in decreased crawling distance and crawling velocity. (A) Actual crawling distance and (B) calculated crawling velocity of neutrophils in response to MIP-2 superfusion (n = 32, 4 mice) or MIP-2–containing gel (n = 57, 5 mice). *P < .05 vs WT activated by MIP-2 superfusion.
Localized extravascular chemokine release results in decreased crawling distance and crawling velocity. (A) Actual crawling distance and (B) calculated crawling velocity of neutrophils in response to MIP-2 superfusion (n = 32, 4 mice) or MIP-2–containing gel (n = 57, 5 mice). *P < .05 vs WT activated by MIP-2 superfusion.
To investigate whether directional intraluminal crawling was MIP-2 specific or if it could be induced by other chemoattractants, the applied gel was loaded with either the chemokine KC (CXCL1) or the analog to the bacterial peptide fMLP, WKYMVm, followed by analysis of crawling directionality. Both chemoattractants resulted in directional intraluminal crawling predominantly toward the ipsilateral side, the side of the vessel facing the gel (61% ± 1% and 68% ± 3%, respectively), suggesting that intravascular gradient formation is a common function for chemotactic molecules, including MIP-2, KC, and WKYMVm.
Chemokines are presented intravascularly by HS on endothelium
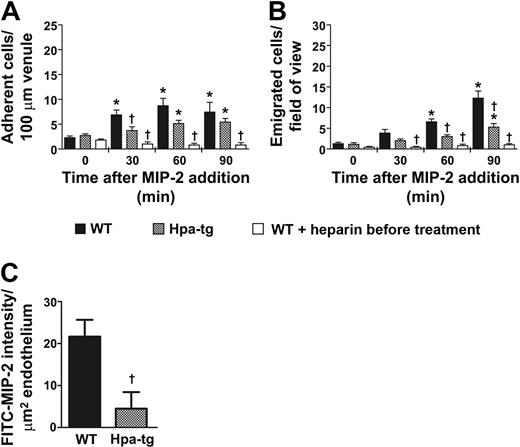
Chemokines have been suggested to bind to HS expressed on endothelial cells.12,16 To determine the role of intravascular HS in chemokine sequestration and leukocyte recruitment, we applied MIP-2 by superfusion (0.5nM) to the cremaster muscle of hpa-tg mice that overexpress heparanase and produce shorter HS side chains.20 A similar effect of MIP-2 on neutrophil rolling and adhesion was observed in WT and hpa-tg mice (Table 1; Figure 3A). However, the number of emigrated cells was decreased by 57% in hpa-tg compared with WT mice after 90 minutes of MIP-2 superfusion (Figure 3B). Despite the observed decrease in emigrated neutrophils in hpa-tg mice, neutrophil recruitment was significantly increased in MIP-2 superfused hpa-tg venules compared with basal levels (time 0, Figure 3A-B) as well as to untreated WT mice (no MIP-2 added to the superfusate, Figure 1A-B, P = .028 and P = .021 for adhesion and emigration at 90 minutes, respectively). Taken together, these results suggest that hpa-tg mice displayed a defect in chemokine sequestration within the postcapillary venules and therefore showed impaired ability to initiate leukocyte diapedesis.
Leukocyte transmigration is dependent on HS sequestration of chemokines on apical venular endothelium. (A) Number of adherent or (B) emigrated neutrophils, in the MIP-2-superfused cremaster muscle of WT (n = 7), hpa-tg mice (n = 7), or in WT pretreated with heparin (0.5 mg; n = 5). (C) In vivo intravascular binding of MIP-2 (expressed as FITC-MIP-2 intensity/ μm2 vessel) 30 minutes after intra-arterial injection of MIP-2 conjugated to FITC in WT and hpa-tg cremasteric venules. *P < .05 compared with time 0 before addition of chemokine. †P < .05 vs WT at the same time period.
Leukocyte transmigration is dependent on HS sequestration of chemokines on apical venular endothelium. (A) Number of adherent or (B) emigrated neutrophils, in the MIP-2-superfused cremaster muscle of WT (n = 7), hpa-tg mice (n = 7), or in WT pretreated with heparin (0.5 mg; n = 5). (C) In vivo intravascular binding of MIP-2 (expressed as FITC-MIP-2 intensity/ μm2 vessel) 30 minutes after intra-arterial injection of MIP-2 conjugated to FITC in WT and hpa-tg cremasteric venules. *P < .05 compared with time 0 before addition of chemokine. †P < .05 vs WT at the same time period.
To investigate whether MIP-2 is sequestered by HS on the endothelium in vivo, HS in WT mice was competitively challenged with close intra-arterial injection of heparin (0.5 mg), an analog of HS with a higher degree of sulfation,28 as we found that heparin-bound MIP-2 with higher affinity than HS in an in vitro assay (data not shown). Heparin pretreatment of WT mice completely prevented the MIP-2-induced leukocyte diapedesis (Figure 3A-B; Table 1), supporting the conclusion that the impaired leukocyte recruitment observed in hpa-tg mice is a result of decreased ability to present chemokines on the endothelium.
Next, we analyzed levels of intravascular chemokine binding in the hpa-tg and WT mice. To enable visualization of intravascularly bound chemokine, MIP-2 was conjugated to the fluorophore FITC. This was followed by close intra-arterial injection of FITC-MIP-2 and anti-CD31 mAb conjugated to Alexa Fluor 555, to the WT cremaster muscle. FITC-MIP-2 was found to be accumulated on venular endothelium, although less binding could be detected in capillaries and arterioles. When this experiment was repeated in hpa-tg mice, much less FITC-MIP-2 could be detected on the venular endothelium compared with WT mice. Quantification of the fluorescent signal in the venules of the 2 mouse strains revealed a 5-fold more FITC-MIP-2 sequestered in WT compared with hpa-tg venules (Figure 3C), in agreement with the observed decrease in emigrated leukocytes (Figure 3B).
Crawling neutrophils follow an intravascular chemokine gradient on HS
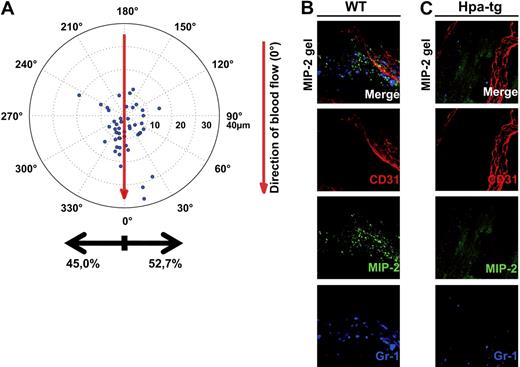
To investigate whether the chemotactic gradient causing directional crawling toward the chemokine source in WT mice was dependent on HS on the vascular endothelium, an MIP-2–containing gel was placed on the exposed cremaster muscle of hpa-tg mice. The crawling neutrophils in hpa-tg cremasteric venules showed no preferred directionality; instead, they started to crawl in apparently random directions after adhesion, resulting in a similar number of cells crawling toward and away from the chemokine gel (Figure 4A). Although the majority of WT neutrophils were displaced toward the gel (Figure 1E), the neutrophils in hpa-tg mice were predominantly displaced with the blood flow and not toward the chemokine gel (Figure 4A). However, no statistically significant difference could be detected for the total distance or crawling velocity between the 2 types of mice (distance: WT 19 ± 2 μm, hpa-tg 22 ± 3 μm; velocity: WT 3 ± 0 μm/s, hpa-tg 3 ± 0 μm/s). The crawling displacement ultimately resulted in more emigrated cells perivascularly on the side of the vessel facing the gel in WT compared with hpa-tg mice (Figure 4B-C). Overall, these results imply that HS play a critical role in sequestering intravascular chemokine gradients during localized extravascular release of chemokines that determines the direction of intraluminal crawling.
Chemokine gradients sequestered by endothelial HS effectively directs crawling leukocytes toward transmigration sites closer to the infection. (A) Displacement of crawling neutrophils (n = 48, 5 mice) from the adhesion point to transmigration site in hpa-tg cremaster muscle activated by an MIP-2-containing gel, placed extravascularly 400 μm from the observed venule (corresponding to 90 degrees in the polar chart). Representative confocal z-projections of (B) WT and (C) hpa-tg cremaster muscle activated with a FITC-MIP-2 (green) loaded gel. Endothelial cell junctions were stained with anti-CD31 mAb (red) and neutrophils with anti–Gr-1 mAb (blue) administered close intra-arterially.
Chemokine gradients sequestered by endothelial HS effectively directs crawling leukocytes toward transmigration sites closer to the infection. (A) Displacement of crawling neutrophils (n = 48, 5 mice) from the adhesion point to transmigration site in hpa-tg cremaster muscle activated by an MIP-2-containing gel, placed extravascularly 400 μm from the observed venule (corresponding to 90 degrees in the polar chart). Representative confocal z-projections of (B) WT and (C) hpa-tg cremaster muscle activated with a FITC-MIP-2 (green) loaded gel. Endothelial cell junctions were stained with anti-CD31 mAb (red) and neutrophils with anti–Gr-1 mAb (blue) administered close intra-arterially.
Venular, but not arterial, endothelium sequester MIP-2, and the chemokine is concentrated in junctional regions
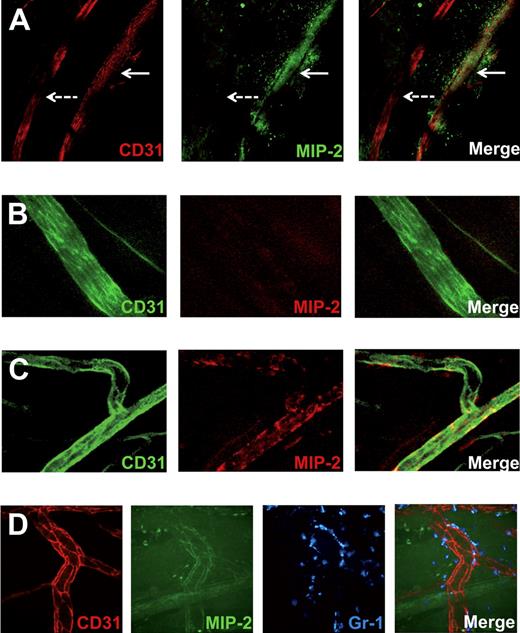
To investigate whether local differences in MIP-2 presentation by the endothelial HS could be detected, FITC-conjugated MIP-2 was added to the gel. To verify that this conjugate is still being transported across the endothelium and can bind CXCR2 on neutrophils, neutrophil recruitment was confirmed in vivo through confocal (Figures 4B, 5D) and by intravital microscopy (data not shown). In hpa-tg mice, a marked decrease in MIP-2 binding compared with WT, both within and outside postcapillary venules, was noted (Figure 4B-C) after application of FITC-MIP-2–loaded gel. Confocal imaging of fixated WT cremaster muscles after exposure to a FITC-MIP-2 gel revealed FITC-MIP-2 binding within postcapillary venules as well as in the adjacent extracellular matrix (Figure 5A). Interestingly, arterioles did not show any MIP-2 binding nor did the perivascular tissue surrounding arterioles (Figure 5A). To further study the differences in arterial and venular chemokine sequestration, MIP-2 was administered into the circulation by close intra-arterial injection followed by injections of antibodies directed toward MIP-2 and CD31, where after the blood was removed and muscle tissue fixated. Confocal microscopy of these muscles revealed that MIP-2 was localized to venular but not arterial endothelium, indicating that HS varies on endothelium of different origin (Figure 5B-C). These observed differences in ability of chemokine sequestration was not the result of different levels of syndecan-1 in arterioles and venules, as demonstrated by anti–syndecan-1 mAb (fluorescence intensity: 57 ± 9 and 60 ± 17 per μm2 endothelium for arterioles and venules, respectively). Using spinning disk confocal in vivo microscopy, FITC-MIP-2 was found to colocalize with anti-CD31 mAb within WT venules, indicating a high junctional MIP-2 sequestration (Figure 5D). This may be the result of junctional transport of MIP-2 from the basolateral to the apical endothelial surface, or simply reflect higher HS concentrations in the junctional regions.
Venular, but not arterial, endothelium sequesters MIP-2, and the chemokine is concentrated in junctional regions. Confocal microscopy images of cremasteric vessels (stained with anti-CD31 mAb). (A) FITC-conjugated MIP-2 (green) administered extravascularly in a gel is sequestered in and around venules (red, continuous arrow) but not in arterioles (red, dashed arrow). (B) MIP-2 administered intra-arterially is not sequestered in arterioles (green), but in (C) venules (green), as visualized by injection of fluorescently labeled anti–MIP-2 mAb (red). (D) Representative in vivo spinning disk confocal images of intravascular sequestration of FITC-labeled MIP-2 (green, administered in a gel) concentrated to endothelial cell junctions (red). Neutrophils were stained with anti–Gr-1 mAb (blue).
Venular, but not arterial, endothelium sequesters MIP-2, and the chemokine is concentrated in junctional regions. Confocal microscopy images of cremasteric vessels (stained with anti-CD31 mAb). (A) FITC-conjugated MIP-2 (green) administered extravascularly in a gel is sequestered in and around venules (red, continuous arrow) but not in arterioles (red, dashed arrow). (B) MIP-2 administered intra-arterially is not sequestered in arterioles (green), but in (C) venules (green), as visualized by injection of fluorescently labeled anti–MIP-2 mAb (red). (D) Representative in vivo spinning disk confocal images of intravascular sequestration of FITC-labeled MIP-2 (green, administered in a gel) concentrated to endothelial cell junctions (red). Neutrophils were stained with anti–Gr-1 mAb (blue).
hpa-tg mice were less efficient in clearing a subcutaneous bacterial infection
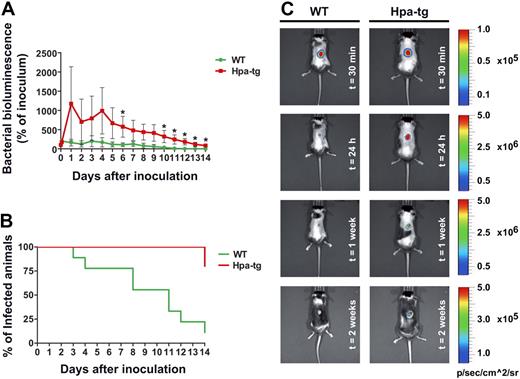
To investigate whether our findings of a defect in chemokine sequestration, nondirectional neutrophil crawling, and ultimately decreased number of emigrated neutrophils in hpa-tg mice would result in a significant defect in immune reaction, we examined bacterial clearance in WT versus hpa-tg mice. Bioluminescent bacteria (S aureus Xen29, 106 CFU) were injected subcutaneously into a shaved back region of WT and hpa-tg mice, and the luminescent area was followed during a period of 2 weeks.11 Already starting on day 1 after infection, the hpa-tg mice showed higher luminescence from the infected area (Figure 6A). Two weeks after infection, the hpa-tg mice still presented 86% of the inoculated amount of bacteria compared with 0% in WT (based on luminescence of the total amount of inoculated bacteria, P = .002, Figure 6A). Although WT mice cleared the infection within a 2-week period, 80% of the hpa-tg mice failed to clear the infection during the same time period (Figure 6B). Representative images of bacterial clearance in WT and hpa-tg mice are presented in Figure 6C.
Random crawling in hpa-tg venules results in a decreased ability to clear bacterial infections. (A) Change in percentage of inoculated bacteria (bacterial bioluminescence) with time in WT (n = 9) and hpa-tg mice (n = 5), after subcutaneous administration of 106 CFU of bioluminescent S aureus (strain Xen 29). *P < .05 vs WT. (B) Clearance rate of S aureus. (C) Representative images of S aureus bioluminescence detection in WT and hpa-tg mice at different time points after inoculation. Please note that the scales differ between time points but are the same at the same time point so that the mice can readily be compared.
Random crawling in hpa-tg venules results in a decreased ability to clear bacterial infections. (A) Change in percentage of inoculated bacteria (bacterial bioluminescence) with time in WT (n = 9) and hpa-tg mice (n = 5), after subcutaneous administration of 106 CFU of bioluminescent S aureus (strain Xen 29). *P < .05 vs WT. (B) Clearance rate of S aureus. (C) Representative images of S aureus bioluminescence detection in WT and hpa-tg mice at different time points after inoculation. Please note that the scales differ between time points but are the same at the same time point so that the mice can readily be compared.
Discussion
It is well established that interstitial leukocytes chemotax along a chemokine gradient toward the site of infection.9,10 This study shows, for the first time, that neutrophils crawling inside blood vessels also chemotax and follow a gradient on the luminal surface of the endothelium. Localized extravascular chemokine release resulted in accelerated leukocyte recruitment from the circulation to the tissue as intraluminal crawling neutrophils were directed toward the chemokine source by a chemotactic gradient immobilized on the luminal endothelium. Establishment of the intravascular gradient was dependent on endothelial HS, and hpa-tg mice, expressing truncated HS side chains, exhibited random intraluminal crawling. Despite similar numbers of adherent neutrophils observed in hpa-tg and WT mice, the altered crawling was translated into decreased number of emigrated cells and ultimately a decreased ability to clear bacterial infections.
Before transmigration, recruited leukocytes were recently observed to crawl a significant distance inside venules.4,5,7,29 The crawling directionality has been suggested to be influenced by both mechanotactic and haptotactic signals because crawling neutrophils in vivo were observed to crawl predominantly perpendicular to the blood flow (mechanotaxis) until a junction was encountered. Crawling directionality was then changed as crawling neutrophils started to follow the junction (haptotaxis).5 The perpendicular crawling was replicated in vitro in the absence of chemokines when shear stress (flow) was applied on randomly crawling neutrophils, supporting the notion that mechanotactic signal(s) affect crawling directionality. However, the aforementioned in vivo observation was made applying homogeneous extravascular chemokine concentrations, a situation that is rarely observed during an established infection. Under experimental conditions, localized chemokine release and exposure of limited venular segments have previously been achieved by applying a chemokine-loaded gel or by microinjection of chemokine(s) close to the observed venule.26,30,31 In the present study, we aimed at mimicking a localized infection by placing a chemokine-releasing gel (MIP-2, CXCL2) on the cremaster muscle of mice at a specific distance from the venule where crawling neutrophils were observed. Indeed, intraluminally crawling neutrophils were directed toward the site of chemokine release and appeared to follow a chemokine gradient along the endothelium. Directional intraluminal crawling was also observed when the gel was loaded with either the chemokine KC (CXCL1) or a synthetic analog of the bacterial peptide fMLP (WKYMVm), indicating that this was not an MIP-2-restricted event. Finally, directional crawling resulted in more leukocytes emigrating in the perivascular tissue at the side of the venule facing the gel. This observation is in accordance with a recent study in which chemokines were applied by microinjection at a distance of 25 to 50 μm from the venule, indicating that an intravascular chemokine gradient exists also when the chemokine is administered by injection.31 Interestingly, the total time of leukocyte recruitment downstream of rolling (adhesion, crawling, and emigration) during localized chemokine release was significantly shorter compared with when the chemokine was added to the superfusate. This indicates that creation of a chemokine gradient by a localized chemokine release influences the speed of leukocyte recruitment from the blood to the tissue by attracting crawling neutrophils to optimal sites for transmigration.
During inflammation, chemokines are produced by activated endothelial cells and leukocytes in the interstitium and must be transported to the luminal surface of endothelial cells for presentation to and activation of rolling leukocytes. Intuitively, chemokines need to be sequestered by the apical surface of the endothelium to avoid being washed away from the site of inflammation by the blood flow. Several in vitro studies using different approaches have shown luminal endothelial immobilization of MIP-1β (immunohistochemistry32 ) and interleukin-8 (IL-8; electron microscopy33 ; immunoprecipitation of human umbilical vein endothelial cells34 ; lung endothelium in vitro19 ). The present study is the first to demonstrate intravascular immobilization of chemokines during in vivo conditions. HS on the endothelial cell surface has been demonstrated to sequester chemokines.19,34 However, the involvement of other endothelial molecules, such as Duffy antigen receptor for chemokines (DARC) in chemokine presentation, cannot be excluded at this point.14,35 DARC deficiency in mice results in down-regulation of CXCR2 on neutrophils because of high levels of circulating chemokines, which precludes studies of neutrophil recruitment by chemokines binding to CXCR2 (eg, KC and MIP-2).36 Yet, normal levels of neutrophils adhered in acute response to the bacterial peptide fMLP in DARC-deficient mice, demonstrating that endothelial DARC is not important in transendothelial transport or sequestration of fMLP.36 In the present study, directed crawling was observed toward the source of a synthetic analog to fMLP, which indicates that the intravascular gradient of bacterial chemoattractant is most probably DARC independent.
To ensure that the directional crawling toward the extravascular chemokine source was indeed induced by intravascular chemokine gradients immobilized by HS, hpa-tg mice were studied compared with WT mice. These mice overexpress heparanase in most tissues, resulting in shorter side chains of HS as well as increased levels of free fragmented HS.20 The free HS fragments were shown to have a higher potency to tether the fibroblast growth factor 2 and its receptor complex.20 However, within the vasculature, free fragmented HS would be washed away by the blood flow, and thus increased binding of chemokines by fragmented HS cannot be expected intravascularly. When FITC-MIP-2 was injected to hpa-tg mice, no staining of arterial and very low staining of venular endothelium were detected, suggesting that shortening of HS side chains on endothelial cells resulting from overexpression of heparanase reduced its capacity to interact with MIP-2. This observation suggests a principal role of HS in endothelial MIP-2 sequestration, although the contribution of other candidate molecules, such as DARC, appears to be limited. This is in agreement with previous studies showing a marked decrease in binding of IL-8 and growth-related gene-α (CXCL1) to the vascular endothelium after heparitinase treatment.33,37 HS has been shown to be a ligand for L-selectin, involved in leukocyte rolling.18,19 A recent study using primary cultured endothelial cells derived from a conditional knockout mouse with selectively deactivated N-acetyl glucosamine N-deacetylase-N-sulfotransferase-1, an enzyme responsible for N-sulfation of HS, demonstrated a significantly higher leukocyte rolling velocity and decreased leukocyte adhesion after tumor necrosis factor-α activation.19 This was attributed to HS functioning as a ligand for L-selectin on leukocytes. In comparison, no alterations in rolling cell velocity were detected in the hpa-tg mice in vivo, possibly because of the previously observed up-regulated sulfation of the truncated HS chains.20 Moreover, the number of adherent neutrophils in response to MIP-2 was similar in the hpa-tg and WT mice, indicating that sufficient amounts of chemokines are presented on the endothelium of the hpa-tg mice to induce integrin activation on leukocytes, resulting in transition from rolling to adhesion and crawling. Yet, the crawling neutrophils could not detect a chemotactic gradient within hpa-tg venules and therefore crawled in random directions. This abnormal crawling resulted in significantly fewer emigrated neutrophils, and the mice exhibited a severe defect in clearing bacterial infection.
Interestingly, MIP-2 could only be detected in postcapillary venules, and no binding was found in arterioles both in hpa-tg and in WT mice. This was not the result of regulated transcytosis or paracellular transport between endothelial cells, as it was also seen when the chemokine was injected into the blood circulation. Even though it is well appreciated that arterial and venular endothelial cells have different functions and meet different challenges, little is known about their diverse appearance. HS has miscellaneous structural features in various tissues and on different cell types.38 HS expressed on arterial and venular endothelium appear to have different structural and/or expression pattern, resulting in different MIP-2-binding properties (Figure 5A-C). This implies that HS varies on endothelium of different origin, accounting for the preferential recruitment of leukocytes occurring in postcapillary venules. In addition, a temporal and spatial control of HS expression on the endothelium may take place and modulate leukocyte recruitment in different organs, thereby contributing to selective leukocyte adherence and transmigration. Indeed, alteration and formation of distinct microenvironments permitting binding and presentation of chemokines within certain tissue compartments have been described during the course of inflammation.39 If the onset of chemokine presentation in discrete areas is the result of altered structure of HS and/or up-regulation of different syndecan expression in the affected region is however not yet known, even though the involvement of syndecan-1 seems to be limited.
HS has also been suggested to be involved in transporting chemokines from the basolateral to the apical surface of endothelial cells. In vitro experiments have detected IL-8 inside endothelial cell caveola, and less luminally sequestered IL-8 was observed after heparitinase treatment or in N-acetyl glucosamine N-deacetylase-N-sulfotransferase-1 null endothelium.19,33 These results, and the observation that no chemokines could be found in the endothelial junctions, led the authors to conclude that chemokines bound to HS are transported in an abluminal-to-luminal direction through transcytosis. However, the methods used in these studies (electron microscopy, cultured endothelium) cannot reveal soluble chemokines passing through junctions. In addition, other studies reported endothelial endocytosis of apically sequestered chemokines to limit inflammation by clearing chemokines from the endothelial surface.40 Thus, the faith of caveolar chemokines is still unknown, as is the route of transport across the endothelial cell layer. In the present study, chemokine binding to endothelium was visualized for the first time in vivo. Decreased binding to the apical endothelial surface after chemokine injection (close intra-arterially), as well as when the chemokine was added extravascularly, could be confirmed in the hpa-tg mice (Figures 3C, 4C). In WT mice, the highest concentration of chemokine was found in the endothelial junctional regions, either reflecting high junctional concentrations of HS or suggesting that the chemokine is transported to these regions paracellularly or longitudinally on the endothelial cell membrane after being transcytosed.
Altogether, our study reveals an intravascular haptotactic chemokine gradient sequestered by HS on endothelial cells. Intraluminal crawling neutrophils follow this gradient, which expedites leukocyte recruitment through efficient diapedesis close to the site of infection.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Monica Eriksson, Petra Gradin, and Sonchita Bagchi for their skilled technical assistance and Björn Petri for excellent advices regarding staining protocols.
This work was supported by the Swedish Medical Research Council (grants 57P-20680, 57X-20675, 72XD-15043, and K2009-67X-21128-01-3), the Swedish Cancer Society (CAN 2008/980, CAN 2009/712), the National Cancer Institute, National Institutes of Health (R01-CA106456), the Royal Swedish Academy of Sciences, Magnus Bergvalls Foundation, the Swedish Society for Medical Research, Clas Groschinskys Foundation, Lars Hiertas Foundation, Harald and Greta Jeanssons Foundation, Åke Wibergs Foundation, and the Wallenberg Foundation.
National Institutes of Health
Authorship
Contribution: S.M. and G.C. designed experiments, performed research, analyzed data, and wrote the paper; E.H. performed research and analyzed data; E.Z. and I.V. contributed with mice and wrote the paper; N.A. designed bacterial experiments; C.R. designed experiments and wrote the paper; J.-P.L. designed experiments, analyzed data, and wrote the paper; and M.P. designed experiments, interpreted data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mia Phillipson, Department of Medical Cell Biology, Uppsala University, PO Box 571, 75123 Uppsala, Sweden; e-mail: mia.phillipson@mcb.uu.se.